Abstract
Infections due to Staphylococcus aureus present a significant health problem in the United States. Between 1990 and 2005, there was a dramatic increase in community-associated methicillin-resistant S. aureus (MRSA), but recent reports suggest that MRSA may be declining. We retrospectively identified S. aureus isolates (n = 133,450) that were obtained from patients in a large integrated health plan between 1 January 1998 and 31 December 2009. Trends over time in MRSA were analyzed, and demographic risk factors for MRSA versus methicillin-susceptible S. aureus (MSSA) were identified. The percentage of S. aureus isolates that were MRSA increased from 9% to 20% between 1998 and 2001 and from 25% to 49% between 2002 and 2005 and decreased from 49% to 43% between 2006 and 2009. The increase in MRSA was seen in blood and in other bacteriological specimens and occurred in all age and race/ethnicity groups, though it was most pronounced in persons aged 18 to <50 years and African-Americans. Hospital onset infections were the most likely to be MRSA (odds ratio [OR], 1.58; confidence interval [CI], 1.46 to 1.70, compared to community-associated cases), but the largest increase in MRSA was in community-associated infections. Isolates from African-Americans (OR, 1.73; CI, 1.64 to 1.82) and Hispanics (OR, 1.11; CI, 1.06 to 1.16) were more likely to be MRSA than those from whites. After substantial increases between 1998 and 2005 in the proportion of S. aureus isolates that were MRSA, the proportion decreased between 2006 and 2009. Hospital onset S. aureus infections are disproportionately MRSA, as are those among African-Americans.
INTRODUCTION
Infections due to Staphylococcus aureus present a substantial health problem in the United States (20, 23). S. aureus is a major cause of hospital-acquired pneumonia and lower respiratory tract infections (37) and the primary cause of surgical-site infections (36) and skin and soft tissue infections (SSTIs) (29) and is now likely the leading cause of invasive bacterial disease (20, 23). Throughout the 1990s, S. aureus infections in hospitalized patients were increasingly caused by methicillin-resistant S. aureus (MRSA), making treatment of these infections more difficult (20, 21, 23, 40). Moreover, between 1990 and 2005, there was an even more dramatic increase in community-associated MRSA (CA-MRSA) infections (1, 5, 10, 11, 19, 27, 32). However, there are indications that MRSA may be on the decline. A recent U.S. study found that the incidence of health care-associated invasive MRSA declined from 2005 to 2008 (18), and an analysis of S. aureus isolates from outpatient pediatric patients with SSTIs found the percentage of isolates that were MRSA was lower in 2008 and 2009 than in 2005 to 2007 (9).
In general, surveillance activity for MRSA has been limited to bloodstream or invasive infections and to health care-associated and hospital onset disease, and there have been few population-based studies. As the epidemiology of S. aureus disease changes, inclusion of community-associated, community onset, and noninvasive disease is important for assessing the magnitude of the burden of disease in the population, for setting priorities for prevention and control, and for creating guidelines for empirical antibiotic treatment.
In this retrospective study covering 12 years, we describe laboratory-confirmed S. aureus infections in a large cohort of persons of all ages, using isolates from sterile and nonsterile sites in both ambulatory and inpatient settings. We describe trends in methicillin resistance of laboratory-confirmed S. aureus isolates and trends in the incidence of S. aureus bloodstream infections and explore the relationship of patient demographics and infection onset type to the likelihood of S. aureus infections being methicillin resistant.
MATERIALS AND METHODS
Setting.
Kaiser Permanente of Northern California (KPNC) is a nonprofit, integrated health care delivery system providing care to over 3 million members. The member population reflects the general population in the Northern California region, although as an insured population, it underrepresents persons with annual incomes less than $25,000 (9% of the KPNC member population 25 to 79 years of age versus 21% of the general Northern California population 25 to 79 years of age) and overrepresents persons with annual incomes greater than $100,000 (30% versus 26%). Although racially diverse, the KPNC population also somewhat underrepresents Latinos/Hispanics (19% versus 22%) and overrepresents African-Americans (8% versus 5.5%) (15). KPNC provides services in more than 15 counties and operates more than 40 outpatient clinics and 18 hospitals throughout Northern California. Laboratory results and diagnostic data from hospital discharges and ambulatory settings, including emergency departments, are archived in databases. KPNC databases contain individual patient records and are readily linked using the patient's unique medical record number, which remains with the patient for life. The study was approved by the KPNC Institutional Review Board. In this retrospective, observational study, clinical decisions regarding diagnosing and testing reflect the usual care by KPNC providers.
Identification of S. aureus infections.
