Abstract
Adenovirus serotype 3 and 7 outbreaks have occurred periodically in northern, eastern, and southern China since 1955, but there has been no report since the adenovirus serotype 7 outbreak first occurred in Hangzhou, China, in 1991. Here we explored the epidemiology and etiology of two adenovirus serotype 3 outbreaks in Hangzhou in 2011. One acute respiratory outbreak was found in Chun'an County, where a total of 371 cases were confirmed in 5 of 23 towns from 4 to 31 May 2011. The outbreak affected 18.57% (13/70) of schools and 14.49% (90/621) of classes. The incidence was 5.18% (371/7,163). The population was distributed among individuals ages 7 to 15 years. No parents or teachers were infected. Another pharyngoconjunctival fever outbreak was discovered in the Chenjinglun Swimming Center located in the Xihu District between 1 and 15 July 2011. A total of 134 cases were confirmed in 900 amateur swimmers, with an incidence of 14.89% (134/900). The ages ranged from 4 to 9 years. The two outbreaks had no severe complications or death. The viruses in 66.67% (10/15) of throat swabs from children with acute respiratory infections and 100% (10/10) of the swabs from children with pharyngoconjunctival fever were confirmed to be adenovirus serotype 3 with 100% homology by PCR. Of these samples, 60.0% (12/20) had a classical characteristic cytopathic effect, presented as grape-like clusters at 72 h after infection in HEp-2 cells. In conclusion, the acute respiratory infection and pharyngoconjunctival fever outbreak in Hangzhou were caused by the completely homologous type 3 adenovirus in subgenus B. Moreover, these outbreaks demonstrated rapid transmission rates, possibly due to close contact and droplet transmission.
INTRODUCTION
Adenoviruses (Ads) are nonenveloped, double-stranded DNA viruses with a 35,000-bp genome belonging to the Adenoviridae family, genus Mastadenovirus. The viral nucleic acids and core proteins are enclosed in an icosahedral capsid, along with the major capsid protein, hexons, and pentons (39). Since the first isolation of adenovirus from children's tonsils in 1953, at least 51 other human serotypes have been identified (18). These serotypes are divided into six subgroups (A to F) on the basis of hemagglutination features, DNA homology, and genomic organization, and subgroup B is further divided into subgroups B1 and B2. Adenoviruses can cause a wide range of infections, mainly in the respiratory tract, gastrointestinal tract, urinary tract and bladder, eyes, liver, etc. (31, 32). In general, one serotype can cause different clinical diseases or different serotypes may also cause the same disease. There are several differences in tissue specificity between different Ad subgroups. Serotype A is frequently used for gene engineering, Ad subgroups B1, C, and E are known to infect the respiratory system, subgroup B2 viruses infect the kidney, a large number of D serotypes have been associated with epidemic keratoconjunctivitis and infection of the ocular system, and subgroup F viruses cause gastroenteritis (51). Humans can develop persistent, long-term immunity against a given serotype, and thus, reinfection with the same subgroup of virus is rare; however, no cross-reactive immunity against different viral serotypes is developed.
Several outbreaks caused by different adenovirus subgroups have been reported worldwide, including recent outbreaks in Argentina (Ad serotype 3 [Ad3], Ad4, Ad7), Brazil (Adl, Ad7, Ad8), Colombia (Ad7), Taiwan (Ad3, Ad4, Ad5) (49), and the former Soviet Union (Ad3, Ad7). Most of these cases were found in foreign military populations (45). In the United States, Ad14 was a rarely reported but emerging adenoviral serotype in Oregon, Washington, Texas, and New York in 2006 and 2007 (7). China has also had many local adenoviral outbreaks since the first pandemic northeast of mainland China, which primarily infected infants under 2 years of age. The epidemic regions were distributed in the north, east, and south of China in cities such as Beijing, Shanghai, Jiangsu, Hangzhou, Shenzhen, Guangzhou, and Shantou. The predominant epidemic strains were serotypes 3 and 7 (2, 8, 9, 16, 22, 28).
Here, we describe two different outbreaks caused by serotype 3 in Hangzhou City in eastern China between May and July 2011. One was an acute respiratory infection that occurred in the county, and the other presented as a pharyngoconjunctival fever that occurred in a swimming training center located in the downtown area of the city. Although the majority of cases presented with mild-moderate clinical symptoms of infection, these outbreaks were characterized by a high transmissibility rate and rapid spread. No adenovirus outbreak had been be reported since the first Ad outbreak caused by serotype 7 in Hangzhou 20 years ago. The origins and characteristics of these two different Ad outbreaks were important factors that we focused on in this research study.
MATERIALS AND METHODS
Case population and exposed population.
The population evaluated in this study included two sections. The first section had a total of 505 patients ranging in age from 7 to 15 years (371 cases with acute respiratory infections and 134 cases with pharyngoconjunctival fever) from two adenovirus serotype 3 outbreaks in Hangzhou, China. Cases were found by active searches by local public health doctors and passive reports from teachers, local clinicians, and patients. The second section had an exposed population of 8,063, including 7,163 teaching staff and students who had close contacts with patients or lived or studied with patients in the same school during the 2 weeks (the longest incubation) of the febrile respiratory disease outbreak (4 to 31 May 2011) and 900 swimmers who took part in training in the same swimming pool within 6 days (incubation, 3 to 6 days) after the first case report in the pharyngoconjunctival fever outbreak.
Case definition. (i) Acute respiratory infection outbreak.
The clinical case definition of an acute respiratory infection was a person who had lived in the initial and nearby towns since the onset of the first case on 4 May 2011 and displayed the following symptoms: temperature of >100.4°F or >38.0°C plus at least one other sign or symptom of respiratory illness, including pharyngitis, cough, expectoration, and asthma, and pneumonia confirmed by lung X ray and computed tomography (CT) scan.
(ii) Pharyngoconjunctival fever outbreak.
From 1 July 2011, all swimming trainees in the Chen Jinglun Sports Center with a temperature of >100.4°F or >38.0°C plus one of the following symptoms or signs were defined as cases. Bronchitis and pneumonia were not included in cases: pharyngitis, rhinitis, conjunctivitis, cervical lymphadenitis, sore throat, or swollen tonsils.
(iii) Severe illness.
We defined severe illness to be a case that resulted in the admission of the patient to an intensive care unit (ICU) or death for serious complications, such as shock, pneumonia, encephalitis, heart, lung, and kidney failure, and sepsis.
(iv) Noncase definition.
The teachers, students, and parents from the exposed population were judged to be not infected with adenovirus if they did not have the corresponding clinical symptoms and signs and the pharyngeal swab was negative for adenovirus DNA.
