Abstract
Blastocystis is a common intestinal parasite of unsettled clinical significance, which is not easily detected by standard parasitological methods. The genus comprises at least 13 subtypes (STs) (which likely represent separate species), 9 of which have been found in humans. Recent data indicate that at least one of the subtypes is associated with intestinal disease. A quantitative TaqMan 5′ nuclease real-time PCR (TaqMan PCR) including an internal process control (IPC) was developed for the detection of Blastocystis and shown to be applicable to genomic DNAs extracted directly from feces. The assay enabled successful amplification of DNAs from all relevant subtypes within the genus (ST1 to ST9). For assay evaluation, 153 samples previously tested by xenic in vitro culture (XIVC) were screened by the TaqMan assay. A total of 49/51 samples positive by XIVC and 13/102 samples negative by XIVC were positive by the TaqMan assay; samples positive by the TaqMan assay and negative by XIVC were subsequently tested by conventional PCR, and amplicons could be identified to the subtype level by sequencing in 69% of the cases. Compared to the TaqMan assay, XIVC had a sensitivity of 79%. This is the first time that a genus-specific, probe-based, internal-process-controlled real-time PCR assay for the detection Blastocystis has been introduced.
INTRODUCTION
Blastocystis is a single-celled intestinal parasite of humans and a vast array of animals. Based on small-subunit (SSU) ribosomal DNA (rDNA) analysis, the genus comprises at least 13 subtypes (STs), 9 of which have been found in humans (29, 32, 34, 36); it is very likely that each subtype represents a separate species (36). In humans, ST3 appears to be the most common subtype, followed in prevalence by ST1, ST2, and ST4 (19, 23, 30–32, 35–38). There apparently is a geographical component to variation in global subtype distribution; for instance, ST4 is rarely reported outside Europe.
Besides Blastocystis being associated with irritable bowel syndrome (IBS) (32), recent data indicate that at least one subtype may be associated with gastrointestinal illness (6, 31). In studies aiming to further explore and clarify the epidemiology and pathogenicity of Blastocystis, accurate identification of carriers and noncarriers in screening situations is essential (34). Recently, various diagnostic methods, including conventional PCR, xenic in vitro culture (XIVC), permanently stained preparations of fixed feces, and microscopy of fecal concentrates, were compared, and PCR and culture were found to be the most sensitive methods and to be almost equally sensitive (30). However, culture results are available only 48 to 72 h after sample submission. Although it involves DNA extraction, molecular detection is faster and enables subsequent subtyping by analysis of sequences obtained from specific PCR products.
The incentive for the application of real-time PCR-based screening platforms in diagnostic parasitology is strong (33). Such assays are advantageous in many ways, primarily due to high specificity and sensitivity and the facts that real-time PCRs are operated in a closed-tube system with minimal risk of contamination and that a cutoff can be set to automatically distinguish positive from negative samples, thus eliminating subjective bias. Only two real-time PCR assays for Blastocystis have been published so far. One targeted an unknown gene and was shown to enable amplification of DNAs from ST1, ST3, and ST4 (11); it is unknown whether the assay enables the detection of Blastocystis strains belonging to other subtypes, and since the gene target is unknown, it is impossible theoretically to determine specificity and sensitivity based on gene copy numbers. Another assay was reported by Poirier et al. (22) and was designed as a genus-specific PCR targeting the SSU rRNA gene, enabling amplification of DNAs from Blastocystis strains belonging to all subtypes so far identified in humans. However, the amplicon was 339 bp long, and generally, significantly shorter amplicons are wanted in diagnostic PCRs to increase sensitivity. Moreover, the assay was based on SYBR green detection of double-stranded DNA and had only 95% specificity. Neither of these two assays included an internal amplification control; for diagnostic PCR assays, testing for potential PCR inhibition in fecal DNA samples that are PCR negative is essential.
The aim of the present study was to design and evaluate a genus-specific TaqMan assay for Blastocystis with an internal amplification control.
(This work was carried out as part of a B.Sc. project performed by Umran Nisar Ahmed [Technical University of Denmark]).
MATERIALS AND METHODS
Primer Design.
