Abstract
Capillaria aerophila, a trichuroid nematode causing pulmonary infections in wild and domestic carnivores, is occasionally and potentially poorly recognized in infections of humans due to clinicopathological mimicry and a lack of accurate, robust laboratory diagnostics. The present work evaluated the efficiency of a DNA-based assay amplifying a partial cytochrome c oxidase subunit 1 (cox1) gene of C. aerophila in the diagnosis of lung capillariosis. Fecal samples from 34 dogs and 10 cats positive at parasitological examination for C. aerophila and other endoparasites (i.e., other lungworms, whipworms, roundworms, hookworms, tapeworms, and/or coccidia) and from 44 animals negative for C. aerophila but positive for other endoparasites were molecularly examined. Of the 44 samples positive for C. aerophila at copromicroscopy, 43 scored positive (i.e., 33/34 dogs and 10/10 cats) in seminested PCR, resulting in a sensitivity of 97 to 100%. Samples that were copromicroscopy negative for C. aerophila although positive for other endoparasites never produced a PCR product or nonspecific amplicons. The specific PCR amplification of C. aerophila (i.e., specificity of 100%) was confirmed by a nucleotide sequence analysis of the cox1 amplicons. The potential implications of the molecular diagnosis of lung capillariosis are discussed.
INTRODUCTION
Capillaria aerophila (syn. Eucoleus aerophilus) is a trichuroid parasitic nematode affecting the respiratory systems of domestic (i.e., dogs and cats) and wild (e.g., foxes and mustelids) carnivores and occasionally of humans (26, 32). The adult lungworms live embedded in the epithelia of the bronchioles, bronchi, and trachea of the definitive host. After mating, the females lay eggs that are coughed, swallowed, and released via feces into the environment, where they undergo further development through the infectious stage. Animals become infected by ingesting environmental embryonated eggs or earthworms, which are considered an intermediate or paratenic host (4, 7, 34). Indeed, the nematode is commonly found in wildlife, but it has also been identified in dogs and/or cats from Spain (20), Germany (12), Portugal (18), Romania (19), and Italy (11, 31).
Pulmonary capillariosis in canine and feline hosts is considered subclinical, although the parasite may cause a chronic bronchitis and symptomatic cases have been recently reported (6, 31, 32). Animals may display minimal respiratory signs (e.g., bronchovesicular sounds) or inflammation, sneezing, wheezing, and chronic dry cough; when bacterial complications occur, the cough may become moist and productive, leading to bronchopneumonia and respiratory failure (31), and additionally, heavy parasite burdens may lead to mortality (6, 14, 26). On occasion, C. aerophila can infect humans, causing bronchitis, coughing, mucoid sputum, presence of blood in the mucous, fever, dyspnea, and pulmonary carcinoma-like masses (1, 10, 16).
Infection by C. aerophila appears to be cosmopolitan, but true knowledge of its distribution in and beyond Eastern Europe and the Mediterranean is lacking. The biological life cycle of the nematode is not clear; thus, there is a need for new information on the impact on pet health and actual zoonotic potential. Such a lack of knowledge is mainly due to limitations inherent to conventional diagnostic methodologies. The diagnosis of canine and feline lung capillariosis relies on the detection of the typical trichuroid eggs through standard fecal floatation (26, 32). This approach is the most common and the least expensive in routine practice, but it presents obstacles in detecting and identifying C. aerophila eggs, which resemble those of other trichuroids (e.g., Trichuris vulpis and Capillaria bohemi) infesting companion animals (32). Also, diagnostic limitations have thus far likely been a factor in evaluation of the actual diffusion of lung capillariosis in humans.
Despite the fact that several DNA-based assays have been recently developed for diagnosing parasitic diseases in pets (15, 17, 29, 30, 32), no molecular research on C. aerophila has been carried out so far. Among different genetic markers used in the last few years for diagnostic purposes, mitochondrial target genes (mtDNA) proved to contain regions useful for diagnosing infections of veterinary and zoonotic concern (8, 27, 30). Thus, the present work presents the assessment of a molecular test based on the specific amplification of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) of C. aerophila and its diagnostic efficiency as evaluated with field-collected samples.
MATERIALS AND METHODS
Sample collection.
