Abstract
Adenovirus serotype 5 (Ad5) vectors and specific neutralizing antibodies (NAbs) generate immune complexes (ICs) which are potent inducers of dendritic cell (DC) maturation. Here we show that ICs generated with rare Ad vector serotypes, such as Ad26 and Ad35, which are lead candidates in HIV vaccine development, are poor inducers of DC maturation and that their potency in inducing DC maturation strongly correlated with the number of Toll-like receptor 9 (TLR9)-agonist motifs present in the Ad vector's genome. In addition, we showed that antihexon but not antifiber antibodies are responsible for the induction of Ad IC-mediated DC maturation.
INTRODUCTION
Replication-deficient adenovirus (Ad) vectors are attractive strategies for gene therapy or vaccination, and they represent one of the leading platforms in the field of HIV vaccines. However, interest in Ad serotype 5 (Ad5) vectors has waned due to the presence of preexisting Ad5 neutralizing antibodies (NAbs) in a large proportion of the population, particularly in developing countries. The presence of Ad5 NAbs and more recently of Ad-specific cellular immunity has been associated with reduced transduction efficacy and a decrease in the magnitude and breadth of HIV-specific CD4 and CD8 T cells in vaccine recipients (9, 31). Ad-specific T cells also recognize epitopes conserved across multiple Ad serotypes (9, 25). Furthermore, a potential correlation between preexisting Ad5 NAb immunity and increased acquisition to HIV infection in vaccine recipients with preexisting Ad5 immunity has been shown in the Step clinical trial (6), which investigated an Ad5 vector vaccine.
To circumvent the challenges of Ad5 preexisting immunity, several strategies have been developed, including vaccine regimens based on rare adenovirus serotypes (e.g., Ad6, Ad11, Ad26, Ad35, and Ad36) (1, 11, 16, 21). These rare Ad serotypes may have substantial biological differences, likely resulting from specific interactions with blood components or cell receptors (38). We and others have shown that Ad vectors derived from various serotypes differentially interact with naïve and memory T cells (2, 27) and human dendritic cells (DCs) (18, 28) and are associated with distinct biological effects in vivo (11, 17). In this regard, we have recently observed that the formation of immune complexes (ICs) composed of Ad vectors and specific NAbs may have, in addition to reduced transduction efficacy, a differential effect on FcR-expressing cells (29). Ad5 ICs have been shown to be potent inducers of DC maturation (as assessed by both costimulatory molecule expression and cytokine production) through FcγR internalization and Toll-like receptor 9 (TLR9) interaction (29). Since Ad IC formation likely occurs in subjects with Ad preexisting immunity (7, 22), we hypothesized (29) that Ad IC-mediated DC maturation may have created more favorable conditions for HIV spread at the port of HIV entry (i.e., mucosal surfaces) and may have contributed to the increased acquisition of HIV infection in vaccinees with preexisting Ad5 immunity in the Step trial.
In the present study, we have evaluated the potency of ICs formed by common and rare Ad-derived vectors (i.e., Ad5, Ad6, Ad26, Ad35, Ad36, and Ad41 vectors) and human plasma to mature human DCs derived from monocytes, as previously described (28). We showed that ICs generated with rare Ad vector serotypes such as Ad26 and Ad35, which are lead candidates in HIV vaccine development, are poor inducers of DCs maturation and that their potency in inducing DC maturation strongly correlated with the number of TLR9-agonist motifs present in the Ad vector's genome. In addition, we showed that antihexon but not antifiber antibodies are responsible for induction of Ad IC-mediated DC maturation.
MATERIALS AND METHODS
Blood mononuclear cell and DC isolation.
Blood samples were obtained from 10 volunteers at the local blood bank (Lausanne, Switzerland). Isolation of blood mononuclear cells and generation of monocyte-derived DCs (MoDCs) were performed as previously described (28).
Adenovirus vectors.
Two E1- and E3-deleted Ad5 vectors (Ad5βgal and Ad5GFP) were used in this study and were previously described (26). Briefly, Adβgal harbors a lacZ expression cassette and was used to assess Ad-mediated DC maturation. Ad5GFP harbors a green fluorescent protein (GFP) expression cassette and was used for the assessment of NAb titers. E1-deleted Ad6-secreted alkaline phosphatase (SEAP), E1- E4- and orf6-deleted Ad26-SEAP, and E1-, E4-, and orf6-deleted Ad36-SEAP vectors were provided by Merck and have already been described (19). E1- and E3-deleted Ad35-luc (16) and E1- and E3-deleted Ad5-luc harboring hypervariable regions 1 to 7 of Ad48 (Ad5HVR1-7) (32) were provided by Dan Barouch. E1-deleted Ad41-GFP (17) and E1-deleted Ad5-luc harboring Ad35 fiber (Ad5F35) (24) were provided by Genvec and have already been described. Ad vector titers were measured in virus physical particles (pp) per cell, as described by Mittereder et al. (23). Ad genome sequences are available in http://www.ncbi.nlm.nih.gov/nuccore under the following accession numbers: Ad5, AY339865.1; Ad6, HQ413315.1; Ad26, EF153474.1; Ad35, AC_000019.1; Ad36, GQ384080.1; and Ad41, DQ315364.
NAb titers.