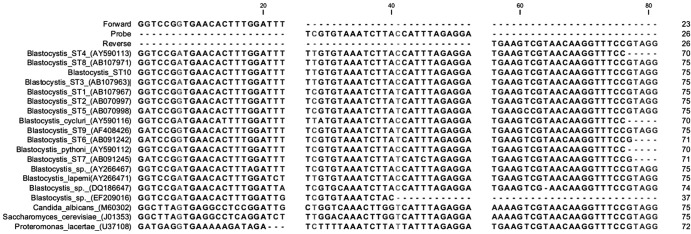
Complete SSU rDNA sequences of Blastocystis sp. ST1 to ST10, other Blastocystis species, and species of taxonomic and differential diagnostic relevance, namely, Proteromonas lacertae, Candida albicans, and Saccharomyces cerevisiae, were aligned (Fig. 1) using MegAlign in DNASTAR (DNASTAR, Madison, WI) and MultAlin (2), and target sequences for genus-specific primer and probes were identified and designed by eye and using Primer Express 2.0 (Applied Biosystems) and Generunner 3.01 (http://www.generunner.net/). All sequences were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/GenBank/), except for the sequence of Blastocystis sp. ST10, which was kindly provided by Graham Clark. Oligonucleotides are shown in Table 1. The forward primer had a mismatch range of 0 to 1 bp in Blastocystis subtypes, 1 to 2 bp in other Blastocystis species, and 10 to 11 bp in non-Blastocystis species. The probe exhibited a mismatch range of 1 to 2 bp in Blastocystis subtypes, 0 to 2 bp in other Blastocystis species, and 4 to 8 bp in non-Blastocystis species. Finally, the reverse primer showed a mismatch range of 0 to 1 bp in Blastocystis subtypes, 0 bp in other Blastocystis species, and 0 to 2 bp in non-Blastocystis species (Fig. 1).
Fig 1.
Alignment of Blastocystis-specific oligonucleotides (forward, probe, and reverse) and SSU rDNAs from Blastocystis sp. ST1 to ST10, other Blastocystis spp., Proteromonas lacertae, Candida albicans, and Saccharomyces cerevisiae. Polymorphic bases are highlighted in gray. Dashes indicate missing or nonexisting bases.
Table 1.
Primers and probes used in the TaqMan assay for amplification of Blastocystis and the internal process control
| Primer or probe | Sequence (5′→3′)a | Nucleotide position |
|---|---|---|
| Primers for TaqMan assay | ||
| Blasto FWD F5 | GGTCCGGTGAACACTTTGGATTT | 1641–1663 in AY244621 sequenceb |
| Blasto R F2 | CCTACGGAAACCTTGTTACGACTTCA | 1734–1759 in AY244621 sequenceb |
| Primers for construction of IPC | ||
| Blasto FWD F5 IPC | GGTCCGGTGAACACTTTGGATTTCCGGGACGTATCATGCT | 13918 in U39284 phage lambda sequence |
| Blasto REV F2 IPC | CCTACGGAAACCTTGTTACGACTTCAACCGCTCAGGCATTTGCT | 14061 in U39284 phage lambda sequence |
| Probes | ||
| Blastocystis probe | FAM-TCGTGTAAATCTTACCATTTAGAGGA-MGBNFQ | 1705–1730 in AY244621b sequence |
| IPC probe | TAMRA-TCCTTCGTGATATCGGACGTTGGCTG–BHQ2 | 14011 in phage lambda sequence |
Boldface corresponds to the phage lambda sequence (GenBank accession number J02459). MGBFQ, minor groove binder and nonfluorescent quencher; and BHQ2, Black Hole Quencher 2 (nonfluorescent quencher).
Sequence may exhibit polymorphism compared to oligonucleotide.
Real-time PCR assay, standard curves, and controls.
To enable the detection of Taq DNA polymerase inhibitors or suboptimal reaction conditions, an internal process control (IPC) was constructed as described previously (7, 8). Briefly, primers for amplification of parts of the phage lambda genome were synthesized with a tail that included the sequence of each of the Blastocystis primers added to the 5′ end of the corresponding phage lambda primer (Table 1). PCR products of 190 bp thus containing the binding sites of the Blastocystis primers were obtained by amplification of 1 ng of purified phage lambda DNA. The amplicons were gel purified, and a 10-fold titration of the IPC was added to separate master mixtures. A dilution of the IPC that had no influence on the cycle threshold (CT) number for purified Blastocystis DNA was used in the assay. The optimum dilution of the IPC was found to be 1 × 10−8. The IPC probe (TAG Copenhagen, Copenhagen, Denmark) was 5′ labeled with 6-carboxytetramethylrhodamine (TAMRA) and quenched with Black Hole Quencher 2 (Table 1). Blastocystis DNA from a strain available in culture was used to generate standard curves.