Using KPNC's Laboratory Utilization and Reporting System, which maintains an account of all laboratory tests performed by KPNC's regional laboratory, we identified all microbiological specimens testing positive for S. aureus that were obtained between 1 January 1997 and 31 December 2009. Virtually all microbiology tests ordered by KPNC providers for KPNC members are performed by the KPNC regional laboratory in Berkeley, CA. Because patients may be tested repeatedly, we adapted the Clinical and Laboratory Standards Institute guideline (originally designed for studies of antibiotic resistance) and recommendations in the literature and included only the first isolate per person per 365-day period (not calendar year) (25, 39). (A sensitivity analysis was performed in which we included only the first isolate per person per 30-day period.) In cases where the same individual had more than one isolate from different sources on the same day (about 2.2% of infections), our selection priority was as follows: blood, cerebrospinal fluid (CSF), bone, body fluid, other miscellaneous bacterial cultures, tissue, respiratory, and urine. The first isolate per person per 365-day period was the “index isolate,” and the date of the index isolate was the “index date” for that case. In order to identify isolates likely to be related to clinically relevant infections, we restricted our analyses to isolates from blood, bone, CSF, body fluid, urine, tissue, respiratory, and other miscellaneous bacterial specimens (such as those taken from abscesses, pustules, boils, wounds, etc.). Screening tests (nares) and genital, stool, catheter tip, and throat cultures were excluded. In addition, we required patients to have been diagnosed with a potentially S. aureus-related clinical syndrome from 7 days before to 7 days after the index date. Clinical syndromes were identified using electronic databases that capture all diagnoses (International Classification of Diseases, 9th Revision, Clinical Modification [ICD9]) received by KPNC patients. The patients could have been diagnosed with more than one clinical syndrome during the applicable period. Only patients who were KPNC members on the index date were included. For analyses restricted to bloodstream infections, the index isolate was the first isolate from a blood specimen per person per 365-day period meeting the above criteria.
Suspect Staphylococcus colonies (catalase-positive, Gram-positive cocci in clusters) were identified by clumping factor and/or protein A or by coagulation of coagulase plasma. Using an 18- to 24-h culture from nonselective medium, isolated S. aureus colonies were selected for antimicrobial susceptibility testing using commercial systems (MicroScan [Siemens Healthcare Diagnostics Inc., Sacramento, CA] or Vitek 2 [bioMérieux, Hazelwood, MO] for urine isolates).
Isolates displaying an oxacillin MIC of ≥4 μg/ml were subjected to additional confirmatory testing for MRSA. A suspension of the suspect isolate was prepared and used to inoculate a BBL Oxacillin Screen agar plate (Becton, Dickinson and Company, Sparks, MD), which was incubated in an ambient-air incubator at 33 to 35°C for 24 h. Isolates were characterized as MRSA if the isolates both grew on the Oxacillin Screen agar plate and displayed oxacillin MIC values of ≥4 μg/ml. This method was used throughout the study period.
Definitions of health care- and community-associated infections.
Using definitions and terminology adapted from the literature on bacterial infections (10, 22, 23, 33) so as to be applicable to the clinical data available to us, we classified infections into one of three mutually exclusive groups: (i) health care associated, hospital onset (HCA-HO); (ii) health care associated, community onset (HCA-CO); and (iii) community associated (CA). HCA-HO infections were those for which the index specimen was obtained during a hospital stay but more than 48 h after admission. HCA-CO infections were those for which the index specimen did not meet the criteria for HCA-HO but did meet one or more of the following criteria: (i) ordered within 365 days of a hospital stay, outpatient surgery, or dialysis; (ii) ordered within 365 days of a known stay at a skilled nursing facility or nursing home. CA infections were those that were neither HCA-HO nor HCA-CO.
Patient characteristics.
Patient age and sex were extracted from KPNC membership databases. Although race/ethnicity is not systematically captured for all members, the race/ethnicity of a majority of patients (about 85%) was identified via a search of electronic medical records, membership databases, and other administrative databases. Patients were classified as Hispanic if their ethnicity was identified as Hispanic in any of these sources. Patient addresses were extracted from membership databases and used to determine the patient's census block group. Using year 2000 U.S. census data, we determined the median census block group income for each patient and classified their index isolate based on the quintile of the median income. Persons whose address information did not map to a valid census block were classified as “unknown.”
Risk factors for MRSA versus methicillin-susceptible S. aureus (MSSA).
We examined the relationship between demographic characteristics and onset type and the risk of MRSA for infections occurring in the years 2006 through 2009. The analytical data set had a record for each S. aureus infection occurring in these 4 years. Odds ratios (OR) were estimated using a multivariate logistic regression where the dependent variable was whether or not the isolate was methicillin resistant and the independent variables were sex, age (treated as a categorical variable with five levels: less than 5, 5 to <18, 18 to <50, 50 to <65, and 65+ years of age), race/ethnicity, quintile of census block income, and onset type. Because some patients had more than one infection between 2006 and 2009 (3.7% of episodes were “repeat” infections), we used a generalized estimating equation approach with an exchangeable covariance structure to account for correlation among records for the same person. The resulting odds ratios represent the odds of testing positive for MRSA given an S. aureus infection compared to that of the reference group.
Calculation of S. aureus bloodstream infection incidence rates.