Ad5 and Ad41 NAb titers of 19 plasma samples were determined by transduction inhibition assays on human embryonic retina (HER; 911 cells [8]) as previously described (26). The NAb titers were expressed as the inverse of the highest dilution of each plasma sample that gave at least 50% reduction of transduction. Ad6, Ad26, and Ad36 NAb titers were provided by Merck and are expressed as the inverse of the highest dilution of each plasma sample that gave at least 50% reduction of secreted alkaline phosphatase (SEAP), as previously described (19). Ad35 NAb titers were provided by Dan Barouch and determined as previously described (3).
Expression of costimulatory molecules and cytokine secretion.
DCs (3 × 105 in 500 μl of complete medium) were exposed to the Ad5βgal, Ad6, Ad26, Ad35, Ad36, or Ad41 vectors alone (2 × 104 pp/cell), or Ad ICs for 36 h. Ad ICs were composed of Ad vector (2 × 104 pp/cell) mixed with 15 μl of plasma for 15 min at room temperature. As negative and positive controls, DCs were either cultured with medium alone or stimulated with TLR7/8 agonist R848 (1 μg/ml; Invivogen). At the end of the incubation period, cells were stained with anti-CD86–allophycocyanin (APC) (clone 2331 [FUN-1]) (BD Biosciences), and expression of the CD86 costimulatory molecule was assessed by flow cytometry on an LSRII four-laser cytometer (405, 488, 532, and 633 nm) and analyzed using FlowJo (version 8.8.6). Supernatants were collected and analyzed for the presence of tumor necrosis factor alpha (TNF-α) by Luminex (Panomics, Italy).
TLR9 inhibition.
DCs were pretreated (2 h at 37°C) with phosphorothioate-protected IRS 869 (TLR9 inhibitor; 5 μM; Sigma) or control oligonucleotide (ODN; Sigma) and then exposed to Ad5, Ad6, Ad26, or Ad36 vector alone, plasma containing serotype-specific NAbs (n = 3 per Ad tested) alone, or Ad ICs for 18 h, as previously described (29). Supernatants were collected and analyzed for the presence of TNF-α by Luminex (Panomics, Italy).
Statistical analyses.
P values were derived from one-way analysis of variance (ANOVA) (Kruskal-Wallis test) followed by Student's t test or using Spearman's rank test for correlations. Statistical calculations were performed in GraphPad Prism version 5.04 as previously described (30).
RESULTS
Differential effects of common versus rare adenovirus vectors forming ICs on DC maturation.
The effects of Ad ICs on DC maturation were assessed by investigating measures of DC maturation such as upregulation of costimulatory molecule expression (i.e., CD86) and production of proinflammatory cytokines (i.e., TNF-α). To exclude the possibility that the effects were due to complement activity, heat-inactivated sera (30 min at 56°C) were used throughout this study.
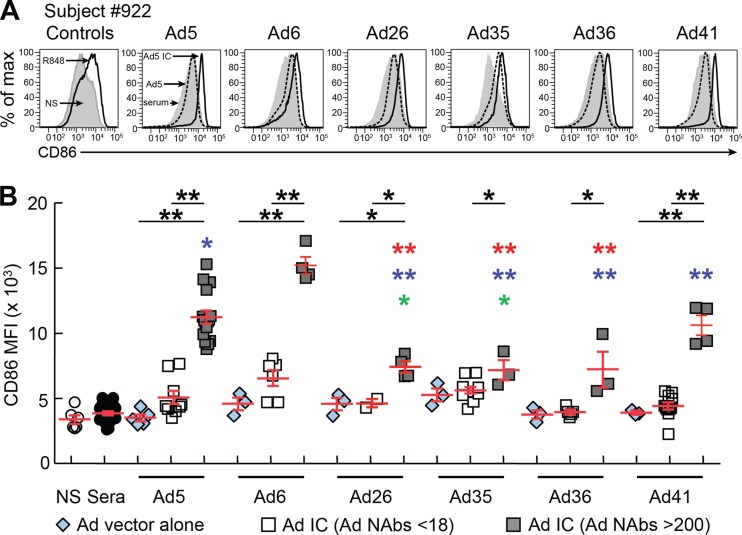
Consistent with previous studies (29), we found that Ad5 vector alone, plasma alone, or Ad5 ICs formed with plasma containing low Ad5-NAb titers (NAb titers < 18), determined as previously described (26), modestly increased CD86 expression on DCs (Fig. 1A and B). Similarly, no significant increase in CD86 expression was observed in DCs exposed to Ad6, Ad26, Ad35, Ad36 or Ad41 vectors alone (Fig. 1B). However, significant increases (P < 0.05) in CD86 expression were observed in the presence of Ad ICs formed with Ad-serotype-specific NAbs (NAb titers > 200) but not with Ad-serotype specific NAbs at titers of <18 (Ad nonneutralizing Abs [NNAbs]) (Fig. 1B). Interestingly, Ad26, Ad35, and Ad36 ICs formed with serotype-specific NAbs, determined as previously described (3, 19), induced significantly lower CD86 expression (P < 0.005) than Ad5 ICs (Fig. 1B).
Fig 1.