Real-time PCRs were performed in 50-μl volumes, and the following reagents were used: 1 μM each of the primers (Table 1), 300 nM each of the two probes (Blastocystis and IPC), 5 U/μl Platinum Taq polymerase (Invitrogen, Taastrup, Denmark) in 20 mM Tris-HCl (pH 8.0), 40 mM sodium chloride, 2 mM sodium phosphate, 0.1 mM dithiothreitol (DTT), stabilizers, 50% (vol/vol) glycerol, 10× PCR buffer minus MgCl2 (200 mM Tris [pH 8.4], 500 mM KCl) (Invitrogen, Taastrup, Denmark), 5 mM MgCl2, dUTP mix (12.5 mM dUTP, 50 mM dGTP, 50 mM dATP, 50 mM dCTP), 5 μl of the appropriate dilution of IPC, 50% glycerol, and water. Samples were processed on an ABI 7500 real-time PCR system instrument with a 96-well block (Applied Biosystems, Nærum, Denmark). The PCR profile consisted of 50°C for 1 s, 95°C for 2 min, and 50 cycles of denaturation at 95°C for 15 s followed by annealing and extension at 60°C for 1 min.
Evaluation and validation of real-time PCR.
DNAs from various subtypes (ST1 to ST9) were tested in the assay to enable confirmation of genus sensitivity. Assay sensitivity was determined by testing a 10-fold dilution series of DNA extracted from 1 million Blastocystis organisms isolated from xenic in vitro culture by gradient centrifugation (38) and eluted in 200 μl (Table 2). Hence, 5 μl of DNA from the undiluted sample was equivalent to 25,000 organisms.
Table 2.
Cycle threshold values for the 10-fold dilution row of DNA from 1 million Blastocystis organisms/200 μl elution buffer
| Probe |
CT values for duplicates at dilution ofa: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 × 100 | 1 × 10−1 | 1 × 10−2 | 1 × 10−3 | 1 × 10−4 | 1 × 10−5 | 1 × 10−6 | 1 × 10−7 | 1 × 10−8 | |
| Blasto | 21.90/21.89 | 25.15/25.22 | 28.67/28.67 | 31.04/30.97 | 34.84/35.11 | 36.82/40.91 | 39.21/38.12 | 40.93/UD | UD/UD |
| IPC | UD/UD | UD/UD | UD/47.26 | 36.56/38.64 | 35.17/35.11 | 35.18/35.70 | 36.76/36.03 | 36.70/34.95 | 35.98/37.15 |
DNAs were tested in duplicates to test for reproducibility. UD, undetermined (i.e., signal absent).
The assay was specificity tested against panel dilutions of fungal DNAs from Candida albicans (ATCC 64548), Candida glabrata (ATCC 90030), Candida parapsilosis (ATCC 22019), Candida tropicalis (UKNEQAS 0527), Candida krusei (ATCC 6258), Geotrichum candidum (UKNEQAS 1911), and Saccharomyces cerevisiae (ATCC 8258). DNAs from the following bacterial ATCC strains were also used for specificity testing: Bacillus cereus (ATCC 14579), Bacillus subtilis (ATCC 6633), Campylobacter coli (ATCC 33559), Enterobacter cloacae (ATCC 13047), Escherichia coli (ATCC 25922), and Proteus mirabilis (ATCC 12453). Tested DNAs from non-ATCC bacterial strains represented Aeromonas caviae, Bacteroides fragilis, Campylobacter jejuni, Campylobacter upsaliensis, Citrobacter freundii, Clostridium difficile, Clostridium perfringens, Clostridium sordelli, Hafnia alvei, Klebsiella pneumoniae, Listeria monocytogenes, Plesiomonas shigelloides, Pseudomonas aeruginosa, Salmonella enteritidis, Salmonella paratyphi, Serratia marcescens, Shigella dysenteriae, Shigella flexneri, Staphylococcus aureus, Staphylococcus pyogenes, Vibrio cholerae serotype Ogawa, Vibrio parahaemolyticus, and Yersinia enterocolitica.