Case diagnosis.
All patients with a clinical illness from 4 May to 31 July 2011 were required to see hospital doctors in the local children's hospital for a physical examination, X-ray scan, and blood test. The diagnosis of adenovirus infection was directly confirmed by viral isolation or PCR assay of the hexon gene.
Study design.
We conducted a retrospective descriptive study of the clinical, laboratory, and radiographic characteristics of 505 patients infected with Ad during the study period (10 May to 31 July 2011). All available medical records were reviewed by 10 clinicians, using a standardized data collection tool. Furthermore, during the outbreak period, 505 patients who met the case definition were required to report to the Hangzhou Center for Disease Control and Prevention (CDC), where epidemiologists and local public health doctors interviewed the reported cases and their parents, caregivers, or teachers using a standard questionnaire. All pharyngeal swabs from patients were submitted to the Hangzhou CDC for testing for Ad DNA by PCR assay, and Ads from a total of 25 representatives of samples from the different regions were subtyped.
Sample collection.
Using the throat sterile swabs provided by the bioMérieux company (catalog no. 482CE), CDC laboratory virologists went to different locations to collect pharyngeal swab specimens from patients with clinical illness meeting the case definition and noncases without clinical symptoms and signs. All samples were transported to the laboratory at 4°C in an ice box, and they were stored at 4°C in a refrigerator until testing for DNA by PCR within 24 h. A total of 15 representative swab specimens from 371 respiratory cases and 10 representative specimens from 134 pharyngoconjunctival fever cases from different areas were used for subtyping. In addition, a total of 30 pharyngeal swab specimens were collected from healthy parents and from the teaching staff, and these samples were used as the control group during the same time period.
Rapid viral DNA extraction and detection by nested PCR.
Adenovirus DNA extraction was performed following the instructions of the kit (catalog no. 51106; Qiagen Company), and 200 μl template was dissolved in double-distilled water and aliquoted into four samples (50 μl/each) that were stored at −80°C. Nested PCR was used to detect the hexon gene of adenovirus. We amplified all Ad serotypes with universal primers from the hexon gene, of which the forward primer sequence was 5′-GCCGAGAAGGGCGTGCGCAGGTA-3′ and the reverse primer sequence was 5′-TACGCCAACTCCGCCCACGCGCGCT-3′. The cycling conditions were as follows: denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 15 s, and extension at 60°C for 45 s. The objective fragment size was 522 bp (bp 930 to 1451; GenBank accession no. FJ943637.1) and was detected by 1.5% agarose electrophoresis. All of the primers and probes were previously reported and synthesized by the Shanghai Bio-Engineer Biology Company (29). The standard adenovirus strains provided by the China CDC were used as a positive control. Saline was used as a negative control (from DNA extraction, the process begins with physiological saline as a negative control).
Viral culture and isolation.
A total of 20 pharyngeal swab specimens that were Ad DNA positive were further cultured in HEp-2 cells maintained in Eagle's medium at 37°C for 14 days. The cell cultures were observed for 3 to 4 weeks. When the cells exhibited a characteristic cytopathic effect (CPE), we identified the serotypes of the isolated adenoviruses using hexon gene type-specific primers, including primers for the hexon genes of the main predominant serotypes, Ad3 and Ad7, in China. The type-specific forward primer sequence for amplification of Ad serotype 3 was 5′-GGTAGAGATGCTGTTGCAGGA-3′; the reverse primer sequence was 5′-CCCATCCATTAGTGTCATCGGT-3′. The amplification size was 502 bp. The type-specific forward primer sequence for PCR Ad serotype 7 was 5′-GGAAAGACATTACTGCAGACA-3′, and the reverse primer sequence was 5′-AATTTCAGGCGAAAAAGCGTCA-3′. The amplification size was 311 bp (52). The adenovirus serotypes were determined by the use of neutralization assays with a type-specific reference antibody. The neutralization concentration was determined against a 100-fold 50% tissue culture infective dose.
Data sequence analysis.
The nucleotide sequences of the amplified products were directly determined by dideoxy sequencing, using an ABI Prism BigDye Terminator cycle sequencing kit. The sequences were determined and compared using the MegAlign software package.
Statistical analysis.
All of the statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS; version 12.0). Categorical variables were compared using the chi-square test and Fisher's exact test. All of the statistical tests were two-sided, and a P value of <0.05 was considered statistically significant.
RESULTS
Distribution of timeline in acute respiratory infection outbreak.
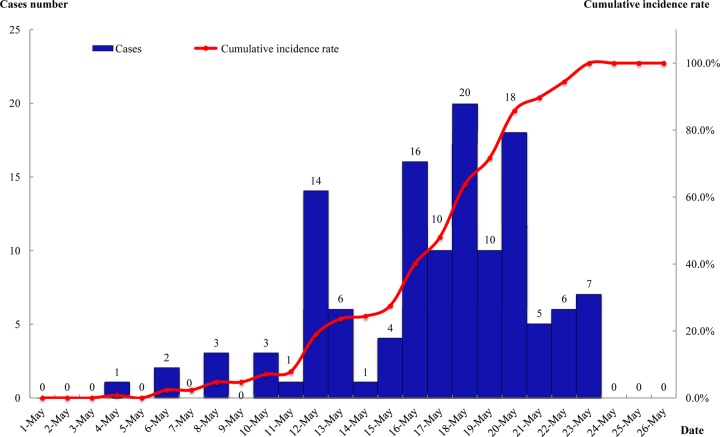
As of 31 May 2011, there were a total of 127 confirmed cases in Hangzhou. The first case was reported on 4 May 2011. The outbreak experienced three stages: the first stage was a sporadic period from 4 to 10 May. The second stage was a sustained-increase period beginning on 11 May, which reached its peak between 16 and 20 May, when there were 74 cases that accounted for 58.26% (74/127) of all infections. The peak did not remain stable because we took a series of control measures, including patient isolation, suspension of classes, sterilization of the environment, and health education. The third stage was a period of decrease though 21 May. The outbreak curve had a single peak (Fig. 1).
Fig 1.
Timeline distribution of 127 acute respiratory cases from an outbreak caused by adenovirus type 3 in the Yaoshan central school, Chun'an County of Hangzhou, from 4 to 31 May 2011.
Distribution of timeline in pharyngoconjunctival fever outbreak.
