Abstract
A single-nucleotide polymorphism (A2254 or G2254) in open reading frame 30 (ORF30) has been linked to the neuropathogenic phenotype of equine herpesvirus-1 (EHV-1). Identification of this polymorphism led to the development of a real-time PCR (rPCR) assay using allelic discrimination (E2) to distinguish between potentially neuropathogenic and nonneuropathogenic EHV-1 strains (G. P. Allen, J. Vet. Diagn. Invest. 19:69–72, 2007). Although this rPCR assay can detect and genotype EHV-1 strains, subsequent studies demonstrated that it lacks the sensitivity for the routine detection of viral nucleic acid in clinical specimens. Therefore, a new allelic discrimination EHV-1 rPCR assay (E1) was developed by redesigning primers and probes specific to ORF30. The E1 and E2 rPCR assays were evaluated using 76 archived EHV isolates and 433 clinical specimens from cases of suspected EHV-1 infection. Nucleotide sequence analysis of ORF30 was used to confirm the presence of EHV-1 and characterize the genotype (A2254 or G2254) in all archived isolates plus 168 of the clinical samples. The E1 assay was 10 times more sensitive than E2, with a lower detection limit of 10 infectious virus particles. Furthermore, all A2254 and G2254 genotypes along with samples from three cases of dual infection (A2254+G2254) were correctly identified by E1, whereas E2 produced 20 false dual positive results with only one actual mixed A2254+G2254 genotype confirmed. Based on these findings, E1 offers greater sensitivity and accuracy for the detection and A/G2254 genotyping of EHV-1, making this improved rPCR assay a valuable diagnostic tool for investigating outbreaks of EHV-1 infection.
INTRODUCTION
Equine herpesvirus-1 (EHV-1) is a double-stranded DNA virus that infects the vast majority of the world's equine populations (4). Almost all domesticated horses are repeatedly exposed to this virus and as a result may experience significant morbidity and even mortality (26). Depending on host and/or viral factors, exposure to EHV-1 can result in respiratory disease, abortion, neonatal deaths, and neurologic disease (equine herpesvirus myeloencephalopathy [EHM]) (10, 12). In a high percentage of infected animals, EHV-1 establishes lifelong latent infections in long-lived cells, including the neurons within the trigeminal ganglia and/or lymphocytes in lymphoreticular tissues associated with the respiratory tract (4). Reactivation of latent virus can lead to recrudescence of disease with associated viral shedding, which may result in transmission of EHV-1 to susceptible horses (4, 12).
Since 2000, there has been a disturbing increase in the number of EHM outbreaks in Europe and North America (6, 7, 19, 31, 34, 35). Within the United States alone, the case fatality rate associated with some of these neurological outbreaks has been reported to be as high as 50% (34). Although it appears that all EHV-1 strains can induce respiratory disease and abortion in pregnant mares, only certain (neuropathogenic) strains have the potential to cause wide-scale outbreaks of EHM (3, 25). Within the past decade, a single-nucleotide polymorphism that appears to be associated with the neuropathogenic or nonneuropathogenic phenotype of EHV-1 has been identified (14, 25). This potential genetic marker is found within open reading frame 30 (ORF30), encoding the viral DNA polymerase, and consists of a single nonsynonymous A-to-G substitution at nucleotide (nt) 2254 (A→G2254), resulting in a change from neutral asparagine to negatively charged aspartic acid at amino acid position 752 (N→D752) (20, 25, 36). EHV-1 isolates with the A2254 genotype have been linked principally to nonneuropathogenic infections, while viruses possessing the G2254 genotype are frequently but not invariably associated with neurologic disease (24, 36). The discovery of this single-nucleotide polymorphism in ORF30 led to the development of a real-time PCR (rPCR) assay using allelic discrimination for the detection and differentiation of potentially neuropathogenic and nonneuropathogenic EHV-1 strains (1, 2).
The clinical signs of EHV-1-related respiratory disease can mimic those caused by other equine viral respiratory pathogens, such as EHV-4, equine influenza virus, equine arteritis virus (EAV), equine rhinitis virus A, and equine adenovirus 1 (29, 30). Similarly, EHV-1-induced abortions and neurologic disease must be differentiated from those caused by other infectious (EAV, EHV-4, West Nile virus, and Sarcocystis neurona) and noninfectious causes (30). When a disease outbreak occurs, confirmation of a provisional clinical diagnosis with a rapid, sensitive, and specific laboratory diagnostic test(s) is vital to ensure that appropriate biosecurity and quarantine measures are implemented without unnecessary delay. Several reports have documented the use of PCR-based assays, both standard and real-time, for the detection of EHV-1 in clinical specimens (2, 8, 9, 13, 18, 21, 32). However, the allelic-discrimination rPCR assay described by Allen (1) has a distinct advantage because it can simultaneously detect and genotype EHV-1 strains. This assay was originally validated using 234 clinical samples (nasal swab and blood samples) and was found to have a specificity of 100% along with a sensitivity of 96.3% for the detection of EHV-1 nucleic acids (1). Subsequent evaluation of clinical samples using the original rPCR assay in several diagnostic laboratories demonstrated that this assay lacks adequate sensitivity for routine diagnostic applications and is prone to generating false dual positive (A2254+G2254) results, seriously compromising its usefulness for A2254/G2254 genotype differentiation (U. B. R. Balasuriya and K. L. Smith, unpublished data; S. Sells and B. Crossley, unpublished data). Additionally, false-negative results are produced in this assay by the presence of a single additional nucleotide substitution within ORF30, at position 2258 (35). Although numerous studies have examined the validity and efficiency of EHV-1 rPCR-based assays as diagnostic and research tools, there remains an urgent need for an assay that will enable reliable detection of EHV-1 coupled with simultaneous A/G2254 genotyping directly from clinical material (16, 21–23, 32, 33). Therefore, the primary objective of this study was to develop a new allelic discrimination EHV-1 rPCR assay, compare its sensitivity and specificity with those of the assay described by Allen (1), and thereby determine which assay is more reliable for detection of EHV-1 nucleic acid in a diagnostic setting.