DNAs used for diagnostic validation of the real-time PCR assay represented 51 samples positive and 102 samples negative for Blastocystis by XIVC from Danish patients submitting stools for parasitological analysis. Culture analyses had been carried out as previously described using Jones' medium supplemented with 10% horse serum (30, 38). Of the 102 XIVC-negative samples, 42 were positive for Dientamoeba fragilis, 1 for Cryptosporidium, and 1 for Entamoeba dispar by in-house real-time PCR assays used for detection of Giardia, Cryptosporidium, Entamoeba histolytica, E. dispar, and D. fragilis (33), and the prevalence of intestinal parasites among the XIVC-positive samples was comparable.
Genomic DNAs from aliquots of fecal samples tested by XIVC were extracted from fresh fecal samples using the NucliSENS easyMAG protocol (bioMérieux, Herlev, Denmark) according to the recommendation of the manufacturer.
In order to validate results obtained by real-time PCR, samples positive by the TaqMan assay and negative by XIVC were subjected to conventional PCR using primers described by Scicluna et al. (25) and Stensvold et al. (38). All products were sequenced unidirectionally. The obtained nucleotide sequences were assigned to subtypes by using BLAST against the Blastocystis database available at www.pubmlst.org/blastocystis (10, 28).
Cohen's kappa index and comparison of means.
Means and medians of cycle threshold (CT) values were calculated and a two-tailed Student t test for comparison of means carried out using software available at http://qudata.com/online/statcalc/ and http://studentsttest.com/. Cohen's kappa index for intertest agreement was calculated (http://olmosantonio.com/diagnostics/kappa/online/calculator.html).
RESULTS
The TaqMan assay allowed amplification of all subtypes included in the study, and no amplification of fungal or bacterial DNA was detected. DNA from 25,000 parasites per reaction was detected at a CT value of 21.89/21.90 in duplicate determinations, and reproducible CT values were obtained down to a 10−4 dilution, which is equivalent to template DNA from 2.5 parasites per reaction (Table 2). DNA from a lower number of parasites was detectable; however, since the number of SSU rRNA gene copies per cell is not known, the absolute detection level of the PCR cannot currently be ascertained.
Forty-nine samples were positive by both XIVC and the TaqMan assay (Table 3), with CT values ranging from 14.03 to 39.52, a mean CT value of 20.48 (standard deviation [SD], 5.85) and a median CT value of 18.76 (interquartile range [IQR], 17.23 to 21.46). Thirteen samples negative by XIVC were positive by the TaqMan assay (Table 3), with CT values ranging from 16.25 to 40.26, a mean CT value of 28.93 (SD, 4.99) and a median CT value of 29.33 (IQR, 26.02 to 31.89). A comparison of the two means gave a P value of 0.00067, which means that samples negative by XIVC and positive by the TaqMan assay were generally characterized by having a smaller amount of Blastocystis-specific DNA than that present in samples positive by both methods.
Table 3.
Comparison of test results for real-time PCR and XIVC and distribution of Blastocystis subtypes
| Test results | No. of samples with ST: |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | —a | Total | |
| Real-time PCR negative | ||||||
| XIVC negative | 0 | 0 | 0 | 0 | 89 | 89 |
| XIVC positive | 0 | 0 | 0 | 0 | 2 | 2 |
| Total | 0 | 0 | 0 | 0 | 91 | 91 |
| Real-time PCR positive | ||||||
| XIVC negative | 3 | 1 | 5 | 0 | 4 | 13 |
| XIVC positive | 18 | 13 | 11 | 5 | 2 | 49 |
| Total | 21 | 14 | 16 | 5 | 6 | 62 |
| Total | 21 | 14 | 16 | 5 | 97 | 153 |
—, not applicable.
The sensitivity and specificity of XIVC compared to the TaqMan assay were 79% and 98%, respectively. Cohen's kappa index was 0.79, indicating substantial intertest agreement.
The 13 samples positive by real-time PCR and negative by XIVC were tested by conventional PCR and sequencing. Using primers amplifying a product of ∼600 bp (25) or ∼300 bp (38), it was possible to amplify 9/13 samples by conventional PCR (ST1, 3 samples; ST2, 1 sample; and ST3, 5 samples); the mean CT value for the 4 samples not amplifiable by conventional PCR was 33.48.
In total, unambiguous sequences were obtained in 56/62 real-time PCR-positive cases. ST1 was seen in 21 cases, ST2 in 14, ST3 in 16, and ST4 in 5. The mean CT values (SDs) for individual subtypes were 19.33 (3.64) for ST1, 19.76 (6.80) for ST2, 23.52 (6.03) for ST3, and 24.36 (9.06) for ST4. Samples positive for ST1 had lower CT values than samples positive for ST3 (P = 0.022).