Differential effects of common versus rare adenovirus vectors forming ICs on DC maturation. MoDCs were differentiated from three individuals and exposed to plasma alone or the Ad5, Ad6, Ad26, Ad35, Ad36, or Ad41 vectors alone or with Ad ICs formed with either nonneutralizing (NNAbs) or neutralizing (NAbs) plasma, as previously described (29). As controls, DCs were either cultured with medium alone (negative control) or stimulated with TLR7/8 agonist R848 (positive control). After 36 h, expression of CD86 was assessed by flow cytometry. (A) Flow cytometric profiles of one representative DC donor (subject 922) expressing CD86 following exposure to plasma alone, Ad vectors, or Ad ICs formed with either NNAbs or NAbs. (B) Cumulative data from 3 subjects of CD86 expression on DCs exposed to plasma alone, Ad ICs formed with NNAbs (NAb titers < 18) or NAbs (NAb titers > 200). Statistical significance (P values) in panel B was obtained using one-way ANOVA (Kruskal-Wallis test) followed by Student's t test. *, P < 0.05; **, P < 0.001. Red, blue, or green asterisks indicate a significant reduction of CD86 expression compared to the levels with Ad5, Ad6, and Ad41 NAb ICs, respectively. A single colored asterisk corresponds to P < 0.05, and the presence of two colored asterisks corresponds to P < 0.005.
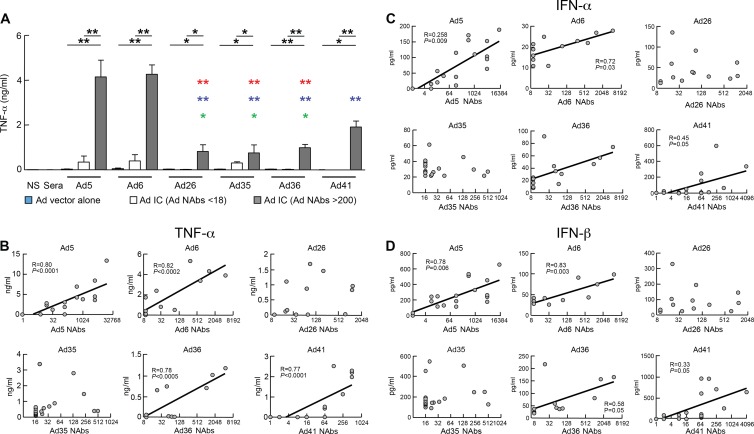
Unstimulated DCs and DCs exposed to Ad5 vector alone or plasma alone did not produce TNF-α (Fig. 2A) (29). However, DCs exposed to Ad ICs formed with Ads and plasma containing Ad-serotype-specific NAbs (NAb titers > 200) significantly induced the production of TNF-α compared to DCs exposed to Ad ICs formed with plasma containing low NAb titers (NAb titers < 18) (Ad5 [P < 0.001], Ad6 [P < 0.001], Ad26 [P < 0.05], Ad35 [P < 0.05], Ad36 [P < 0.05], or Ad41 [P < 0.001]) (Fig. 2B). Interestingly, Ad26, Ad35, and Ad36 induced lower levels of TNF-α production than Ad5 and Ad6, while Ad41 induced intermediate levels of TNF-α production (Fig. 2B). Furthermore, the levels of TNF-α production directly correlated with the titers of serotype-specific NAbs for Ad5 (P < 0.0001), Ad6 (P < 0.0002), Ad36 (P < 0.0005), and Ad41 (P < 0.0001) but not for Ad26 and Ad35, and the induction of TNF-α production was strictly dependent on the specificity of the NAbs (Fig. 2B, Fig. 3, and Tables 1, 2, and 3). Of note, as previously shown for Ad5 ICs (29), Ad ICs induced production of other cytokines, such as type I interferons (IFNs). The levels of IFN-α and IFN-β directly correlated with the titers of serotype-specific NAbs for Ad5 (P = 0.009 and P = 0.006), Ad6 (P = 0.03 and P = 0.003), Ad36 (P < 0.05 and P < 0.05), and Ad41 (P < 0.05 and P < 0.05) but not for Ad26 and Ad35 (Fig. 2C and D).
Fig 2.
Ad26 ICs, Ad35 ICs and Ad36 ICs induce lower levels of TNF-α production than Ad5 IC-stimulated DCs. MoDCs were exposed to plasma alone, Ads alone, or Ad ICs formed with NNAbs or NAbs as described in the legend to Fig. 1. After 36 h, TNF-α production in the supernatants was determined by Luminex. (A) Cumulative data of TNF-α production by DCs exposed to Ad5, Ad6, Ad26, Ad35, Ad36, or Ad41 ICs formed with either NNAbs (NAb titers < 18) or neutralizing plasma (NAb titers > 200). (B) TNF-α production induced by Ad ICs correlates with Ad NAb titers to Ad5, Ad6, Ad36, and Ad41 but not to Ad26 or Ad35. Neutralizing plasma-forming immune complexes with Ads induce IFN-α (C) and IFN-β (D) production by DCs. Statistical significance (P values) in panel A was obtained using one-way ANOVA (Kruskal-Wallis test) followed by Student's t test. *, P < 0.05; **, P < 0.001. Red, blue, or green asterisks indicate a significant reduction of TNF-a production compared to that of the Ad5, Ad6, and Ad41 NAb ICs, respectively. A single colored asterisk corresponds to P < 0.05, and the presence of two colored asterisks corresponds to P < 0.005. Statistical significance (P values) in panels B, C, and D was obtained using Spearman's rank correlations.
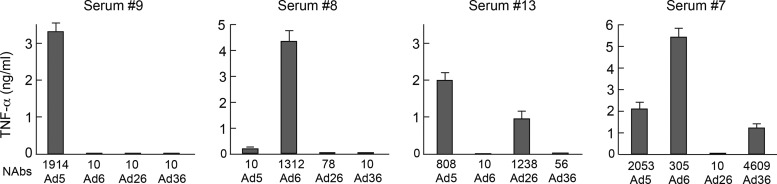
Fig 3.
