Abstract
We sought to determine the relationship between two recent additions to the murine leukemia virus (MLV) ecotropic subgroup: Mus cervicolor isolate M813 and Mus spicilegus endogenous retrovirus HEMV. Though divergent in sequence, the two viruses share an Env protein with similarly curtailed VRA and VRB regions, and infection by both is restricted to mouse cells. HEMV and M813 displayed reciprocal receptor interference, suggesting that they share a receptor. Expression of the M813 receptor murine sodium-dependent myo-inositol transporter 1 (mSMIT1) allowed previously nonpermissive cells to be infected by HEMV, indicating that mSMIT1 also serves as a receptor for HEMV. Our findings add HEMV as a second member to the MLV subgroup that uses mSMIT1 to gain entry into cells.
TEXT
The diversity of receptor usage by murine leukemia viruses (MLVs) and their close relatives reflects an active recent evolutionary history. All MLV subgroups identified to date have related env genes and use structurally similar but unrelated small-molecule transporters as receptors (18). M813, an exogenous MLV isolated from Mus cervicolor, represents a novel MLV subgroup that uses murine sodium-dependent myo-inositol transporter 1 (mSMIT1) for entry (10). Mus spicilegus endogenous retrovirus HEMV (20) is one of the few gammaretroviruses that has yet to have its receptor identified (for a review, see reference 18).
HEMV was initially identified in our laboratory by a comprehensive analysis of endogenous MLV sequences from various Mus subspecies (21). Analysis of the long terminal repeats (LTRs) of multiple endogenous MLV-related proviruses from wild-type-derived mice revealed differences exceeding the scope of the previous standard classification of proviruses found in inbred strains (4–7, 17, 21). The provirus containing an unusual LTR found only in the genome of M. spicilegus was named HEMV, and further analyses showed that the locus represented an intact provirus (21). HEMV bears hallmarks of an ancient endogenous retrovirus, in that it is fixed in its host species, the sequences of gag and pol occupy positions near the root of the MLV phylogenetic tree, and the LTR has the simplest structure of all known MLVs (21). However, further study revealed that it is a recent insertion into the M. spicilegus genome and that the cloned HEMV provirus is capable of producing infectious virus (20).
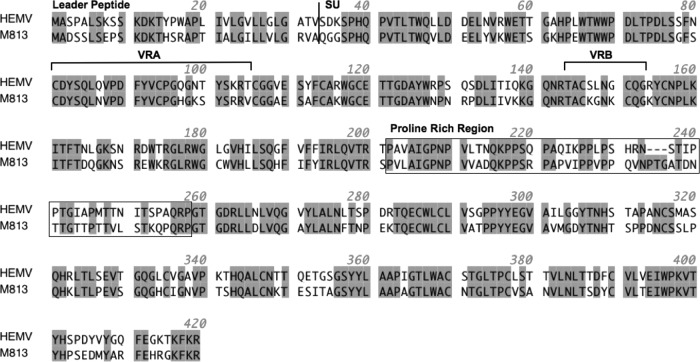
The coding sequence of the HEMV env gene is substantially shorter than that of most other gammaretroviruses, due to differences in the VRA and VRB regions of SU that encode the receptor-binding domain. Among other known gammaretroviruses, this property was seen only in M813, whose VRA and VRB regions are up to 18 and 8 amino acids shorter than those of other gammaretroviruses, respectively. Though highly divergent in other regions of the genome, including TM, HEMV and M813 share VRA and VRB regions similar in length (Fig. 1). Overall, HEMV and M813 env genes show 69% identity at the nucleotide level, corresponding to 72% at the protein level, comparable to the degree of amino acid identity in the VRA (76%) and VRB (70%) regions. The most prominent differences between the two Env proteins are a three-residue gap in HEMV relative to M813 in the proline-rich region and a single mismatched cysteine at residue 181 in M813 (Fig. 1). Closer examination of the SU phylogenetic tree revealed that, although the two viral SU regions share a branch in the tree, the branches leading to the viruses from their last common node are quite long, suggesting a distant relationship (20).
Fig 1.