We calculated incidence rates for the subset of laboratory-confirmed S. aureus infections that were identified from blood specimens. These infections tend to represent especially severe cases, and we expected the propensity for microbiological testing of these infections to be stable over time. The denominator used for calculating incidence rates of S. aureus bloodstream infections was KPNC member years. We identified all persons who were members of KPNC at any time between 1 January 1998 and 31 December 2009. KPNC membership systems track membership on a monthly basis, and people could enter and leave the health plan throughout this time. We summarized S. aureus bloodstream infections and member months for each calendar year and calculated annual incidence rates by dividing the number of cases by member years. To examine trends, we standardized all annual incidence rates to the 2009 KPNC age and sex population using direct adjustment.
RESULTS
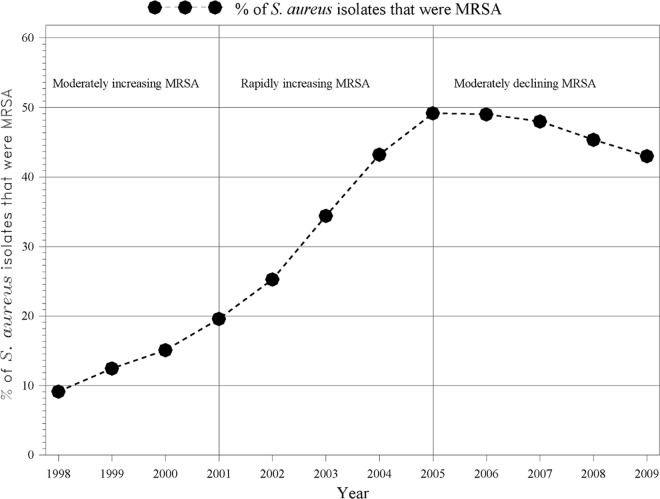
We identified 133,450 culture-confirmed S. aureus infections during the period 1998 to 2009. Based on visual analysis of the trend in the percentage of isolates positive for MRSA, we defined three distinct time periods: (i) moderately increasing MRSA period (1998 to 2001), where the percentages of infections that were MRSA were 9, 12, 15, and 20 in each year, respectively; (ii) rapidly increasing MRSA period (2002 to 2005), where the percentages with MRSA were 25, 34, 43, and 49 in each year, respectively; and (iii) moderately declining MRSA period (2006 to 2009), where the percentages with MRSA were 49, 48, 45, and 43 in each year, respectively (Fig. 1). The trend in the percentage of S. aureus isolates that were MRSA was statistically significantly different in each of the three periods, with an average increase of 3.4% per year (confidence interval [CI], 2.7% to 4.1%) from 1998 to 2001, 8.1% (CI, 7.3% to 8.8%) from 2002 to 2005, and −2.1% (CI, −2.8% to −1.3%) from 2006 to 2009.
Fig 1.
Percentage of S. aureus isolates that were MRSA, 1998 to 2009.
Table 1 describes the S. aureus infections by time period. The percentages of isolates that were MRSA were 14% in 1998 to 2001, 41% in 2002 to 2005, and 46% in 2006 to 2009. As a share of all laboratory-confirmed S. aureus infections, those that were health care associated declined over time (10% to 4% for hospital onset and 35% to 22% for community onset), while the share that were community associated increased substantially (55% to 74%). The share of infections where the index isolate was from a blood specimen declined from 9% to 3%. When analyzing the first relevant isolate per person per 30-day period (rather than per 365-day period as described above), the percentages of isolates that were MRSA were 14%, 38%, and 44% in the periods 1998 to 2001, 2002 to 2005, and 2006 to 2009, respectively.
Table 1.