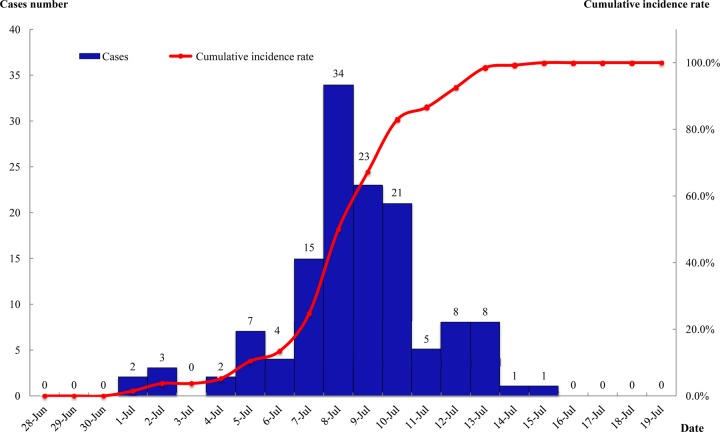
The earliest onset was found on 1 July 2011, and the last case was confirmed on 15 July. The outbreak attained a high peak from 7 to 10 July, when there were a total of 93 confirmed cases, accounting for 69.40% of all the cases. The epidemic curve showed a single peak (Fig. 2).
Fig 2.
Timeline distribution of 134 pharyngoconjunctival fever cases from an outbreak caused by adenovirus type 3 in the Chenjinglun Swimming Center located in the Xihu District of Hangzhou from 1 to 15 July 2011.
Regional distribution in acute respiratory infection outbreak.
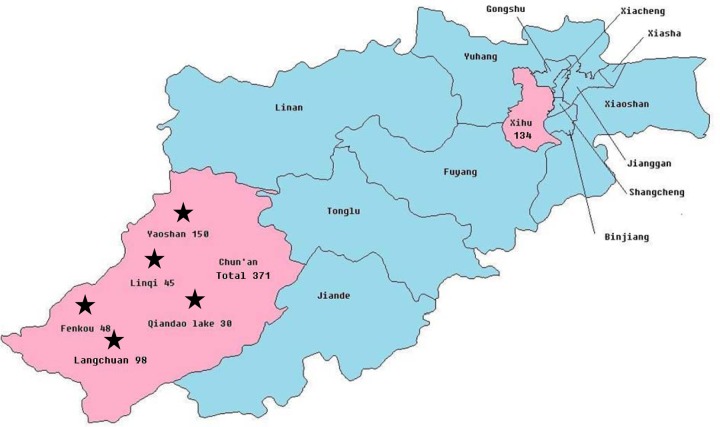
Hangzhou, the capital of Zhejiang Province, has 13 districts/counties, including the 8 districts of Shangcheng, Xiacheng, Jianggan, Gongshu, Xihu, Binjiang, Xiaoshan, and Yuhang and the 5 counties of Jiande, Fuyang, Lin'an, Tonglu, and Chun'an. This adenovirus outbreak occurred in the suburb of Chun'an, situated southwest of Hanghzhou, with 23 towns and a population of 336,843. Of these, 21.74% (5/23) of the towns had an adenovirus 3 outbreak from 4 to 31 May 2011. The outbreak occurred in 13 of 70 schools (18.57%, 13/70) across 5 towns. However, there were 14.49% (90/621) classes with reported cases. A total of 371 cases were confirmed from the exposed population of 7,163, and the incidence was 5.18% (371/7 163). The outbreak affected a population that was mainly associated with elementary schools (30%, 6/20), kindergartens (13.95%, 6/43), and junior high schools (14.28%, 1/7) (Fig. 3).
Fig 3.
Regional distribution of outbreak of 371 acute respiratory cases in 5 towns from Chun'an County from 4 to 31 May 2011 and outbreak of 134 pharyngoconjunctival fever cases in the Xihu District of Hangzhou from 1 to 15 July 2011, all caused by adenovirus type 3.
Regional distribution in pharyngoconjunctival fever outbreak.
The Chenjinglun Swimming Center is located in the Xihu District in the center of Hangzhou City. There are four swimming pools, including two outdoor pools. One of the outdoor pools was 16 m in length and 25 m in width with a depth of 1.1 to 1.5 m, and the other was irregular with an area of approximately 300 m2 and a depth of 1.2 m. The water in these two pools can be exchanged via a water circulation system. The two indoor swimming pools were standard pools that were 50 m by 25 m with a depth of 2 m. The water cannot be exchanged between these two pools. All 134 patients that were positive for Ad type 3 were trained using the outdoor swimming pool, and no cases were found among indoor swimming pool users.
Population distribution in acute respiratory infection outbreak.
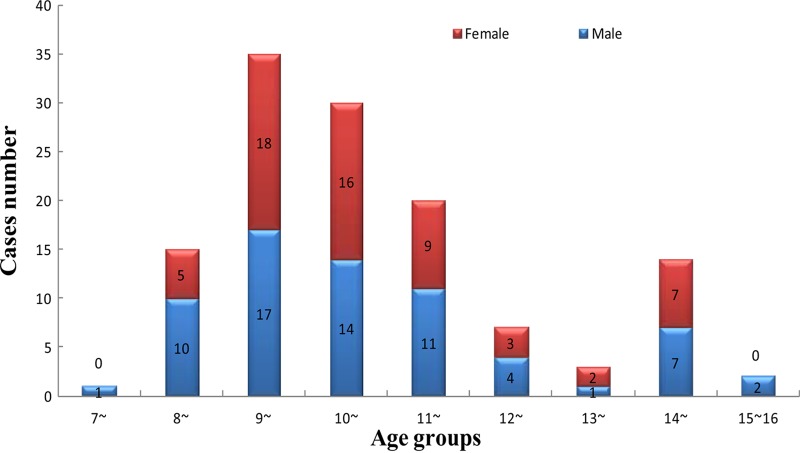
The largest outbreak was found in the Yaoshan Town central school, where there were 33 staff and 442 students, including 6 grades and 14 classes. All of the students came from 11 nearby areas comprised of local residents without moving populations. A total of 127 confirmed cases were reported, and the incidence was 28.73% (127/442). These included 67 male cases (30.18%, 67/222) and 60 female cases (27.27%, 60/220). The male-to-female ratio was 1.12:1. The gender distribution had no statistically significant difference between male and female students (χ2 = 0.46, P > 0.05). The average age was 10.29 years, and ages ranged from 7 to 15 years. Moreover, there were 72 students absent during the entire month of May. None of the teachers or parents associated with individual cases was found to be infected. The oldest cases were 15 years of age, and the youngest were 7 years of age. The age distribution was as follows: 7 years old, 0.79% (1/127); 8 years old, 11.81% (15/127); 9 years old 27.56% (35/127); 10 years old, 23.62% (30/127); 11 years old, 15.75% (20/127); 12 years old, 5.51% (7/127); 13 years old, 2.36% (3/127); 14 years old, 11.02% (14/127); and 15 years old, 1.57% (2/127) (Fig. 4).