Ten adult stages of C. aerophila collected from red foxes and beech marten from different countries were kindly provided by different colleagues. Individual fecal samples were collected from 34 dogs and 10 cats diagnosed to be infected by C. aerophila alone or by other endoparasites using a standard flotation procedure (24). These animals were from central (site 1, Marche region; site 2, Abruzzo region) and southern (site 3, Apulia region) Italy. Eggs of C. aerophila in feces of the 44 infected animals were identified by their barrel-like shape with asymmetrical bipolar mucoid plugs, densely striated outer shell with a network of anastomosis ridges, and typical size (34). Stool samples from 22 dogs and 22 cats negative for C. aerophila but positive for other nematodes were also collected.
Parasitic ova different from C. aerophila retrieved at copromicroscopic examination of 69 fecal samples were identified according to morphological keys (24, 26). After morphological identification of parasitic elements, an aliquot of 3 to 5 g of each fecal sample was subjected to a flotation technique as previously described (28) to concentrate the eggs, and an aliquot of 200 μl of supernatant for each sample was stored at −20°C before molecular testing.
Molecular procedures. (i) Characterization of a region internal to the cox1 gene of Capillaria aerophila.
Adult stages of C. aerophila were individually processed for DNA extraction. DNA samples were subjected to a PCR specific for a 344-bp-long region internal to the cox1 gene using the degenerated set of primers Cox1NEMF (forward, 5′-CCTGAGGTTTATATTYTWRTT-3′) and Cox1NEMR (reverse, 5′-CCTGTTARRCCTCCRATACT-3′) designed on the basis of Capillarinae consensus sequences available in the GenBank according to the criteria of Sharrocks (22).
PCR mixtures (50 μl) contained 50 pmol of each primer, 4 μl of DNA extract, 25 μl of Ready Mix REDTaq (Sigma, St. Louis, MO), and distilled water provided by the same manufacturer. PCRs were performed in a thermal cycler (2700; Applied Biosystems, Foster City, CA) using the following cycling protocol: 10 min at 95°C; 40 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min; and a final extension at 72°C for 10 min. Amplicons were electrophoresed in a 1.6% (wt/vol) agarose gel, stained with Gel Red 10.000X (Biotium, Inc.), purified using a QIAquick gel extraction kit (Qiagen GmbH, Hilden, Germany), and then sequenced directly using a Taq DyeDeoxyTerminator cycle sequencing kit (v.2; Applied Biosystems Inc.).
(ii) Diagnostic seminested PCR assay.
All supernatant samples from canine and feline feces were subjected to three freeze-thaw cycles (with liquid nitrogen for 5 min and at 95°C for 5 min) and then to the genomic DNA extraction using a QIAamp DNA stool minikit (Qiagen GmBH). DNA extracts were then subjected to a seminested PCR protocol to amplify a diagnostic region within the cox1 gene of C. aerophila. In the first step, primers Cox1NEMF and Cox1NEMR were used, while in the second round, the forward primer CaerInt2F (5′-GAAGCCTTAATAACTATTTCAGG-3′) within the aforementioned C. aerophila 344-bp-long cox1 region, designed following the criteria of Sharrocks (22), was used together with primer Cox1NEMR to achieve specific amplification of a 299-bp-long fragment (pcox1).
PCR mixtures (50 μl) contained 100 pmol of each primer in both steps, 4 μl of DNA extract in the first step and 5 μl of template in the second step, 25 μl of Ready Mix REDTaq (Sigma, St. Louis, MO), and distilled water provided by the same manufacturer. PCRs were performed in a thermal cycler (2700; Applied Biosystems, Foster City, CA) using the following cycling protocol: 10 min at 95°C; 40 cycles at 94°C for 1 min, 48°C (first step) or 52°C (second step) for 1 min, and 72°C for 1 min; and a final extension at 72°C for 10 min. Amplicons were electrophoresed in a 1.6% (wt/vol) agarose gel, stained with Gel Red 10.000X (Biotium, Inc.), purified using a QIAquick gel extraction kit (Qiagen, GmbH, Hilden, Germany), and then sequenced directly using a Taq DyeDeoxyTerminator cycle sequencing kit (v.2; Applied Biosystems Inc.).
(iii) Molecular analysis.