The two samples positive by XIVC and negative by real-time PCR tested negative by the conventional PCR after repeated efforts with multiple DNA dilutions. Results from IPC analysis showed that inhibition in these two samples was not an issue.
DISCUSSION
Accurate diagnostic tools are of vital significance in clinical and epidemiological studies of Blastocystis. So far, PCR has been used mostly for characterization purposes (1, 3–6, 12–14, 16–21, 23–27, 29, 31, 32, 35, 37, 40–42), although a few diagnostic PCRs have been published, two of which are based on real-time PCR technology (11, 22).
A major challenge in the development of genus-specific Blastocystis PCRs is the genetic diversity seen within the genus, which limits the number of potential targets in the SSU rRNA gene. The pairwise genetic distance of Blastocystis subtypes amounts to at least 14.8% across the SSU rRNA gene (29), and some conserved regions are likely to be conserved in other genera as well, which hampers identification of oligonucleotide target regions.
Compared to previously published real-time PCR assays (11, 22), the present one has the advantage of probe-based detection, which increases assay specificity. Based on confirmatory sequencing, the TaqMan assay did not produce any false positives, and this is probably due to the fact that primers and probe sequences were highly specific. The real-time PCR assay developed by Poirier et al. (22) generated 8/186 false positives and had a specificity of 95%. Specificity testing of previously published diagnostic PCRs has included testing against other intestinal parasites, such as Entamoeba, Dientamoeba, Giardia, and Cryptosporidium (22), or even bacteria (11), but it is also highly relevant to evaluate the assay against a panel of fungi such as Candida, Geotrichum, and Saccharomyces, which are common components of the fecal flora (9, 15) and which differ from Blastocystis by only about 20% at the SSU rDNA level.
A previous comparison between XIVC and conventional PCR (amplifying 550 bp) revealed a nonsignificant difference in sensitivity in favor of PCR (30). Although the sensitivity of the TaqMan assay is higher than that of the XIVC, it is not immediately comparable to the data presented by Poirier et al. (22), who found that the sensitivity of XVIC was only 53% compared to their SYBR green assay. Importantly, Poirier et al. (22) used Jones' medium supplemented with antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin), while we used Jones' medium without adding antibiotics. It is not unlikely that these antibiotics will indirectly suppress the growth of Blastocystis by a reduction of bacteria, despite the fact that anaerobic chambers were used.
Samples with CT values of ≤35 are most likely indicative of active, ongoing infestation. CT values of >35 possibly represent samples with relatively few Blastocystis organisms, and it could be speculated that there is no active Blastocystis infection going on in the patients from whom those samples came. It is possible that these patients had been exposed to nonviable Blastocystis (detectable by real-time PCR but not by XIVC) or that they were clearing an infection. CT values of >40 may primarily reflect unspecific amplification of a target present in the DNA samples that is of non-Blastocystis origin.
Whether parasite intensity is linked to clinical outcome of Blastocystis infections remains unclear. It is known that Blastocystis shedding exhibits day-to-day variation (39). The present data obtained by real-time PCR analysis confirmed that Blastocystis-positive fecal samples exhibit a range in CT values from 12 to 40. Such a span of CT values likely reflects vast differences in relative parasite load. Using real-time PCR, Poirier et al. (22) did not find any correlation between high intensity and symptoms, but the study was limited with regard to sample size. In the present study, all samples were from patients submitting stools for parasitological analysis due to travel-associated or persistent diarrhea. Future studies should aim to investigate whether differences in symptoms and the severity of these are associated with differences in CT values. If low CT values are associated with diarrhea and/or other symptoms, epidemiological cutoff values could be determined and used in the clinical management of Blastocystis-positive patients.
The overall subtype distribution reflected the usual subtype distribution seen in Danish cohorts (23, 30, 32, 35, 37, 38) and indicates that assay detection is independent of subtype. ST1 samples had lower CT values than ST3 samples, indicating that ST3 infections might be lighter in parasite load. However, larger data sets are needed to confirm this hypothesis and allow speculation on its clinical implications.