Fig 4.
Age group distribution of 127 adenovirus-confirmed cases in the Yaoshan central school, Chun'an County of Hangzhou, from 4 to 31 May 2011.
Population distribution in pharyngoconjunctival fever outbreak.
The Chenjinglun Swimming Center had 8 coaches, 48 staff members, and 1,300 trainee children 6 to 10 years old that were divided into 40 classes. One group with 900 amateurs from kindergarten and primary schools were training in the outdoor swimming pool. Another group with 400 professional swimmers was training in the indoor swimming pool. A total of 134 cases were consistent with the case definition, with an overall incidence of 14.89% (134/900). All of the patients were from the amateur group trained in the outdoor pool. No professional athletes, staff, or teachers that worked with the patients became infected. Approximately 80.60% (108/134) of the total reported cases were found in training between 5 p.m. and 6 p.m. There were 65 male and 69 female cases, and the ratio of males to females was 0.94:1. The age distribution ranged from 4 to 9 years, with the 5- to 7-year-old category accounting for 90.30% (121/134) of the total number of reported cases. The average age was 6.85 years.
Clinical characteristics in acute respiratory infection outbreak.
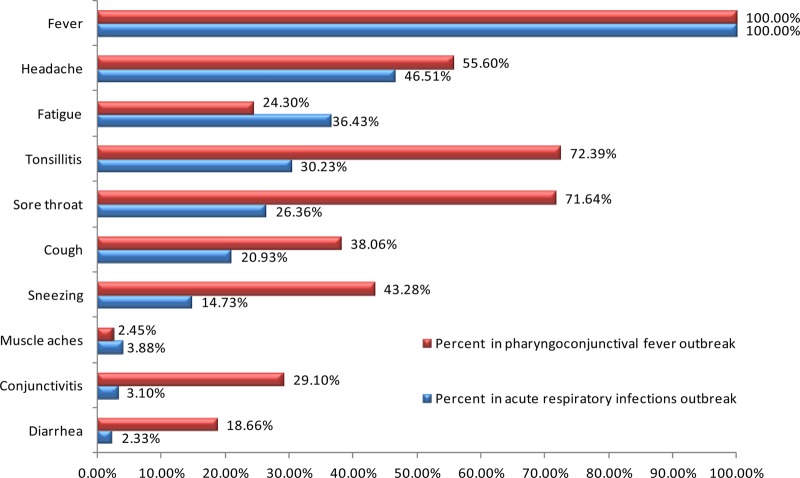
A total of 127 confirmed cases were analyzed and collated. There were no severe cases or deaths among these cases. The most common symptoms included fever (100.00%, 127/127), headache (46.51%, 59/127), fatigue (36.43%, 46/127), tonsillitis (30.23%, 38/127), sore throat (26.36%, 33/127), cough (20.93%, 27/127), sneezing (14.73%, 19/127), muscle aches (3.88%, 5/127), conjunctivitis (3.10%, 4/127), and diarrhea (2.33%, 3/127).
All cases had a high fever for several days. The average clinical stay was 7 to 10 days. Laboratory testing showed that the total number of white blood cells was not significantly elevated during the early period, but the leukocyte and neutrophil counts increased in 3.1% (4/127) of patients with coinfections involving bacteria during the later clinical period. A total of 15 patients (11.81%, 15/127) were hospitalized for persistent fever without serious complications. Another 112 cases (88.19%, 112/127) did not need hospitalization. The ratio of hospitalized cases versus those treated but not admitted was 1:7.5 (15/112). The clinical symptoms of patients infected with adenovirus type 3, causing 127 acute respiratory infections and 134 pharyngoconjunctival fever cases from two outbreaks in Hangzhou in 2011, are shown in Fig. 5, which does not include data related to antibiotic treatment.
Fig 5.
Clinical symptoms of infection with adenovirus type 3, causing 127 acute respiratory infections and 134 pharyngoconjunctival fever cases from two outbreaks in Hangzhou in 2011.
Clinical characteristics in pharyngoconjunctival fever outbreak.
A total of 134 cases presented with fever, and 63.43% (85/134) of patients had a high fever (temperature over 40°C). Symptoms among the cases were fever (100.00%, 134/134), tonsillitis (72.39%, 97/134), sore throat (71.64%, 96/134), headache (55.60%, 75/134), sneezing (43.28%, 58/134), cough (38.06%, 51/134), conjunctivitis (29.10%, 39/134), fatigue (24.30%, 33/134), diarrhea (18.66%, 25/134), and muscle aches (2.45%, 3/134) (Fig. 5). The clinical course usually lasted approximately 7 days. A total of 14 patients were hospitalized without severe complications, such as pneumonia and heart failure.
Pathogenic characteristics of acute respiratory infection outbreak.
A total of 15 pharyngeal swab specimens were collected from acutely ill cases, and 30 specimens were collected from the healthy control population. All pharyngeal swabs were used to detect the hexon gene of adenovirus DNA. The positive rates of detection were 66.67% (10/15) and 0% (0/30), respectively, and the difference between the two groups was statistically significant (χ2 = 22, P < 0.01).
Pathogenic characteristics of pharyngoconjunctival fever outbreak.
