Abstract
Efforts to identify all persons infected with HIV in the United States are driven by the hope that early diagnosis will lower risk behaviors and decrease HIV transmission. Identification of HIV-infected people earlier in the course of their infection with HIV antigen/antibody (Ag/Ab) combination assays (4th-generation HIV assays) should help achieve this goal. We compared HIV RNA nucleic acid test (NAT) results to the results of a 4th-generation Ag/Ab assay (Architect HIV Ag/Ab Combo [HIV Combo] assay; Abbott Diagnostics) in 2,744 HIV antibody-negative samples. Fourteen people with acute HIV infection (HIV antibody negative/NAT positive) were identified; the HIV Combo assay detected nine of these individuals and was falsely negative in the remaining five. All five persons missed by the HIV Combo assay were in the stage of exponential increase in plasma virus associated with acute HIV infection (3, 7, 20, 35, 48). In contrast, most acutely infected persons detected by the HIV Combo assay demonstrated either a plateauing or decreasing plasma viral load. The HIV Combo assay also classified as positive five other samples which were negative by NAT. Taken together, the HIV Combo assay had a sensitivity of 73.7% and a specificity of 99.8%. Using published data, we estimated secondary transmission events had HIV infection in these five individuals remained undiagnosed. Screening of our population with NAT cost more than screening with the HIV Combo assay but achieved new diagnoses that we predict resulted in health care savings that far exceed screening costs. These findings support the use of more sensitive assays, like NAT, in HIV screening of populations with a high prevalence of acute HIV infection.
INTRODUCTION
An estimated 1.1 million persons are currently living with HIV in the United States, and 56,000 new infections occur every year (19). The majority of persons with HIV are aware of their status (75 to 80%), yet the incidence of HIV infection in the United States has remained relatively unchanged for the last decade (40). Acute HIV infection is a critical driver of HIV transmission, accounting for 10 to 50% of new infections (12, 17, 31). Estimates suggest that transmission rates in the first 6 months of infection are 5.5 to 26 times higher than those in established disease (7, 21–23, 39, 47), probably due to higher plasma viral loads (pVLs) or increased infectivity of virus (3, 7, 21, 36, 48). Because many people are unaware of their status during the earliest stages of infection and thus do not access HIV prevention services, acute infection will likely continue to be a major driver of new HIV infections (11, 23, 24, 32).
The most widely used tests to diagnose HIV infection depend on the detection of antibodies (Abs) to HIV (10). However, the ability of antibody tests to identify HIV infection is limited in early disease. The increased sensitivity associated with HIV antibody third-generation enzyme immunoassays (EIAs) has improved the diagnostic yield of HIV screening during the first 3 to 5 weeks after HIV infection, when the antibody response is still developing, i.e., the window period of acute infection (37). However, diagnosis of HIV infection during acute and very early infection is more reliably established by measurement of HIV RNA or the HIV antigen (Ag) p24. The p24 antigen is a viral core protein often detectable in the blood when HIV RNA increases to greater than 4 log10 HIV RNA copies/ml (10, 16). Although RNA tests have improved sensitivity to identify acute and early HIV infections, the high cost of these assays and the delayed time to generate results (average, 7 to 14 days) have limited their widespread use. To decrease these costs, HIV nucleic acid tests (NATs) are most often used in pooled strategies in high-risk populations (4, 22, 37, 38).
