Abstract
We previously reported that long-term rhesus cytomegalovirus (RhCMV) excretion in infected macaques was related to UL/b′ coding content. Acute biopsy specimens of the inoculation sites from the previous study have now been analyzed to determine whether there were acute phenotypic predictors of long-term RhCMV infection. Only in animals displaying acute endothelial tropism and neutrophilic inflammation was RhCMV excretion detected. The results imply that vaccinating against these early viral determinants would significantly impede long-term RhCMV infection.
TEXT
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus with adult seroprevalence rates of 50 to >90% (5). The rate of congenital infection in the United States is ∼0.6% (14), indicating that the preponderant mechanism for spread of HCMV in the population is horizontal transmission. It is generally thought that during primary infection, HCMV replicates locally within epithelial and endothelial cells and fibroblasts. Local replication is followed by transmission to leukocytes for the hematogenous spread of virions to multiple cell types throughout the body, particularly sites of virus shedding (e.g., salivary glands, mammary glands, genitourinary tract) (3, 20). Primary HCMV infection is mostly subclinical in an immunocompetent host. However, HCMV establishes a lifelong latency following resolution of acute infection. Latent viral genomes can reactivate to produce virus that can be transmitted to those most at risk for HCMV infection, such as pregnant women. The acute interactions between HCMV and a naïve individual are likely instrumental in defining the number and tissue distribution of long-term HCMV-infected cells, the kinetics and specificities of adaptive immune responses, and the frequency and magnitude of viral shedding. HCMV can infect multiple cell types in vivo, including epithelial and endothelial cells, macrophages, and fibroblasts (20, 23). Since certain cell types may assume a greater role in supporting primary HCMV replication, vaccine targeting of particular HCMV cell tropism-determining proteins should limit local replication and greatly restrict the long-term pattern of HCMV infection.
The phenotype of long-term rhesus cytomegalovirus (RhCMV) shedding in bodily fluids is dependent on the genotype of the strain of RhCMV used for primary infection in naïve animals (18). Animals inoculated with RhCMV strains containing a full-length UL/b′ region (UCD52 and UCD59) persistently shed high titers of virus in saliva and urine that are similar to those from shedding in animals naturally exposed to wild-type (WT) RhCMV (RhCMV-WT). Inoculation with the 68-1 strain of RhCMV, which lacks genes encoding proteins for epithelial/endothelial cell tropism and proteins potentially involved in leukocyte chemoattraction (12, 15, 17, 18, 21), is characterized by pronounced attenuation of shedding (18). This study was initiated to determine whether the patterns of acute infection predicted differences in persistent infection.
To assess how coding content differences within the UL/b′ region may alter primary RhCMV infection, early virus-host interactions were analyzed in skin biopsy specimens obtained from two previous studies (1, 18) in which RhCMV-seronegative rhesus macaques (Macaca mulatta) had been inoculated subcutaneously with RhCMV 68-1 (n = 3), RhCMV UCD52 (n = 6), or RhCMV UCD59 (n = 1). Cytomegalic cells were present in all skin biopsy specimens at 7 days postinoculation, accompanied by moderate infiltration of inflammatory cells admixed with various degrees of tissue necrosis (Fig. 1). Both UCD52 and UCD59 induced moderate inflammation composed predominantly of neutrophils (Fig. 1A; only UCD52 is shown), while inflammation induced by RhCMV 68-1 was essentially mononuclear, with negligible numbers of neutrophils at the site of inoculation (Fig. 1B). This particular phenotype of 68-1 has been observed in other studies (1).