All sequences were determined in both orientations, and the quality of individual electropherograms was verified by eye. Sequences were aligned using BioEdit software 7.0 (13) and then compared with each other and with those of the Capillarinae cox1 gene available in GenBank using the nucleotide-nucleotide Basic Local Alignment Search Tool (BLAST) (2). Subsequently, pairwise comparisons of sequence differences (D) were made using the formula D = 1 − (M/L), where M is the number of alignment positions at which the two sequences have a base in common and L is the total number of alignment positions over which the two sequences are compared (9). The open reading frames (ORFs) were confirmed by conceptual translation of all nucleotide sequences into amino acid sequences using the invertebrate mitochondrial code MEGA5 software (25).
Nucleotide sequence accession numbers.
Nucleotide sequence data for pcox1 of C. aerophila have been registered in the GenBank database under accession numbers JQ905052 to JQ905059.
RESULTS
Parasitological examination.
Of the 34 dogs positive for C. aerophila eggs (Fig. 1), 19 were also positive for other endoparasites, i.e., whipworms, roundworms, and/or hookworms (Fig. 2), Capillaria bohemi (Fig. 3), or tapeworms and coccidians (Table 1). Of the 10 cats with lung capillariosis, 6 were positive for other endoparasites, i.e., Aelurostrongylus abstrusus, roundworms, hookworms, tapeworms, and/or coccidia (Table 1). The 44 animals negative for C. aerophila eggs were positive for whipworms (only dogs), other helminths, and coccidia as well (Table 1).
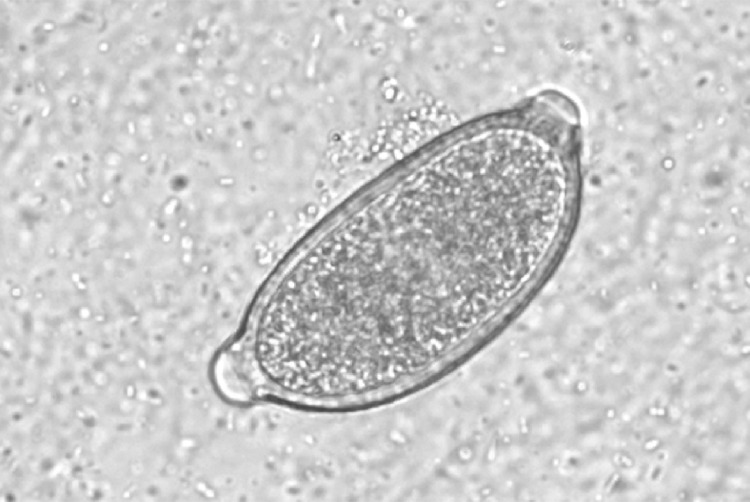
Fig 1.
Capillaria aerophila egg (canine sample D15).
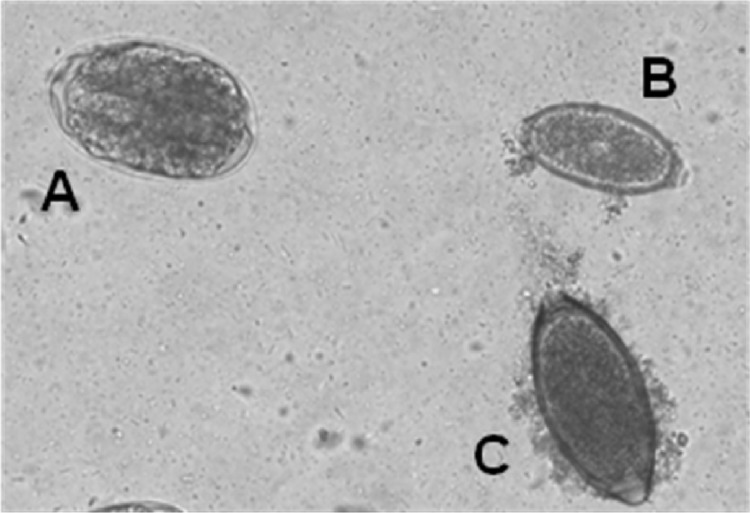
Fig 2.
Ancylostoma caninum (A), Capillaria aerophila (B), and Trichuris vulpis (C) eggs (canine sample 6).