TNF-α production by DCs is induced by serotype-specific NAbs forming ICs. Four representative sera with distinct Ad-serotype specific NAbs were mixed with Ad5, Ad6, Ad26, or Ad36 to form ICs and exposed to DCs. After 36 h of incubation, supernatants were collected and TNF-α production was determined by Luminex.
Table 1.
Ad5 and Ad41 NAb titers
| Plasma sample no. | Ad NAb titera |
|
|---|---|---|
| Ad5 | Ad41 | |
| 1 | 4,096 | 4,096 |
| 2 | 1 | 0 |
| 3 | 128 | 64 |
| 4 | 128 | 2 |
| 5 | 16 | 64 |
| 6 | 16,000 | 256 |
| 7 | 128 | 512 |
| 8 | 8 | 0 |
| 9 | 4,096 | 8 |
| 10 | 16 | 64 |
| 11 | 4,096 | 16 |
| 12 | 32 | 2 |
| 13 | 4,096 | 0 |
| 14 | 8 | 64 |
| 15 | 0 | 8 |
| 16 | 1,024 | 8 |
| 17 | 512 | 16 |
| 18 | 0 | 128 |
| 19 | 8 | 0 |
| 20 | 512 | ND |
Ad5 and Ad41 NAb titers were determined on 911 cells and are expressed as the inverse of the highest dilution of each plasma sample that gave at least 50% reduction of transduction. ND, not determined.
Table 2.
Ad5, Ad6, Ad26, and Ad36 NAb titers
| Plasma sample no. | Ad NAb titera |
|||
|---|---|---|---|---|
| Ad5 | Ad6 | Ad26 | Ad36 | |
| 1 | 110 | 23 | 204 | 1,691 |
| 2 | 10 | 10 | 784 | 10 |
| 3 | 4,609 | 93 | 22 | 89 |
| 4 | 54 | 671 | 90 | 49 |
| 5 | 743 | 32 | 1,194 | 10 |
| 6 | 2,117 | 10 | 38 | 10 |
| 7 | 2,053 | 305 | 10 | 4,609 |
| 8 | 10 | 1,312 | 78 | 10 |
| 9 | 1,914 | 10 | 10 | 10 |
| 10 | 10 | 4,609 | 21 | 23 |
| 11 | 227 | 10 | 73 | 1,054 |
| 12 | 1,575 | 10 | 20 | 71 |
| 13 | 808 | 10 | 1,238 | 56 |
Ad5, Ad6, Ad26, and Ad36 NAb titers are expressed as the inverse of the highest dilution of each plasma sample that gave at least 50% reduction of secreted alkaline phosphatase (SEAP) activity.
Table 3.
Ad-serotype specific NAbs forming immune complexes with Ad vectors induce TNF-α production by DCs
| Plasma sample no. | TNF-α production (pg/ml) witha: |
||||
|---|---|---|---|---|---|
| Plasma alone | Ad5 IC | Ad6 IC | Ad26 IC | Ad36 IC | |
| 1 | 13 | 751 | 2,409 | 1,462 | 449 |
| 2 | 18 | 29 | 149 | 26 | 27 |
| 3 | 7 | 5,265 | 516 | 165 | 18 |
| 4 | 5 | 248 | 3,396 | 1,691 | 423 |
| 5 | 10 | 421 | 817 | 826 | 35 |
| 6 | 7 | 2,976 | 403 | 17 | 12 |
| 7 | 6 | 2,056 | 5,372 | 12 | 1,200 |
| 8 | 5 | 172 | 4,350 | 15 | 10 |
| 9 | 9 | 3,328 | 12 | 12 | 15 |
| 10 | 7.5 | 260 | 3,933 | 1,107 | 364 |
| 11 | 10 | 1,153 | 1,766 | 873 | 349 |
| 12 | 8 | 3,229 | 9 | 121 | 20 |
| 13 | 12 | 2,011 | 6 | 960 | 26 |
Thirteen plasma samples harboring distinct Ad-serotype specific NAbs (Table 2) were mixed with Ad5, Ad6, Ad26, or Ad36 to form ICs and exposed to DCs. Following 36 h of incubation, supernatants were collected and TNF-α production (pg/ml) was monitored by Luminex.
