Abstract
We have recently reported that a group of human adenoviruses (HAdVs) uses desmoglein 2 (DSG2) as a receptor for infection. Among these are the widely distributed serotypes HAdV-B3 and HAdV-B7, as well as a newly emerged strain derived from HAdV-B14. These serotypes do not infect rodent cells and could not up until now be studied in small-animal models. We therefore generated transgenic mice containing the human DSG2 locus. These mice expressed human DSG2 (hDSG2) at a level and in a pattern similar to those found for humans and nonhuman primates. As an initial application of hDSG2-transgenic mice, we used a green fluorescent protein (GFP)-expressing HAdV-B3 vector (Ad3-GFP) and studied GFP transgene expression by quantitative reverse transcription-PCR (qRT-PCR) and immunohistochemistry subsequent to intranasal and intravenous virus application. After intranasal application, we found efficient transduction of bronchial and alveolar epithelial cells in hDSG2-transgenic mice. Intravenous Ad3-GFP injection into hDSG2-transgenic mice resulted in hDSG2-dependent transduction of epithelial cells in the intestinal and colon mucosa. Our findings give an explanation for clinical symptoms associated with infection by DSG2-interacting HAdVs and provide a rationale for using Ad3-derived vectors in gene therapy.
INTRODUCTION
Human adenoviruses (HAdVs) have been classified into six species (HAdV-A to HAdV-F) currently containing 55 serotypes. Most adenovirus (Ad) serotypes utilize the coxsackie-adenovirus receptor (CAR) as a primary attachment receptor. However, this is not the case for species B Ad serotypes. Species B Ads form two genetic clusters, B1 (HAdV-B3, -B7, -B16, -B21, and -B50) and B2 (HAdV-B11p, -B14, -B34, and -B35) (84). Recently, we have suggested a new grouping of species B Ads based on their receptor usage (82). The members of group 1 (HAdV-B16, -B21, -B35, -B50) nearly exclusively utilize CD46 as a receptor; the members of group 2 (HAdV-B3, -B7, -B14) use a receptor that was unknown until recently (receptor X), group 3 (HAdV-B11p) preferentially interacts with CD46 but also utilizes receptor X if CD46 is blocked or absent. This novel receptor usage-based grouping system has been supported by studies from various groups (27, 50, 65, 66). A newly emerged, pathogenic strain, Ad14-p1, uses the same receptor as prototype HAdV-B14 (89).
HAdV-B3 and -B7 are considered to be widely distributed human pathogens (14, 52, 59). Studies from the United States show that HAdV-B3 and -B7 infections occur more often in adolescents and adults (23, 92), while studies from Europe and Asia indicate that these serotypes are also common in children (32, 45, 90). Since 2005, several outbreaks of HAdV-B14p1 in military facilities and civic communities have been reported in various countries, including the United States, Europe, and Asia (8, 48, 78). Ads, including HAdV-B3, enter the body via the mouth/nose and generally cause respiratory diseases. The respiratory tract epithelium is also the preferred site of viral replication. Subsequent virus-induced cytolysis results in release and viremic spread of progeny virus. HAdVs can therefore be found in other organs and are readily detectable in the stool of infected patients. While it is generally accepted that HAdV-B3, -B7, and -B14 cause respiratory symptoms, a number of studies also describe a series of symptoms that can be explained by Ad infection of other tissues. Among those is the gastrointestinal (GI) tract. For example, after cough and shortness of breath, the main symptoms associated with HAdV-B14p1 infection in a recent community outbreak in Oregon were vomiting and diarrhea (44). There are several reports from different Asian countries that HAdV-B3 infection in children is associated with acute gastroenteritis (10, 33, 37, 45, 46).
We have recently identified desmoglein 2 (DSG2) as receptor X and shown that it serves as a receptor for HAdV-B3, -B7, -B11, -B14, and -B14p1 (82, 88). Our findings have been recently confirmed by others (79). DSG2 is a calcium-binding transmembrane glycoprotein that is primarily localized in desmosomes of epithelial junctions in the basal layer of stratified and simple epithelia of airway, GI, and urinary tracts (13). In addition, DSG2 can be found in nonepithelial tissues such as cardiac muscle and the dendritic reticulum of lymphatic follicles (63). In humans, genetic DSG2 defects can cause arrhythmogenic ventricular cardiomyopathy (58). In two recent studies, we have reported on the mechanism used by HAdV-B3 and subviral particles to breach epithelial barriers formed by intercellular junctions. In vitro, HAdV-B3 binding to DSG2 triggered pathways involved in epithelial-to-mesenchymal transition (EMT), leading to transient intercellular junction opening (88). This effect was also achieved with recombinant, DSG2-interacting proteins derived from HAdV-B3, specifically the Ad3 penton-dodecahedra (PtDd) and a self-dimerizing recombinant Ad3 fiber knob protein, which was originally called Ad3-K/S/Kn (87) and is now known as JO-1 (junction opener 1) (5). In mouse models, intravenous injection of JO-1 triggered junction opening in epithelial xenograft tumors through at least two potentially connected mechanisms: (i) cleavage of the DSG2 extracellular domain and disruption of DSG2 dimers between neighboring epithelial cells and (ii) intracellular signaling that leads to a transient decrease of E-cadherin and potentially other junction proteins, leading to changes in the membrane distribution of cellular receptors (5). Notably, JO-1-triggered changes translated directly into increased intratumoral penetration and therapeutic efficacy of anti-tumor monoclonal antibodies (5), as well as large chemotherapeutic drugs.