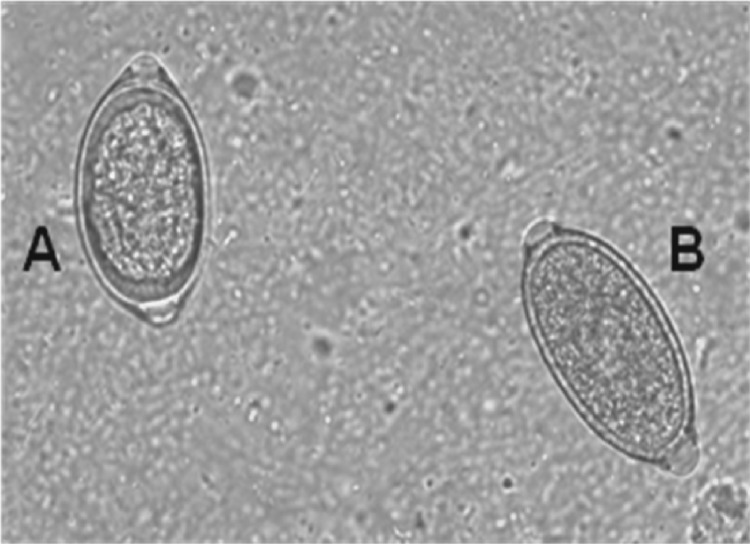
Fig 3.
Capillaria bohemi (A) and Capillaria aerophila (B) eggs (canine sample 8).
Table 1.
Fecal samples, collected from dogs and cats positive or negative for Capillaria aerophila and other endoparasites, used to validate a diagnostic molecular assay specific for lung capillariosis
| Capillaria aerophila result and animal species | No. of positive samples/total (% positive) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capillaria bohemi | Trichuris vulpis | Toxocara canis | Ancylostoma caninum | Isospora canis | Aelurostrongylus abstrusus | Toxocara cati | Ancylostoma tubaeforme | Isospora felis | Toxascaris leonina | Uncinaria stenocephala | Dipylidium caninun | |
| Positive | ||||||||||||
| Dog | 1/34 (2.9) | 13/34 (38.2) | 3/34 (8.8) | 7/34 (20.6) | 1/34 (2.9) | —a | — | — | — | 0/34 (0) | 2/34 (5.9) | 2/34 (5.9) |
| Cat | — | — | — | — | — | 4/10 (40) | 1/10 (10) | 1/10 (10) | 2/10 (20) | 1/10 (10) | 0/10 (0) | 1/10 (10) |
| Negative | ||||||||||||
| Dog | 0/22 (0) | 14/22 (63.6) | 5/22 (27.7) | 7/22 (31.8) | 3/22 (13.6) | — | — | — | — | 1/22 (4.5) | 0/22 (0) | 2/22 (9.1) |
| Cat | — | — | — | — | — | 8/22 (36.4) | 10/22 (45.5) | 1/22 (4.5) | 3/22 (13.6) | 4/22 (18.2) | 0/22 (0) | 4/22 (18.2) |
—, not found in this host.
Molecular identification and analysis.
All samples but one from the 34 dogs (97%) and all samples from cats (100%) with lung capillariosis and other infections (Table 1) scored positive in the seminested PCR for an amplicon of ∼300 bp. All samples collected from animals negative for C. aerophila and positive for other parasites (Table 1) were PCR negative. Sequencing of all 43 PCR products generated by the second round with the primer set CaerInt2F-Cox1NEMR confirmed their identity as C. aerophila compared each other and with cox1 sequences obtained from adult nematodes previously identified morphologically and provided by colleagues.