The present assay has a built-in IPC, which distinguishes it from previously published PCR assays. In the current evaluation, inhibition or suboptimal conditions appeared not to be a problem. The assay does not enable accurate subtyping by sequencing of PCR products; although the amplicon spans a hypervariable region, it is relatively small compared to the amplicon size usually recommended for subtyping (25, 30).
In conclusion, we have developed a highly applicable TaqMan assay for sensitive and specific screening for Blastocystis ST1 to ST9 of large numbers of DNAs extracted directly from human fecal samples. We believe that this method will prove to be an invaluable tool in all studies aiming at accurately identifying carriers and noncarriers of Blastocystis. Once DNAs have been found to be positive, these can be subjected to the genus-specific PCR published by Scicluna et al. (25) for subtyping.
ACKNOWLEDGMENTS
We thank Lis Lykke Wassmann, Gitte Jensen, and Birthe Dohn (Statens Serum Institut) for excellent technical assistance, Graham Clark (London School of Hygiene and Tropical Medicine) for providing the ST10 sequence, Maiken Cavling Arendrup, Rasmus Hare Jensen, and Søren Persson (Statens Serum Institut) for providing fungal and bacterial DNAs for specificity testing, and Jørgen Skov Jensen (Statens Serum Institut) for professional advice.
Footnotes
Published ahead of print 14 March 2012
REFERENCES
- 1. Abd-Alla MD, Wahib AA, Ravdin JI. 2000. Comparison of antigen-capture ELISA to stool-culture methods for the detection of asymptomatic Entamoeba species infection in Kafer Daoud, Egypt. Am. J. Trop. Med. Hyg. 62:579–582 [DOI] [PubMed] [Google Scholar]
- 2. Corpet F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dogruman-Al F, Dagci H, Yoshikawa H, Kurt O, Demirel M. 2008. A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol. Res. 103:685–689 [DOI] [PubMed] [Google Scholar]
- 4. Dogruman-Al F, et al. 2009. Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem. Inst. Oswaldo Cruz 104:724–727 [DOI] [PubMed] [Google Scholar]
- 5. Dogruman-Al F, Yoshikawa H, Kustimur S, Balaban N. 2009. PCR-based subtyping of Blastocystis isolates from symptomatic and asymptomatic individuals in a major hospital in Ankara, Turkey. Parasitol. Res. 106:263–268 [DOI] [PubMed] [Google Scholar]
- 6. Domínguez-Márquez MV, Guna R, Muñoz C, Gómez-Muñoz MT, Borrás R. 2009. High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain). Parasitol. Res. 105:949–955 [DOI] [PubMed] [Google Scholar]
- 7. Jensen JS, Björnelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen JS, Borre MB, Dohn B. 2003. Detection of Mycoplasma genitalium by PCR amplification of the 16S rRNA gene. J. Clin. Microbiol. 41:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jobst D, Kraft K. 2006. Candida species in stool, symptoms and complaints in general practice—a cross-sectional study of 308 outpatients. Mycoses 49:415–420 [DOI] [PubMed] [Google Scholar]
- 10. Jolley KA, Maiden MC. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones MS, et al. 2008. Detection of Blastocystis from stool samples using real-time PCR. Parasitol. Res. 103:551–557 [DOI] [PubMed] [Google Scholar]
- 12. Jones MS, et al. 2009. Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol. Res. 104:341–345 [DOI] [PubMed] [Google Scholar]
- 13. Li LH, et al. 2007. Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol. Res. 102:83–90 [DOI] [PubMed] [Google Scholar]
- 14. Li LH, et al. 2007. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol. Int. 56:281–286 [DOI] [PubMed] [Google Scholar]
- 15. Macura AB, Witalis J. 2010. Fungi isolated from the stool in patients with gastrointestinal disorders in 2005-2009. Przegl. Epidemiol. 64:313–317 [PubMed] [Google Scholar]
- 16. Meloni D, et al. 2011. Molecular subtyping of Blastocystis sp. isolates from symptomatic patients in Italy. Parasitol. Res. 109:613–619 [DOI] [PubMed] [Google Scholar]
- 17. Navarro C, et al. 2008. High prevalence of Blastocystis sp. in pigs reared under intensive growing systems: frequency of ribotypes and associated risk factors. Vet. Parasitol. 153:347–358 [DOI] [PubMed] [Google Scholar]