HAdV-B3, -B7, -B11, and -B14 do not infect rodent cells (88) and, so far, could not be studied in small-animal models. We therefore generated transgenic mice containing approximately 90 kb of the human DSG2 (hDSG2) locus. hDSG2-transgenic mice were fully characterized in terms of transgene insertion site, copy number, hDSG2 mRNA expression, and hDSG2 protein tissue distribution. As an initial application of hDSG2-transgenic mice, we used a green fluorescent protein (GFP)-expressing Ad serotype 3 virus (Ad3-GFP) and studied in vivo transduction based on GFP transgene expression in tissues as well as Ad3-GFP-associated pathology after intranasal and intravenous Ad3-GFP application.
MATERIALS AND METHODS
Cell lines.
Mouse mammary carcinoma (MMC) cells were established from a spontaneous tumor in a neu-tg mouse (39). MMC cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 2 mM l-glutamine (Gln), 100 U/ml penicillin (P), and 100 μg/ml streptomycin (S). To create MMC-hDSG2 cells, MMC cells were transduced with a lentivirus vector expressing the human DSG2 cDNA under the control of the EF1a promoter (88). hDSG2-positive cells were isolated by fluorescence-activated cell sorter (FACS) 1 week later.
Vero cells (ATTC, CCL-81) are epithelial kidney cells from Cercopithecus aethiops. Vero cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS, P/S, and Gln.
Adenovirus.
Ad3-GFP is a wild-type HAdV-B3-based vector containing a cytomegalovirus (CMV)-GFP expression cassette inserted into the E3 region (88). It was propagated in HeLa cells and purified, and its titer was determined as described previously (88). Ad5/35-GFP and Ad5-GFP are Ad5 vectors containing Ad35 and Ad5 fibers, respectively, and a CMV-GFP expression cassette (69). The viral particle number was measured spectrophotometrically based on the optical density at 260 nm (OD260). The infectious unit titer was calculated based on the number of plaques that formed at day 10 after infection of 293 cells as described earlier (69). The ratio of viral particles to PFU was 15:1 for all viruses. The generation of JO-1 (also known as Ad3-K/S/Kn) has been described previously (87). Recombinant Ad3 penton-dodecahedral (PtDd) protein complexes were produced in insect cells and purified as described elsewhere (20). For in vitro Ad transduction studies, cells were exposed to Ad vectors at the indicated multiplicities of infection (MOIs) for 1 h and washed, and GFP expression was measured by flow cytometry 18 h later.
DSG2 siRNA.
A set of DSG2-specific small interfering RNA (siRNA) was synthesized by Dharmacon (Thermo Scientific). The target sequences were CAAUAUACCUGUAGUAGAA, GAGAGGAUCUGUCCAAGAA, CCUUAGAGCUACGCAUUAA, and CCAGUGUUCUACCUAAAUA. Control siRNA was purchased from Qiagen (Valencia, CA). siRNA transfection was performed using HyperFect transfection reagent (Qiagen).
Generation of hDSG2-transgenic mice.