Fourth-generation HIV Ag/Ab combination assays offer a high-throughput platform that can potentially identify both acute and established HIV infections without the need for pooling strategies (4, 15). Of the currently available assays, the FDA-approved Architect HIV Ag/Ab Combo (HIV Combo) assay (Abbott Diagnostics) has shown promise as a screening tool with a reported sensitivity of 100% and specificity of 95 to 99% when evaluated on p24 antigen and HIV antibody clinical standards as well as panels of fresh and frozen clinical specimens (2, 25, 30, 33). The HIV Combo assay is a chemiluminescent magnetic microparticle-based immunoassay that uses recombinant antigens derived from the transmembrane proteins of HIV-1 groups M and O and HIV-2 for antibody detection and monoclonal anti-p24 for antigen detection (25). However, when applied to samples from acutely infected individuals (HIV serology negative but HIV RNA positive), the HIV Combo assay detects only 62 to 80% of infected individuals whose pVL is less than 4.48 log10 HIV RNA copies/ml (15, 35). To evaluate if the HIV Combo assay would be suitable to screen a population with a high incidence of acute HIV infection (34), we retrospectively evaluated the HIV Combo assay in comparison to HIV NAT.
MATERIALS AND METHODS
Study population and testing algorithm.
The San Diego Early Test program was approved by the local Human Research Protections Program and enrolled participants between July 2008 and July 2010. This program is a prospective HIV screening program designed to identify and stage persons with unknown HIV infection status (34). At enrollment, participants underwent a point-of-care rapid antibody test (Oraquick Advance rapid HIV; OraSure Technologies, Inc., Bethlehem PA). If this test was positive, then confirmation and HIV staging included Western blotting (Cambridge Biotech, distributed by Ortho-Clinical Diagnostics, Inc., United Kingdom), testing of pVL (Cobas Amplicor HIV-1 test; Roche Molecular Systems, Pleasanton, CA), and detuned HIV EIA (Vironostika LS EIA [bioMérieux Inc., Durham, NC] and Vitros LS EIA [Ortho-Clinical Diagnostics, Inc., United Kingdom]). Persons with a negative rapid HIV screening test submitted blood plasma for individual-donation HIV NAT (ID-NAT; Procleix HIV-1/HCV or Procleix Ultrio HIV-1/HCV/HBV assay; Chiron/GenProbe Inc., Emeryville/San Diego, CA) by the American Red Cross. An HIV pVL (Cobas Amplicor HIV-1 test; Roche Molecular Systems, Pleasanton CA) confirmed all positive ID-NAT results, as did documentation of subsequent HIV seroconversions by antibody testing (Oraquick Advance rapid HIV; OraSure Technologies, Inc., Bethlehem PA). No further testing was performed on samples that were rapid test negative and ID-NAT negative. Literature reports that the HIV-1 viral load limit of detection (LOD) for the Amplicor HIV-1 assay is 1.37 to 1.49 log10 copies/ml (41), and the manufacturer package insert reports that the Procleix HIV-1 NAT LOD is 2 log10 copies/ml. All study participants had plasma samples banked at the time of the Early Test (day 0) for confirmatory testing and assay validation. Participants found to be HIV infected were followed with specimens collected and banked at regular study intervals, as previously described (34).
Architect HIV Ag/Ab Combo assay evaluation.
Using banked samples collected from the San Diego Early Test program, we then evaluated the performance of the HIV Combo assay on participants with negative HIV antibody results. Specifically, laboratory staff from Abbott Diagnostics performed the HIV Combo assays per the manufacturer's instructions on the collected samples in a blinded fashion. Published literature suggests that the HIV-1 viral load LOD for the HIV Combo assay is ∼4.48 log10 copies/ml (15, 35).
Cost analysis.