Relationship between HEMV and M813 Env proteins. A comparison of the SU regions of HEMV and M813 Env proteins is shown. The leader peptide, VRA and VRB domains, and the proline-rich region are indicated; the sequence represents the region upstream of the furin cleavage site.
The species tropism of HEMV is best described as strictly ecotropic, reflecting its ability to infect only cells of mouse origin, including M. spicilegus (Table 1) (20). M813 has been reported to infect mouse and rat cells, although infection of the latter is 3 orders of magnitude less efficient (16). To better understand the receptor usage of HEMV and M813, we examined the species tropism of both viruses.
Table 1.
HEMV and M813 host ranges are restricted to mouse cells
| Cell line | Titer (IU/ml) of viral pseudotypea |
Description | |||
|---|---|---|---|---|---|
| HEMV | M813 | MoMLV | 10A1 | ||
| Murine | |||||
| NIH 3T3 | 3.2 × 105 | 1.5 × 105 | 3.6 × 105 | 5.1 × 105 | NIH—Swiss embryonic fibroblasts |
| MMK | 7.0 × 105 | 1.2 × 105 | 4.8 × 105 | 3.2 × 105 | Mus musculus molossinus kidney cells |
| SC1 | 1.7 × 105 | 1.9 × 105 | 3.0 × 105 | 2.3 × 105 | Feral mouse embryonic cells |
| M. dunni | 1.4 × 106 | 3.4 × 105 | 2.0 × 105 | 4.8 × 105 | Mus dunni tail fibroblasts |
| M. spicilegus | 1.7 × 105 | 4.1 × 104 | 1.7 × 105 | 1.3 × 105 | Mus spicilegus tail fibroblasts |
| MC3T3 | 2.1 × 105 | 7.3 × 104 | 2.9 × 105 | 2.7 × 105 | Mus musculus calvaria osteoblasts |
| Rat | |||||
| Rat1 | <1 | <1 | 8.5 × 104 | 2.8 × 105 | Rat embryonic fibroblasts |
| REF | <1 | <1 | 1.9 × 102 | 7.2 × 105 | Rat embryonic fibroblasts |
| Hamster | |||||
| E36 | <1 | <1 | <1 | 2.8 × 105 | Chinese hamster lung cells |
| CHO-K1 | <1 | <1 | <1 | <1 | Chinese hamster ovary cells |
| Primate | |||||
| COS1 | <1 | <1 | <1 | 8.7 × 104 | African green monkey kidney fibroblasts |
| 293T | <1 | <1 | <1 | 4.4 × 105 | Human kidney epithelial cells |
| 293TmCAT1 | <1 | <1 | 4.0 × 105 | 2.5 × 105 | 293T cells expressing mCAT1 receptor |
| Avian | |||||
| DF1 | <1 | <1 | <1 | 2.4 × 105 | Chicken fibroblasts |
| QT6 | <1 | <1 | <1 | 1.7 × 105 | Quail fibrosarcoma cells |
| Feline | |||||
| FEF | <1 | <1 | <1 | 3.4 × 105 | Feline embryonic fibroblasts |
| Canine | |||||
| D17 | <1 | <1 | <1 | 2.3 × 105 | Canine osteosarcoma cells |
The MLV-based pLacPuro vector construct was cotransfected with clones expressing the indicated env genes. Titers were determined by X-Gal staining. The standard deviations for titers on the order of 105 ranged between 1.25 × 105 and 3.76 × 105, those on the order of 104 ranged between 2.40 × 104 and 7.42 × 104, and those on the order of 103 ranged between 2.19 × 103 and 4.81 × 103. The data represent the averages of the results of at least two independent experiments.
The M813 env gene was constructed from the published sequence (16) by linking regularly staggered 120-bp oligonucleotides in sequential PCRs and cloning into pCR2.1-TOPO (Invitrogen) to create pM813-TOPO. Mismatches from the published sequence (GenBank accession no. AF327437) were repaired by site-directed mutagenesis (Stratagene). The HEMV env gene from pHEMV18 (21), the M813 env gene from pM813-TOPO, and the 10A1 env gene from pB6 (13) were cloned into pSV-psi-minus-E-MLV (12) to create constructs psi-HEMV, psi-M813, and psi-10A1, respectively. Cotransfection of each construct with pLacPuro (15) or MLV-green fluorescent protein (MLV-GFP) (14) into 293T cells resulted in the production of single-round infectious LacZ-positive (LacZ+) PuroR (puromycin-resistant) or GFP+ viruses bearing the specified env genes. Following infection of target cells, titers of the virus inocula were determined either by counting LacZ+ (staining blue with X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside]) cells under a light microscope or by quantification of GFP expression via flow cytometry.