Bacterial artificial chromosome (BAC) clone CTD-2233I9 was purchased from Invitrogen (Carlsbad, CA). Based on the plasmid backbone pBeloBAC11, it contains the genome region from position 29054158 to position 29143265 of human chromosome 18. BAC DNA was prepared using the NucleoBond BAC Xtra Maxi EF (endotoxin-free) kit (Clontech, Mountain View, CA) following the manufacturer's instructions. PCR with primers specific to different regions of the BAC DNA was performed to confirm the identity and intactness of the transgene. To further verify the expression of hDSG2, BAC DNA was transfected into MMC cells with Lipofectamine 2000 (Invitrogen) and cells were stained with hDSG2 monoclonal antibody (MAb) (clone 6D8; AbD Serotec, Raleigh, NC) 4 days after transfection. To prepare DNA for injection, purified BAC DNA was digested with NotI and heat inactivated at 80°C for 20 min. Digestion products were then purified by Sepharose 4B-CL chromatography (GE Healthcare, San Francisco, CA) and eluted with injection buffer (endotoxin free; 100 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.1 mM EDTA [pH 8.0]). Fractions were run on a pulse-field electrophoresis gel, and the DNA fragment containing hDSG2 locus was isolated from the gel and used for pronuclear microinjection into mouse embryos. This procedure was performed by the Transgenic Resources Program at the University of Washington.
Genotyping of hDSG2-transgenic mice.
Mice were genotyped by PCR analysis using genomic DNA from mouse tail tip samples lysed with DirectPCR(Tail) (Viagen, Los Angeles, CA) by following the manufacturer's protocol. The following PCR primer pairs were used: 1F (5′-GAT AAC CTA AGG TAG CTG TCA AAA TCG GGT CAC TG-3′) and 1R (5′-CAT TCC TCC CAT CAC TGA TCT TCC ATC CTA TTC TTT TTG C-3′), 2F (5′-CAC TCT GAC ACA GAT TTC AAT AGC TTT GAC-3′) and 2R (5′-CTG CCT CAC TTT CTA AGT TCA AGC AAC AAT G-3′), 3F (5′-CTC TGC TAT CTG CAG GAA AGC ACC ATA GAC-3′) and 3R (5′-CAG GCT CCA GAG ATA CGA TTC TAT AGG AA-3′), 4F (5′-CAG TCA TTC TTG GTT ATG TAT GTG TGT ACT AAC-3′) and 4R (5′-TTC AAT AAA ACT GCA ACA ACC AAT AGA AGC-3′), 5F (5′-GAT TTG TAC TTA CAT TCT AGG GCT ATG CTC TAC-3′) and 5R (5′-AGC AGT TGA GCC AAG AGT CGT GGG TAT CCA GTC GT-3′). These five primer pairs covered several different regions within the hDSG2 locus.
Inverse PCR for analysis of integration site.
Inverse PCRs were used to find the transgene integration site. To identify the transgene/chromosome junctions, 5 μg of cellular DNA isolated from mouse fibroblasts was digested with XbaI and religated under conditions that promote intramolecular reactions. The ligation mixture was subjected to nested PCR (30 cycles each) using LongAmp Taq DNA polymerase (NEB, Ipswich, MA). The following primer pairs were used: RP4 (5′-AGA CTG CCC TGA GGA ACA GAT GCC AAC-3′) and FP4 (5′-GGA ATG ACA TGT CAG GAA GCA TAG AAC AG-3′); RP4 and FP1 (5′-TCC AAG CAG ACA AAG AGT GAT ATT GTG-3′). The PCR fragments were cloned into pGEMT-Easy (Promega, Fitchburg, WI), and junctions were sequenced using BAC DNA-specific primers.
hDSG2 copy number.
The plasmid LV41-DSG2 (DSG2 cDNA accession number BC099657; Capital Bioscience) was diluted to generate a standard curve with 2 × 101 to 2 × 106 copies per ml. Using two primer pairs, this standard was run in duplicate on a 7900HT fast real-time PCR system (Applied Biosystems/Life Technologies). The mouse genome (100 ng, corresponding to 16,667 cells, as there are 6 pg of DNA per diploid mouse cell) was analyzed with the two primer pairs. The threshold cycle (CT) values versus log copy numbers were plotted, and the trendline equation was used to calculate the number of copies of hDSG2 per cell. The following primers were used: 5′-AAG GTA CAG TTG TGG TCA CTG AAA-3′, 5′-CCA TGA CGT AAG GTA CAT CTT GAA-3′, 5′-ACT GAA AAT TCT ATG ACG GCT AGG-3′, and 5′-TAA CTC TGG TGG AGG ATG TGG TTA-3′.
hDSG2 DNA FISH.
To isolate fibroblasts from transgenic mice, small tail cuts were incubated in collagenase 1 mg/ml at 37°C for 20 min, followed by incubation with 0.05% trypsin for 20 min. Cells were cultured in DMEM, supplemented with 10% FBS, P/S, and amphotericin B (Fungizone). To arrest cells in metaphase, colcemid (100 ng/ml; Invitrogen, Carlsbad, CA) was added and cells were cultured overnight. Metaphase chromosomes were hybridized with an hDSG2 probe derived from BAC-CTD-2233I9. The DNA was labeled using a nick translation reagent kit (Abbott Molecular Inc., Des Plaines, CA). Fluorescence in situ hybridization (FISH) was performed following the protocol from Cytocell Technologies for Aquarius probes (Rainbow Scientific Inc., Windsor, CT).