MATERIALS AND METHODS
Cells.
Fetal equine dermis (KyED) cells were maintained as confluent monolayers in 150-cm2 culture flasks using Eagle's minimal essential medium (EMEM; Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum and gentamicin (50 μg/ml; Invitrogen) as previously described (5). In this study, the KyED cells were used between passages 9 and 12. A low-passage-number cell line, rabbit kidney-13 (RK-13, passage level 194 to 204; ATCC CCL-37; American Type Culture Collection, Manassas, VA), was maintained in EMEM (Mediatech, Inc., Herndon, VA) supplemented with 10% ferritin-supplemented bovine calf serum (HyClone Laboratories, Inc., Logan, UT), 1% penicillin and streptomycin (10,000 IU/ml and 10,000 μg/ml; Mediatech), and 0.1% amphotericin B (1,000 μg/ml; Sigma-Aldrich, St. Louis, MO).
Archived viruses.
Viral nucleic acids were extracted from archived, cell culture-isolated EHV-1 and EHV-4 strains from the United States (1941 to 2006) and Europe (United Kingdom, 1981; France, 2004 to 2006) that had been stored as either lyophilized or frozen (−70°C) tissue culture fluid (TCF) stocks (n = 76). Working stocks of EHV-1 strains from the United States and the United Kingdom were generated in confluent monolayers of KyED cells as previously described (35) and were confirmed as either EHV-1 (n = 38) or EHV-4 (n = 16) by DNA sequencing (G. P. Allen, unpublished data). EHV-1 strains from France were isolated in RK-13 cells and identified as EHV-1 (n = 22) by sequencing ORF30 at the Gluck Equine Research Center, Lexington, KY (Y. Li and U. B. R. Balasuriya, unpublished data).
Clinical samples.
A total of 433 clinical specimens, comprising 260 nasal swabs and 173 buffy coat samples, were included in this study. Of these, 168 were samples from EHV outbreaks or single cases of infection that occurred in Kentucky between 2001 and 2006. These samples were initially identified as positive for EHV-1 or EHV-4 by nested PCR, using reaction conditions and primers from a previously published study (11). In addition, the genotype (A2254 or G2254) of each EHV-1 isolate was determined by sequencing ORF30 (G. P. Allen, unpublished data). The remaining clinical samples (n = 265) were collected from outbreaks and submitted between 2008 and 2011 to the University of Kentucky Veterinary Diagnostic Laboratory or to the Gluck Equine Research Center for routine diagnostic investigation.
Viral nucleic acid purification.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation over Ficoll-Plaque Plus (Amersham Biosciences, Piscataway, NJ) from buffy coats of heparinized blood collected from horses (3). The PBMC layer was collected and washed twice with PBS (pH 7.4) by centrifugation, and the final cell pellet was resuspended at 1 × 106 cells/ml in PBS (pH 7.4). These cells were used for virus isolation and DNA extraction.
DNA extraction from archived and clinical materials was performed using four different methods. All purified archived viruses from the United States and the United Kingdom (1941 to 2006) were treated with proteinase K (20 mg/ml in Tris-EDTA [TE buffer] containing 50% glycerol [pH 8.0]; Invitrogen) prior to deproteination with phenol-chloroform and DNA extraction (5). For the 2001–2006 clinical specimens, a High-Pure PCR template preparation kit (Roche, Indianapolis, IN) was used to extract DNA from nasal swabs, while a Wizard genomic DNA purification system (Promega, Madison, WI) was used for all buffy coat samples. In both cases, nucleic acids were extracted according to the manufacturer's instructions and stored at −20°C.
In addition to the techniques outlined above, clinical materials collected between 2008 and 2011, along with cell culture EHV-1 isolates from France (2004 to 2006), were processed utilizing a KingFisher 96 automatic nucleic acid extraction machine (Thermo Fischer Scientific, Inc., Waltham, MA) in conjunction with a MagMax-96 viral RNA isolation kit (Applied Biosystems, Foster City, CA). This nucleic acid extraction kit is recommended for both DNA and RNA isolation from clinical specimens by the manufacturer. Starting material for the automated extraction procedure consisted of 50 μl of clarified supernatant (13,800 × g for 2 min). The DNA was eluted in 50 μl of elution buffer, divided into 25-μl aliquots, and stored at −20°C.
PCR amplification and sequencing of ORF30.