Characteristics of S. aureus infections, Kaiser Permanente of Northern Californiaa
| Characteristic | Valueb |
|||
|---|---|---|---|---|
| Moderately increasing MRSA (1998–2001) | Rapidly increasing MRSA (2002–2005) | Moderately declining MRSA (2006–2009) | All (1998–2009) | |
| Total no. of infections | 21,242 (100) | 37,875 (100) | 74,333 (100) | 133,450 (100) |
| Isolate type | ||||
| MRSA | 3,016 (14) | 15,449 (41) | 34,436 (46) | 52,901 (40) |
| MSSA | 18,226 (86) | 22,426 (59) | 39,897 (54) | 80,549 (60) |
| Sex | ||||
| Female | 9,301 (44) | 17,046 (45) | 34,870 (47) | 61,217 (46) |
| Male | 11,941 (56) | 20,829 (55) | 39,463 (53) | 72,233 (54) |
| Age (yr) | ||||
| Under 5 | 1,251 (6) | 2,014 (5) | 5,461 (7) | 8,726 (7) |
| 5 to <18 | 2,108 (10) | 4,088 (11) | 10,980 (15) | 17,176 (13) |
| 18 to <50 | 6,584 (31) | 13,406 (35) | 28,526 (38) | 48,516 (36) |
| 50 to <65 | 4,824 (23) | 7,958 (21) | 14,847 (20) | 27,629 (21) |
| 65+ | 6,475 (30) | 10,409 (27) | 14,519 (20) | 31,403 (24) |
| Mean age (SD) (yr) | 49 (25) | 47 (25) | 42 (25) | 45 (25) |
| Race/ethnicityc | ||||
| Asian | 1,645 (8) | 2,982 (8) | 6,204 (8) | 10,831 (8) |
| African-American | 1,852 (9) | 3,639 (10) | 7,210 (10) | 12,701 (10) |
| Hispanic | 2,602 (12) | 5,137 (14) | 11,912 (16) | 19,651 (15) |
| Other or unknown race | 2,803 (13) | 5,799 (15) | 12,268 (17) | 20,870 (16) |
| White | 12,340 (58) | 20,318 (54) | 36,739 (49) | 69,397 (52) |
| Onset type | ||||
| Health care associated, hospital onset | 2,152 (10) | 3,469 (9) | 3,159 (4) | 8,780 (7) |
| Health care associated, community onset | 7,395 (35) | 11,098 (29) | 16,312 (22) | 34,805 (26) |
| Community associated | 11,695 (55) | 23,308 (62) | 54,862 (74) | 89,865 (67) |
| Specimen type of index isolated | ||||
| Blood | 1,837 (9) | 2,207 (6) | 2,267 (3) | 6,311 (5) |
| Other | 19,405 (91) | 35,668 (94) | 72,066 (97) | 127,139 (95) |
Infections were defined as the first positive S. aureus isolate per patient per 365-day period and patients who were diagnosed with a potentially S. aureus-related clinical syndrome from 7 days before to 7 days after the index date. Only isolates from blood, bone, CSF, body fluid, urine, tissue, respiratory, and other miscellaneous bacterial specimens (such as those taken from abscesses, pustules, boils, and wounds) were included.
Data are number (%) of S. aureus infections unless otherwise indicated.
Race was not systematically collected for all patients. Electronic medical records, membership databases, and other administrative databases were searched for indications of race. Persons with high utilization have more chances to have race captured in the electronic systems. Patients were classified as Hispanic if their ethnicity was identified as Hispanic in any of these sources.
Specimen type of the first positive S. aureus isolate per person per 365-day period. If an isolate from a nonblood specimen preceded an isolate from a blood specimen (within 365 days), only the nonblood isolate was included in the table.
Skin and soft tissue infections were by far the most common clinical syndrome (Table 2). The pattern of increasing and then stabilizing (or declining) MRSA was seen in nearly all clinical syndromes. The same pattern was seen in all age groups, with the largest increase in the 18 to <50 year olds, and in all race/ethnicity groups, with the largest increase in African-Americans. The precipitous increase in the percentage of staphylococcal infections that were MRSA was largely related to community-associated infections, where the percentage increased from about 6% from 1998 to 2001 to 46% from 2006 to 2009. However, consistent with the overall trend seen in Fig. 1, between 2006 and 2009 the percentage of isolates that were MRSA declined modestly for every age (except 0 to 5 year olds) and race/ethnicity group and onset type (not shown).
Table 2.
S. aureus infections by time period, clinical syndrome, demographics, and onset type, Kaiser Permanente of Northern Californiaa
| Characteristics | Value |
|||||
|---|---|---|---|---|---|---|
| Moderately increasing MRSA (1998–2001) |
Rapidly increasing MRSA (2002–2005) |
Moderately decreasing MRSA (2006–2009) |
||||
| No. of infections | % MRSA | No. of infections | % MRSA | No. of infections | % MRSA | |
| Clinical syndromesb | ||||||
| Necrotizing fasciitis/toxic shock | 37 | 27 | 78 | 37 | 103 | 49 |
| Deep organ abscess | 317 | 28 | 607 | 51 | 796 | 48 |
| Skin and soft tissue infection | 15,718 | 13 | 30,775 | 42 | 66,382 | 48 |
| Pneumonia or lung related | 2,023 | 33 | 3,169 | 51 | 3,003 | 50 |
| Joint or bone infection | 1,947 | 14 | 2,668 | 30 | 3,496 | 34 |
| Urinary tract infection | 2,548 | 30 | 4,094 | 49 | 4,561 | 48 |
| Ear, nose, throat infections | 1,695 | 5 | 1,929 | 18 | 2,884 | 25 |
| Shock | 307 | 34 | 794 | 51 | 982 | 50 |
| Fever | 749 | 18 | 1,252 | 34 | 1,332 | 35 |
| Other selected syndromesc | 446 | 27 | 626 | 45 | 687 | 42 |
| Age (yr) | ||||||
| Under 5 | 1,251 | 5 | 2,014 | 32 | 5,461 | 46 |
| 5 to <18 | 2,108 | 5 | 4,088 | 34 | 10,980 | 41 |
| 18 to <50 | 6,584 | 8 | 13,406 | 44 | 28,526 | 50 |
| 50 to <65 | 4,824 | 13 | 7,958 | 37 | 14,847 | 44 |
| 65+ | 6,475 | 26 | 10,409 | 44 | 14,519 | 45 |
| Race/ethnicity | ||||||
| Asian | 1,645 | 10 | 2,982 | 29 | 6,204 | 35 |
| African-American | 1,852 | 19 | 3,639 | 54 | 7,210 | 60 |
| Hispanic | 2,602 | 12 | 5,137 | 41 | 11,912 | 48 |
| Other or unknown race | 2,803 | 10 | 5,799 | 43 | 12,268 | 48 |
| White | 12,340 | 15 | 20,318 | 40 | 36,739 | 45 |
| Onset type | ||||||
| Health care associated, hospital onset | 2,152 | 41 | 3,469 | 58 | 3,159 | 56 |
| Health care associated, community onset | 7,395 | 20 | 11,098 | 40 | 16,312 | 45 |
| Community associated | 11,695 | 6 | 23,308 | 39 | 54,862 | 46 |
Infections were defined as the first positive S. aureus isolate per patient per 365-day period and patients who were diagnosed with a potentially S. aureus-related clinical syndrome from 7 days before to 7 days after the index date. Only isolates from blood, bone, CSF, body fluid, urine, tissue, respiratory, and other miscellaneous bacterial specimens (such as those taken from abscesses, pustules, boils, and wounds) were included.