In the San Diego Early Test program, study participants disclosed demographics, exposure risk categories, and sexual behaviors. Using provided risk behavior data, we calculated probable new transmission events from the acutely infected participants who were identified by ID-NAT but missed by the HIV Combo assay. Parameter estimates for this analysis were derived from published studies of the effect of sexual behavior and stage of HIV infection on transmission risk (42, 47). Since all acutely infected participants in the current study identified themselves as men who have sex with men (MSM), we used the published transmission risk of unprotected receptive anal intercourse (URAI; probability, 0.014 transmissions/URAI; 95% confidence interval [CI], 0.002 to 0.025) to conservatively estimate potential secondary transmission events (1). This rate was modified to account for potentially different rates of HIV transmission during different stages of infection in reference to transmission rates in heterosexual HIV-discordant couples (transmission rates, 0.0082/coital act in the first 2.5 months after seroconversion, 0.0039/coital act in the next 2.5 to 6 months, and 0.0015/coital act in the following 6 to 15 months [47]). To evaluate the cost of the predicted new transmissions had the HIV Combo assay been the only HIV screening tool used, the discounted lifetime treatment cost of $385,200 per person infected with HIV was used (44). In this study, total ID-NAT costs $14 per test. However, others have reported the cost of this assay (including technician time and equipment) to be $14 to $49 per test (14, 22, 46); thus, costs for ID-NAT are reported as a range. The cost for the HIV Combo assay is reported to be $4.86 per test. Additional costs that would be necessary for both tests (i.e., confirmatory testing with EIA and Western blotting and labor costs to notify persons of their serostatus and partner services) are not included in the cost analyses.
Viral dynamics slope analysis.
To account for repeated measures, mixed-effect modeling (27, 45) was used to determine whether the change in pVL over time was different for acutely infected patients who tested positive using the HIV Combo assay versus those who did not test positive. The outcome was the base 10 logarithm of the pVL at baseline and at a follow-up visit that was no more than 10 days from the baseline. The Combo assay result and the number of days from baseline to the second pVL measure were included in the model as fixed effects, and the patient was included as the random effect. The interaction between the Combo assay result and days relative to baseline was included to test the difference in the slopes.
RESULTS
Testing results.
Between July 2008 and July 2010, 7,176 participants were screened for HIV. Of these participants, 133 were rapid test positive and 28 were ID-NAT positive. Of the 7,176 participants screened, 2,755 had stored samples. By excluding plasma samples that were rapid test positive by prospective screening, a total of 2,744 individual samples were included in the retrospective analysis of the HIV Combo assay. Fourteen HIV-infected persons (0.5%) were identified by prospective ID-NAT screening with appropriate confirmatory testing. There were no false-positive tests for HIV NAT. In the 2,744 HIV rapid test-negative plasma samples, HIV NAT demonstrated a specificity of 100%.
The HIV Combo assay identified 9 of the 14 acutely infected persons, with simultaneous pVLs ranging from 3.4 to 6.6 log10 HIV RNA copies/ml. The pVLs of the five acutely infected persons who were not detected by the HIV Combo assay ranged from 1.8 to 5.1 log10 HIV RNA copies/ml (mean, 4.5 log10 HIV RNA copies/ml). The HIV Combo assay was also reactive in five additional samples that were negative by rapid test and HIV NAT. Repeat tests of three of these participants at later time points were also negative; the remaining two did not return for a retest. We considered these tests consistent with false-positive results for the HIV Combo assay. In the HIV rapid test-negative samples, the HIV Combo assay demonstrated a sensitivity of 73.7% and a specificity of 99.8%, giving it a positive predictive value of 73.8% and a negative predictive value of 99.8% (Table 1).
Table 1.
Summary of relevant HIV Combo assay resultsa
| Patient identifier | Result by: |
HIV RNA load (log10 copies/ml) | ||
|---|---|---|---|---|
| HIV rapid test | Nucleic acid test | HIV Combo assay | ||
| 1 | NR | R | NR | 4.7 |
| 2 | NR | R | NR | 3.0 |
| 3 | NR | R | NR | 2.7 |
| 4 | NR | R | NR | 5.1 |
| 5 | NR | R | NR | 1.8 |
| 6 | NR | R | R | 6.2 |
| 7 | NR | R | R | 6.6 |
| 8 | NR | R | R | 5.4 |
| 9 | NR | R | R | 5.8 |
| 10 | NR | R | R | 4.8 |
| 11 | NR | R | R | 4.6 |
| 12 | NR | R | R | 5.4 |
| 13 | NR | R | R | 3.4 |
| 14 | NR | R | R | 6.2 |
| 15 | NR | NR | R | NA |
| 16 | NR | NR | R | NA |
| 17 | NR | NR | R | NA |
| 18 | NR | NR | R | NA |
| 19 | NR | NR | R | NA |
NR, nonreactive; R, reactive; NA, not available.
