Abstract
We studied the presence of primary resistance to raltegravir (RAL), natural polymorphisms, and selection pressure on HIV-1 integrase. We found a high frequency of integrase polymorphisms related to the resistance to RAL and sequence stability. Further studies are needed to determine the importance of these polymorphisms to RAL resistance.
TEXT
Raltegravir (RAL) is the first integrase (IN) inhibitor to have been approved and is indicated for HIV-1 patients who are resistant to multiple antiretroviral agents (4, 8, 11). Studies have shown that three point mutations with or without secondary mutations can lead to RAL resistance: N155H (L74M, E92Q, G163R), Q148H/K/R (E138K, G140S/A), and Y143R (4). Transmission of resistant HIV-1 viruses is a concern and can affect therapeutic strategies. It is not clear whether genetic polymorphisms influence the antiretroviral efficacy of RAL. In this study, we examined the frequency of transmitted resistance mutations and naturally occurring polymorphisms in HIV-1 B and non-B subtypes and the selective pressure on IN domains. A total of 100 HIV-1-infected patients with highly active antiretroviral therapy (HAART) failure were included in our study (HIV RNA ≥ 5,000 copies/ml and T CD4 cell count ≤ 350 cells/mm3). Each patient was naïve with respect to IN inhibitors, and all patients received different HAART schemes. A total of 34% of the patients were receiving 2 nucleoside reverse transcriptase inhibitors (NRTI) plus 1 protease inhibitor (PI), 22% 2 NRTI plus 1 non-NRTI (NNRTI), 16% 2 NRTI plus 2 PIs, 10% 3 NRTI plus 1 PI, 7% 2 NRTI plus 1 NNRTI plus 1 PI, 3% 1 NRTI plus 1 NNRTI plus 1 PI, 2% 3 NRTI plus 1 NNRTI, 2% 2 NRTI plus 3 PIs, 1% 3 NRTI, 1% 1 NRTI plus 1 PI, 1% 1 NRTI plus 1 NNRTI plus 1 PI, and 1% 2 NRTI plus sulfamethoxazole and trimethoprim (Bactrim). Samples were collected between 2006 and 2007. The study was approved by the Ethics Committees and the Institutional Review Board of the Federal University of Sao Paulo (no. 0595/09). We analyzed three functional IN domains: the N-terminal domain (NTD; amino acids 1 to 50), the catalytic core domain (CCD; 51 to 212), and the C-terminal domain (CTD; 213 to 288). HIV-1 RNA was extracted from plasma by the use of a QIAamp viral RNA minikit, and reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) and nested PCR to amplify 1,085 bp of the IN gene. The sequencing was performed using an ABI Prism 3130 Genetic Analyzer (Applied Biosystems Inc., Foster City, CA).
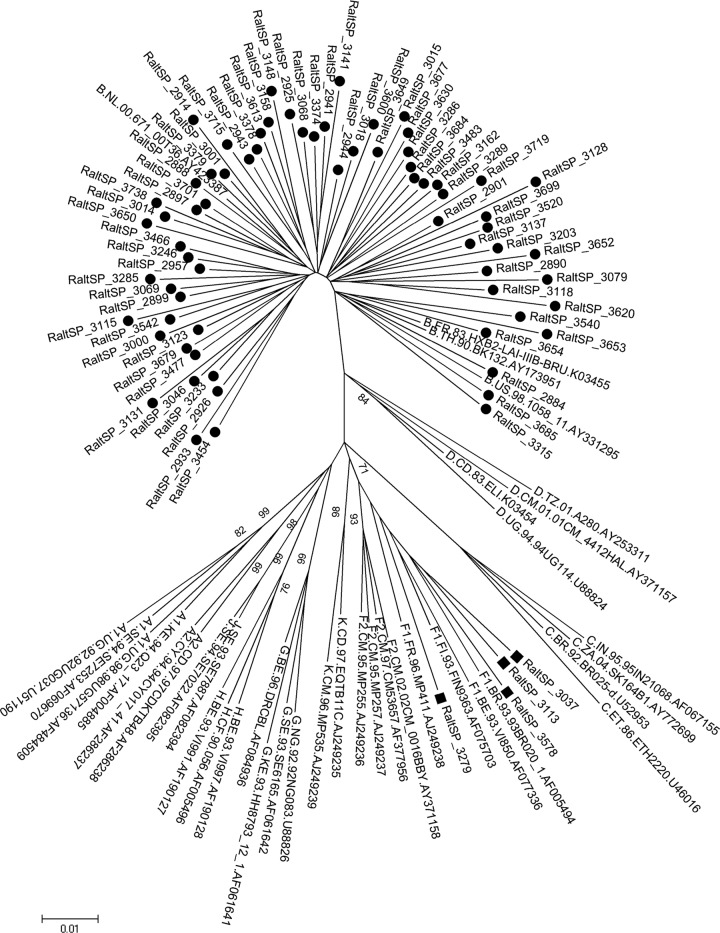
The sequences were analyzed using Sequencher version 4.2 software (Gene Code, Ann Arbor, MI). Alignments were performed using Bioedit software (Carlsbad, CA). The mutations were determined using the National Agency for Aids Research (11). Polymorphisms were examined according to Low et al. (7). The Nei-Gojobori method was used to calculate the ratio of nonsynonymous to synonymous evolutionary changes (dS/dN) (3). Each subtype was mapped to a phylogenetic tree (bootstrap with 1,000 replications). The recombinant strains were analyzed using the Rega HIV subtyping tool. Phylogenetic relationships between the individual sequence types were determined using the neighbor-joining algorithm of MEGA version 4 software (MEGA4: Molecular Evolutionary Genetics Analysis). Frequencies of resistance mutations were calculated using the Fisher exact test (Minitab version 16). The mean CD4+ T-cell count and median HIV-1 load were 102.65 cells/mm3 (range, 2 to 249) and 4.66 log10 copies/ml (range, 3.70 to 5.87), respectively. Among the subjects in the study, 43 were females and 60 were born in the city of São Paulo. The overall analysis revealed that 85% of patients were infected with subtype B and 6% with