Clinical syndromes were based on ICD9 codes received by the patient from 7 days before to 7 days after the S. aureus specimen was obtained. Patients could have more than one clinical syndrome, and thus, the numbers add up to more than the total number of infections.
Includes meningitis, intracranial abscess, tracheostomy infection, esophagostomy infection, gastrostomy infection, nonhealing surgical wounds, acute pericarditis, acute endocarditis, acute myocarditis, other diseases of the pericardium, arterial embolism, and thrombosis.
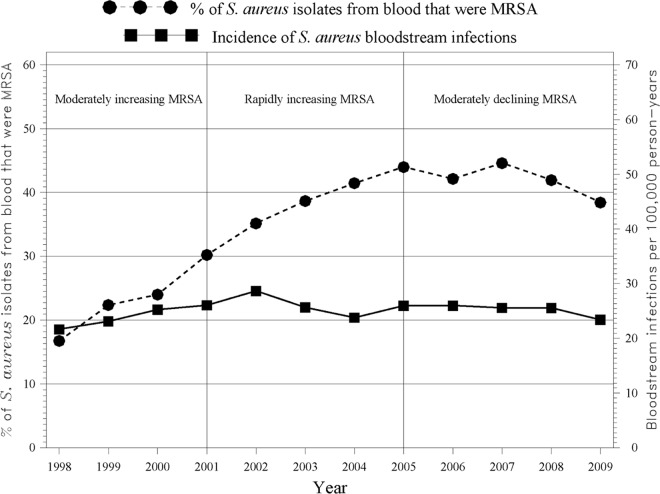
Multivariate logistic regression indicated that, among S. aureus infections, isolates in persons 65 years of age and older and persons 18 to <50 years of age were more likely to be MRSA than those from persons in other age groups (Table 3). Isolates from African-Americans (OR, 1.73; CI, 1.64 to 1.82) and Hispanics (OR, 1.11; CI, 1.06 to 1.16) were more likely to be MRSA than those from whites, while isolates from Asians were less likely to be MRSA than those from whites (OR, 0.69; CI, 0.65 to 0.73). Isolates from persons living in less affluent census blocks were more likely to be MRSA than those from persons living in more affluent census blocks, and hospital onset isolates were more likely to be MRSA than community-associated isolates (OR, 1.58; CI, 1.46 to 1.70). Restricting the analysis to bloodstream infections, the percentage of MRSA isolates increased from about 17% to 44% between 1998 and 2005, after which it stabilized at around 40% (Fig. 2). Although the percentage of S. aureus bloodstream infections that were MRSA increased over time, the incidence rate of S. aureus bloodstream infections (both MRSA and MSSA) remained nearly constant at around 27 infections per 100,000 person years. When analyzing the first relevant blood isolate per person per 30-day period, the incidence rate of S. aureus bloodstream infections (all onset types) was very similar to the rate when using the first isolate per person per 365-day period (25.7 infections per 100,000 in 2009 using the 30-day rule versus 23.4 per 100,000 using the 365-day rule).
Table 3.