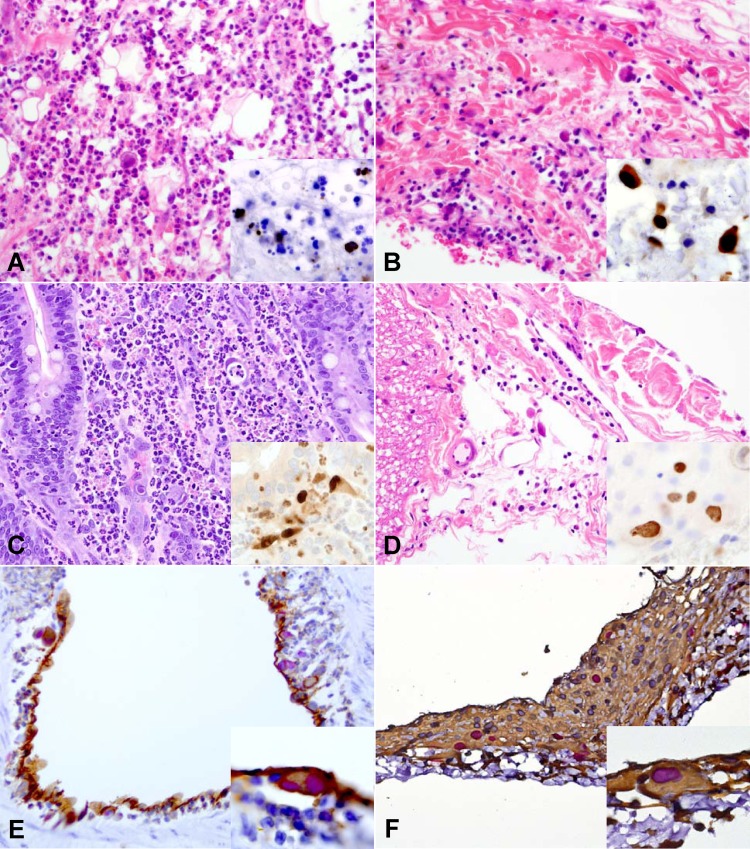
Fig 1.
Histopathologic features and immunostaining of skin biopsy specimens collected from rhesus macaques experimentally inoculated with RhCMV UCD52 (A) or RhCMV 68-1 (B), macaques with RhCMV-WT reactivation in the context of SIV immunosuppression (jejunum [C] and pulmonary artery [E]), and macaques with reactivated RhCMV 68-1 in SIV/RhCMV-coinfected animals (D and F; spinal cord meninges). (A and C) Tissue sections from animals inoculated with RhCMV UCD52 or RhCMV-WT that were stained with hematoxylin and eosin (H&E) and RhCMV-IE1 immunohistochemistry (IHC) (insets) show diffuse regions of inflammation characterized by a large accumulation of neutrophils expanding the tissue and causing tissue necrosis. (B and D) In contrast to infection with RhCMV UCD52 and RhCMV-WT, acute (B) and chronic (D) inflammation in tissue sections collected from animals inoculated with RhCMV 68-1 lacked neutrophils and was composed predominantly of mononuclear cells. (E) Direct RhCMV-WT infection of endothelial cells during RhCMV reactivation, confirmed by RhCMV-IE1 immunoreactivity (inset; magenta) in endothelial cells (CD31, brown) lining pulmonary arteries. (F) RhCMV 68-1 immunoreactivity (RhCMV IE-1, magenta; inset) in fibroblasts (vimentin, brown). Magnification of large images, ×400; magnification of inset images, ×600.
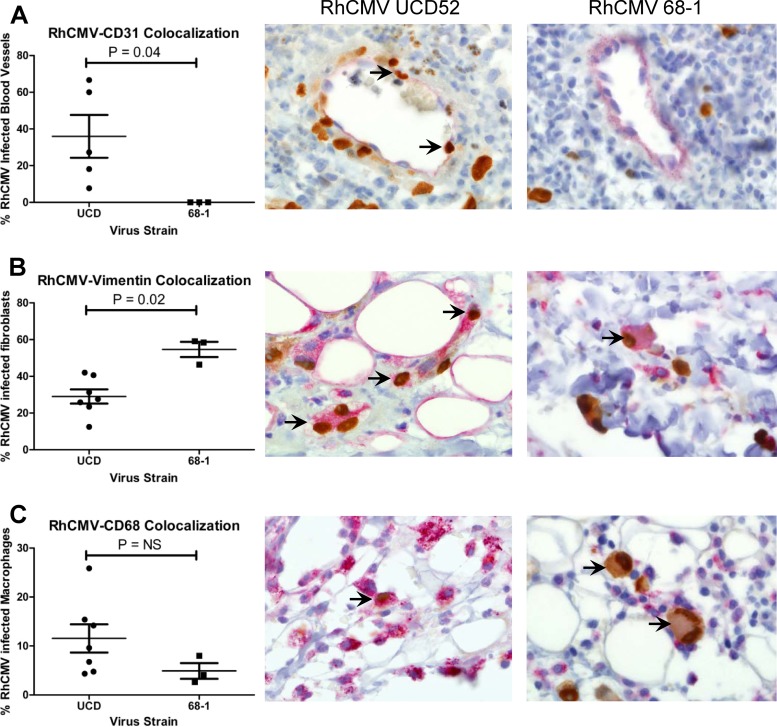
The manifestations of HCMV infection depend on viral dissemination to multiple tissues, and viral tropism is an important determinant in viral spread within the infected host (23). Because of this, we asked whether interstrain differences in the coding capacity of the UL/b′ region would predict interstrain differences in cell tropism during acute RhCMV infection. Double immunohistochemistry, using antiserum for the RhCMV immediate-early (IE1) protein and cell-specific markers for endothelial (CD31) and mesenchymal (vimentin) cells and macrophages (CD68), was performed to identify the cell types supporting RhCMV infection. The total numbers of all RhCMV and RhCMV double-labeled cells were counted, and the percentage of colocalization of the two markers was calculated by dividing the number of double-labeled cells by the number of