The molecular analysis showed no insertions or deletions in any of the sequences. Eight pcox1 sequence types (designated haplotypes I to VIII) were detected among the 43 sequences determined. The nucleotide sequence variation among all 8 haplotypes, upon pairwise comparison, ranged from 0.4 to 5.5% (mean, 1.9%) (Table 2). The most prevalent haplotypes were I, II, and III, followed by the other 5 haplotypes. In particular, 32 sequences obtained (27 from dogs and 5 from cats) were identical to each other (haplotype I). The remaining 11 sequences differed from haplotype I by a number of mutations (from 1 to 11). Table 3 reports the number of sequences obtained for each of the haplotypes I to VIII and the nucleotide A-G and T-C transitions and transversions and their residue numbers for haplotypes II to VIII compared to haplotype I. Most nucleotide alterations were synonymous (70%), with the exception of two nonsynonymous nucleotide substitutions at the 2nd (A→G) and 59th (T→G) positions, which resulted in two amino acid alterations (i.e., K→S and L→R, respectively). The comparison of the sequences generated here with those for Capillarinae available in the GenBank, showed maximum identity of 87% (haplotypes I to VII) to 88% (haplotype VIII) with the cox1 gene of Capillaria sp. 1 isolate C1DvC23 from the Australian Oriental quall (Dasyurus viverrinus) (accession number AJ288164.1). When sequences were compared with those obtained from adult C. aerophila parasites from different hosts and European countries used to preliminarily characterize the cox1 gene of the nematode, 100% homology was found between haplotype I and sequences obtained from parasites collected from foxes (Vulpes vulpes) in Serbia and Romania. Also, haplotype II was identical to the cox1 sequence from one C. aerophila adult collected from a fox in Serbia, while haplotype III showed identity with C. aerophila adults retrieved at the necropsy of a fox and a beech marten (Martes foina) in Portugal. Haplotype IV matched sequences from C. aerophila collected from foxes in Romania and Portugal (not shown), while the remaining haplotypes did not display 100% homology with sequences from adults collected from wildlife.
Table 2.
Pairwise comparison of sequences differences (%) among the cox1 sequence haplotypes (HI to HVIII) representing Capillaria aerophila isolates from 33 dogs and 10 cats from Italy
| Haplotype | Sequence difference (%) from: |
||||||
|---|---|---|---|---|---|---|---|
| HI | HII | HIII | HIV | HV | HVI | HVII | |
| HII | 0.4 | ||||||
| HIII | 0.4 | 0.8 | |||||
| HIV | 0.4 | 0.8 | 0.8 | ||||
| HV | 0.4 | 0.8 | 0.8 | 0.8 | |||
| HVI | 0.4 | 0.8 | 0.8 | 0.8 | 0.8 | ||
| HVII | 1.6 | 1.2 | 2 | 1.2 | 2 | 2 | |
| HVIII | 4.3 | 4.3 | 4.7 | 4.7 | 4.7 | 4.7 | 5.5 |
Table 3.
Residue positions of nucleotide transitions (A-G and T-C) and transversions in haplotypes II to VIII compared to haplotype I of Capillaria aerophila sequences from 33 dogs and 10 cats from Italy
| Haplotype | na | Nucleotide at positionb: |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 51 | 57 | 63 | 75 | 82 | 83 | 84 | 96 | 114 | 156 | 174 | 177 | 183 | 225 | 244 | 270 | ||
| HI | 32 | A | A | T | C | C | C | T | T | T | C | T | A | T | C | G | T | T |
| HII | 3 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . |
| HIII | 2 | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . |
| HIV | 1 | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . |
| HV | 1 | . | . | . | . | . | . | G | . | . | . | . | . | . | . | . | . | . |
| HVI | 1 | G | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| HVII | 2 | . | . | . | . | . | . | . | . | . | . | C | . | . | . | A | C | C |
| HVIII | 1 | . | T | C | T | T | T | . | G | . | T | . | T | A | A | T | . | . |
Number of sequences belonging to the indicated haplotype.
A dot indicates that the nucleotide is the same as in HI.
DISCUSSION
The efficiency of a seminested PCR for the specific molecular identification of C. aerophila in naturally infected dogs and cats has been demonstrated here. Specific amplicons were also generated from fecal samples containing eggs from closely related trichuroids as well as from other common endoparasites affecting dogs and cats, resulting in an overall specificity of 100%. Additionally, the seminested PCR displayed an assay sensitivity of up to 100% in the unequivocal molecular identification of C. aerophila eggs.
The molecular assay proposed here may greatly contribute to the diagnosis of pulmonary capillariosis, which cannot be achieved by clinical examination due to the many other conditions with overlapping clinical pictures in dogs and cats (e.g., viral, bacterial, and mycotic diseases, allergic conditions, foreign bodies, nasopharyngeal polyps, and lung cancers) (32). Although confirmatory copromicroscopic findings based on the detection of typical trichuroid C. aerophila eggs are pivotal in corroborating a clinical suspicion, their morphological identification is challenging.