Cost of a missed acute infection diagnosis.
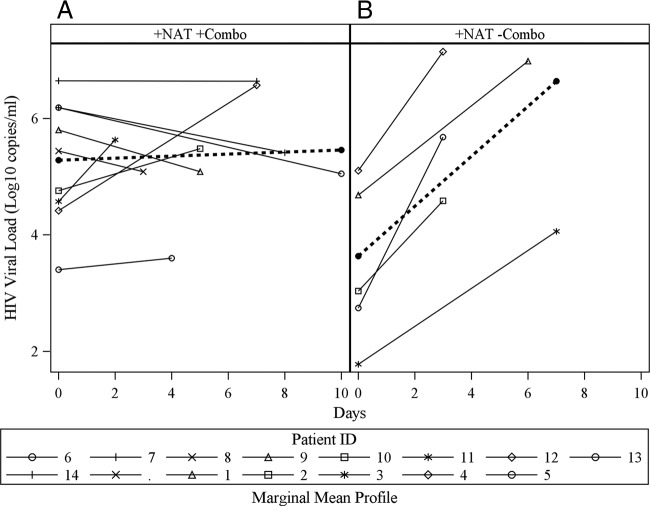
Quantitative pVL trends demonstrated that the nine acutely infected individuals identified by both the HIV Combo assay and NAT demonstrated either stable or decreasing pVLs between day 0 and days 2 to 10, a dynamic that was confirmed by a mean slope that was not significantly different from 0 (P = 0.759) (Fig. 1A). These data are in contrast to those for the five acutely infected persons missed by the HIV Combo assay, all of whom were in the ramp-up phase of viral replication and had pVLs significantly increased from those at the time of diagnosis (P = 0.0099) (Fig. 1B) (10). Comparison of the means of the slopes between acutely infected persons missed by the HIV Combo assay and acutely infected persons whose infection was diagnosed by the HIV Combo assay clarified that these two populations have significantly different HIV dynamics (P = 0.0031). The five false-negative samples by HIV Combo assay were all from MSM who in the past month had a median of 3 (range, 2 to 20) partners, 2 (range, 0 to 2) episodes of protected insertive anal intercourse (PIAI), 2 episodes (range, 0 to 2) of protected receptive anal intercourse (PRAI), 0 acts (range, 0 to 9) of unprotected insertive anal intercourse (UIAI), and 0 acts (range, 0 to 18) of unprotected receptive anal intercourse (URAI) (Table 2). If risk behaviors remained unchanged, missed HIV diagnoses of these five persons were predicted to result in 2.31 (95% CI, 0.33 to 4.13) transmissions in the first 2.5 months, 1.23 (95% CI, 0.18 to 2.21) transmissions from months 2.5 to 6, and 1.01 (95% CI, 0.14 to 1.8) transmissions during months 6 to 12 for a total of 4.55 (95% CI, 0.65 to 8.13) new HIV transmissions over 1 year (Table 3) (44, 47).
Fig 1.
(A) Plasma VL at study entry (day 0) and 2 to 10 days later in acutely infected persons detected by both NAT and HIV Combo assay; (B) plasma VL over time for the five acutely infected persons missed by the HIV Combo assay (day 0 is the day of HIV detection by NAT).
Table 2.