RhCMV antigen-positive cells. The percentage of blood vessels containing RhCMV IE1 antigen-positive cells was calculated by dividing the number of RhCMV-positive blood vessels by the total number of blood vessels in the field. Animals inoculated with UCD52 or UCD59 demonstrated RhCMV IE1 expression in endothelial cells with a frequency that was significantly greater than that in 68-1-infected animals (P = 0.0357) (Fig. 2A). Conversely, there was significant skewing of infectivity for fibroblasts in 68-1-infected animals compared to that in UCD52/UCD59-inoculated animals (P = 0.0167) (Fig. 2B). UCD52/UCD59 infection of CD68+ macrophages was slightly higher than that in 68-1-infected animals, although the difference did not reach statistical significance (P = 0.12). Since RhCMV 68-1 does not encode RhUL128 and RhUL130, the mechanism for demonstrable infectivity of macrophages is not known, since a functional HCMV UL128 complex is critical for efficient infection of human macrophages (23). In vivo infection of rhesus macrophages has been previously demonstrated in RhCMV-68-1-infected macaques (16), and infection of macrophages may be through a gB-mediated fusion.
Fig 2.
Semiquantitative spectral image analysis and immunophenotyping of RhCMV-infected cells in skin biopsy specimens collected from rhesus macaques experimentally inoculated with RhCMV UCD52 and UCD59 (circles) or RhCMV 68-1 (squares). Sections of the skin biopsy specimens were coimmunolabeled against RhCMV-IE1 (brown) and endothelial cells (CD31) (A), fibroblasts (vimentin) (B), and macrophages (CD68) (C) (red). The percentages (mean ± standard error of the mean [SEM]) of RhCMV-infected blood vessels (A), fibroblasts (B), and macrophages (C) are presented. Double-labeled RhCMV-infected cells are indicated by arrows. Data were pooled from analysis of 10 photomicrographs of one skin biopsy specimen per animal. Statistical analysis was performed by using the Mann-Whitney test comparing RhCMV UCD infection with RhCMV 68-1 infection. The representative immunohistochemical images shown are from an animal inoculated with RhCMV UCD52 (images on left) and an animal inoculated with RhCMV 68-1 (images on right). Magnification, ×600. NS, not significant (P = 0.12).