As previously reported, both HEMV and M813 were able to infect all mouse cell lines tested, all derived from several different Mus species, whereas nonmurine cells, including human, simian, feline, canine, avian, and hamster cells, were not infected by either virus (Table 1) (16, 20). In contrast to published data, we found M813 to be incapable of infecting rat cells, despite using at least one cell line identical to a cell line used in the previous studies (Rat1 cells; Table 1). Even though the published infection efficiency was very low in this species, it was within the sensitivity of the described assay as set by infection levels of truly resistant target cells. The reason for this discrepancy is unknown, but we believe it unlikely to be due to low M813 titers, since an infection with several orders of magnitude less efficiency would still yield detectable titers. 10A1 pseudotypes were able to infect all cell lines tested, with the exception of CHO-K1, while those with the ecotropic (Moloney) MLV Env were restricted to mouse and some rat cell lines, as expected (Table 1).
The similarities in the SU regions of the env proteins of HEMV and M813, as well as the identical host ranges seen in our experiments, prompted us to use interference assays to test whether the two viruses were members of the same subgroup; i.e., used the same receptor for infection. HEMV and M813 env genes were cloned into pNCS (3) to generate replication-competent recombinant viruses with the specified Env proteins. NIH 3T3 cells chronically infected with these viruses, as well as viruses with a variety of other receptor specificities, were then challenged with the LacZ+ PuroR HEMV, M813, 10A1, or ecotropic pseudotyped virions. M813 exhibited the same interference pattern as reported in the literature (16), completely interfering with itself. No significant drop in titers of either M813 or HEMV was observed when the cells were preinfected with members of other MLV subgroups, including ecotropic, polytropic, amphotropic, and 10A1 MLVs (Table 2), implying that both M813 and HEMV use a receptor different from that used by any of the tested subgroups. When NIH 3T3 cells were infected with replication-competent M813, HEMV was completely blocked from infection. Likewise, chronic infection of NIH 3T3 cells with HEMV abolished M813 infection (Table 2). This type of reciprocal interference is often seen with viruses that use the same domain(s) of a common entry receptor. Prior infection with HEMV or M813 did not have any effect on infection by 10A1 or ecotropic virus, suggesting that they use a separate receptor for entry.
Table 2.
Receptor interference between MLV Env proteins
| Preinfecting virus | Relative titer of challenge virusa |
|||
|---|---|---|---|---|
| HEMV | M813 | Ecotropic | 10A1 | |
| None | 1 | 1 | 1 | 1 |
| HEMV | <3.1 × 10−6 | <6.5 × 10−6 | 0.42 | 1.51 |
| M813 | <3.1 × 10−6 | <6.5 × 10−6 | 2.03 | 0.13 |
| Ecotropic | 0.54 | 0.17 | <2.6 × 10−6 | 0.73 |
| Amphotropic | 0.50 | 0.98 | 0.64 | 0.84 |
| Polytropic | 0.15 | 0.26 | 0.20 | 0.13 |
| 10A1 | 1.08 | 0.24 | 0.32 | <1.96 × 10−4 |
Data were calculated as the ratio of titers on preinfected cells and on uninfected cells. The data represent the averages of the results of at least two independent experiments. The variation between experiments was no more than 64% of the mean value in each case.