Animal studies.
All experiments involving animals were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Washington (permit number A3464-01). The specific experiments are covered by IACUC protocol number 3108-01. For intranasal Ad application, mice were sedated and held in an upright position and virus in a total volume of 50 μl was instilled into both nostrils. Intravenous Ad injection was performed through the tail vein in a total volume of 200 μl. In vivo images were taken with an IVIS Spectrum instrument (Xenogen Corporation/Caliper Life Sciences, Inc.). Mouse blood was analyzed using a HemaVet 950FS machine. Biochemical blood analyses were performed by the University of Washington Medical Center.
Immunohistochemistry.
Paraffin sections of the human samples were obtained from the University of Washington, Department of Pathology. Macaca fascicularis tissues were provided from the Washington National Primate Research Center. Tissues were either fixed in 10% neutral buffered formalin and processed for paraffin sections or embedded in optimal cutting temperature (OCT) compound for frozen sections. Tissues from hDSG2-transgenic mice were treated the same way. For hDSG2 staining, paraffin sections were subjected to antigen retrieval by antigen unmasking solution (Vector Labs, Burlingame, CA) using a Pascal pressure cooker (DakoCytomation, Carpinteria, CA). Polyclonal goat anti hDSG2 1:50 (R&D Systems, Inc., Minneapolis, MN) were used on paraffin sections, followed by the ImmPress reagent kit (Vector Labs). Frozen sections were fixed with methanol-acetone (1:1) and incubated with goat anti-hDSG2 antibodies followed by Alexa Fluor-conjugated donkey anti-goat immunoglobulin G (IgG; 1:200; Molecular Probes, Eugene, OR). Fluorescein isothiocyanate (FITC)-conjugated mouse anti-E-cadherin (BD Biosciences, San Jose, CA) was used on frozen sections. For GFP staining, paraffin sections were incubated with monoclonal anti-GFP 1:400 (Clontech, Mountain View, CA), followed by the Klear Mouse DAB kit (Golden Bridge International Inc., Mukilteo, WA). For frozen sections, the tissues were fixed with 10% neutral formalin, dehydrated with 5%, 10%, and 20% sucrose, and then embedded in OCT. For detection of Doxil, tissues were fixed with 4% paraformaldehyde and incubated with anti-polyethylene glycol (anti-PEG) antibody AGP4 (74), followed by Alexa Fluor 568-conjugated goat anti-mouse IgM antibody. For detection of Ad3 penton, a polyclonal rabbit antibody was used (19). The hexon antibody was from Millipore (Billerica, MA). Images were taken by a Leica DMLB microscope (Wetzlar, Germany), using a Leica DFC300FX digital camera and Leica Application Suite version 2.4.1 R1 software (Heerbrugg, Germany). Confocal images were taken with a Zeiss LSM 510 confocal microscope using Zeiss 510 software (Carl Zeiss MicroImaging, LLC, Thornwood, NY). Quantification of transduced cells was done on 20 independent areas from at least three sections taken at different depths of the tissue.
The anti-rat Neu antibody 7.16.4 was generously provided by Mark Greene (University of Pennsylvania).
Transmission electron microscopy (TEM) of platelets.
Platelets were isolated 1 h after Ad3 injection. Platelets were fixed in half-strength Karnovsky fixative (pH 7.3) and then processed for transmission electron microscopy. Processed grids were evaluated and photomicrographed with a Phillips 410 electron microscope operated at 80 kV (magnification, ×21,000).
hDSG2 enzyme-linked immunosorbent assay (ELISA).
Rabbit polyclonal anti-hDSG2 antibody (catalog no. AF947; R&D Systems) was used as a capture antibody (2 μg/ml). Mouse monoclonal anti-hDSG2 antibody 6D8 (MCA2272; AbD Serotec) in combination with goat anti-mouse IgG-horseradish peroxidase (HRP) (BD Pharmingen, San Jose, CA) was used for detection.
DSG2 mRNA quantification in human tissues.
Multi-tissue cDNA (MTC) panels (Human Immune System MTC panel, Human MTC panel I, and Human MTC panel II) were acquired from Clontech. These panels are fully normalized to the mRNA expression levels of four housekeeping genes (α-tubulin, β-actin, GAPDH [glyceraldehyde-3-phosphate dehydrogenase], and phospholipase A2). cDNA was used as a template for PCR with the following primers: DSG2 fw, 5′-ATC TGT GTC TTC TAG GCA GGC GCA AAA-3′; DSG2 rev, 5′-ACA CCG TGG TGT TCC TAG CCG TCA TA-3′; GAPDH fw, 5′-TGC ACC ACC AAC TGC TTA GC-3′; GAPDH rev, 5′-GGC ATG GAC TGT GGT CAT GAG-3′.