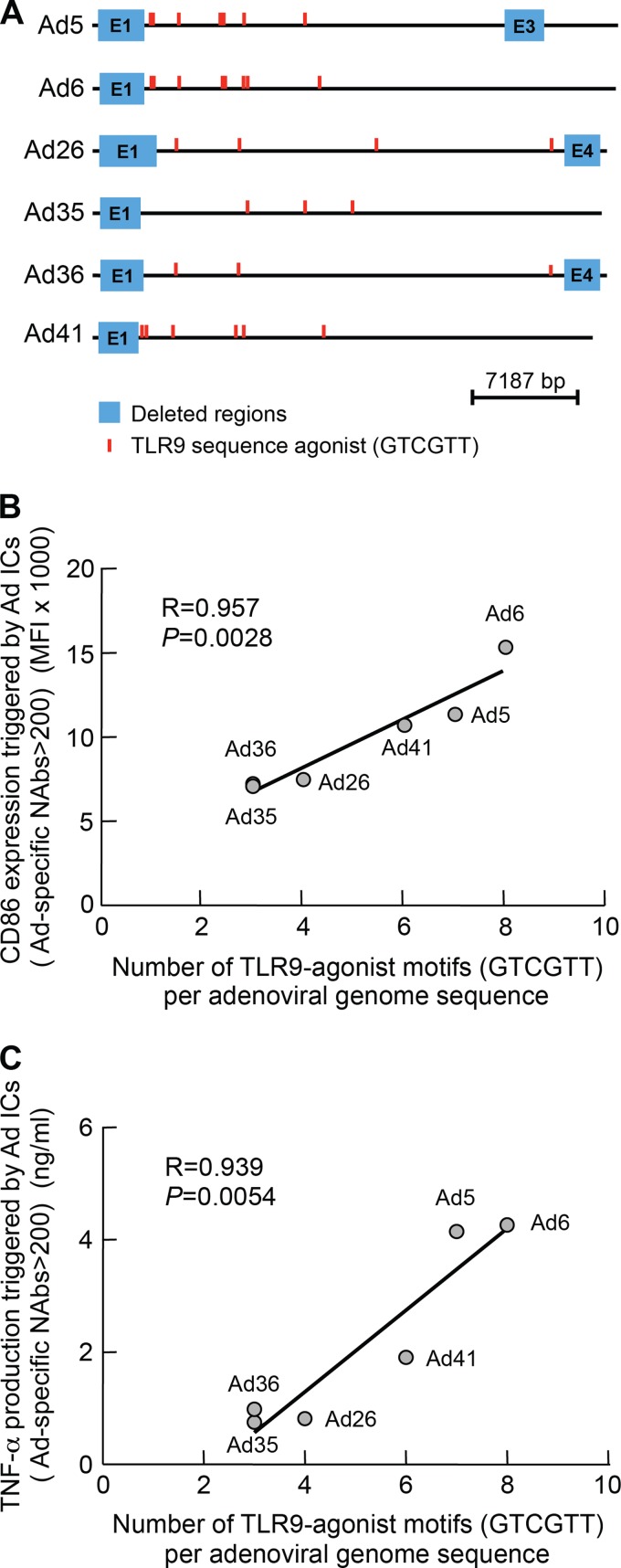
The number of TLR9-agonist motifs (GTCGTT) in adenoviral genome sequences directly correlates with the level of DC maturation.
We have recently shown that Ad5 ICs formed with ΔE1 Ad5 vector (vector deleted in the E1 regions), but not with the helper-dependent Ad5 vector (HD-Ad5) (vector deleted in all viral genes), triggered DC maturation, underscoring the role of Ad5 DNA genome in Ad5 IC-mediated DC maturation (29). We therefore first assessed the role of Ad DNA-TLR9 interaction in Ad IC-mediated DC maturation (assessed by TNF-α production) with Ad ICs formed with common versus rare Ad serotypes (i.e., Ad5, Ad6, Ad26, and Ad36). The results obtained clearly indicate that TLR9 antagonist (IRS 869) significantly reduced TNF-α production by Ad5 IC-, Ad6 IC-, Ad26 IC-, and Ad36 IC-mediated DC maturation (Fig. 4), supporting the role of TLR9 in Ad IC-mediated DC maturation. This observation is consistent with previous studies that showed that Ad DNA interaction with TLR9 triggers DC maturation (15, 29).
Fig 4.
Ad IC-mediated DC maturation requires TLR9 interaction. MoDCs were stimulated with Ad5, Ad6, Ad26, or Ad36 IC (with three distinct sera containing NAbs) in the presence or absence of TLR9 inhibitor (IRS 869). At the end of the stimulation period, supernatants were analyzed for the presence of TNF-α. Results are expressed as the percentages of the cytokine levels measured in cultures treated with TLR9 inhibitor versus those found in control cultures (absence of inhibitors). The experiment described above was performed in triplicate. Statistical significance (P values) was obtained using Student's t test.
We then investigated whether specific regions of the Ad5 genome were involved in DC maturation. Thus, we determined the presence of GTCGTT motifs that were shown to stimulate human TLR9-expressing cells (4, 14) within each Ad vector sequence genome. We found that the number of GTCGTT motifs varied among different Ad vector serotypes (Fig. 5A). Interestingly, the number of GTCGTT motifs present in each Ad vector genome sequence correlated with the potency of Ad IC-mediated DC maturation (i.e., CD86 expression and TNF-α production; P = 0.0028 and P = 0.0054, respectively) (Fig. 5B and C).
Fig 5.
The number of TLR9 agonist motifs (GTCGTT) in adenoviral genome sequences directly correlates with the level of DC maturation. Schematic representation of GTCGTT motif positions in Ad5, Ad6, Ad26, Ad35, Ad36, and Ad41 genome sequences (A). The number of TLR9 agonist sequences (GTCGTT) in Ad5, Ad6, Ad26, Ad35, Ad36, and Ad41 genome sequences directly correlates with the level of DC maturation as assessed by CD86 expression (B) and TNF-α production (C). Statistical significance (P values) in panels B and C was obtained using Spearman's rank correlations.
CD46 signaling does not interfere with Ad IC-mediated DC maturation.
