Abstract
We describe here the clinical significance of coinfection with Theileria orientalis and Babesia ovata in cattle. Anemia status in a herd of dairy cattle in Japan was investigated in relation to infection with these parasites. Our findings indicate that while B. ovata infection might not be the primary cause of anemia in the cattle, it may contribute to the clinical development of anemia in animals coinfected with both B. ovata and T. orientalis.
TEXT
The benign group of Theileria parasites (Theileria sergenti, Theileria buffeli, and Theileria orientalis) and Babesia ovata constitute the only bovine piroplasms that are known to be endemic in Japan (1, 7, 13). T. orientalis is not considered to be highly pathogenic; however, clinical signs of anemia have sometimes been observed in cattle affected by this parasite (5, 8). In contrast, previous studies of splenectomized calves indicate that only immunocompromised animals exhibit anemia-related clinical signs when they are infected with B. ovata (3). Both T. orientalis and B. ovata are transmitted to the bovine host by Haemaphysalis longicornis, which is an ixodid tick vector (6). Therefore, mixed infections with the two parasites are expected to be very common in areas of endemicity (3). Although several researchers have focused on the clinical consequences of infection with each of these parasites alone (3, 5), the pathobiology of mixed infection with T. orientalis and B. ovata has not been evaluated. Infections with T. orientalis and B. ovata can be diagnosed by examination of Giemsa-stained thin blood smears under a light microscope. However, the lack of sensitivity and difficulty of species differentiation have limited the use of this technique (2). Therefore, PCR methods are employed because of their greater sensitivity and specificity (11). Although several PCR assays have been developed for diagnosis of T. orientalis infection (8, 11), microscopy remains the only available technique for detecting B. ovata.
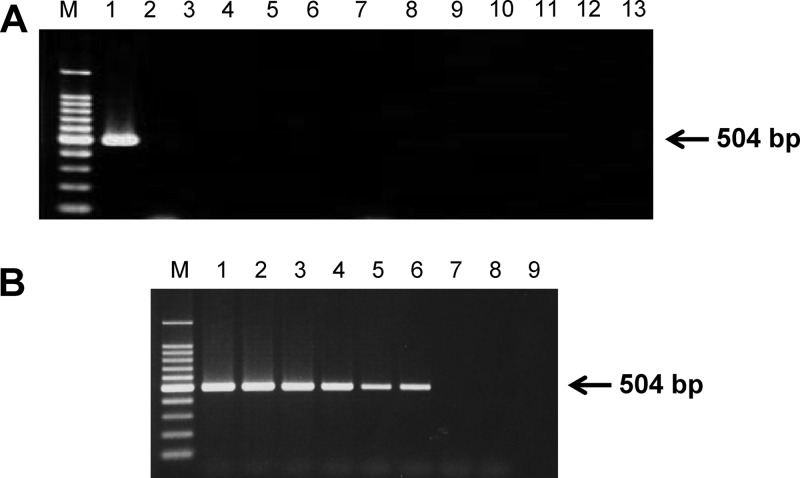
In the current study, we developed a novel PCR assay for detection of B. ovata in DNA samples extracted from infected bovine blood. A set of forward (5′-GATACGAGGCTGTCGGTAGC-3′) and reverse (5′-AGTATAGGTGAGCATCAGTG-3′) primers was designed to amplify a 504-bp fragment of the B. ovata Apical membrane antigen 1 (AMA-1) gene (GenBank accession number AB634843). One microliter of template DNA was added to 25 μl of a reaction mixture containing 2.5 μl of 10× PCR buffer (Applied Biosystems, Branchburg, NJ), 200 μM (each) deoxynucleoside triphosphates (dNTP) (Applied Biosystems), 0.8 μM (each) primer, 1 U Taq polymerase (Applied Biosystems), and double-distilled water. PCRs were conducted using, a Veriti thermal cycler (Applied Biosystems). Optimized cycle conditions were used for the PCR: briefly, 95°C for 5 min, followed by 45 cycles each of which consisted of a denaturing step at 95°C for 30 s, an annealing step at 56°C for 1 min, and an extension step at 72°C for 1 min. After a final elongation step at 72°C for 10 min, agarose gel electrophoresis and ethidium bromide staining were followed by visualization of amplicons under UV light. The AMA-1 PCR amplified a 504-bp product from B. ovata DNA; no amplicons were produced using DNA derived from 11 other bovine blood pathogens (Fig. 1A). Furthermore, the assay was sensitive enough to detect 100 fg genomic DNA from B. ovata (Miyake strain) in vitro culture (Fig. 1B).
Fig 1.