The reciprocal interference between HEMV and M813 implied usage of a common entry receptor. To test this possibility directly, cDNA of the mSMIT1 gene, the entry receptor for M813, was amplified from an NIH 3T3 cDNA library (11) and cloned into pLPCX retroviral expression vector (Clontech) to create pLPCX-mSMIT. mSMIT-transducing virus was made by cotransfecting 293T cells with pLPCX-mSMIT, pMLVgagpol, and a vesicular stomatitis virus G (VSV-G) expression vector. Canine Cf2 Th cells were then infected either with mSMIT-transducing virus or with virus made with “empty” pLPCX and selected with puromycin (10 μg/ml). Puromycin-resistant colonies were expanded and challenged by the use of GFP-expressing MLV pseudotypes, and the titers were determined to give matched infectious units on NIH 3T3 cells. Infection was scored by flow cytometry. The expression of mSMIT1 rendered nonpermissive Cf2 Th cells susceptible to infection by both HEMV and M813 (Table 3), confirming mSMIT1 to be a receptor for HEMV. Moloney MLV (MMLV), capable of infecting only murine cells, remained unable to infect Cf2 Th cells, whereas 10A1-MLV, which has a broad host range, was able to infect canine cells regardless of mSMIT1 expression. Infectious units were reduced for M813 due to rapid inactivation of this pseudotype over the course of the experiment (data not shown).
Table 3.
HEMV and M813 use mSMIT1 as a receptor
| Target cell | % (±SD) GFP-positive cellsa |
||||
|---|---|---|---|---|---|
| HEMV | M813 | Ecotropic | 10A1 | No envelope | |
| NIH 3T3 | 75.3 ± 1.3 | 7.3 ± 0.8 | 72.5 ± 0.3 | 70.1 ± 1.4 | 0.9 ± 0.3 |
| Cf2 Th LPCX (“empty”) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 65.0 ± 3.1 | 0.9 ± 0.1 |
| Cf2 Th LPCX-mSMIT | 45.4 ± 2.7 | 12.2 ± 1.7 | 0.1 ± 0.1 | 69.1 ± 2.7 | 0.9 ± 0.1 |
Data represent percent infection of 10,000 gated viable cells of 1 × 105 cells infected, the threshold was set at 1.0% fluorescein isothiocyanate (FITC) in uninfected cells. The data represent the averages of the results of three independent experiments.
The possibility that HEMV and M813 might use the same receptor was suggested by the similarity and phylogenetic relationship of their SU proteins, relative to other gammaretroviruses, and by the unusually short receptor-binding regions (VRA and VRB) shared by these viruses. They also have a common host range: unlike other MLVs, they infect only cells from Mus species. Although only partial sequences are available for the M813 genome (limited to env and portions of pol and the LTR), available evidence suggests that the two viruses are not closely related outside SU. Unlike SU, the TM region is relatively well conserved among gammaretroviruses and has been previously used to establish viral relationships, most often congruent with reverse transcriptase (RT) (1). Phylogenetic analysis places the M813 TM well away from that HEMV, after the branching of feline leukemia virus (FeLV) (20). The LTRs of the two viruses share about 77% sequence identity.
Three major hypotheses as to how HEMV and M813 came to belong to the same subgroup despite the obvious divergence of most of their genomes present themselves. The first is that HEMV and M813 share an ancestor that, although distantly related, used an ancestral form of mSMIT1 and that their divergence reflects evolution as an exogenous virus infecting different host species in different parts of the world while preserving the use of the same receptor. The second hypothesis is that the two viruses independently arrived in this subgroup through convergent evolution, the two SU proteins being the result of jumping similar evolutionary hurdles. The third possibility is that the two viruses evolved separately from a distant gammaretrovirus ancestor and acquired the same receptor-binding domain by a relatively recent recombination event. It is worth noting that the discordance between the SU and TM regions suggests the possibility of at least one recombination event in the evolutionary history of these viruses. Such events are well documented to alter the host range of MLVs, such as the 10A1 virus (8), as well as being found to do so during replication of endogenous ecotropic viruses in some strains of mice (2, 9). In this case, recombination would require that all elements of the final virus be present either as exogenous or endogenous viruses in a single host. Given the rather different geographic ranges of M. spicilegus and M. cervicolor in eastern Europe and southern Asia, where these two viruses were isolated, it is likely that other, intermediate viruses exist. Identifying pol and TM regions intermediate to those positions might prove fruitful with respect to understanding this relationship.