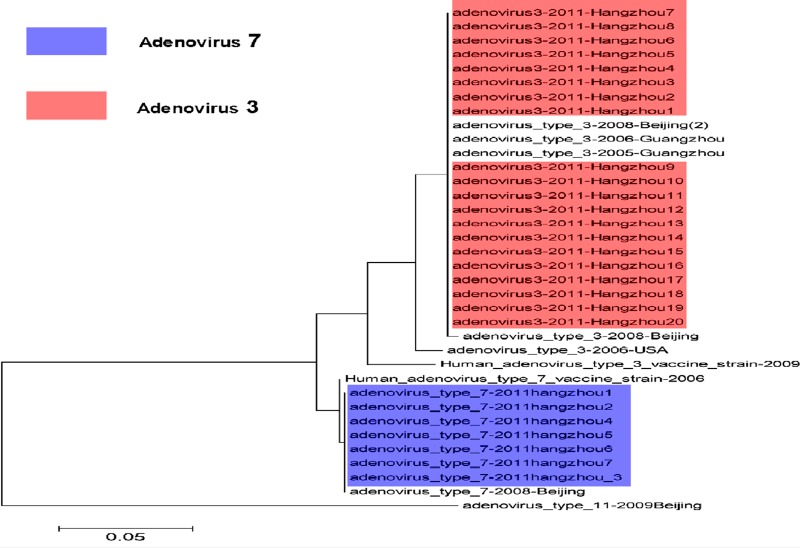
A total of 10 and 30 pharyngeal swab specimens were collected from pharyngoconjunctival fever and healthy students, respectively. The 40 samples were tested for the presence of adenovirus DNA by PCR. The results showed that all samples from the cases were positive and 7 samples were strongly positive. The positive rates were 100% (10/10) and 0% (0/30) in pharyngoconjunctival fever and healthy students, respectively. There were obvious differences between the two groups (P = 0, P < 0.01, Fisher's exact test). The 20 hexon gene sequences from the two outbreaks were searched for in GenBank with BLAST analysis and identified as adenovirus serotype 3. The nucleotide identity between the 20 sequences was 100%, with 98.7% to 100% nucleotide identity to serotype 3 strains obtained from other regions, including Beijing, Guangzhou, and the United States, detected. The nucleotide identity to a representative strain from serotype 11 was only 65.7% (Fig. 6). In addition, samples from the two outbreaks were also tested by PCR for other pathogens known to cause acute respiratory diseases (ARDs), such as influenza viruses H1N1, H3N2, H2N2, and B, parainfluenza virus, respiratory syncytial virus, and severe acute respiratory syndrome coronavirus. They were all negative.
Fig 6.
Phylogenetic analysis of the hexon gene sequences isolated from acute respiratory infection and pharyngoconjunctival fever cases from two outbreaks in Hangzhou and from other regions. Purple, adenovirus type 7 isolated from the adenovirus surveillance; pink, adenovirus type 3 isolated from two outbreaks.
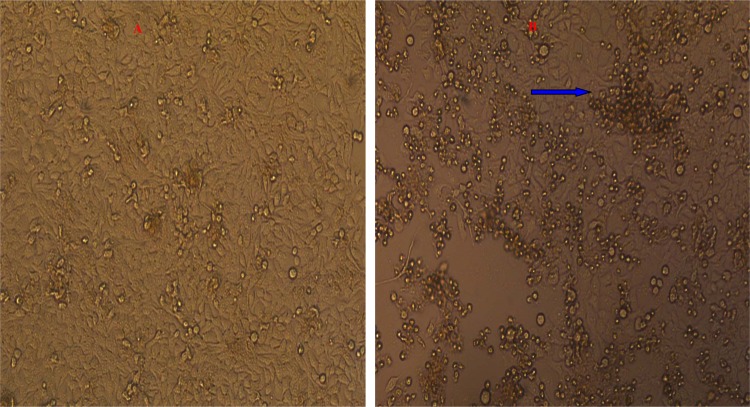
Furthermore, 20 positive pharyngeal swab specimens were inoculated into HEp-2 cell cultures. CPE was observed in cultures derived from 60.0% (12/20) of the pharyngeal swab specimens. The typical CPE characteristics of adenovirus were observed under an inverted microscope, where infected cells began to swell, became rounded, and developed into grape-like clusters 72 h after infection (Fig. 7). The 50% tissue culture infective doses (CID50s) of the isolated viral strains were between 10−4 and 10−5. The presence of adenovirus and adenovirus type were further confirmed, and the virus was found to be serotype 3 by the adenovirus neutralization test.
Fig 7.
HEp-2 cells were found to exhibit a classical CPE when inoculated with throat swabs from the two outbreaks. The CPE presented as grape-like clusters at 72 h after infection. Magnification, ×100. (A) Control; (B) CPE; blue arrow, characteristic cytopathic effect (CPE) site.
Adenovirus emergency surveillance.
The Hangzhou government built the adenovirus emergency pathogen surveillance system in Chun'an, Tonglu, located in the suburbs, and the provincial children's emergency hospital in the center of the city since the adenovirus outbreak was reported in schools in the suburbs of Hangzhou. The results showed that the rates of adenoviral DNA-positive individuals with influenza-like illness (ILI) were 44.44% (8/18), 7.69% (2/26), 0% (0/15), 17.64% (3/17), and 0% (0/14) in June, July, August, September, and October 2011, respectively. The total positive rate was 13.54% (13/96). In addition, 7 hexon gene sequences from patients with influenza illness were confirmed to be serotype 7 (Fig. 6).
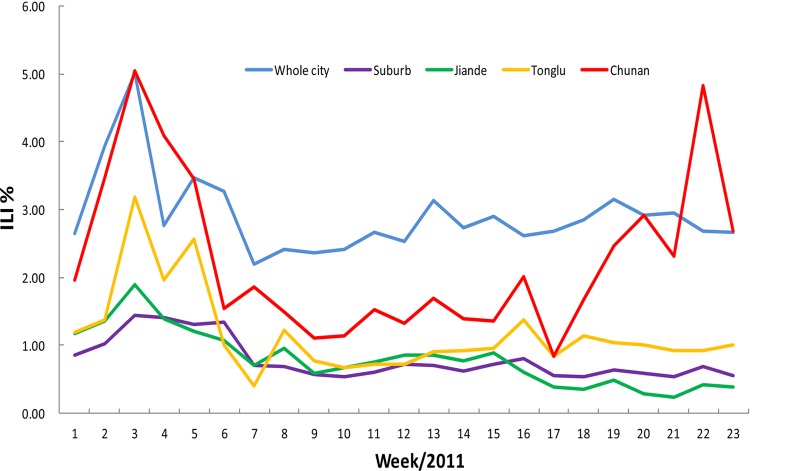
The influenza surveillance showed that the percent ILI in the entire city fluctuated approximately 2.58%, with the urban ILI (2.00%) being higher than that of the suburbs (1%), including Tonglu, Jiande, and Chun'an Counties. Generally, the percent ILI maintained a stable curve, except for the epidemic peak that occurred earlier in the year, but from week 17, the rate of influenza-like illness sharply increased from 0.99% to 4.95% in Chun'an County due to the adenovirus outbreak (Fig. 8).
Fig 8.
The adenovirus outbreak influenced the percent of ILI in Hangzhou, 2011.
DISCUSSION
Ad3 and Ad7 have usually been associated with outbreaks of acute respiratory tract infections. Adenovirus-associated acute respiratory disease (AARD) is most often reported in the military in the Americas, Europe, and Asia. The dominant serotypes were Ad3a2, with occasional outbreaks of Ad3a4, Ad3a5, and Ad3a6 occurring in China between 1962 and 1988 (29). These outbreaks were associated with recruits from different regions of China that were housed together for long periods of time during intense training at military training centers. They were faced with close living quarters, crowding, physical and mental stress, and meteorological conditions, which were thought to be factors involved in these AARD outbreaks (5, 10, 12, 13, 14, 27, 47).