ORF30 and the flanking sequences (forward primer, 5′-GACATGGATATACCAACGGTTAGT-3′ [nt 51,401 to 51,424]; reverse primer, 5′-TTTAAAGCTAAATCTAAACACGCCC-3′ [55,206 to 55,230]; sequences are numbered according to GenBank accession number AY464052; 3,830 bp) of all archived specimens and 168 of the clinical samples were amplified with EHV-1-specific primers using Phusion Hot Start DNA polymerase (New England BioLabs, Ipswich, MA). The 50-μl PCR mixture for each reaction contained 10 μl of 5× Phusion HF buffer, 1 μl of 10 mM deoxynucleoside triphosphate (dNTP) mix, 0.5 μl of Phusion Hot Start DNA polymerase, 34 μl of RNase-free water, 1.0 μl of the forward primer, 1.0 μl of the reverse primer (final concentration of each primer, 400 nM), and 2.5 μl of the template DNA. The PCR amplification was performed in an Eppendorf thermal cycler using the following amplification parameters: 98°C for 30 s, followed by 35 cycles at 98°C for 10 s, 55.7°C for 30 s, and 72°C for 2 min with a final extension of 72°C for 10 min. The PCR products were analyzed on a 1% agarose gel and purified using a QIAquick gel extraction kit (Qiagen, Valencia, CA). Both sense and antisense strands were sequenced with a Prism Ready Reaction DyeDeoxy Terminator cycle sequencing kit (MWG Operon, Huntsville, AL). Sequence data were analyzed with CodonCode (Codon Code Corp., Dedham, MA) and VectorNTI (Invitrogen) software. PCR products exhibiting sequence ambiguities at position 2254 were cloned into a pDrive cloning vector using a Qiagen PCR cloning kit (Qiagen), according to the manufacturer's instructions. A minimum of six molecular clones were sequenced from each PCR amplicon using both sense and antisense primers specific for EHV-1 ORF30.
Duplex rPCR assays E1 and E2.
A new set of EHV-1 ORF30-specific primers and probes were designed to target the conserved regions of ORF30 using Primer Express software v3.0 (Applied Biosystems) (Table 1). The relative positions of the primers and probes within ORF30 utilized in each assay are shown in Fig. 1. For a 25-μl reaction mixture, 5 μl of viral DNA was combined with 20 μl of master mix, composed of the following: 1.25 μl of the A2254 primer-probe mix (400 nM [each] primer and 200 nM probe), 1.25 μl of the G2254 primer-probe mix (400 nM [each] primer and 175 nM probe), 12.5 μl of QuantiTect Multiplex PCR Master Mix (Qiagen), and 5 μl of nuclease-free water. Using an ABI 7500 fast real-time PCR system (Applied Biosystems), the following thermocycling conditions were used with the fast 7500 mode: initial denaturation at 95°C for 15 min, followed by 40 cycles at 95°C for 15s and 58°C for 1 min. The previously published E2 allelic discrimination rPCR assay was performed as originally reported, with the exception that the cycle number was reduced from 55 to 40 (1).
Table 1.
EHV-1 ORF30 specific primer/probe sets used in E1 and E2 allelic-discrimination rPCR assays
| Assay | Primer or probe (sense) | Probe specificitya | Location (nt)b | Sequence (5′–3′) |
|---|---|---|---|---|
| E1 | E1Fwd (positive) | NA | 2229–2245 | TCT GGC CGG GCT TCA AC |
| E1Rev (negative) | NA | 2276–2294 | TTT GGT CAC CCA CCT CGA A | |
| E1PrA2254 (positive) | A2254 | 2247–2262 | 5HEX-ATC CGT CAA CTA CTC G-BHQ2a | |
| E1PrG2254 (positive) | G2254 | 2247–2262 | 6∼FAM-ATC CGT CGA CTA CTC G-BHQ1 | |
| E2 | E2Fwd (positive) | NA | 2204–2218 | CCA CCC TGG CGC TCG |
| E2Rev (negative) | NA | 2328–2348 | AGC CAG TCG CGC AGC AAG ATG | |
| E2PrA2254 (positive) | A2254 | 2246–2261 | VIC-CAT CCG TCA ACT ACT C-MGB | |
| E2PrG2254 (positive) | G2254 | 2248–2261 | 6-FAM-CAT CCG TCG ACT ACT C-MGB |
NA, not applicable.
Nucleotides are numbered according to GenBank accession number AY464052.
Fig 1.
Positions of the E1 and E2 primers and probes within ORF30. The E1 assay produces an amplicon of 66 bp, whereas the E2 amplicon is 145 bp.
Each rPCR assay included a control without DNA (20 μl of master mix plus 5 μl of nuclease-free water), along with EHV-1 DNA samples containing known G2254 and A2254 genotypes. Results were presented as a plot of PCR cycle number versus the accumulated level of fluorescence (Rn) from each of the reporter probes. If the plot line of a given test sample did not enter the exponential phase by cycle threshold (CT) 39, the sample was considered negative. The specificity of both assays for EHV-1 was confirmed by using nucleic acid extracted from TCFs containing either EHV-2, EHV-3, EHV-4, or EHV-5.
Determination of the analytical sensitivity of the rPCR assays.