subsubtype F1. Phylogenetic analysis classified 9% of the sequences as BF1 recombinants. Figure 1 shows the phylogenetic tree map of the 80 full-length sequences. Overall data among the NTD, CCD, and CTD of the IN region showed dS/dN values > 1. As expected, the residues involved in catalytic activity and zinc binding were fully conserved. No transmission of mutations associated with resistance to RAL was observed in the 100 sequences; however, we did find a secondary mutation, G163R/E/V/Q (9%), and the following polymorphisms related to reduced sensitivity to RAL in vitro: V72I (59%), L74I/M (3%), T97A (1%), T125A/V/M/Q (36%), V151I (18%), M154L (3%), M154I (1%), K156N/R (14%), E157Q (1%), V165I (10%), V201I (54%), I203M (5%), T206S (13%), and S230N/G (8%) (Fig. 2). The V201I and T125A polymorphisms showed increased frequencies in non-B clades, which reached levels of 93% and 46% in the non-B subtypes versus 47% and 30% in the B subtypes, respectively. The M154I was present only in the non-B clades (6.7%), unlike S230N, which was present only in the B subtypes at a frequency of 9.4%. The T206S and L74I polymorphisms did not correlate with a subtype. Despite the absence of mutations associated with transmission of resistance to RAL, we found a high frequency of polymorphisms, previously described (8), related to the reduced sensitivity to RAL in vitro. We also observed the G163R polymorphism, which is capable of restoring viral fitness associated with N155H (12). It is unknown whether G163E/V/Q has a similar role in the IN gene. In fact, in other studies realized with Brazilian samples, a high proportion of patients presenting with accessory mutations and natural polymorphisms was revealed (1, 9). On the other hand, in another Brazilian study, the authors observed low levels of mutations associated with transmitted resistance to RAL (6). The dS/dN values indicated an absence of selection pressure. Because the patients in our study were naïve with respect to IN inhibitors, it is possible that inhibitors that target other HIV-1 enzymes indirectly applied selection pressure on the IN gene, as observed by Ceccherini-Silberstein et al. (3). Their study found a higher frequency of select IN polymorphisms in AIDS-associated retrovirus (ARV)-treated patients than in drug-naïve patients. We noticed a high frequency of non-B strains, including BF recombinants, which are more polymorphic than the B subtype (5). Garrido et al. found that polymorphisms such as T206S, L74I, T125A, and V201I were significantly more frequent in non-B strains than in the clade B viruses, unlike M154I, E157Q, and S230R/N, which were found more frequently in the B clade (5). That result is in agreement with our findings, with the exceptions of L74I and T206S, which did not correlate with a subtype, and M154I, which was absent in the B clade. Despite the associations between the genetic polymorphisms and the HIV-1 subtypes that we observed, note that we analyzed only a few non-B samples. We also observed that the BF recombinants were not restricted to the city of Santos, where they were first reported and showed an increased frequency, but were also present in other regions of Brazil (2). According to our results, the HIV-1 IN gene is a polymorphic gene and may influence the genetic barrier to RAL treatment. We did not find mutations in residues critical for HIV-1 IN activity, and IN stabilization was maintained. We conclude that RAL would benefit patients who are naïve with respect to integrase inhibitors and who have failed multiple ARV regimens. Further studies are needed to determine the importance of these polymorphisms in reducing the genetic barrier to RAL resistance, especially in HIV-1 non-B subtypes.