Babesia ovata-specific AMA-1 PCR assay development. A new PCR method was developed for the diagnostic detection of B. ovata, based on the B. ovata AMA-1 gene. (A) PCR specificity. Lanes 1 to 13 represent genomic DNA samples from B. ovata, Babesia bigemina, Babesia bovis, Theileria annulata, T. orientalis, Trypanosoma brucei gambiense, Trypanosoma evansi, T. theileri, Anaplasma marginale, Anaplasma bovis, Anaplasma centrale, Anaplasma phagocytophilum, and normal bovine blood, respectively. (B) PCR sensitivity. 10-fold serial dilutions of B. ovata genomic DNA extracted from in vitro culture. Lanes 1 to 8 represent 10 ng/μl, 1 ng/μl, 100 pg/μl, 10 pg/μl, 1 pg/μl, 100 fg/μl, 10 fg/μl, and 1 fg/μl, respectively. An uninfected bovine DNA sample was included as a negative control (lane 9). M, 100-bp DNA marker ladder (both panels).
Blood samples were collected from 94 randomly selected dairy cattle from a dairy farm within the Shin-Hidaka district in Japan on 19 June 2009. Microscopic examination of Giemsa-stained thin blood smears indicated that 29 (30.8%) and 3 (3.2%) of the animals were positive for infection with T. orientalis and B. ovata, respectively. However, when DNA from the blood samples was screened using a previously described PCR method based on the gene encoding the major piroplasm surface protein (MPSP-PCR) (8) and our newly developed AMA-1 PCR assay, 47 (50%) and 23 (24.5%) of the animals were found to be infected with T. orientalis and B. ovata, respectively (Table 1). All of the blood samples that were microscopy positive were also found to be positive using the two PCR assays (i.e., MPSP-PCR for T. orientalis and AMA-1 PCR for B. ovata). Sequencing analysis confirmed that all of the B. ovata-specific amplicons had nucleotide sequences identical to those of the B. ovata AMA-1 gene (GenBank accession number AB634843). The degree of anemia in the animals was determined by red blood cell (RBC) counts, hemoglobin (Hb) concentrations, and hematocrits (HCT), using a Celltac (Nihon Kohden, Tokyo, Japan) automated hematological analyzer (8). An animal was considered to be anemic if the RBC, Hb, or HCT value was less than 5 × 106 RBCs/μl, 8 g/dl, or 24%, respectively (4).
Table 1.
Diagnostic results obtained for 94 field grazing cattle using T. orientalis MPSP-PCR and B. ovata AMA-1 PCR assays
| Infection typea | No. (%) of positive animals | No. of anemic animals | Anemia rate (%) |
|---|---|---|---|
| Noninfected | 38 (40.4) | 0 | 0 |
| T. orientalisb | 33 (35.1) | 6 | 18.2 |
| B. ovatab | 09 (9.6) | 0 | 0 |
| T. orientalis and B. ovata | 14 (14.9) | 6 | 42.9 |
Animals were categorized in 4 groups, which consisted of noninfected, T. orientalis-infected, B. ovata-infected, or coinfected animals, based on the infection types.
Animals were infected with a single parasite species.
Animals were categorized in four groups according to their infection profiles (Table 1). All of the noninfected animals had normal values for all three hematological parameters (i.e., RBC count, Hb, and HCT); hence, other agents that could have contributed to the anemia among the animals could be ruled out. In addition, all the DNA samples were PCR screened for the presence of Anaplasma species as described previously (9, 12). Because only a single animal in the noninfected category was found to be infected with Anaplasma bovis, possible involvement of anaplasmosis as a cause of the anemia could be excluded. We found that the anemic animals were infected with T. orientalis alone or with T. orientalis and B. ovata (Table 1). Among the anemic cattle, all animals were positive for T. orientalis by microscopy, while B. ovata was detected on a blood smear from a single animal. In agreement with findings of a previous study (3), none of the animals that were infected only with B. ovata were anemic. The anemia rates among the coinfected animals (42.9%) were significantly higher than those for animals infected with T. orientalis (18.2%) alone (Table 1). Among the anemic animals, three exhibited low values for all three hematological parameters; two of these animals were found to be coinfected with both of the parasites (data not shown). The mean RBC count of the animals (n = 33) infected only with T. orientalis (5.90 × 106/μl) was lower than that of the noninfected animals (7.29 × 106/μl), while the RBC count of the coinfected animals (n = 14) (5.64 × 106/μl) was similar to that obtained for those animals infected with T. orientalis alone (Table 2). In contrast, a significant reduction of the mean Hb concentration was observed only in the coinfected animals (10.3 g/dl), compared with results for the noninfected animals (11.6 g/dl), whereas T. orientalis infection alone did not alter the mean Hb concentration (11.2 g/dl). A similar observation was also made for the mean HCT value, in which only the coinfected animals (27.8%) showed a lower HCT value than the noninfected animals (32.0%) (Table 2).
Table 2.