To determine whether the acute pattern of infection with RhCMV was similar to the long-term reactivation of RhCMV, the cell types supporting reactivated RhCMV and the nature of the inflammatory infiltrate were analyzed in animals infected long-term with RhCMV and simian immunodeficiency virus (SIV). This analysis included two monkeys coinfected with RhCMV 68-1 and SIV from a previous study (22) and 100 historical cases of wild-type RhCMV reactivation in monkeys coinfected with SIV (the latter of which are summarized in Table 1). RhCMV-WT reactivation was found in almost every major organ system, with the preponderance equally distributed within the cardiovascular, pulmonary, and nervous system compartments. Like the acute response following UCD52 and UCD59 inoculations, 88% of RhCMV-WT cases were noted for marked neutrophilic infiltrates (Fig. 1C). RhCMV-WT reactivations occurred in the context of simian AIDS in monkeys infected long-term with RhCMV-WT, indicating that RhCMV-WT reactivation during immune deficiency mirrored the histological pattern of acute infection with UCD52 and UCD59. Evidence of direct RhCMV-WT infection of endothelial cells was confirmed in four cases by the presence of inclusion bodies or immunohistochemistry in endothelial cells (Fig. 1E). In animals experimentally coinfected with RhCMV 68-1 and SIV, RhCMV reactivation was confirmed in the thymus and spinal cord of two animals euthanized at 38 and 53 weeks after primary RhCMV infection, respectively. The histopathologic pattern and cellular tropism of RhCMV reactivation reflected the patterns of acute RhCMV 68-1 infection (Fig. 1D). RhCMV 68-1 reactivation induced a predominantly mononuclear cell inflammation, and RhCMV immunoreactivity was confined to fibroblasts and macrophages with no evidence of endothelial cell infection (Fig. 1F).
Table 1.
Organ involvement in natural RhCMV infection in SIV-coinfected rhesus macaques at the NPRCa
| Organ system or type of inflammationb | No. of casesc |
|---|---|
| Organ system | |
| Pulmonary | 37 |
| Cardiovascular | 7 |
| GId | 39 |
| Nervous | 37 |
| Urogenital | 22 |
| Lymphatic | 7 |
| Othere | 11 |
| Type of inflammation | |
| Neutrophilicf | 88 |
| Nonneutrophilicg | 8 |
| No inflammation | 4 |
n = 100. Samples were collected between 1997 and 2010.
Case identification is based on histopathologic examination with observation of typical cytomegalovirus inclusion and immunohistochemistry when available.
Numbers include those animals with RhCMV lesions observed in multiple organ systems; each lesion was separately counted. If >1 organ had a prominent neutrophilic infiltrate, that animal was classified as neutrophilic.
GI, gastrointestinal.
Includes 3 livers, 1 pancreas, 1 lacrimal gland, 1 adrenal gland, and 5 oral cavities (tongue, salivary gland, and labial mucosa).
Indicates neutrophils as the predominant type of inflammatory cell in the lesion.
Usually superimposed by other opportunistic infections. Very few cytomegalic cells were noted in these cases.
Recruitment of neutrophils to the site of acute RhCMV infection and long-term reactivation is a novel finding and suggests that extravasated neutrophils may play a role in intrahost dissemination of progeny virions. This response is not unique to RhCMV. Neutrophilic infiltrates are often observed in the proximity of cytomegalic cells in tissue samples from individuals with HCMV disease (4, 6, 9, 13, 19). In vitro experiments have demonstrated that infected endothelial cells can transfer virions to neutrophils migrating through the endothelial layer, a process that is dependent on a functional UL128 complex of proteins (UL128, UL130, UL131, gH/gL) (2, 7, 8, 10, 11). Neutrophils may act as a “Trojan horse” to facilitate virus dissemination from the primary site of infection to distal sites. This remains speculative and requires additional study.
In summary, there are differences in the acute patterns of infection between strains of RhCMV that differ in genetic coding content, with distinctions in cell tropism and the nature of the inflammatory response to viral infection. Differences in the phenotypes of acute infection were notably similar to the different phenotypes of AIDS-induced RhCMV reactivation. Based on pronounced differences in the long-term shedding between UCD52/UCD59 and 68-1 (18), one implication of preferential tropism for endothelial cells and/or recruitment of neutrophils from the periphery during acute infection is that long-term shedding patterns may be a downstream consequence of the earliest virus-host interactions during primary infection. Accordingly, vaccine strategies that target these earliest interactions should profoundly change the long-term virus-host relationship. The results of this current study suggest that inclusion of vaccine antigens targeting the UL128 complex of proteins should greatly inhibit the acute infection of endothelial and epithelial cells, with commensurate changes in the long-term pattern of persistent infection.
ACKNOWLEDGMENTS
We thank Andrew Miller, Charles Bailey, and the NPRC histopathology core for helpful discussions and technical assistance.
Grants from the NIH (AI063356 to P.A.B. and D.J.D., P51 OD011107 to the CNPRC, and RR00168 and RR07000 to the NPRC) and the Margaret Deterding Infectious Disease Research Support Fund (to P.A.B.) supported this study.