Fig 1.
Phylogenetic analysis of integrase sequences encompassing 288 amino acids. The tree was constructed using the neighbor-joining (NJ) method and MEGA version 4 software. Bootstrap analysis was performed with 1,000 replications. Filled circles represent the subtype B sequences, and filled squares represent the F1 subtype.
Fig 2.
Amino acid alignment of integrase sequences showing main secondary mutations and polymorphisms. The header shows the HXB2 amino acid consensus obtained from the Los Alamos HIV sequence database. •, no amino acid exchange in relation to HXB2 consensus sequence AF033819.
ACKNOWLEDGMENTS
We thank Daniela Teixeira and Erika Fusuma for organization of the samples and Antonio Charlys da Costa for administrative assistance and secretarial help. We are also grateful to the patients enrolled in this study.
This work was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (grant 09/05712-3 to S.V.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare that no competing interests exist.
Footnotes
Published ahead of print 7 March 2012
REFERENCES
- 1. Arruda LB, Fonseca LA, Duarte AJ, Casseb J. 2010. Genetic diversity on the integrase region of the pol gene among HIV type 1-infected patients naive for integrase inhibitors in Sao Paulo City, Brazil. AIDS Res. Hum. Retroviruses 26:105–107 [DOI] [PubMed] [Google Scholar]
- 2. Canducci F, et al. 2010. Genotypic/phenotypic patterns of HIV-1 integrase resistance to raltegravir. J. Antimicrob. Chemother. 65:425–433 [DOI] [PubMed] [Google Scholar]
- 3. Ceccherini-Silberstein F, et al. 2010. Specific HIV-1 integrase polymorphisms change their prevalence in untreated versus antiretroviral-treated HIV-1-infected patients, all naive to integrase inhibitors. J. Antimicrob. Chemother. 65:2305–2318 [DOI] [PubMed] [Google Scholar]
- 4. Fransen S, et al. 2009. Loss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathways. J. Virol. 83:11440–11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garrido C, et al. 2010. Integrase variability and susceptibility to HIV integrase inhibitors: impact of subtypes, antiretroviral experience and duration of HIV infection. J. Antimicrob. Chemother. 65:320–326 [DOI] [PubMed] [Google Scholar]
- 6. Gräf T, et al. 2011. HIV-1 genetic diversity and drug resistance among treatment naive patients from Southern Brazil: an association of HIV-1 subtypes with exposure categories. J. Clin. Virol. 51:186–191 [DOI] [PubMed] [Google Scholar]
- 7. Low A, et al. 2009. Natural polymorphisms of human immunodeficiency virus type 1 integrase and inherent susceptibilities to a panel of integrase inhibitors. Antimicrob. Agents Chemother. 53:4275–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY [Google Scholar]
- 9. Passaes CB, et al. 2009. Lack of primary mutations associated with integrase inhibitors among HIV-1 subtypes B, C, and F circulating in Brazil. J. Acquir. Immune Defic. Syndr. 51:7–12 [DOI] [PubMed] [Google Scholar]
- 10. Shafer RW, Schapiro JM. 2008. HIV-1 drug resistance mutations: an updated framework for the second decade of HAART. AIDS Rev. 10:67–84 [PMC free article] [PubMed] [Google Scholar]
- 11. Sichtig N, et al. 2009. Evolution of raltegravir resistance during therapy. J. Antimicrob. Chemother. 64:25–32 [DOI] [PubMed] [Google Scholar]
- 12. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]