However, a small number of outbreaks were reported among students and younger children in educational environments who were living, studying, and training in groups, resembling conditions of a military environment (6, 35, 36, 53). Only a few studies found low rates among 15- to 25-year-old students in 1960, 1978, and 1997 in the United States (4, 21, 33, 34). In Japan, Ad7 outbreaks of febrile respiratory disease were linked to high school dormitories in the mid-1990s and again in 2003 (6).
This study demonstrated that an acute respiratory infection outbreak in 2011 was caused by adenovirus type 3 (B subgroup) and encompassed over 13 schools and 90 classes across 5 towns. A total of 371 cases in an exposed population of 7,163 were confirmed. The regional distribution was remote mountainous areas, and no cases were found in the downtown area of the city. This outbreak had a large influence on the society because of the large number of children affected and the wide geographic areas involved. Furthermore, this outbreak spread rapidly, clustered in classes and dormitories, and had a high incidence rate. In all, there were special epidemic characteristics in this outbreak: a wide range of geographic areas were involved; a high incidence was found in schools, resulting in a large number of patients in a short time; and children less than 15 years old were the infected population. Spreading was controlled after class closures and a series of other measures, and respiratory tract infectious diseases were recorded. However, disease transmission patterns, which include droplets, person-to-person contact, or the fecal-oral route, need to be further studied.
In this outbreak, most of the cases showed mild-moderate clinical symptoms with acute, persistent high fever and respiratory signs. Laboratory testing showed that the white blood cell count was not significantly elevated during the early period, but the leukocyte and neutrophil counts increased later in 3.1% (4/127) of patients coinfected with bacteria, with the increase confirmed by sputum culture and blood culture. According to the patients' clinical signs, routine blood testing results, and other epidemic characteristics, we can assume that this was a viral outbreak. However, further PCR diagnosis from throat swabs is needed to confirm the type of virus outbreak (11, 54). No serious extrapulmonary complications, such as lymphadenopathy, bleeding diathesis, anemia, hepatomegaly, splenomegaly, exanthema, neurological signs, renal signs, or death, were discovered. The lack of these symptoms suggests that this infection is involved with the upper and not the lower respiratory tract with little or no viremia. We also noted that all of the infections were found in preschool and school-age children with an average age of 10 years, but their parents and teachers who were living or staying with them were not infected. We hypothesized that this phenomenon was likely caused by children without sufficient immunity because they live in a mountainous area and had little contact with the outside world after birth. Further epidemiological investigation and serological detection of adenovirus antibodies are needed to confirm this supposition. Sun et al. reported that the seroprevalence and antibody titers of adenovirus type 5 among the general population in the south of China increased with age and that the proportions of people with high-level Ad5 neutralizing antibodies (titers > 1,000) were 30.56%, 30.77%, 48.08%, 52.27%, and 54.35% in different age groups of <12, 13 to 20, 21 to 31, 32 to 40, and 41 to 72 years, respectively (48). Another Chinese serum epidemiological survey also showed that 70% to 80% of children under 5 years of age had antibodies against more than one adenovirus subtype and were positive for Ad types 1, 2, and 5 but were rarely positive for Ad types 3, 4, and 7 (1). That research supported the idea that seropositivity increased with age and may contribute to the children's increased susceptibility.
When the outbreak occurred, our government undertook a series of measures to protect public health, including isolating and treating patients at home, conducting a body check in the morning, providing ventilation and disinfection support, overseeing aggressive treatment of patients, helping to provide early detection of cases, providing school health education, and supporting hospital infection control. We conducted emergency surveillance of fever and respiratory tract infections in Chun'an County and the surrounding counties to identify clustered cases as soon as possible and also found some sporadic cases. With these measures, along with the closing of the schools and the approaching summer holiday, in which the students were dismissed until 20 June, the outbreak was completely controlled.
Another, swimming-related pharyngeal conjunctival fever outbreak was found in 900 swimmers (<10 years old). The epidemic characteristics were apparent aggregation, fast disease spread, and similar symptoms, and the number of cases quickly appeared within a short time, with the incidence being high. As a result of the training class being conducted in the outdoor swimming pool, disinfection became a problem because the environmental temperature was too high; the residual chlorine in the water became volatile much faster, making it difficult to achieve the appropriate disinfecting effect. Another factor played a role in the pool disinfection problem. The swimming classes were comprised of large numbers of trained students; each class had more than 300 people in a small swimming pool, which caused frequent contact between students. Furthermore, the swimmers became more susceptible to virus due to the sudden change of climate temperature at that time. The following measures of prevention and control were proposed over time. First, the Chenjinglun Swimming Center was closed starting on 15 July. Second, screening for fever, sore throat, and other symptoms was conducted for all trainees and trainers, and when these were found, a visit or a call was made to persuade parents to immediately take their children to the hospital. Third, physical examinations of healthy training students were performed before they were allowed back into the water. Fourth, health education and health promotion work was performed through communication with the parents, customers, and the media to eliminate a public pandemic. At the same time, we developed a surveillance system for detecting respiratory tract infection cases in all hospitals of Hangzhou.
He et al. and others have reported that there was an acute conjunctivitis outbreak caused by Ad3a2 in Shenzhen City in the south of China in the summer of 1997 (17, 37). Hangzhou City's earliest adenovirus outbreak was reported in 1991 and was caused by adenovirus serotype 7; there were no additional reports of Ad outbreaks, until now. These two outbreaks took place from May to July 2011, and both were caused by the same serotype, adenovirus serotype 3. This showed that adenovirus type 3 is the major epidemic strain in Hangzhou City. In addition, adenovirus type 3 has been reported in other cities in China. These two outbreaks were found in younger children, but no infections were found in adults. In addition, there were different symptoms and modes of transmission in the two outbreaks. The adenovirus infectious source has not been identified, even though adenovirus can infect humans, poultry, cattle, dog, rats, pigs, and monkeys. It was once thought that aviadenovirus or fowl adenovirus (FAV) could not infect mammals, including humans, because of strict host specificity; however, this idea has been refuted (28). In the future, we should monitor adenovirus transmission between birds and animals, and future studies of adenovirus in poultry should determine whether unique or similar strains circulate within that population (2).