Risk behavior characteristics in the past month of individuals missed by HIV Combo assaya
| Patient identifier | No. of partners | No. of instances of: |
|||
|---|---|---|---|---|---|
| PIAI | PRAI | UIAI | URAI | ||
| 1 | 10 | 2 | 0 | 9 | 0 |
| 2 | 3 | 1 | 0 | 0 | 0 |
| 3 | 2 | 2 | 2 | 0 | 0 |
| 4 | 20 | 2 | 2 | 3 | 3 |
| 5 | 2 | 0 | 2 | 0 | 18 |
| Total | 37 | 7 | 6 | 12 | 21 |
PIAI, protected insertive anal intercourse; PRAI, protected receptive anal intercourse; UIAI, unprotected insertive anal intercourse; URAI, unprotected receptive anal intercourse. Patients 1 and 4 accounted for all of the estimated new transmissions.
Table 3.
Estimated costsa
| Patient identifier or parameter | Estimated no. of HIV transmissions |
|||
|---|---|---|---|---|
| 0-2.5 mo | 2.5-6 mo | 6-12 mo | Total | |
| 1 | 1.73 | 0.93 | 0.76 | 3.41 |
| 2 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0.58 | 0.31 | 0.25 | 1.14 |
| 5 | 0 | 0 | 0 | 0 |
| Total no. of new cases/time period | 2.31 | 1.23 | 1.01 | 4.55 |
| Total cost ($)/time period | 889,812 | 473,796 | 389,052 | 1,753,739 |
Estimates are for 12 months following HIV testing and presume that a missed HIV diagnosis results in continued risk behaviors.
Using estimates of the lifetime cost of HIV care in the United States, these 4.55 infections would cost $1,753,739 (95% CI, $279,218 to $2,440,074) (44). Previous research has observed that 76% of persons diagnosed with acute and early HIV infection (i.e., roughly four of the five of these acutely infected persons can be predicted to practice safer sex) eliminated risk of onward transmission entirely when evaluated at 12 weeks postdiagnosis (18). Taking into account the behaviors of the five study participants identified by ID-NAT but not the HIV Combo assay, 0 to 3.41 new transmissions may have been prevented within the first year of diagnosis, saving up to $1,313,532 (Table 3). The greatest number of predicted transmissions with their translated cost occurs in the first 2.5 months from infection (Table 3), demonstrating the increased risk of sexual transmission observed in acute infection (47). Prevention of these infections would likely prevent additional transmissions and continue to result in cost savings, but we did not consider those secondary prevented transmissions in this cost calculation. In comparison, using the HIV Combo assay to evaluate this entire cohort would have cost $13,335 and ID-NAT would have cost $38,416 to 134,897 (14, 46). The difference between these screening costs remains a fraction of the potential savings from averted new transmissions. Previous papers have evaluated the cost-effectiveness of ID-NAT compared to other methods. In this article, we used costs to illustrate the potential impact of missed diagnoses of acute HIV infection (14, 46).
DISCUSSION
In this high-risk population screened for HIV infection, the HIV Combo assay detected 64% of acutely infected persons, consistent with previous published reports (15). However, the magnitude of the pVL for two of the five specimens that were not detected by HIV Combo assay was higher than what has been reported in other studies (4.7 and 5.1 log10 HIV RNA copies/ml) (15, 35). All five of the HIV-infected persons missed by the HIV Combo assay were in the stage of exponential increase in plasma virus associated with acute HIV infection, a stage of infection that carries an increased risk of sexual HIV transmission (3, 7, 21, 36, 48). In contrast, most of the acutely infected persons detected by the HIV Combo assay demonstrated either plateauing or decreasing pVLs (Fig. 1). This is due to the fact that the HIV Combo assay detects p24 Ag (25), which is not usually detectable in HIV infection until >4 log10 HIV RNA copies/ml is achieved (16). Thus, although assays for HIV RNA and HIV p24 antigen both improve identification of acutely infected persons (before HIV antibody is produced), their ability to do so is not equivalent.