Risk factors for methicillin-resistant S. aureus among isolates positive for S. aureus, Kaiser Permanente of Northern California, 2006–2009
| Patient characteristic | OR (CI) of MRSA vs. MSSA among S. aureus isolates (n = 74,333)a |
|---|---|
| Sex | |
| Female | 1.05 (1.02, 1.08) |
| Male | Reference |
| Age (yr) | |
| Under 5 | 1.02 (0.96, 1.09) |
| 5 to <18 | 0.84 (0.79, 0.88) |
| 18 to <50 | 1.20 (1.15, 1.26) |
| 50 to <65 | 0.97 (0.92, 1.02) |
| 65+ | Reference |
| Race/ethnicity | |
| Asian | 0.69 (0.65, 0.73) |
| African-American | 1.73 (1.64, 1.82) |
| Hispanic | 1.11 (1.06, 1.16) |
| Other/unknown | 1.10 (1.05, 1.15) |
| White | Reference |
| Census block income quintile | |
| Unknown | 1.38 (1.29, 1.47) |
| 1st (lowest income) | 1.54 (1.46, 1.62) |
| 2nd | 1.39 (1.32, 1.46) |
| 3rd | 1.25 (1.19, 1.31) |
| 4th | 1.14 (1.09, 1.20) |
| 5th (highest income) | Reference |
| Onset type | |
| Health care associated, hospital onset | 1.58 (1.46, 1.70) |
| Health care associated, community onset | 0.96 (0.92, 1.00) |
| Community associated | Reference |
Odds ratios were estimated using a multivariate logistic regression. The analytic data set had a record for each S. aureus isolate. The dependent variable was whether or not the isolate was methicillin resistant, and the independent variables were gender, age, race/ethnicity, income quintile based on census block, and onset type. Reference, reference group for other odds ratios within a characteristic.
Fig 2.
S. aureus bloodstream infections: percent MRSA and incidence rate.
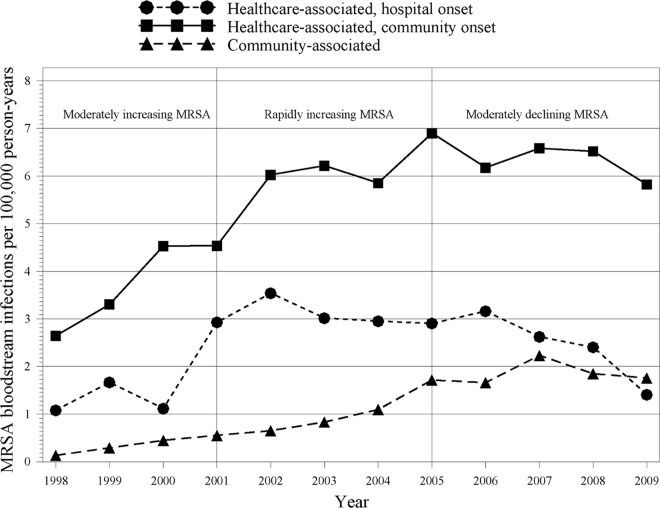
In analyses restricted to MRSA bloodstream infections, we found that the incidence rates of community onset and community-associated bloodstream MRSA infections increased between 1998 and about 2007 (Fig. 3). In contrast, the incidence of hospital onset bloodstream MRSA infections peaked in 2002 and declined thereafter.
Fig 3.
Incidence of bloodstream MRSA infections by onset type.
DISCUSSION
During the 12-year study period from 1998 to 2009, we identified over 133,000 culture-confirmed S. aureus infections and documented a 5-fold increase in the percentage of index isolates that were MRSA. This increase in MRSA occurred most rapidly between 2002 and 2005, after which the percentage of isolates that were MRSA modestly declined. The increase in the percentage of MRSA isolates was seen in blood and, to a greater extent, in other bacteriological specimens and occurred in all ages and race/ethnicity groups, though it was most pronounced in persons aged 18 to <50 years and in African-Americans. Although hospital onset infections were the most likely to be MRSA, the largest increase in MRSA was among community-associated infections.
Although the percentage of S. aureus infections that were MRSA increased significantly between 1998 and 2009 among both bloodstream and nonbloodstream infections, the incidence of bloodstream infections remained fairly constant. Thus, among bloodstream infections, MRSA appears to have merely replaced, to some extent, MSSA. This finding is similar to that of a Canadian study that reported a fairly constant incidence of S. aureus bloodstream infections in Calgary from 2000 to 2006 (about 2 cases per 10,000 person years) while also noting that a higher proportion was due to MRSA (24). We focused our incidence rate analysis on bloodstream infections because they are usually the most serious infections and because bloodstream infection rates are less likely to be affected by changes in the practice of obtaining cultures.
There are surprisingly few published studies that present data on all types of S. aureus infections in a general population in the last decade. Most S. aureus epidemiology studies have focused exclusively on MRSA isolates, have been restricted to invasive disease and isolates from hospitalized patients, and/or have been in special populations.(3, 5, 7, 8, 10, 14, 16, 16–18, 20, 23, 25, 26). Nevertheless, numerous studies reported that in the United States by the mid-2000s, MRSA accounted for 40 to 70% of S. aureus infections (13, 16, 20, 28, 30, 31, 41).
As far as we know, ours is the first study showing that in a very large and diverse population, the percentage of S. aureus isolates that were MRSA began to decrease after 2006. This was true for nearly all ages and races we examined, for blood and nonblood isolates, and for both health care-associated and community-associated infections. These results are consistent with recent national data showing that the incidence of health care-associated invasive MRSA infections appears to have declined after 2005 (18).