Although it is common for distantly related viruses to retain the use of cognate receptors, for instance, in the case of GaLV and FeLV-B (19), and although incremental changes in receptor usage are achievable both in vivo and in vitro, as seen in coreceptor usage by HIV-1 and HIV-2, understanding how a true subgroup switch occurs remains elusive. The establishment of HEMV and M813 as members of the same subgroup may have important implications for the evolution and spread of gammaretroviruses. Their relationship represents an experimentally tractable model for a subgroup's origin and evolutionary history. Investigating how the two viruses came to be members of the same subgroup depends upon further exploration of wild mouse populations, the examination of carrier species, and, finally, the possible identification of more members of this subgroup.
ACKNOWLEDGMENTS
This work was supported by grant R37 CA 089441 to J.M.C. J.M.C. was a Research Professor of the American Cancer Society, with support from the F. M. Kirby Foundation.
Footnotes
Published ahead of print 28 March 2012
REFERENCES
- 1. Benit L, Dessen P, Heidmann T. 2001. Identification, phylogeny, and evolution of retroviral elements based on their envelope genes. J. Virol. 75:11709–11719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosselman RA, van Straaten F, Van Beveren C, Verma IM, Vogt M. 1982. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J. Virol. 44:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colicelli J, Goff SP. 1988. Isolation of an integrated provirus of Moloney murine leukemia virus with long terminal repeats in inverted orientation: integration utilizing two U3 sequences. J. Virol. 62:633–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frankel WN, Coffin JM. 1994. Endogenous nonecotropic proviruses mapped with oligonucleotide probes from the long terminal repeat region. Mamm. Genome 5:275–281 [DOI] [PubMed] [Google Scholar]
- 5. Frankel WN, Stoye JP, Taylor BA, Coffin JM. 1990. A linkage map of endogenous murine leukemia proviruses. Genetics 124:221–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frankel WN, Stoye JP, Taylor BA, Coffin JM. 1989. Genetic analysis of endogenous xenotropic murine leukemia viruses: association with two common mouse mutations and the viral restriction locus Fv-1. J. Virol. 63:1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frankel WN, Stoye JP, Taylor BA, Coffin JM. 1989. Genetic identification of endogenous polytropic proviruses by using recombinant inbred mice. J. Virol. 63:3810–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han JY, et al. 1997. Identification of envelope protein residues required for the expanded host range of 10A1 murine leukemia virus. J. Virol. 71:8103–8108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartley JW, Wolford NK, Old LJ, Rowe WP. 1977. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc. Natl. Acad. Sci. U. S. A. 74:789–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hein S, et al. 2003. Sodium-dependent myo-inositol transporter 1 is a cellular receptor for Mus cervicolor M813 murine leukemia virus. J. Virol. 77:5926–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitamura T, et al. 1995. Efficient screening of retroviral cDNA expression libraries. Proc. Natl. Acad. Sci. U. S. A. 92:9146–9150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mann R, Mulligan RC, Baltimore D. 1983. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell 33:153–159 [DOI] [PubMed] [Google Scholar]
- 13. Ott D, Friedrich R, Rein A. 1990. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J. Virol. 64:757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perron MJ, et al. 2004. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl. Acad. Sci. U. S. A. 101:11827–11832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfeiffer JK, Topping RS, Shin NH, Telesnitsky A. 1999. Altering the intracellular environment increases the frequency of tandem repeat deletion during Moloney murine leukemia virus reverse transcription. J. Virol. 73:8441–8447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prassolov V, et al. 2001. Mus cervicolor murine leukemia virus isolate M813 belongs to a unique receptor interference group. J. Virol. 75:4490–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stoye JP, Coffin JM. 1988. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J. Virol. 62:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tailor CS, Lavillette D, Marin M, Kabat D. 2003. Cell surface receptors for gammaretroviruses. Curr. Top. Microbiol. Immunol. 281:29–106 [DOI] [PubMed] [Google Scholar]
- 19. Takeuchi Y, et al. 1992. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J. Virol. 66:1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tipper CH, Bencsics CE, Coffin JM. 2005. Characterization of hortulanus endogenous murine leukemia virus, an endogenous provirus that encodes an infectious murine leukemia virus of a novel subgroup. J. Virol. 79:8316–8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomonaga K, Coffin JM. 1999. Structures of endogenous nonecotropic murine leukemia virus (MLV) long terminal repeats in wild mice: implication for evolution of MLVs. J. Virol. 73:4327–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]