Viral DNA purified from serial 10-fold dilutions (10−1 to 10−8) of TCF, containing either EHV-1 strain A183 (G2254 neuropathogenic genotype [17]) or T220 (A2254 nonneuropathogenic genotype [3]), was used to ascertain the analytical sensitivity of each rPCR assay. The TCF was clarified by microcentrifugation at 13,800 × g for 2 min, with 50 μl of the resultant supernatant being used for DNA extraction, utilizing a MagMax-96 viral RNA isolation kit (Applied Biosystems) in conjunction with a MagMax Express particle processor (Applied Biosystems), according to the manufacturer's instructions. Viral DNA from each of the serial dilutions was eluted in 50 μl of elution buffer, and 5 μl was tested in triplicate with the E1 and E2 assays. This determination was repeated independently twice on different days. The plaque number in the highest dilution was used to calculate the number of infectious particles that can be detected by each assay.
Statistical methods.
The samples that were verified by sequencing were used as a gold standard in the calculations of sensitivity and specificity. Confidence intervals (CI) were calculated using the Clopper-Pearson approach. All confidence intervals reported in this study were at the 95% level. Hypothesis tests regarding equal sensitivity between assays were conducted using Fisher's exact test.
RESULTS
Development of E1 allelic discrimination rPCR assay.
The primer and probe sets (Fig. 1) designed in this study were highly specific for EHV-1 and did not cross-react with any of the other equine herpesviruses (EHV-2, EHV-3, EHV-4, and EHV-5) under the same assay conditions. Two primers (E1Fwd and E1Rev) designed for a highly conserved region of ORF30 of EHV-1 yielded a significantly smaller PCR product (66 bp) than those in the E2 assay (145 bp). The new probes specific for the nonneuropathogenic and neuropathogenic genotypes (E1PrA2254 and E1PrG2254, respectively) are different from the probes described for the E2 assay, although there is significant overlap, since they are directed against the same region of ORF30 (Fig. 1). The E1 PCR assay was optimized using DNA extracted from TCF containing EHV-1 A183 and T220, with different primer and probe combinations tested over a range of concentrations using QuantiTect multiplex PCR master mix reagents (Qiagen) in a series of checkerboard assays. The optimal primer and probe concentrations producing the highest specificity and sensitivity for detection and discrimination of A2254 and G2254 genotypes were selected for the final assay described in Materials and Methods.
Analytical sensitivities of E1 and E2.
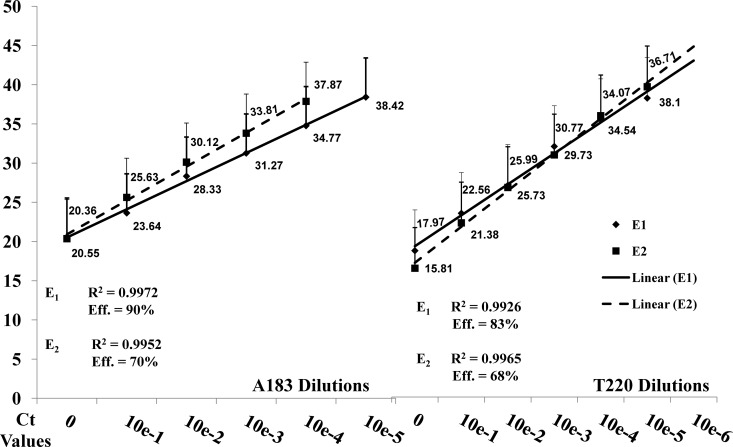
The analytical sensitivities of the E1 and E2 assays were determined using DNA purified from 10-fold dilutions of TCF containing EHV-1 strains A183 (titer, 1.5 × 105 PFU/ml) and T220 (5 × 106 PFU/ml). The two viruses were titrated in equine endothelial cells, and 50 μl from each dilution was used for DNA extraction. Detection of A183 (G2254) and T220 (A2254) EHV-1 DNA was linear for TCF dilutions from 100 to 10−6, with intra- and interassay variability of less than 1%. The detection limits of the E2 assay were 100 infectious virus particles (average CT = 37.87) for A183 and 10 infectious virus particles (average CT = 38.10) for T220. By comparison, the E1 assay was capable of detecting 10 infectious virus particles of each strain (A183 CT = 38.42; T220 CT = 36.71). The coefficients of determination (R2) and amplification efficiencies ([10(−1/slope) − 1] × 100) were calculated for each data set. For A183, E1 yielded an amplification efficiency of 90% and an R2 value of 0.9972. For E2, the amplification efficiency was 70%, and R2 was 0.9952. With the T220 dilutions, E1 produced an amplification efficiency of 83%, and R2 was 0.9926. The amplification efficiency for E2, however, was only 68%, with an R2 value of 0.9965 (Fig. 2).
Fig 2.
Regression analysis of the A183 and T220 serial dilutions with E1 and E2. The average CT values for each dilution were generated from two runs of each assay and then used to plot a linear trend line. The coefficients of determination (R2) and amplification efficiencies are listed, along with average CT values for each dilution.
Evaluation of E1 and E2 rPCR assays using archived TCF and clinical samples.