Adenovirus in China is not a statutorily reportable infectious disease, and these viruses have not been incorporated into the monitoring system; therefore, information regarding seasonal and population distributions as well as the high-risk and pathogenic characteristics for the current epidemic is not clear. The Hangzhou City government has developed adenovirus surveillance, including epidemiological and pathogenic surveillance, based on the current influenza monitoring system.
An adenovirus subgroup vaccine of a different subtype has been used in the United States and other countries, including Britain, the Soviet Union, the Netherlands, Canada, France, and Finland, where it has successfully protected military trainees against Ad infection. Ad vaccines have a very good record of preventing infection with the same type of adenovirus. However, due to the number of different predominant serotypes that have been observed, it is time to develop an effective long-term vaccination strategy (3, 19, 40, 41, 42, 43, 44, 46, 47, 50, 51). Although there has been a report that vaccination against Ad4 and Ad7 was a successful preventive measure in controlling an AARD outbreak in military trainees in Taiwan and in mainland China, there are no readily available vaccines that can be used. Therefore, in the future we must strengthen vaccine research and development for Ad3, which is the most frequent serotype found in China (15, 20, 22, 23, 24, 25, 26, 30, 38).
ACKNOWLEDGMENTS
This work was supported by the Science Research Foundation of Medicine in Zhejiang Province, China (2011KYB076), and the Hangzhou Health Bureau (2011A057).
We declare that no conflicts of interest exist.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Aberle SW, et al. 2003. Adenovirus DNA in serum of children hospitalized due to an acute respiratory adenovirus infection. Infect. Dis. 187:311–314 [DOI] [PubMed] [Google Scholar]
- 2. Bao CJ, et al. 2007. Possible spread of adenovirus type 3 from poultry to humans: indirect evidence from an outbreak in China. J. Nanjing Med. Univ. 21:324–332 [Google Scholar]
- 3. Belov AB, Ogarkov PI. 2005. Epidemiology and prophylaxis of influenza and other acute respiratory infections occurred in the military collectives. Voen. Med. Zh. 326:32–38, 80 [PubMed] [Google Scholar]
- 4. Brůcková M, Wadell G, Sundell G, Syrùcek L, Kunzová L. 1980. An outbreak of respiratory disease due to a type 5 adenovirus identified as genome type 5a. Acta Virol. 24:161–165 [PubMed] [Google Scholar]
- 5. Burov SA, Chukhlovin BA, Maslennikova LK. 1961. Experience in serodiagnosis of adenovirus diseases in military personnel. Voen. Med. Zh. 12:37–39 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 1998. Civilian outbreak of adenovirus acute respiratory disease—South Dakota, 1997. MMWR Morb. Mortal. Wkly. Rep. 47:567–570 [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2007. Acute respiratory disease associated with adenovirus serotype 14—four states, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 56:1181–1184 [PubMed] [Google Scholar]
- 8. Chen HL, et al. 2004. Respiratory adenoviral infections in children: a study of hospitalized cases in southern Taiwan in 2001-2002. J. Trop. Pediatr. 50:279–284 [DOI] [PubMed] [Google Scholar]
- 9. Chuang YY, et al. 2003. Severe adenovirus infection in children. J. Microbiol. Immunol. Infect. 36:37–40 [PubMed] [Google Scholar]
- 10. de Albuquerque MC, da Siiva FM, Soares CC, Volotao Ede M, Santos N. 2003. Adenoviruses isolated from civilian and military personnel in the city of Rio de Janeiro, Brazil. Rev. Inst. Med. Trop. Sao Paulo 45:233–236 [DOI] [PubMed] [Google Scholar]
- 11. Duan PR, Zhao H, Peng SY. 2000. Serological analysis and detection of multi-virus during the prevalence of Flu A3 in Beijing. Immunol. J. 16:127–129 [Google Scholar]
- 12. Evans AS. 1975. Serologic studies of acute respiratory infections in military personnel. Yale J. Biol. Med. 48:201–209 [PMC free article] [PubMed] [Google Scholar]
- 13. Evans AS, et al. 1973. Acute respiratory infections in different ecologic settings. I. Argentine military recruits. Am. Rev. Respir. Dis. 108:1311–1319 [DOI] [PubMed] [Google Scholar]
- 14. Evans AS, Jeffrey C, Niederman JC. 1971. The risk of acute respiratory infections in two groups of young adults in Colombia, South America: a prospective seroepidemiologic study. Am. J. Epidemiol. 93:463–471 [DOI] [PubMed] [Google Scholar]
- 15. Hashem A, et al. 2012. Subcutaneous immunization with recombinant adenovirus expressing influenza A nucleoprotein protects mice against lethal viral challenge. Hum. Vaccin. Immunother. 8:1–7 [DOI] [PubMed] [Google Scholar]
- 16. Hatherill M, et al. 2004. Evolution of an adenovirus outbreak in a multidisciplinary children's hospital. J. Paediatr. Child Health 40:449–454 [DOI] [PubMed] [Google Scholar]
- 17. He Y, Zhao J, Yang H. 2000. Viral etiology study of acute conjunctivitis. Chin. J. Exp. Clin. Virol. 14:40–43 [PubMed] [Google Scholar]
- 18. Hierholzer JC. 1973. Further subgrouping of the human adenoviruses by differential hemagglutination. J. Infect. Dis. 128:541–550 [DOI] [PubMed] [Google Scholar]
- 19. Hitchcock G, Tyrrell DA, Bynoe ML. 1960. Vaccination of man with attenuated live adenovirus. J. Hyg. (Lond.) 58:277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Julkunen I, Lehtomaki K, Hovi T. 1986. Immunoglobulin class-specific serological responses to adenovirus in respiratory infections of young adult men. J. Clin. Microbiol. 24:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz S, Badger F, Denison AB, Denny FW, Jr, Jordan WS., Jr 1959. Search for illness due to adenovirus type 4 among college dormitory freshmen. Proc. Soc. Exp. Biol. Med. 101:592–594 [DOI] [PubMed] [Google Scholar]