Footnotes
Published ahead of print 4 April 2012
REFERENCES
- 1. Abel K, et al. 2008. A heterologous DNA prime/protein boost immunization strategy for rhesus cytomegalovirus. Vaccine 26:6013–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barry PA, Alcendor DJ, Power MD, Kerr H, Luciw PA. 1996. Nucleotide sequence and molecular analysis of the rhesus cytomegalovirus immediate-early gene and the UL121-117 open reading frames. Virology 215:61–72 [DOI] [PubMed] [Google Scholar]
- 3. Boppana SB, Fowler KB. 2007. Persistence in the population: epidemiology and transmission, p 795–813 In Arvin A, et al. (ed), Human herpesviruses: biology, therapy and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom: [PubMed] [Google Scholar]
- 4. Cagle PT, Allen TC, Barrios R. 2007. Pulmonary pathology, 2nd ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 5. Cannon MJ, Schmid DS, Hyde TB. 2010. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 20:202–213 [DOI] [PubMed] [Google Scholar]
- 6. Duggan MA, Pomponi C, Robboy SJ. 1986. Pulmonary cytology of the acquired immune deficiency syndrome: an analysis of 36 cases. Diagn. Cytopathol. 2:181–186 [DOI] [PubMed] [Google Scholar]
- 7. Gerna G, et al. 2003. Rescue of human cytomegalovirus strain AD169 tropism for both leukocytes and human endothelial cells. J. Gen. Virol. 84:1431–1436 [DOI] [PubMed] [Google Scholar]
- 8. Gerna G, Percivalle E, Sarasini A, Baldanti F, Revello MG. 2002. The attenuated Towne strain of human cytomegalovirus may revert to both endothelial cell tropism and leuko- (neutrophil- and monocyte-) tropism in vitro. J. Gen. Virol. 83:1993–2000 [DOI] [PubMed] [Google Scholar]
- 9. Granter SR, Doolittle MH, Renshaw AA. 1996. Predominance of neutrophils in the cerebrospinal fluid of AIDS patients with cytomegalovirus radiculopathy. Am. J. Clin. Pathol. 105:364–366 [DOI] [PubMed] [Google Scholar]
- 10. Grundy JE, Lawson KM, MacCormac LP, Fletcher JM, Yong KL. 1998. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J. Infect. Dis. 177:1465–1474 [DOI] [PubMed] [Google Scholar]
- 11. Hahn G, et al. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78:10023–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. 2003. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 77:6620–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holland GN, et al. 1983. Acquired immune deficiency syndrome. Ocular manifestations. Ophthalmology 90:859–873 [DOI] [PubMed] [Google Scholar]
- 14. Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17:253–276 [DOI] [PubMed] [Google Scholar]
- 15. Lilja AE, Shenk T. 2008. Efficient replication of rhesus cytomegalovirus variants in multiple rhesus and human cell types. Proc. Natl. Acad. Sci. U. S. A. 105:19950–19955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lockridge KM, et al. 1999. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 73:9576–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oxford KL, et al. 2008. Protein coding content of the ULb′ region of wild-type rhesus cytomegalovirus. Virology 373:181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oxford KL, et al. 2011. Open reading frames carried on UL/b′ are implicated in shedding and horizontal transmission of rhesus cytomegalovirus in rhesus monkeys. J. Virol. 85:5105–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pepose JS, Holland GN, Nestor MS, Cochran AJ, Foos RY. 1985. Acquired immune deficiency syndrome: pathogenic mechanisms of ocular disease. Ophthalmology 92:472–484 [DOI] [PubMed] [Google Scholar]
- 20. Plachter B, Sinzger C, Jahn G. 1996. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 46:195–261 [DOI] [PubMed] [Google Scholar]
- 21. Rivailler P, Kaur A, Johnson RP, Wang F. 2006. Genomic sequence of rhesus cytomegalovirus 180.92: insights into the coding potential of rhesus cytomegalovirus. J. Virol. 80:4179–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sequar G, et al. 2002. Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis. J. Virol. 76:7661–7671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinzger C, Digel M, Jahn G. 2008. Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 325:63–83 [DOI] [PubMed] [Google Scholar]