In an effort to identify the 250,000 persons in the United States who are unaware that they are living with HIV, the CDC recommends routine HIV testing (5). The widespread introduction of a user-friendly, low-cost, point-of-care assay for HIV infection will likely increase access and reduce barriers to HIV testing in the United States. However, as health care programs implement expanded HIV testing, strategies to most effectively identify individuals with acute HIV infection should not be overlooked (5, 8, 9). As a recent mathematical model suggests, biannual screening with 4th-generation Ag/Ab assays like the HIV Combo assay may be one way to improve the diagnosis of HIV infection, especially in high-risk populations (28), but still does not close the window of acute HIV infection (6). The limitation of the HIV Combo assay and other 4th-generation HIV Ag/Ab assays to detect acute HIV infection lies with their employ of the p24 antigen, with a limit of detection of 11 to 18 pg/ml (29) or an HIV viral load of 4.5 to 4.7 log10 copies/ml (26, 43). Unlike the improvements in antibody detection observed in the new 4th-generation HIV Ag/Ab assays, the limitations of the p24 antigen in the diagnosis of acute HIV infection have not been overcome (6, 13, 43). Identification of HIV infection during the acute phase of infection provides an opportunity to significantly reduce the risk of HIV transmission by providing education, safer-sex counseling, enrollment in care, and in some cases, initiation of antiretroviral therapy at a time when transmission risk may be greatest (7, 21–23, 39, 47). An accurate diagnosis of acute HIV infection is particularly relevant, as the impact of pre- and posttest counseling on risk behaviors may be eroding (20). Inclusion of the HIV p24 antigen in 4th-generation assays like the HIV Combo assay does allow the detection of some persons with acute HIV infection. However, as illustrated, failure to identify even a small number of acutely infected persons could result in ongoing HIV transmission and significant health care costs. ID-NAT testing in our population (MSM) proved beneficial. Thus, for testing programs that evaluate persons at risk for HIV (as opposed to non-risk-based screening), the use of ID-NAT or pooled NAT should be strongly considered. Similarly, future work evaluating the impact of novel strategies of HIV testing and linkage to care should optimize diagnostic ability by utilizing NAT-based methods.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AI69432, AI043638, MH62512, MH083552, AI077304, AI36214, AI047745, AI74621, and AI080353), the James B. Pendleton Charitable Trust, and RN07-SD-702 from the California HIV Research Program and was partially supported by grant KL2 RR031978 from the National Center for Research Resources, which is now at the National Center for Advancing Translational Sciences. No funding was received from Abbott Diagnostics.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We thank Abbot Diagnostics, and we specifically thank Catherine Brennan for performing the HIV Ag/Ab Combo assay testing of the study samples and for her thoughtful comments.
This article is original, and no form of it has ever been presented.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Baggaley RF, White RG, Boily MC. 2010. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int. J. Epidemiol. 39:1048–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baleriola C, et al. 8 April 2011. Infectious disease screening of blood specimens collected post-mortem provides comparable results to pre-mortem specimens. Cell Tissue Bank. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 3. Bar KJ, et al. 2010. Wide variation in the multiplicity of HIV-1 infection among injection drug users. J. Virol. 84:6241–6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Branson BM. 2010. The future of HIV testing. J. Acquir. Immune Defic. Syndr. 55(Suppl. 2):S102–S105 [DOI] [PubMed] [Google Scholar]
- 5. Branson BM, et al. 2006. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recommend. Rep. 55(RR-14):1–17 [PubMed] [Google Scholar]