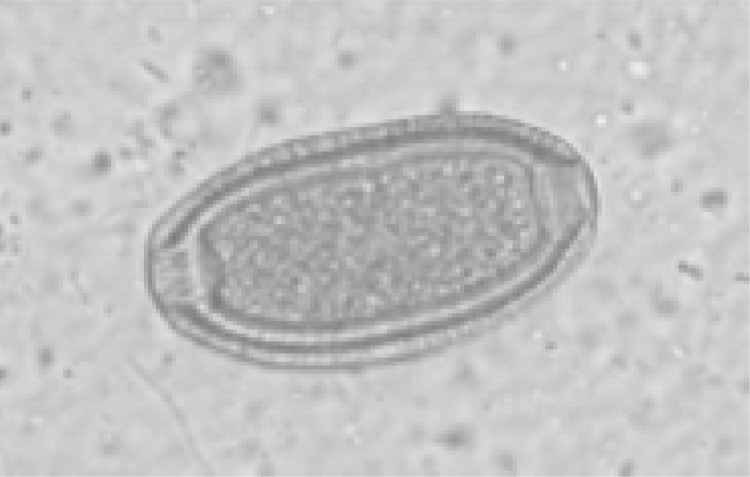
Most often, a copromicroscopical identification of C. aerophila eggs may be complicated, not only in mixed infection caused by this parasite and other helminthes (Table 1 and Fig. 2) but also, and especially, in mixed infection caused by more than one trichuroid at the same time, as in 14 dogs (Table 1 and Fig. 2 and 3). In fact, C. aerophila eggs in canine samples are often misdiagnosed as those of the canine intestinal whipworm T. vulpis and the nasal capillarid C. boehmi, which have very similar morphometric and morphological features (32, 34). Although the identification of C. aerophila eggs in cats may be easy because of the extremely limited distribution of feline whipworms in only a few geographical areas of North and South America (5, 34), eggs of C. aerophila are barely differentiable from those of pseudoparasitic trichuroids. In fact, as demonstrated in one animal examined here, cats may shed in their feces other trichuroid eggs with similar shape, such as those of Capillaria annulata or Capillaria hepatica (Fig. 4) from prey birds and rodents, respectively (5, 26).
Fig 4.
Capillaria hepatica egg (feline sample 12).
It is noteworthy that morphometric and morphological measurements and appraisal are indeed difficult but they also are not routinely performed when trichuroid eggs are retrieved in a stool sample, with the common misconception that T. vulpis is the only nematode that sheds these eggs in dog and cat feces (33, 34).
The occurrence of haplotype I in dogs and cats from Italy as well as in wildlife from other European regions indicates that some C. aerophila populations are shared between wild and domestic carnivores. In particular, Vulpes vulpes and Martes foina may contribute to the spreading of C. aerophila in areas where it previously was not endemic and in companion animals. This epidemiological pattern is similar to that recorded for the cardiopulmonary worm Angiostrongylus vasorum as a likely effect of the increase of fox populations in periurban and urban areas and of movements of wild and companion animals around regions (21, 32, 37). More studies are warranted for evaluating the distribution of C. aerophila in wildlife and pets cohabiting the same geographic areas in order to elucidate the phylogeography of different parasite populations.
The DNA-based method presented here is a powerful tool for holistic studies on C. aerophila by providing a basis for a better understanding of poorly known aspects of the biology, epidemiology, pathogenesis, and taxonomy of this parasite. Topics that deserve to be better investigated include the actual role of C. aerophila in causing lung diseases in humans and the role of earthworms and paratenic hosts in its life history. Indeed, studies aiming to evaluate the diffusion of C. aerophila in humans would be valuable. In fact, thus far only 12 cases of infection have been published in the literature (1, 3, 10, 16, 23, 35, 36). However, it is likely that the disease is underdiagnosed, as the symptoms reported in the literature actually overlap those of a plethora of respiratory diseases which may be self-limiting or may resolve after nonspecific treatments. The application of a reliable test in regional or national parasitology laboratories would be a powerful tool to diagnose human infections by C. aerophila.