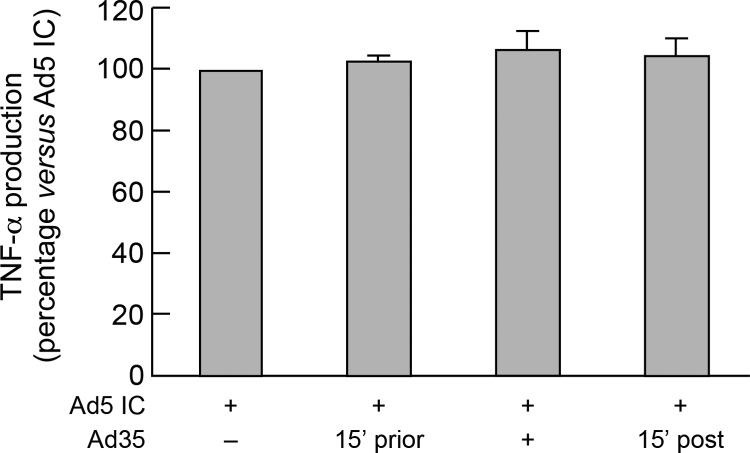
The cellular attachment protein for Ad belonging to the subgroups A, C (including Ad5 and Ad6), D (including Ad36), and E and F (including Ad41) is mediated by coxsackievirus-adenovirus receptor (CAR) interactions, while CD46 is used for some Ads belonging to subgroup B (including Ad35), and D (including Ad26) (1, 5, 10, 33–36, 40). Since both Ad26 ICs and Ad35 ICs triggered a reduced DC maturation compared to Ad5 ICs, we therefore investigated the potential role of Ad-CD46 interaction on Ad IC-mediated DC maturation. To address this issue, DCs were exposed to Ad35 (which binds only to CD46) either prior to, at the time of, or post-Ad5 IC stimulations. The results indicate that CD46 signaling did not interfere with Ad5 IC-mediated DC maturation (assessed by TNF-α production) (Fig. 6).
Fig 6.
CD46 signaling does not interfere with Ad IC-mediated DC maturation. MoDCs were stimulated with Ad5 IC formed with 3 distinct sera containing NAbs (Ad5 NAbs, n = 512) in the presence or absence of Ad35 (pre-Ad5 IC stimulation, poststimulation, or at the time of stimulation). Following 36 h of incubation, supernatants were collected and analyzed for the presence of TNF-α. Results are expressed as the percentage of the cytokine levels measured in cell culture exposed to Ad35 versus those found in Ad5 IC alone. The experiment described above was performed in triplicate. Statistical significance (P values) was obtained using Student's t test.
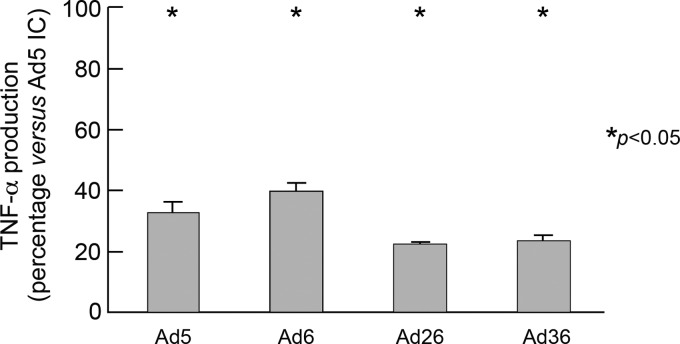
Antihexon but not antifiber antibodies are responsible for inducing Ad IC-mediated DC maturation.
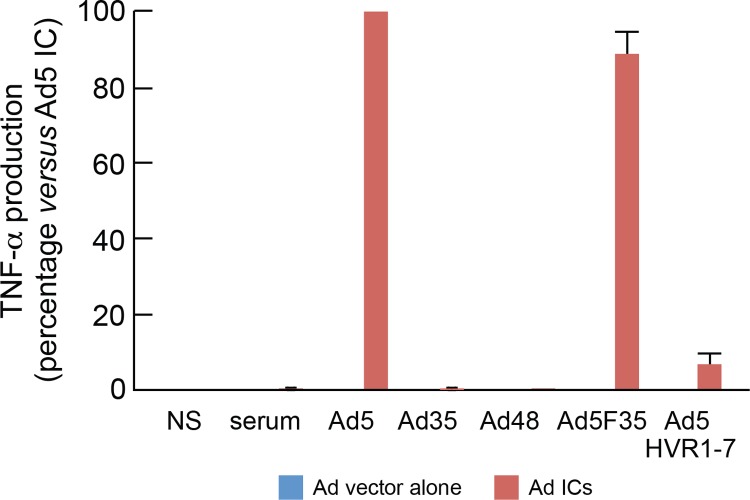
Ad5 neutralization may be mediated by either antifiber antibodies through the aggregation of the virions (extracellular neutralization) or by antihexon antibodies through the blocking of the endosomal escape or dissociation (intracellular neutralization) (39). Next we investigated which type of NAbs (i.e., antifiber or antihexon) induced DC maturation. DCs were exposed to ICs formed by plasma containing Ad5 NAbs (NAb titer of 512), and Ad5, or Ad5F35 vector (chimeric Ad5 vector engineered with Ad35 fiber) or Ad5HVR1-7 vector (chimeric Ad5 vector engineered with the hypervariable regions [HVR] 1 to 7 of Ad48) (24, 32). Therefore, the role of Ad5 antihexon selective neutralizing activity in Ad5 IC-mediated DC maturation can be assessed following the formation of Ad5HVR1-7 ICs, while the role of antifiber neutralizing activity in Ad5 IC-mediated DC maturation can by assessed by the formation of Ad5F35 ICs. Of note, the plasma samples tested were Ad35 and Ad48 seronegative (NAb titer < 16). The results obtained showed that about 90% of Ad5 IC-mediated DC maturation activity as assessed by TNF-α production was observed with Ad5F35 ICs, thus indicating that antihexon antibodies directed to hypervariable regions 1 to 7 of the hexon protein were almost exclusively responsible for DC maturation (Fig. 7).