- 6. Branson BM, Stekler JD. 2012. Detection of acute HIV infection: we can't close the window. J. Infect. Dis. 205:521–524 [DOI] [PubMed] [Google Scholar]
- 7. Brenner BG, et al. 2007. High rates of forward transmission events after acute/early HIV-1 infection. J. Infect. Dis. 195:951–959 [DOI] [PubMed] [Google Scholar]
- 8. Campsmith ML, Rhodes PH, Hall HI, Green TA. 2010. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J. Acquir. Immune Defic. Syndr. 53:619–624 [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention 2011. Results of the expanded HIV testing initiative—25 jurisdictions, 2007-2010., MMWR Morb. Mortal. Wkly. Rep. 60:805–810 [PubMed] [Google Scholar]
- 10. Cohen MS, Gay CL, Busch MP, Hecht FM. 2010. The detection of acute HIV infection. J. Infect. Dis. 202(Suppl. 2):S270–S277 [DOI] [PubMed] [Google Scholar]
- 11. Colfax GN, et al. 2002. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS 16:1529–1535 [DOI] [PubMed] [Google Scholar]
- 12. Crepaz N, Marks G. 2002. Towards an understanding of sexual risk behavior in people living with HIV: a review of social, psychological, and medical findings. AIDS 16:135–149 [DOI] [PubMed] [Google Scholar]
- 13. Daar ES, et al. 2001. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann. Intern. Med. 134:25–29 [DOI] [PubMed] [Google Scholar]
- 14. Davidson T, Ekermo B, Gaines H, Lesko B, Akerlind B. 2011. The cost-effectiveness of introducing nucleic acid testing to test for hepatitis B, hepatitis C, and human immunodeficiency virus among blood donors in Sweden. Transfusion 51:421–429 [DOI] [PubMed] [Google Scholar]
- 15. Eshleman SH, et al. 2009. Detection of individuals with acute HIV-1 infection using the ARCHITECT HIV Ag/Ab Combo assay. J. Acquir. Immune Defic. Syndr. 52:121–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiebig EW, et al. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871–1879 [DOI] [PubMed] [Google Scholar]
- 17. Fisher JD, Smith LR, Lenz EM. 2010. Secondary prevention of HIV in the United States: past, current, and future perspectives. J. Acquir. Immune Defic. Syndr. 55(Suppl. 2):S106–S115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox J, et al. 2009. Reductions in HIV transmission risk behaviour following diagnosis of primary HIV infection: a cohort of high-risk men who have sex with men. HIV Med. 10:432–438 [DOI] [PubMed] [Google Scholar]
- 19. Hall HS, et al. 2008. Estimation of HIV incidence in the United States. JAMA 300:520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heijman T, et al. 2012. Less decrease in risk behaviour from pre-HIV to post-HIV seroconversion among MSM in the combination antiretroviral therapy era compared with the pre-combination antiretroviral therapy era. AIDS 26:489–495 [DOI] [PubMed] [Google Scholar]
- 21. Hollingsworth TD, Anderson RM, Fraser C. 2008. HIV-1 transmission, by stage of infection. J. Infect. Dis. 198:687–693 [DOI] [PubMed] [Google Scholar]
- 22. Hutchinson AB, et al. 2010. Cost-effectiveness of pooled nucleic acid amplification testing for acute HIV infection after third-generation HIV antibody screening and rapid testing in the United States: a comparison of three public health settings. PLoS Med. 7:e1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacquez J, Koopman J, Simon C, Longini IJ. 1994. Role of the primary infection in epidemics of HIV infection in gay cohorts. J. Acquir. Immune Defic. Syndr. 7:1169–1184 [PubMed] [Google Scholar]
- 24. Koopman JS, et al. 1997. The role of early HIV infection in the spread of HIV through populations. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14:249–258 [DOI] [PubMed] [Google Scholar]
- 25. Kwon JA, et al. 2006. Performance evaluation of three automated human immunodeficiency virus antigen-antibody combination immunoassays. J. Virol. Methods 133:20–26 [DOI] [PubMed] [Google Scholar]
- 26. Layne SP, et al. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189:695–714 [DOI] [PubMed] [Google Scholar]