The performance of the two rPCR assays was compared initially using DNA extracted from archived TCF (n = 76), containing either EHV-1 or EHV-4 nucleic acid. Of the 54 U.S./United Kingdom specimens, all EHV-1-positive samples were identified in the E1 assay with no cross-reactivity with EHV-4 samples (Table 2). Furthermore, the E1 assay accurately distinguished between the A2254 (17/17) and G2254 (21/21) polymorphisms present in ORF30. In contrast, the E2 assay failed to detect viral nucleic acid in two of the EHV-1-positive samples. With the archived French TCF, E1 successfully detected all 22 EHV-1 strains, while E2 failed to detect 1 of these isolates (Table 2). Overall, the specificity for both assays in terms of the archived cell isolated specimen group was 100% (CI, 79% to 100%). E1 demonstrated a greater diagnostic sensitivity than E2 (E1 sensitivity = 100%; CI, 94% to 100%; E2 sensitivity = 95%; CI, 86% to 99%), although the difference was not statistically significant (P = 0.49).
Table 2.
E1 and E2 real-time PCR results
| Specimen type and source (n) | No. of specimens with result |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E1 |
E2 |
|||||||||
| EHV-1 rPCR |
EHV1 genotype |
EHV-1 rPCR |
EHV1 genotype |
|||||||
| Negative | Positive | A2254 | G2254 | A2254+G2254 | Negative | Positive | A2254 | G2254 | A2254+G2254 | |
| Archived EHV TCF (76) | ||||||||||
| USA & UK (54) | 16a | 38 | 17 | 21 | 0 | 18a | 36 | 17 | 19 | 0 |
| France (22) | 0 | 22 | 15 | 7 | 0 | 1 | 21 | 14 | 7 | 0 |
| Clinical samples (2001–2011) (433) | 229b | 204 | 107 | 94 | 3 | 289b | 144 | 70 | 53 | 21 |
| Nasal swabs (260) | 151 | 109 | 60 | 46 | 3 | 178 | 82 | 42 | 19 | 21 |
| Buffy coat cells (173) | 78 | 95 | 47 | 48 | 0 | 111 | 62 | 28 | 34 | 0 |
Sixteen samples that were positive for EHV-4 by standard PCR were included as negative controls.
Forty-three samples that were positive for EHV-4 by DNA sequencing were included as negative controls.
Second, we used viral DNA extracted from 168 EHV-positive clinical samples (2001 to 2006) that had been sequenced to determine the specificity and sensitivity of the E1 and E2 assays. Previous direct sequencing of ORF30 demonstrated that of the 168 clinical samples, 125 were EHV-1 (60 [48%] A2254, 65 [52%] G2254), while the remaining 43 samples were EHV-4. Neither of the rPCR assays produced false-positive results in the presence of EHV-4 DNA (specificity, 100%; CI, 92% to 100%). The E1 assay successfully identified 110 of the 125 EHV-1-positive clinical samples (54 A2254, 56 G2254) (88%; CI, 81% to 93%). In contrast, the sensitivity of E2 was significantly lower (P < 0.001) with only 72 of the 125 EHV-1 samples identified correctly (33 A2254, 39 G2254) (58%; CI, 48% to 66%). The genotypes of all PCR-positive samples for both assays agreed perfectly with the direct sequencing results. The comparative sensitivities of the two assays are displayed in Fig. 3. In the case of both rPCR assays, the overall sensitivity scores for the 168 sequenced clinical samples were significantly reduced by the inclusion of 109 buffy coat samples. These buffy coats were shown to contain EHV-1 DNA via an ultrasensitive sequence capture nested PCR assay (2). However, 94 of these 109 samples were positive for EHV-1 DNA in the E1 assay, whereas only 56 gave positive reactions in E2.
Fig 3.
Sensitivities of E1 and E2 for EHV-1 (allele-specific forms [left and center columns] and overall [right column]) in sequenced clinical and archived samples. Lines indicate 95% confidence intervals; dots indicate point estimates. The P values test the hypothesis that E1 and E2 have equal sensitivities in the given sample.
The PCR results of all 433 clinical samples (2001 to 2006 [168 samples] and 2008 to 2011 [265 samples]) tested by both E1 and E2 are given in Table 2. Of these, 204 (43%) were identified as EHV-1 positive by the E1 assay, with 107 A2254 (58%) and 94 G2254 (41%) genotypes. Interestingly, 3 samples (2%) tested positive for both A2254 and G2254 genotypes (A2254+G2254 [dual genotype]) in the E1 assay (Fig. 4). In contrast, the E2 assay identified only 144 (35%) of these samples as EHV-1: 70 (53%) A2254, 53 (34%) G2254, and 21 (14%) A2254+G2254. Only one (F55-R4 [see below]) of the 21 samples that were dual positive in the E2 assay gave the same results in the E1 assay. Comparison of the CT values of the sequenced clinical samples and the archived viral specimens are given in Table 3.
Fig 4.
Amplification plot for F35-R3 as an example of a dual positive result produced by E1. The graph lines with slight arches are the reaction results for F35-R3, and the graph lines which remain in the baseline phase are water controls. The CT values for this specimen were 38.75 (A2254) and 36.99 (G2254).
Table 3.
CT values and genotypes of select archived and clinical specimens for E1 and E2
| Specimen | Genotype (CT) according to: |
||
|---|---|---|---|
| E1 | E2 | Sequencing data | |
| Archived | |||
| A2 | G2254 (28.90) | G2254 (31.06) | G2254 |
| A9 | A2254 (24.02) | A2254 (25.30) | A2254 |
| A32 | A2254 (18.34) | A2254 (24.57) | A2254 |
| Clinical | |||
| X1 | A2254 (36.99) | Negativea | A2254 |
| X15 | G2254 (35.80) | G2254 (38.06) | G2254 |
| X38 | G2254 (31.73) | G2254 (34.31) | G2254 |
False negative.