- 22. Kidd AH, et al. 1996. Rapid subgenus identification of human adenovirus isolates by a general PCR. J. Clin. Microbiol. 34:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laitinen LA, Miettinen AK, Kuosma E, Huhtala L, Lehtomaki K. 1992. Lung function impairment following mycoplasmal and other acute pneumonias. Eur. Respir. J. 5:670–674 [PubMed] [Google Scholar]
- 24. Lehtomaki K. 1988. Clinical diagnosis of pneumococcal, adenoviral, mycoplasmal and mixed pneumonias in young men. Eur. Respir. J. 1:324–329 [PubMed] [Google Scholar]
- 25. Lehtomaki K. 1988. Rapid etiological diagnosis of pneumonia in young men. Scand. J. Infect. Dis. Suppl. 54:1–56 [DOI] [PubMed] [Google Scholar]
- 26. Lehtomaki K, et al. 1986. Rapid diagnosis of respiratory adenovirus infections in young adult men. J. Clin. Microbiol. 24:108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lehtomaki K, et al. 1988. Etiological diagnosis of pneumonia in military conscripts by combined use of bacterial culture and serological methods. Eur. J. Clin. Microbiol. Infect. Dis. 7:348–354 [DOI] [PubMed] [Google Scholar]
- 28. Li MD, Wang YM. 2004. Practice of infectious diseases, 3rd ed, p 361–365 People's Medical Publishing House, Beijing, China [Google Scholar]
- 29. Li QG, Zheng QJ, Liu YH, Wadell G. 1996. Molecular epidemiology of adenovirus types 3 and 7 isolated from children with pneumonia in Beijing. J. Med. Virol. 49:170–177 [DOI] [PubMed] [Google Scholar]
- 30. Lin KH, et al. 2004. A two decade survey of respiratory adenovirus in Taiwan: the reemergence of adenovirus types 7 and 4. J. Med. Virol. 73:274–279 [DOI] [PubMed] [Google Scholar]
- 31. Lukashok SA, Horwitz MS. 1998. New perspectives in adenoviruses. Curr. Clin. Top. Infect. Dis. 18:286–305 [PubMed] [Google Scholar]
- 32. Mei YF, Wadell G. 1996. Epitopes and hemagglutination binding domain on subgenus B: 2 adenovirus fibers. J. Virol. 70:3688–3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mogabgab WJ. 1968. Acute respiratory illnesses in university (1962-1966), military and industrial (1962-1963) populations. Am. Rev. Respir. Dis. 98:359–379 [DOI] [PubMed] [Google Scholar]
- 34. Mogabgab WJ. 1968. Mycoplasma pneumoniae and adenovirus respiratory illnesses in military and university personnel, 1959-1966. Am. Rev. Respir. Dis. 97:345–358 [DOI] [PubMed] [Google Scholar]
- 35. National Institute of Infectious Disease 2004. Adenovirus and pharyngoconjunctival fever, 2003. Infect. Agents Surveill. Rep. 25:94–95 [Google Scholar]
- 36. Ozawa S, et al. 1998. An outbreak of adenovirus type 7 in a dormitory of a high school. Rinsho To Uirusu Clin. Virol. 26:226–237 [Google Scholar]
- 37. Pan HM, et al. 2003. Report on the outbreak caused by adenovirus in children in indoor pool. J. Prev. Med. Info. 3:237–238 [Google Scholar]
- 38. Raty R, Kleemola M, Melen K, Stenvik M, Julkunen I. 1999. Efficacy of PCR and other diagnostic methods for the detection of respiratory adenoviral infections. J. Med. Virol. 59:66–72 [PubMed] [Google Scholar]
- 39. Rux JJ, Burnett RM. 2004. Adenovirus structure. Hum. Gene Ther. 15:1167–1176 [DOI] [PubMed] [Google Scholar]
- 40. Selivanov AA. 1975. Etiology and specific prophylaxis of respiratory diseases in military personnel. Voen. Med. Zh. 10:45–49 [PubMed] [Google Scholar]
- 41. Selivanov AA, Boldasov VK, Kovaleva TP, Shaposhnikova RP, Morozenko MA. 1970. Combined immunization against influenza and other viral respiratory infections. Vestn. Akad. Med. Nauk SSSR 25:33–37 [PubMed] [Google Scholar]
- 42. Selivanov AA, et al. 1968. Epidemiologic effectiveness of live enteral adenovirus and influenza vaccine on susceptible groups of adults. Vestn. Akad. Med. Nauk SSSR 23:35–41 [PubMed] [Google Scholar]
- 43. Selivanov AA, Pleshanova RA, Skryabina EA, Smorodintsev AA. 1964. Testing the effectiveness of live adenovirus vaccine. I. Reactogenic properties. Acta Virol. 21:263–270 [PubMed] [Google Scholar]
- 44. Selivanov AA, Pleshanova RA, Smorodintsev AA. 1964. Testing the effectiveness of live adenovirus vaccine. II. Immunogenic properties. Acta Virol. 21:271–276 [PubMed] [Google Scholar]
- 45. Sivan AV, Lee T, Binn LN, Gaydos JC. 2007. Adenovirus-associated acute respiratory disease in healthy adolescents and adults: a literature review. Mil. Med. 172:1198–1203 [DOI] [PubMed] [Google Scholar]
- 46. Smorodintsev AA, et al. 1969. Live vaccines against influenza, adenoviruses, measles, and mumps. Arch. Environ. Health 18:105–114 [DOI] [PubMed] [Google Scholar]
- 47. Smorodintsev AA, Selivanov AA. 1959. Results of immunization of volunteers with live attenuated adenovirus vaccine type IV, V and VII (preliminary communication). Vopr. Virusol. 4:648–652 [PubMed] [Google Scholar]
- 48. Sun C, et al. 2011. Epidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern China. Vaccine 29:3837–3841 [DOI] [PubMed] [Google Scholar]
- 49. Tai FH, Grayston JT, Johnston PB, Woolridge RL. 1960. Adenovirus infections in Chinese Army recruits on Taiwan. J. Infect. Dis. 107:160–164 [DOI] [PubMed] [Google Scholar]
- 50. van der Veen J, Abarbanel MF, Oei KG. 1969. Vaccination with live type 4 adenovirus: evaluation of antibody response and protective efficacy. Antonie Van Leeuwenhoek 35:239–241 [DOI] [PubMed] [Google Scholar]
- 51. van der Veen J, Prins A. 1960. Studies of the significance of the recall phenomenon in the antibody response to adenovirus vaccine and infection. J. Immunol. 84:562–584 [PubMed] [Google Scholar]
- 52. Xu W, Erdman DD. 2001. Type-specific identification of human adenovirus 3, 7, and 21 by a multiplex PCR assay. J. Med. Virol. 64:537–542 [DOI] [PubMed] [Google Scholar]
- 53. Yamadera S, Yamashita K, Akatsuka M, Kato N, Inouye S. 1998. Trend of adenovirus type 7 infection, an emerging disease in Japan. A report of the National Epidemiological Surveillance of Infectious Agents in Japan. Jpn. J. Med. Sci. Biol. 51:43–51 [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y, Bergelson JM. 2005. Adenovirus receptors. J. Virol. 79:12125–12131 [DOI] [PMC free article] [PubMed] [Google Scholar]