Mean red blood cell counts and hemoglobin and hematocrit values for the different categories of animals
| Infection type | Mean value ± confidence interval |
||
|---|---|---|---|
| RBC count (×106/μl) | Hb concn (g/dl) | HCT value (%) | |
| Noninfected | 7.29 ± 0.29 | 11.6 ± 0.31 | 32.0 ± 0.91 |
| T. orientalis | 5.90 ± 0.35a | 11.2 ± 0.49 | 30.1 ± 1.39 |
| B. ovata | 7.24 ± 0.63 | 11.4 ± 0.44 | 31.7 ± 1.45 |
| T. orientalis and B. ovata | 5.64 ± 0.56a | 10.3 ± 0.88a | 27.8 ± 2.33a |
Statistically significant reduction in the mean value obtained compared to that for the noninfected animals (P < 0.01).
Based on these findings, we suggest that B. ovata might be a risk factor for the induction of clinical anemia when animals are coinfected with T. orientalis, although B. ovata is unlikely to be the sole agent causing anemia in the cattle population in regions of endemicity. In common with other Babesia parasites, B. ovata induces intravascular hemolysis, as was evident from the hemoglobinuria detected when splenectomized cattle were infected with the parasite (3). In contrast, the anemia induced by T. orientalis is thought to be due to erythrophagocytosis of red blood cells (10). Therefore, we assume that the combination of these two different mechanisms for anemia could have potentiated the serious development of clinical anemia among the animals that were coinfected with B. ovata and T. orientalis. In this report, we have described the effects T. orientalis and B. ovata infections have on the anemia status of a cattle population. Further studies using a larger sample size are now necessary to confirm our findings. In addition, in vivo experiments to study the effects of coinfection with both parasite species in susceptible animals are a high priority.
ACKNOWLEDGMENTS
We thank all of the staff from the study farm in Hokkaido for their kind cooperation. In addition, we thank Hiroko Yamamoto for her excellent technical assistance.
This study was supported by a program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), by a grant from the Global COE Program from the Japanese Ministry of Education, Science, Sports, Culture and Technology, and by Grants-in Aid for Scientific Research from the Japan Society for Promotion of Science (JSPS), Japan.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Arai S, et al. 1998. Antigenic and genetic diversities of Babesia ovata in persistently infected cattle. J. Vet. Med. Sci. 60:1321–1327 [DOI] [PubMed] [Google Scholar]
- 2. Böse R, Jorgensen WK, Dalgliesh RJ, Friedhoff KT, de Vos AJ. 1995. Current state and future trends in the diagnosis of babesiosis. Vet. Parasitol. 57:61–74 [DOI] [PubMed] [Google Scholar]
- 3. Fujinaga T. 1981. Bovine babesiosis in Japan: clinical and clinico-pathological studies on cattle experimentally infected with Babesia ovata. Nihon Juigaku Zasshi 43:803–813 [DOI] [PubMed] [Google Scholar]
- 4. Jain K. 1993. Essentials of veterinary hematology. Blackwell Publishing, Hoboken, NJ [Google Scholar]
- 5. McFadden AM, et al. 2011. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N. Z. Vet. J. 59:79–85 [DOI] [PubMed] [Google Scholar]
- 6. Minami T, Ishihara T. 1980. Babesia ovata sp.n. isolated from cattle in Japan. Nat. Inst. Anim. Health Q. (Tokyo) 20:101–113 [PubMed] [Google Scholar]
- 7. Onuma M, Kakuda T, Sugimoto C. 1998. Theileria parasite infection in East Asia and control of the disease. Comp. Immunol. Microbiol. Infect. Dis. 21:165–177 [DOI] [PubMed] [Google Scholar]
- 8. Ota N, et al. 2009. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J. Vet. Med. Sci. 71:937–944 [DOI] [PubMed] [Google Scholar]
- 9. Parola P, et al. 2000. Detection of Ehrlichia in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 94:707–708 [DOI] [PubMed] [Google Scholar]
- 10. Sugimoto T, Fujisaki K. 2002. Non-transforming Theileria parasites of ruminants, p 93–106 In Dobbelaere D, McKeever D. (ed), World class parasites, vol 3, 1st ed Theileria Kluwer Academic Publishers, Norwell, MA [Google Scholar]
- 11. Tanaka M, et al. 1993. Detection of Theileria sergenti infection in cattle by polymerase chain reaction amplification of parasite-specific DNA. J. Clin. Microbiol. 31:2565–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warner CK, Dawson JE. 1996. Genus- and species-level identification of Ehrlichia species by PCR and sequencing, p 100–105 In Persing DHH. (ed), PCR protocols for emerging infectious diseases. ASM Press, Washington, DC [Google Scholar]
- 13. Yokoyama N, et al. 2011. Genotypic diversity of Theileria orientalis detected from cattle grazing in Kumamoto and Okinawa prefectures of Japan. J. Vet. Med. Sci. 73:305–312 [DOI] [PubMed] [Google Scholar]