Sequence confirmation of dual ORF30 genotypes and resolution of discrepancies between the E1 and E2 assays.
Direct sequence analysis of the PCR products from three clinical samples (F14-R2, F35-R3, and F55-R4) that were positive for both genotypes in the E1 assay demonstrated sequence ambiguity at nt 2254, consistent with the presence of both virus genotypes. This finding was further confirmed by sequencing individual molecular clones derived from the PCR products (Table 4). Interestingly, molecular clones from all three samples showed another variable site at nt 2258 within the probe binding region of ORF30. Of the three samples that were dually positive in E1, only one (F55-R4) produced similar results in the E2 assay. Additional direct sequencing of ORF30 PCR products from a limited number of samples that gave dually positive results in the E2 assay failed to identify any ambiguities in the probe binding region.
Table 4.
Sequence analysis of molecular clones derived from the samples that had dual genotypes (A2254 and G2254) in the E1 assay
| Sample | No. of clones | ORF30 nucleotides at position: |
|
|---|---|---|---|
| 2254 | 2258 | ||
| F14-R2 | 7 | 5 G, 2 A | 5 C, 2 A |
| F35-R3 | 6 | 5 G, 1 A | 3 C, 3 A |
| F55-R4 | 17 | 16 G, 1 A | 14 C, 3 T |
DISCUSSION
The results from this study demonstrate that the newly developed E1 assay provides at least a 10-fold-higher sensitivity than the E2 assay and is therefore more appropriate for the detection of EHV-1 viral nucleic acid in clinical specimens. This increased sensitivity probably results from the fact the E1 assay involves a shorter amplicon length than E2, leading to increased efficiency and/or less susceptibility to secondary DNA structural effects (Fig. 1).
During these experiments, both rPCR assays were evaluated using a range of sample types and nucleic acid extraction techniques. The archived samples from the United States and the United Kingdom were isolated in cell culture prior to nucleic acid extraction with phenol-chloroform to remove protein contaminants. As the viruses in these samples were amplified by propagation in mammalian cells, they are predicted to contain greater quantities of viral DNA than nasal swabs or blood samples. Therefore, it is not surprising that while a slightly higher number of EHV-1-positive reactions were observed in the E1 assay (100%) than in E2 (95%), these did not result in statistically significant differences between the two tests. Although both rPCR tests performed well with archived TCF samples, EHV-1 isolation in cell culture is time-consuming and may be possible only for relatively short periods following exposure (4). For example, it has been demonstrated that certain strains of virus can be isolated from nasal swabs only up to 5 days postinfection, in contrast to rPCR, which can detect viral nucleic acid in nasal swabs after 21 days or more (28). While important for viral characterization and molecular epidemiological studies, virus isolation is not suited for the often urgent need for rapid diagnosis of EHV-1 infections. To meet this requirement during EHM and/or EHV abortion outbreaks, diagnostic assays must be capable of rapidly detecting EHV-1 nucleic acid directly in clinical specimens.
The data presented in this study suggest that E2, with an overall success rate of just 58% when nasal swab or buffy coat samples are used, is ill suited for the routine diagnosis of EHV-1 infections. Alternatively, the E1 assay is significantly more sensitive (88%), although it too was unable to detect EHV-1 in 15 whole blood samples shown to contain EHV-1 DNA by a magnetic-bead-based sequence capture nested PCR described by Allen et al. (2). This technique was specifically designed to detect EHV-1 DNA that is in low abundance in lymph nodes and buffy coat cells during clinically quiescent periods and relies upon oligonucleotide hybridization coupled with biotin-streptavidin magnetic-bead capture technology. As a result, this very powerful technology has a detection threshold limit well above those of conventional nested PCR and rPCR (2). Therefore, it is possible that the E1 assay was unable to detect EHV-1 DNA in the 15 buffy coat samples simply because of the low copy number present, which is not surprising considering that the amount of virus present in the blood is highly dependent on what stage of infection the virus is in when the sample is taken (e.g., latent or cell-associated viremia) (4). Alternatively, blood is known to contain inhibitors, including heme, lactoferrin, and immunoglobulins that when combined with low nucleic acid copy numbers can prevent detection by PCR-based methods. Furthermore, recent studies have demonstrated that at least some PCR inhibitors present in whole blood are not completely removed by common nucleic extraction techniques, including the MagMax-96 viral RNA isolation kit (15). Studies are under way to determine if, as shown by Das et al. (15), improvements in detection of low levels of EHV-1 DNA in blood can be achieved by modification of the MagMax-96 viral RNA isolation protocol by including additional high-salt washes.
The detection of both A2254 and G2254 genotypes in the same clinical specimen confirms previous findings and raises many questions about the impact that at least two simultaneously replicating virus strains can have on viral pathogenesis, latency and reactivation (32). While the ability to identify multiple genotypes within clinical samples represents a significant step in our understanding of the dynamics associated with in vivo EHV-1 replication events, false dual positive results are very detrimental in any diagnostic situation. Clearly, the E1 assay, with no evidence of false dual positive results coupled with the accurate detection of actual A2254+G2254 infections, performed significantly better than the E2 assay, which yielded 20 false dual positive results among the samples tested (Table 2). This result confirms observations by diagnosticians in the field. However, the false dual positive results generated by E2 occurred only with clinical samples processed using the MagMax-96 viral RNA isolation kit, suggesting a possible correlation between these aberrant reactions and the method employed for nucleic acid extraction. Although further studies are required to confirm this observation, it should be noted that the accuracy of ORF30 genotyping in the E1 assay was completely independent of the sample preparation technique.