- 27. Littell RC, Henry PR, Ammerman CB. 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231 [DOI] [PubMed] [Google Scholar]
- 28. Long EF. 2011. HIV screening via fourth-generation immunoassay or nucleic acid amplification test in the United States: a cost-effectiveness analysis. PLoS One 6:e27625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ly TD, Ebel A, Faucher V, Fihman V, Laperche S. 2007. Could the new HIV combined p24 antigen and antibody assays replace p24 antigen specific assays? J. Virol. Methods 143:86–94 [DOI] [PubMed] [Google Scholar]
- 30. Malm K, von Sydow M, Andersson S. 2009. Performance of three automated fourth-generation combined HIV antigen/antibody assays in large-scale screening of blood donors and clinical samples. Transfus. Med. 19:78–88 [DOI] [PubMed] [Google Scholar]
- 31. Marks G, Burris S, Peterman TA. 1999. Reducing sexual transmission of HIV from those who know they are infected: the need for personal and collective responsibility. AIDS 13:297–306 [DOI] [PubMed] [Google Scholar]
- 32. Marks G, Crepaz N, Senterfitt JW, Janssen RS. 2005. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J. Acquir. Immune Defic. Syndr. 39:446–453 [DOI] [PubMed] [Google Scholar]
- 33. Miedouge M, Greze M, Bailly A, Izopet J. 2011. Analytical sensitivity of four HIV combined antigen/antibody assays using the p24 WHO standard. J. Clin. Virol. 50:57–60 [DOI] [PubMed] [Google Scholar]
- 34. Morris SR, et al. 2010. Evaluation of an HIV nucleic acid testing program with automated Internet and voicemail systems to deliver results. Ann. Intern. Med. 152:778–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pandori MW, et al. 2009. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J. Clin. Microbiol. 47:2639–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pao D, et al. 2005. Transmission of HIV-1 during primary infection: relationship to sexual risk and sexually transmitted infections. AIDS 19:85–90 [DOI] [PubMed] [Google Scholar]
- 37. Patel P, et al. 2006. Detection of acute HIV infections in high-risk patients in California. J. Acquir. Immune Defic. Syndr. 42:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel P, et al. 2010. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006-2008. Arch. Intern. Med. 170:66–74 [DOI] [PubMed] [Google Scholar]
- 39. Prabhu V, Hutchinson A, Farnham P, Sansom S. 2009. Sexually acquired HIV infections in the United States due to acute-phase HIV transmission: an update. AIDS 23:1792–1794 [DOI] [PubMed] [Google Scholar]
- 40. Prejean J, et al. 2011. Estimated HIV incidence in the United States, 2006-2009. PLoS One 6:e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pyne MT, Brown KL, Hillyard DR. 2010. Evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test and identification of rare polymorphisms potentially affecting assay performance. J. Clin. Microbiol. 48:2852–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quinn TC, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N. Engl. J. Med. 342:921–929 [DOI] [PubMed] [Google Scholar]
- 43. Rosenberg NE, et al. 2012. Detection of acute HIV infection: a field evaluation of the Determine(R) HIV-1/2 Ag/Ab Combo test. J. Infect. Dis. 205:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schackman BR, et al. 2006. The lifetime cost of current human immunodeficiency virus care in the United States. Med. Care 44:990–997 [DOI] [PubMed] [Google Scholar]
- 45. Vangeneugden T, Laenen A, Geys H, Renard D, Molenberghs G. 2004. Applying linear mixed models to estimate reliability in clinical trial data with repeated measurements. Control. Clin. Trials 25:13–30 [DOI] [PubMed] [Google Scholar]
- 46. van Hulst M, et al. 2008. Cost-effectiveness of HIV screening of blood donations in Accra (Ghana). Value Health 11:809–819 [DOI] [PubMed] [Google Scholar]
- 47. Wawer MJ, et al. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191:1403–1409 [DOI] [PubMed] [Google Scholar]
- 48. Xiridou M, Geskus R, de Wit J, Coutinho R, Kretzschmar M. 2004. Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS 18:1311–1320 [DOI] [PubMed] [Google Scholar]