Fig 7.
Ad5 IC-mediated TNF-α production is due to anti-hypervariable region 1 to 7 NAbs but not to antifiber antibodies. MoDCs were stimulated with 3 distinct sera alone containing NAbs (Ad5 NAbs; n = 512), Ad5, Ad35, Ad48, Ad5F35, or Ad5HVR1-7 vectors alone or Ad ICs formed with the plasma and vectors described above. Following 36 h of incubation, supernatants were collected and analyzed for the presence of TNF-α. Results are expressed as the percentage of the cytokine levels measured in cell culture exposed to Ad5 ICs versus those found in Ad35, Ad48, Ad5F35, or Ad5HVR1-7 ICs. The experiment described above was performed in triplicate.
DISCUSSION
In 2007, the phase IIb test-of-concept efficacy study called Step, which evaluated a trivalent Ad5-Gag/Pol/Nef vaccine candidate, was prematurely terminated due to the lack of efficacy of the vaccine (6, 20). Unexpectedly, uncircumcised vaccine recipients with higher titers of Ad5 NAbs (Ad5 titers > 18) showed increased susceptibility to HIV infection compared to vaccine recipients with lower titers (<18) of Ad5 NAbs and/or circumcised and placebo recipients (6).
Several hypotheses were proposed to explain the increased acquisition of HIV infection among vaccine recipients of the Step trial. In this regard, we showed that DCs exposed to Ad5 ICs but not Ad5 vector alone induced a strong maturation of DCs that enhanced HIV infection of DC-CD4 T cell coculture (29). We also demonstrated that Ad5 IC-mediated DC maturation required Ad5 IC internalization through FcγRII receptor followed by TLR9 engagement (29). We hypothesized that Ad IC-mediated DC maturation may create more favorable conditions for HIV spreading in CD4 T cells at the mucosal level in vaccinees with preexisting Ad5 immunity in the Step trial. Of note, the central role of DCs in transmitting HIV to antigen-specific CD4 T cells was later supported by Gringhuis et al. (13). Furthermore, the results from the Step trial have further underscored the potential emergence of safety issues in subjects with preexisting immunity against viral vector-based vaccines. It is therefore of paramount importance to determine the immunological effects that may be triggered in subjects with preexisting immunity when they reencounter adenovirus following vaccination with Ad vector-based vaccines.
In the present study, we extended our previous observations with Ad5 to investigate the potential impact of preexisting humoral immunity to Ad vectors based on rare Ad serotypes such as Ad6, Ad26, Ad35, Ad36, and Ad41. For these purposes, we have used an in vitro experimental system that allows us to determine the effect of Ad antibody preexisting immunity on DC maturation. Rare Ad serotype vectors such as Ad26 and Ad35 are being used to develop vaccines in the fields of HIV, tuberculosis, and malaria research.
Of interest, we have demonstrated major biological differences in rare Ad serotypes such as Ad26, Ad35, and Ad36 ICs and Ad5 and Ad6 ICs in terms of the ability to stimulate maturation of DCs. Ad26, Ad35, and Ad36 ICs were poor inducers of DC maturation compared to Ad5 and Ad6 ICs. We previously shown that Ad DNA-TLR9 interaction was responsible for DC maturation mediated by Ad5 ICs. In the present study, we provide evidence that the same TLR9 mechanism is operating in the DC maturation mediated by rare Ad IC serotypes.
Almost a decade ago, two independent studies showed that a particular hexamer motif (GTCGTT) had a strong capacity to stimulate human TLR9-expressing cells (4, 14). We therefore investigated whether there was a relationship between the number of hexamer motifs (GTCGTT) within common and rare Ad-derived vector genomes (i.e., Ad5, Ad6, Ad26, Ad35, Ad36, and Ad41 vectors) and their ability to induce DC maturation. We found that the number of GTCGTT motifs, which varied among different Ad genome sequences, correlated with the potency of Ad ICs to induce DC maturation. Of note, these motifs are located outside the E1, E3, and E4 genes, which are the genes being deleted in the different Ad5 vector HIV vaccine candidates evaluated in different clinical trials.
Ad5, Ad6, Ad36, and Ad41 utilized CAR as a cellular receptor, while Ad26 and Ad35 utilize CD46 (1, 5, 10, 33–36, 40). It is therefore possible that the biological differences observed between common and rare Ad serotypes in stimulating DC maturation resulted from a different receptor usage rather than from the number of GTCGTT motifs and TLR9 interaction. However, three observations in the present study support the importance of the number of TLR9-agonist motifs as primary mechanism to explain the potency of the different Ad ICs in the maturation of DCs. First, Ad36 utilizes CAR, and it is a poor inducer of DC maturation, like Ad35 and Ad26, which utilize CD46. Therefore, the difference between Ad36 and Ad5 or Ad6 relies on the number of TLR9-agonist motifs and not on receptor usage. Second, treatment of DCs with Ad35, which potentially delivers via CD46 an inhibitory signal to DCs, did not prevent DC maturation mediated by Ad5 ICs that utilize CAR. Third, Ad5F35 ICs (Ad5 pseudotyped with Ad35 fiber), which can interact with CD46 and harbor an equal number of TLR9 agonist motifs to Ad5 (n = 7), had similar potency in mediating DC maturation (assessed by TNF-α production) compared to Ad5 ICs. Therefore, CD46 signaling did not interfere with Ad IC-mediated DC maturation via CAR.