Overall, this study has demonstrated that E1 is significantly more sensitive than E2 for the detection of EHV-1 in clinical samples. Furthermore, it produced fewer false dual positives, regardless of the DNA extraction procedure employed, and is therefore better suited than E2 for use in a routine diagnostic setting. In such an environment, an allelic-discrimination rPCR assay directed against ORF30 has the advantage over rPCR assays targeting other EHV-1 genes (e.g., gB and gD genes [3, 16, 18, 27, 32]) because it can both detect and discriminate between the A2254 and G2254 genotypes present in clinical samples. Although recent studies have suggested that possession of G2254 is not always associated with a neuropathogenic phenotype (31), additional data from field cases of EHM are required before this issue can be fully clarified. The widespread use of a more sensitive and more specific ORF30-based allelic-discrimination assay, coupled with thoroughly documented clinical histories, will play an important role in generating this essential information. However, regardless of the genotype detected, stringent quarantine and biosecurity practices should be implemented immediately to curtail the spread of EHV-1.
ACKNOWLEDGMENTS
This study was supported by the Grayson Jockey Club Research Foundation Inc., Lexington, KY, along with gifts and contracts to Udeni B. R. Balasuriya at the Gluck Equine Research Center, Department of Veterinary Science, University of Kentucky, Lexington, KY.
Footnotes
Published ahead of print 4 April 2012
REFERENCES
- 1. Allen GP. 2007. Development of a real-time polymerase chain reaction assay for rapid diagnosis of neuropathogenic strains of equine herpesvirus-1. J. Vet. Diagn. Invest. 19:69–72 [DOI] [PubMed] [Google Scholar]
- 2. Allen GP, et al. 2008. Prevalence of latent, neuropathogenic equine herpesvirus-1 in the Thoroughbred broodmare population of central Kentucky. Equine Vet. J. 40:105–110 [DOI] [PubMed] [Google Scholar]
- 3. Allen GP, Breathnach CC. 2006. Quantification by real-time PCR of the magnitude and duration of leucocyte-associated viraemia in horses infected with neuropathogenic vs. non-neuropathogenic strains of EHV-1. Equine Vet. J. 38:252–257 [DOI] [PubMed] [Google Scholar]
- 4. Allen GP, Kydd JH, Slater JD, Smith KC. 2004. Equid herpesvirus-1 (EHV-1) and -4 (EHV-4) infections, p 829–859 In Coetzer JAW, Tustin RC. (ed), Infectious diseases of livestock, 2nd ed, vol 2 Oxford University Press Southern Africa, Cape Town, South Africa [Google Scholar]
- 5. Allen GP, O'Callaghan DJ, Randall CC. 1977. Genetic relatedness of equine herpesvirus types 1 and 3. J. Virol. 24:761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen GP, Timoney PJ. 2008. Recent advances in our understanding of equine herpesvirus-1 (EHV-1) myeloencephalopathy, p 373–380 In Richey M. (ed), Proceedings of the 111th Annual Meeting of the United States Animal Health Association Richardson Printing, Kansas City, MO [Google Scholar]
- 7. APHIS 2011. Equine herpesvirus (EHV-1): final situation report. U.S. Department of Agriculture, Washington, DC [Google Scholar]
- 8. Ballagi-Pordany A, Klingeborn B, Flensburg J, Belak S. 1990. Equine herpesvirus type 1: detection of viral DNA sequences in aborted fetuses with the polymerase chain reaction. Vet. Microbiol. 22:373–381 [DOI] [PubMed] [Google Scholar]
- 9. Bell SA, et al. 2006. Temporal detection of equine herpesvirus infections of a cohort of mares and their foals. Vet. Microbiol. 116:249–257 [DOI] [PubMed] [Google Scholar]
- 10. Boehmer PE, Nimonkar AV. 2003. Herpes virus replication. IUBMB Life 55:13–22 [DOI] [PubMed] [Google Scholar]
- 11. Borchers K, Slater J. 1993. A nested PCR for the detection and differentiation of EHV-1 and EHV-4. J. Virol. Methods 45:331–336 [DOI] [PubMed] [Google Scholar]
- 12. Borchers K, Thein R, Sterner-Kock A. 2006. Pathogenesis of equine herpesvirus-associated neurological disease: a revised explanation. Equine Vet. J. 38:283–287 [DOI] [PubMed] [Google Scholar]
- 13. Carvalho R, Passos LM, Martins AS. 2000. Development of a differential multiplex PCR assay for equine herpesvirus 1 and 4 as a diagnostic tool. J. Vet. Med. 47:351–359 [DOI] [PubMed] [Google Scholar]
- 14. Crabb BS, et al. 1995. A type-specific serological test to distinguish antibodies to equine herpesviruses 4 and 1. Arch. Virol. 140:245–258 [DOI] [PubMed] [Google Scholar]
- 15. Das A, Beckham TR, McIntosh MT. 2011. Comparison of methods for improved RNA extraction from blood for early detection of classical swine fever virus by real-time reverse transcription polymerase chain reaction. J. Vet. Diagn. Invest. 23:727–735 [DOI] [PubMed] [Google Scholar]