- 18. Noël C, et al. 2005. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 43:348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ozyurt M, et al. 2008. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol. Int. 57:300–306 [DOI] [PubMed] [Google Scholar]
- 20. Parkar U, et al. 2007. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134:359–367 [DOI] [PubMed] [Google Scholar]
- 21. Parkar U, et al. 2010. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet. Parasitol. 169:8–17 [DOI] [PubMed] [Google Scholar]
- 22. Poirier P, et al. 2011. Development and evaluation of a real-time PCR assay for detection and quantification of blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J. Clin. Microbiol. 49:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rene BA, Stensvold CR, Badsberg JH, Nielsen HV. 2009. Subtype analysis of Blastocystis isolates from Blastocystis cyst excreting patients. Am. J. Trop. Med. Hyg. 80:588–592 [PubMed] [Google Scholar]
- 24. Santín M, Gómez-Muñoz MT, Solano-Aguilar G, Fayer R. 2011. Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol. Res. 109:205–212 [DOI] [PubMed] [Google Scholar]
- 25. Scicluna SM, Tawari B, Clark CG. 2006. DNA barcoding of blastocystis. Protist 157:77–85 [DOI] [PubMed] [Google Scholar]
- 26. Souppart L, et al. 2010. Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitol. Res. 106:505–511 [DOI] [PubMed] [Google Scholar]
- 27. Souppart L, et al. 2009. Molecular epidemiology of human Blastocystis isolates in France. Parasitol. Res. 105:413–421 [DOI] [PubMed] [Google Scholar]
- 28. Stensvold CR, Alfellani M, Clark CG. 2012. Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infect. Genet. Evol. 12:263–273 [DOI] [PubMed] [Google Scholar]
- 29. Stensvold CR, et al. 2009. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 39:473–479 [DOI] [PubMed] [Google Scholar]
- 30. Stensvold CR, Arendrup MC, Jespersgaard C, Mølbak K, Nielsen HV. 2007. Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn. Microbiol. Infect. Dis. 59:303–307 [DOI] [PubMed] [Google Scholar]
- 31. Stensvold CR, Christiansen DB, Olsen KE, Nielsen HV. 2011. Blastocystis sp. subtype 4 is common in Danish Blastocystis-positive patients presenting with acute diarrhea. Am. J. Trop. Med. Hyg. 84:883–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stensvold CR, et al. 2009. Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol. Infect. 137:1655–1663 [DOI] [PubMed] [Google Scholar]
- 33. Stensvold CR, Nielsen HV. 2012. Comparison of microscopy and PCR for the detection of intestinal parasites in Danish patients supports incentive for molecular screening platforms. J. Clin. Microbiol. 50:540–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stensvold CR, Nielsen HV, Mølbak K, Smith HV. 2009. Pursuing the clinical significance of Blastocystis—diagnostic limitations. Trends Parasitol. 25:23–29 [DOI] [PubMed] [Google Scholar]
- 35. Stensvold CR, et al. 2011. The prevalence and clinical significance of intestinal parasites in HIV-infected patients in Denmark. Scand. J. Infect. Dis. 43:129–135 [DOI] [PubMed] [Google Scholar]
- 36. Stensvold CR, et al. 2007. Terminology for Blastocystis subtypes—a consensus. Trends Parasitol. 23:93–96 [DOI] [PubMed] [Google Scholar]
- 37. Stensvold CR, et al. 2007. Blastocystis: subtyping isolates using pyrosequencing technology. Exp. Parasitol. 116:111–119 [DOI] [PubMed] [Google Scholar]
- 38. Stensvold R, Brillowska-Dabrowska A, Nielsen HV, Arendrup MC. 2006. Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction. J. Parasitol. 92:1081–1087 [DOI] [PubMed] [Google Scholar]
- 39. Vennila GD, et al. 1999. Irregular shedding of Blastocystis hominis. Parasitol. Res. 85:162–164 [DOI] [PubMed] [Google Scholar]
- 40. Yoshikawa H, et al. 2004. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol. Res. 92:22–29 [DOI] [PubMed] [Google Scholar]
- 41. Yoshikawa H, Wu Z, Nagano I, Takahashi Y. 2003. Molecular comparative studies among Blastocystis isolates obtained from humans and animals. J. Parasitol. 89:585–594 [DOI] [PubMed] [Google Scholar]
- 42. Yoshikawa H, et al. 2009. Molecular characterization of Blastocystis isolates from children and rhesus monkeys in Kathmandu, Nepal. Vet. Parasitol. 160:295–300 [DOI] [PubMed] [Google Scholar]