Finally, we showed that antihexon but not antifiber antibodies are responsible for inducing DC maturation. Since antihexon NAbs may prevent capsid disassembly and nuclear entry of viral DNA by cross-linking the viral particles (37), it is likely that Ad5 ICs may stabilize the capsid at endosomal pH (pH 6), which might prevent at least in part the endosomal escape of Ad5 DNA (12).
In conclusion, the present study provides new insights into the biological differences between Ad serotypes and their potential interaction with the host immune system in the presence of preexisting immunity. However, the potential role of Ad IC-mediated DC maturation in creating favorable conditions for HIV spread in vivo remains to be determined. Notwithstanding the limited knowledge of the tropism of the rare Ad serotypes in vivo, one may speculate that the lack of induction of DC maturation by Ad26 and Ad35 ICs together with the low seroprevalence may render rare Ad serotype vectors an attractive platform for the vaccine field.
ACKNOWLEDGMENTS
We thank Sandy Ibanez and Aurélie Gennetier for Ad5 vector preparations.
This work was funded by the Swiss National Science Foundation.
The authors declare they have no conflicting financial interests.
Footnotes
Published ahead of print 4 April 2012
REFERENCES
- 1. Abbink P, et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams WC, et al. 2011. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc. Natl. Acad. Sci. U. S. A. 108:7499–7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barouch DH, et al. 2011. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29:5203–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer S, et al. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. U. S. A. 98:9237–9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergelson JM, et al. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323 [DOI] [PubMed] [Google Scholar]
- 6. Buchbinder SP, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cichon G, et al. 2001. Complement activation by recombinant adenoviruses. Gene Ther. 8:1794–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fallaux FJ, et al. 1996. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther. 7:215–222 [DOI] [PubMed] [Google Scholar]
- 9. Frahm N, et al. 2012. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J. Clin. Invest. 122:359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaggar A, Shayakhmetov DM, Lieber A. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408–1412 [DOI] [PubMed] [Google Scholar]
- 11. Geisbert TW, et al. 2011. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against Ebolavirus challenge. J. Virol. 85:4222–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greber UF, Willetts M, Webster P, Helenius A. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477–486 [DOI] [PubMed] [Google Scholar]
- 13. Gringhuis SI, et al. 2010. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat. Immunol. 11:419–426 [DOI] [PubMed] [Google Scholar]
- 14. Hartmann G, et al. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617–1624 [DOI] [PubMed] [Google Scholar]
- 15. Iacobelli-Martinez M, Nemerow GR. 2007. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 81:1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemckert AA, et al. 2005. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-Ad5 immunity. J. Virol. 79:9694–9701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemiale F, et al. 2007. Novel adenovirus vaccine vectors based on the enteric-tropic serotype 41. Vaccine 25:2074–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lore K, et al. 2007. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J. Immunol. 179:1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mast TC, et al. 2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950–957 [DOI] [PubMed] [Google Scholar]
- 20. McElrath MJ, et al. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michael NL. 2012. Rare serotype adenoviral vectors for HIV vaccine development. J. Clin. Invest. 122:25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mistchenko AS, et al. 1992. Participation of immune complexes in adenovirus infection. Acta Paediatr. 81:983–988 [DOI] [PubMed] [Google Scholar]
- 23. Mittereder N, March KL, Trapnell BC. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nanda A, et al. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 79:14161–14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. 2002. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum. Gene Ther. 13:1167–1178 [DOI] [PubMed] [Google Scholar]
- 26. Perreau M, Guerin MC, Drouet C, Kremer EJ. 2007. Interactions between human plasma components and a xenogenic adenovirus vector: reduced immunogenicity during gene transfer. Mol. Ther. 15:1998–2007 [DOI] [PubMed] [Google Scholar]
- 27. Perreau M, Kremer EJ. 2005. Frequency, proliferation, and activation of human memory T cells induced by a nonhuman adenovirus. J. Virol. 79:14595–14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perreau M, et al. 2007. Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: implications for clinical efficacy. J. Virol. 81:3272–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perreau M, Pantaleo G, Kremer EJ. 2008. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 205:2717–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perreau M, et al. 2011. Modulation of human memory T-cell function by different antigen-presenting cells. Eur. J. Immunol. [Epub ahead of print.] doi:10.1002/eji.201142094 [DOI] [PubMed] [Google Scholar]
- 31. Priddy FH, et al. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46:1769–1781 [DOI] [PubMed] [Google Scholar]
- 32. Roberts DM, et al. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239–243 [DOI] [PubMed] [Google Scholar]
- 33. Roelvink PW, et al. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909–7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segerman A, et al. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183–9191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Segerman A, Mei YF, Wadell G. 2000. Adenovirus types 11p and 35p show high binding efficiencies for committed hematopoietic cell lines and are infective to these cell lines. J. Virol. 74:1457–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sirena D, et al. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varghese R, Mikyas Y, Stewart PL, Ralston R. 2004. Postentry neutralization of adenovirus type 5 by an antihexon antibody. J. Virol. 78:12320–12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waddington SN, et al. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409 [DOI] [PubMed] [Google Scholar]
- 39. Wohlfart C. 1988. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62:2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu E, et al. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]