Numerous studies have reported that African-American race and low income are risk factors for MRSA infection (14, 18, 23, 25, 38). Our results support these findings. Even in the most current period of stable or declining MRSA, we found that, among persons with an S. aureus infection, African-Americans and persons living in lower-income census blocks were at increased risk of MRSA infection after adjusting for other demographic factors and infection onset type. In addition, our findings indicate that Hispanics have a higher risk of MRSA infection and that Asians have a lower risk of MRSA infection than whites.
Limitations.
Although the rates of MRSA bloodstream infections in our population were about one-third higher than those reported for the Calgary, Canada, population (24), they were substantially lower than those reported by Kallen et al. (18) for a U.S. population and may not be representative of national rates of MRSA bloodstream infections. In part, the difference is methodological, in that we used a more conservative 365-day isolate deduplication algorithm (compared to 30 days) and required patients to have received an S. aureus-related diagnosis. In addition, although KPNC enrolls persons covered by Medicaid and other dues subsidy programs, enrollment is generally through employment, and therefore, the KPNC population likely underrepresents certain populations thought to be at increased risk of MRSA (e.g., incarcerated persons, intravenous drug users, military personnel, the very poor, and the uninsured) (4, 6, 27). Nevertheless, our study population of over 3 million is more representative of the general population than are studies of those special populations. Although we used standard algorithms to classify infections as health care associated and community acquired, we could not establish with certainty the setting in which the S. aureus strains originated. In addition, we had limited information on nursing home status, which was used in the algorithm to define health care-associated infection. Members were assumed to be in nursing homes if their home address matched the known address of a licensed nursing home.
Conclusions.
It has been suggested that, if the emergence of MRSA follows the same trend as beta-lactamase-positive S. aureus, the percentage of community-associated S. aureus infections caused by MRSA strains may approach 100% (6). However, this study, along with several other recent studies (9, 18), indicates a possible interruption in that trend. Culture and susceptibility testing remain important, because in general, beta-lactam antibiotics are still considered the first line in staphylococcal infections that are susceptible, and knowledge of local resistance patterns of S. aureus can help guide empirical therapy. As for future trends in S. aureus, it remains to be seen whether a stabilization or reduction in the proportion of S. aureus isolates that are MRSA will correspond to an absolute decrease in S. aureus infections. The emergence and rapid spread of CA-MRSA may have been related to the emergence of strains possessing certain fitness characteristics, such as carrying Panton-Valentine leukocidin genes (2, 12, 34), rather than to methicillin resistance. There is some evidence that these characteristics are becoming associated with MSSA, making those strains more competitive against MRSA strains (35). Thus, continued surveillance of S. aureus epidemiology is important.
ACKNOWLEDGMENTS
We thank Robin L. Young for providing us with information on antibiogram analysis and laboratory procedures used to culture S. aureus. We also thank Margaret Kelly for her contributions to the style of the manuscript.
This work was supported by GlaxoSmithKline.
G.T.R. received research support from GlaxoSmithKline, Pfizer, and Merck. R.B. received research funding from GlaxoSmithKline, Pfizer, Merck, Sanofi Pasteur, and Novartis. J.A.S. is an employee of GlaxoSmithKline.
Footnotes
Published ahead of print 14 March 2012
REFERENCES
- 1. Adam HJ, et al. 2009. Community-associated methicillin-resistant Staphylococcus aureus: prevalence in skin and soft tissue infections at emergency departments in the Greater Toronto Area and associated risk factors. CJEM 11:439–446 [DOI] [PubMed] [Google Scholar]
- 2. Baba T, et al. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819–1827 [DOI] [PubMed] [Google Scholar]
- 3. Boucher HW, Corey GR. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S344–S349 [DOI] [PubMed] [Google Scholar]
- 4. Charlebois ED, et al. 2002. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco. Clin. Infect. Dis. 34:425–433 [DOI] [PubMed] [Google Scholar]
- 5. Crum NF, et al. 2006. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am. J. Med. 119:943–951 [DOI] [PubMed] [Google Scholar]
- 6. David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23:616–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis SL, et al. 2007. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J. Clin. Microbiol. 45:1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daxboeck F, Assadian O, Apfalter P, Koller W. 2004. Resistance rates of Staphylococcus aureus in relation to patient status and type of specimen. J. Antimicrob. Chemother. 54:163–167 [DOI] [PubMed] [Google Scholar]