ACKNOWLEDGMENTS
We are grateful to all friends and colleagues who have provided fecal samples and adult specimens of Capillaria aerophila: Emanuela Di Giulio, Andrea Rosati, Donato Raele, Luigi Petrucci, Dusan Lalosevic, Eric Morgan, Andrei D. Mihalca, Ludovina Padre, Gary Conboy, and Luis Madeira de Carvalho. We thank Marietta Grazietta for revising the English of the text.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Aftandelians R, Raafat F, Taffazoli M, Beaver PC. 1977. Pulmonary capillariasis in a child in Iran. Am. J. Trop. Med. Hyg. 26:64–71 [DOI] [PubMed] [Google Scholar]
- 2. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananina NO. 1958. Thominx infection of the lungs. Sovetskaya Med. 22:136–137 [PubMed] [Google Scholar]
- 4. Anderson RC. 2000. Nematode parasites of vertebrates. Their development and transmission, p 163–164 CAB International, Wallingford, United Kingdom [Google Scholar]
- 5. Bowman DD. 2000. Respiratory system parasites of the dog and cat. I. Nasal mucosa and sinuses, and respiratory parenchyma. International Veterinary Information Service, Ithaca, NY: http://www.ivis.org/advances/Parasit_Bowman/ddb_resp/ivis.pdf [Google Scholar]
- 6. Burgess H, Ruotsalo K, Peregrine AS, Hanselman B, Abrams-Ogg A. 2008. Eucoleus aerophilus respiratory infection in a dog with Addison's disease. Can. Vet. J. 49:389–392 [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell BG. 1991. Trichuris and other trichinelloid nematodes of dogs and cats in the United States. Compen. Contin. Educ. Pract. Vet. 13:769–778 [Google Scholar]
- 8. Carlsgart J, Roepstorff A, Nejsum P. 2009. Multiplex PCR on single unembryonated Ascaris (roundworm) eggs. Parasitol. Res. 104:939–943 [DOI] [PubMed] [Google Scholar]
- 9. Chilton NB, Gasser RB, Beveridge I. 1995. Differences in a ribosomal DNA sequence of morphologically indistinguishable species within the Hypodontus macropi complex (Nematoda: Strongyloidea). Int. J. Parasitol. 25:647–651 [DOI] [PubMed] [Google Scholar]
- 10. Coudert J, Despeignes J, Battesti R. 1972. A propos d'un cas de capillariose pulmonaire. Bull. Soc. Pathol. Exot. 65:841–848 [PubMed] [Google Scholar]
- 11. Di Cesare A, et al. 2011. Canine and feline infections by cardiopulmonary nematodes in central and southern Italy. Parasitol. Res. 109(Suppl. 1):S87–S96 [DOI] [PubMed] [Google Scholar]
- 12. Epe C, Coati N, Schnieder T. 2004. Results of parasitological examinations of faecal samples from horses, ruminants, pigs, dogs, cats, hedgehogs and rabbits between 1998 and 2002. Dtsch. Tierarztl. Wochenschr. 6:243–247 [PubMed] [Google Scholar]
- 13. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 14. Holmes PR, Kelly JD. 1973. Capillaria aerophila in the domestic cat in Australia. Aust. Vet. J. 49:472–473 [DOI] [PubMed] [Google Scholar]
- 15. Jefferies R, Morgan ER, Helm J, Robinson M, Shaw SE. 2011. Improved detection of canine Angiostrongylus vasorum infection using real-time PCR and indirect ELISA. Parasitol. Res. 109:1577–1583 [DOI] [PubMed] [Google Scholar]
- 16. Lalosević D, Lalosević V, Klem I, Stanojev-Jovanović D, Pozio E. 2008. Pulmonary capillariasis miming bronchial carcinoma. Am. J. Trop. Med. Hyg. 78:14–16 [PubMed] [Google Scholar]
- 17. Latrofa MS, et al. 2012. A duplex real-time polymerase chain reaction assay for the detection of and differentiation between Dirofilaria immitis and Dirofilaria repens in dogs and mosquitoes. Vet. Parasitol. 185:181–185 [DOI] [PubMed] [Google Scholar]
- 18. Madeira de Carvalho LM, Pereira da Fonseca LM, Gomes L, Meireles JM. 2009. Lungworms in domestic and wild carnivores in Portugal: rare parasites or rarely diagnosed? In Proceedings of the Bayer Angiostrongylosis Forum, 19th Annual Congress of the European College of Veterinary Internal Medicine—Companion Animals, Porto, Portugal [Google Scholar]