- 16. Diallo IS, Hewitson G, Wright L, Rodwell BJ, Corney BG. 2006. Detection of equine herpesvirus type 1 using a real-time polymerase chain reaction. J. Virol. Methods 131:92–98 [DOI] [PubMed] [Google Scholar]
- 17. Doll ER. 1953. Intrauterine and intrafetal inoculations with equine abortion virus in pregnant mares. Cornell Vet. 43:112–121 [PubMed] [Google Scholar]
- 18. Galosi CM, et al. 2001. A polymerase chain reaction for detection of equine herpesvirus-1 in routine diagnostic submissions of tissues from aborted foetuses. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:341–346 [DOI] [PubMed] [Google Scholar]
- 19. Goehring LS, van Winden SC, van Maanen C, Sloet van Oldruitenborgh-Oosterbaan MM. 2006. Equine herpesvirus type 1-associated myeloencephalopathy in The Netherlands: a four-year retrospective study (1999–2003). J. Vet. Intern. Med. 20:601–607 [DOI] [PubMed] [Google Scholar]
- 20. Goodman LB, et al. 2007. A point mutation in a herpesvirus polymerase determines neuropathogenicity. PLoS Pathog. 3:e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussey SB, et al. 2006. Detection and quantification of equine herpesvirus-1 viremia and nasal shedding by real-time polymerase chain reaction. J. Vet. Diagn. Invest. 18:335–342 [DOI] [PubMed] [Google Scholar]
- 22. Leutenegger CM, et al. 2008. Detection of EHV-1 neuropathogenic strains using real-time PCR in the neural tissue of horses with myeloencephalopathy. Vet. Rec. 162:688–690 [DOI] [PubMed] [Google Scholar]
- 23. Malik P, Palfi V, Balint A. 2010. Development of a new primer-probe energy transfer method for the differentiation of neuropathogenic and non-neuropathogenic strains of equine herpesvirus-1. J. Virol. Methods 169:425–427 [DOI] [PubMed] [Google Scholar]
- 24. Mumford JA, et al. 1994. Abortigenic and neurological disease caused by experimental infection with equid herpesvirus-1, p 261–275 In Nakajima H, Plowright W. (ed), Equine infectious diseases VII. Proceedings of the Seventh International Conference on Equine Infectious Diseases R&W Publications, Newmarket, United Kingdom [Google Scholar]
- 25. Nugent J, et al. 2006. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 80:4047–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel JR, Heldens J. 2005. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)—epidemiology, disease and immunoprophylaxis: a brief review. Vet. J. 170:14–23 [DOI] [PubMed] [Google Scholar]
- 27. Perkins GA, Goodman LB, Dubovi EJ, Kim SG, Osterrieder N. 2008. Detection of equine herpesvirus-1 in nasal swabs of horses by quantitative real-time PCR. J. Vet. Intern. Med. 22:1234–1238 [DOI] [PubMed] [Google Scholar]
- 28. Perkins GA, et al. 2009. Investigation of the prevalence of neurologic equine herpes virus type 1 (EHV-1) in a 23-year retrospective analysis (1984–2007). Vet. Microbiol. 139:375–378 [DOI] [PubMed] [Google Scholar]
- 29. Powell DG. 1991. Viral respiratory disease of the horse. Vet. Clin. North Am. Equine Pract. 7:27–52 [DOI] [PubMed] [Google Scholar]
- 30. Powell DG, et al. 1988. The application of advanced molecular techniques to investigate epizootics of infectious disease in the equine population. Acta Vet. Scand. Suppl. 84:337–339 [PubMed] [Google Scholar]
- 31. Pronost S, Cook RF, Fortier G, Timoney PJ, Balasuriya UB. 2010. Relationship between equine herpesvirus-1 myeloencephalopathy and viral genotype. Equine Vet. J. 42:672–674 [DOI] [PubMed] [Google Scholar]
- 32. Pusterla N, et al. 2011. Surveillance programme for important equine infectious respiratory pathogens in the U. S. A. Vet. Rec. 169:12. [DOI] [PubMed] [Google Scholar]
- 33. Pusterla N, et al. 2009. Characterization of viral loads, strain and state of equine herpesvirus-1 using real-time PCR in horses following natural exposure at a racetrack in California. Vet. J. 179:230–239 [DOI] [PubMed] [Google Scholar]
- 34. Slater JD, et al. 2006. Report of the equine herpesvirus-1 Havermeyer Workshop, San Gimignano, Tuscany, June 2004. Vet. Immunol. Immunopathol. 111:3–13 [DOI] [PubMed] [Google Scholar]
- 35. Smith KL, et al. 2010. The increased prevalence of neuropathogenic strains of EHV-1 in equine abortions. Vet. Microbiol. 141:5–11 [DOI] [PubMed] [Google Scholar]
- 36. Van de Walle GR, et al. 2009. A single-nucleotide polymorphism in a herpesvirus DNA polymerase is sufficient to cause lethal neurological disease. J. Infect. Dis. 200:20–25 [DOI] [PubMed] [Google Scholar]