- 9. Diamantis ML, Ortega-Loayza AG, Morrell DS. 2011. Update on the characterization of Staphylococcus aureus skin infections in a pediatric dermatology tertiary health care outpatient facility: antibiotic susceptibility patterns and decreased methicillin resistance. J. Am. Acad. Dermatol. 64:440–441 [DOI] [PubMed] [Google Scholar]
- 10. Dietrich DW, Auld DB, Mermel LA. 2004. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 113:e347–e352 [DOI] [PubMed] [Google Scholar]
- 11. Farley JE. 2008. Epidemiology, clinical manifestations, and treatment options for skin and soft tissue infection caused by community-acquired methicillin-resistant Staphylococcus aureus. J. Am. Acad. Nurse Pract. 20:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Francis JS, et al. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100–107 [DOI] [PubMed] [Google Scholar]
- 13. Frazee BW, et al. 2005. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann. Emerg. Med. 45:311–320 [DOI] [PubMed] [Google Scholar]
- 14. Fridkin SK, et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 15. Gordon NP. 2012. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2007 California Health Interview Survey. Kaiser Permanente Division of Research, Oakland, CA: http://www.dor.kaiser.org/external/chis_non_kp_2007/ [Google Scholar]
- 16. Hulten KG, et al. 2006. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr. Infect. Dis. J. 25:349–353 [DOI] [PubMed] [Google Scholar]
- 17. Jungk J, Como-Sabetti K, Stinchfield P, Ackerman P, Harriman K. 2007. Epidemiology of methicillin-resistant Staphylococcus aureus at a pediatric healthcare system, 1991–2003. Pediatr. Infect. Dis. J. 26:339–344 [DOI] [PubMed] [Google Scholar]
- 18. Kallen AJ, et al. 2010. Health care-associated invasive MRSA infections, 2005–2008. JAMA 304:641–648 [DOI] [PubMed] [Google Scholar]
- 19. King MD, et al. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309–317 [DOI] [PubMed] [Google Scholar]
- 20. Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 13:1840–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klevens RM, et al. 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin. Infect. Dis. 42:389–391 [DOI] [PubMed] [Google Scholar]
- 22. Klevens RM, et al. 2006. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg. Infect. Dis. 12:1991–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klevens RM, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 24. Laupland KB, Ross T, Gregson DB. 2008. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J. Infect. Dis. 198:336–343 [DOI] [PubMed] [Google Scholar]
- 25. Liu C, et al. 2008. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin. Infect. Dis. 46:1637–1646 [DOI] [PubMed] [Google Scholar]
- 26. Lucero CA, et al. 2009. Evaluating the potential public health impact of a Staphylococcus aureus vaccine through use of population-based surveillance for invasive methicillin-resistant S. aureus disease in the United States. Vaccine 27:5061–5068 [DOI] [PubMed] [Google Scholar]
- 27. Miller LG, Kaplan SL. 2009. Staphylococcus aureus: a community pathogen. Infect. Dis. Clin. North Am. 23:35–52 [DOI] [PubMed] [Google Scholar]
- 28. Miller LG, et al. 2007. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin. Infect. Dis. 44:471–482 [DOI] [PubMed] [Google Scholar]
- 29. Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. 2007. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn. Microbiol. Infect. Dis. 57:7–13 [DOI] [PubMed] [Google Scholar]
- 30. Moran GJ, Amii RN, Abrahamian FM, Talan DA. 2005. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg. Infect. Dis. 11:928–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran GJ, et al. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 32. Morin CA, Hadler JL. 2001. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J. Infect. Dis. 184:1029–1034 [DOI] [PubMed] [Google Scholar]
- 33. Naimi TS, et al. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976–2984 [DOI] [PubMed] [Google Scholar]
- 34. O'Hara FP, et al. 2008. A geographic variant of the Staphylococcus aureus Panton-Valentine leukocidin toxin and the origin of community-associated methicillin-resistant S. aureus USA300. J. Infect. Dis. 197:187–194 [DOI] [PubMed] [Google Scholar]
- 35. Orscheln RC, et al. 2009. Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin. Infect. Dis. 49:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petti CA, Sanders LL, Trivette SL, Briggs J, Sexton DJ. 2002. Postoperative bacteremia secondary to surgical site infection. Clin. Infect. Dis. 34:305–308 [DOI] [PubMed] [Google Scholar]
- 37. Richards MJ, Edwards JR, Culver DH, Gaynes RP. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887–892 [DOI] [PubMed] [Google Scholar]
- 38. Sattler CA, Mason EO, Jr, Kaplan SL. 2002. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococcus aureus infection in children. Pediatr. Infect. Dis. J. 21:910–917 [DOI] [PubMed] [Google Scholar]
- 39. Shannon KP, French GL. 2002. Validation of the NCCLS proposal to use results only from the first isolate of a species per patient in the calculation of susceptibility frequencies. J. Antimicrob. Chemother. 50:965–969 [DOI] [PubMed] [Google Scholar]
- 40. Shorr AF. 2007. Epidemiology and economic impact of meticillin-resistant Staphylococcus aureus: review and analysis of the literature. Pharmacoeconomics 25:751–768 [DOI] [PubMed] [Google Scholar]
- 41. Young DM, et al. 2004. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch. Surg. 139:947–951 [DOI] [PubMed] [Google Scholar]