- 19. Mircean V, Titilincu A, Vasile C. 2010. Prevalence of endoparasites in household cat (Felis catus) populations from Transylvania (Romania) and association with risk factors. Vet. Parasitol. 171:163–166 [DOI] [PubMed] [Google Scholar]
- 20. Miro G, et al. 2004. Prevalence of antibodies to Toxoplasma gondii and intestinal parasites in stray, farm and household cats in Spain. Vet. Parasitol. 3:249–255 [DOI] [PubMed] [Google Scholar]
- 21. Morgan ER, et al. 2005. Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol. 21(2):49–51 [DOI] [PubMed] [Google Scholar]
- 22. Sharrocks AD. 1994. The design of primers for PCR, p 5–11 In Griffin HG, Griffin R. (ed), PCR technology: current innovations. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 23. Skrjabin KI, Shikhovalova NP, Orlov IV. 1957. Essentials of nematology. VI. Trichocephalidae and Capillaridae of animals and man and diseases caused by them. Academy of Sciences of USSR, Moscow, USSR [Google Scholar]
- 24. Sloss MW, Kemp RL, Zajac AM. 1994. Veterinary clinical parasitology, 6th ed, p 17–44 Iowa State University Press, Ames, IA [Google Scholar]
- 25. Tamura K, et al. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor MA, Coop RL, Wall RL. 2007. Veterinary parasitology. 3rd ed, p 395–396 Blackwell Publishing, Oxford, United Kingdom [Google Scholar]
- 27. Testini G, et al. 2011. New insights into the morphology, molecular characterization and identification of Baylisascaris transfuga (Ascaridida, Ascarididae). Vet. Parasitol. 175:97–102 [DOI] [PubMed] [Google Scholar]
- 28. Traversa D, et al. 2004. Semi-nested PCR for the specific detection of Habronema microstoma or Habronema muscae DNA in horse faeces. Parasitology 129:733–739 [DOI] [PubMed] [Google Scholar]
- 29. Traversa D, Iorio R, Otranto D. 2008. Diagnostic and clinical implications of a nested PCR specific for ribosomal DNA of the feline lungworm Aelurostrongylus abstrusus (Nematoda, Strongylida). J. Clin. Microbiol. 46:1811–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Traversa D, Otranto D. 2009. Biotechnological advances in the diagnosis of little-known parasitoses of pets. Parasitol. Res. 104:209–216 [DOI] [PubMed] [Google Scholar]
- 31. Traversa D, Di Cesare A, Milillo P, Iorio R, Otranto D. 2009. Infection by Eucoleus aerophilus in dogs and cats: is another extra-intestinal parasitic nematode of pets emerging in Italy? Res. Vet. Sci. 87:270–272 [DOI] [PubMed] [Google Scholar]
- 32. Traversa D, Di Cesare A, Conboy G. 2010. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit. Vectors 3:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Traversa D. 2011. Are we paying too much attention to cardio-pulmonary nematodes and neglecting old-fashioned worms like Trichuris vulpis? Parasit. Vectors 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Traversa D, et al. 2011. New insights into morphological and biological features of Capillaria aerophila (Trichocephalida, Trichuridae). Parasitol. Res. 1:S97–S104 [DOI] [PubMed] [Google Scholar]
- 35. Vilella JM, Desmaret MC, Rouault E. 1986. Capillariose à Capillaria aerophila chez un adulte? Med. Mal. Infect. 1:35–36 [Google Scholar]
- 36. Volkov VE, Pak EM. 1973. A case of Thominx aerophilus complicated by asthmatic bronchitis. Voen. Med. Zh. 5:84 [Google Scholar]
- 37. Wandeler P, Funk RL, Largiader CR, Gloor S, Breitenmoser U. 2003. The city-fox phenomenon: genetic consequences of a recent colonization of urban habitat. Mol. Ecol. 12:647–656 [DOI] [PubMed] [Google Scholar]