Samples were collected after cycles 22, 26, 30, 34, and 38 and analyzed on agarose gels. Intensity of signals was measured. Signal intensity of different tissues was compared to that of skeletal muscle (the tissue with the lowest DSG2 expression level).
DSG2 mRNA quantification in monkey and transgenic mouse tissues.
Tissues were homogenized using TissueRuptor from Qiagen, and total RNA was extracted using a miRCURY RNA isolation kit (cell and plant) from Exiqon (Woburn, MA). Reverse transcription was performed on 1 μg of total RNA using the Quantitect reverse transcription (RT) kit (Qiagen) to evaluate the expression levels of human DSG2 in DSG2 transgenic mice (tg-mice) and monkey DSG2 and GFP in DSG2 tg-mice. The expression of the housekeeping gene GAPDH was used as a reference. The quantitative PCR (qPCR) was run, in triplicate, using a SensiMix SYBR kit (Quantace, Taunton, MA) with a 7900HT fast real-time PCR system (Applied Biosystems/Life Technologies, Carlsbad, CA). The following primers were used: human DSG2 fw, 5′-ATG ACG GCT AGG AAC ACC AC-3′; DSG2 rev, 5′-TCA GGT ACA TTG GAA ACA TGA AA-3′; mouse GAPDH fw, 5′-CGT CCC GTA GAC AAA ATG GT-3′; mouse GAPDH rev, 5′-TCA ATG AAG GGG TCG TTG AT-3′; monkey DSG2 fw, 5′-TCA TGA GCC TTA CAG ACA CAC A-3′; monkey DSG2 rev, 5′-CTT CTC AGG ATT GTT TGC ATA GC-3′; monkey GAPDH fw, 5′-ATC CTG GGC TAC ACT GAG CA-3′; monkey GAPDH rev, 5′-TTG ATG GTA CAC GAC AAG GTG-3′; GFP fw, 5′-TAT ATC ATG GCC GAC AAG CA-3′; GFP rev, 5′-GAA CTC CAG CAG GAC CAT GT-3′.
ΔCT values were normalized for GAPDH expression, and the log eΔCT was used to compare DSG2 mRNA levels between tissues. The value for the lowest expression (skeletal muscle) was taken as 1.
Flow cytometry of blood cells.
Blood was collected in EDTA, and erythrocytes were lysed using PharmLyse (BD Biosciences) for 15 min at room temperature. The remaining cells were washed with 2% FBS/phosphate-buffered saline (PBS). Fc block was added, and the mixture was incubated at room temperature for 10 min. The antibodies listed below were added and incubated at room temperature in the dark for 30 min, followed by two washes with 2% FBS/PBS: FITC anti-mouse CD41 (no. 133903; BioLegend, SanDiego, CA), PE-Cy7 anti-mouse CD45 (no. 103113; BioLegend), FITC anti-human CD41 (no. 303703; BioLegend), and phycoerythrin (PE) anti-human CD45 (no. 557059; BD Biosciences, San Jose, CA). Mouse Fc block was from BD Biosciences. Human Fc block was from Miltenyi Biotech (Cambridge, MA).
Mouse cytokine array.
Tissues were homogenized in complete lysis buffer M (Roche, San Francisco, CA) using TissueRuptor (Qiagen). The total protein concentration was determined using the protein assay reagent from Bio-Rad, Hercules, CA. For each sample, 100 μg was assayed and the cytokine array panel A (R&D Systems, Minneapolis, MN) was used according to the manufacturer's suggestions. For the development of the assay, ECL Plus (GE Healthcare) was used together with Amersham Hyperfilm ECL (GE Healthcare). The developed films were scanned and intensities were quantified with SigmaGel (Jandel Scientific, San Rafael, CA).
Permeability assay.
A total of 5 × 105 MMC-hDSG2 cells were seeded in 12-mm transwell inserts (0.4-μm-pore-size polyester [PET] membrane; Corning, NY) and cultured for >20 days until transepithelial resistance was stable. Culture medium was changed every 2 to 3 days. The cells were exposed to hDSG2 ligands (20 μg/ml) in adhesion medium (DMEM, 1% FBS, 2 mM MgCl2, 20 mM HEPES) for 15 min at room temperature. Next, 1 mCi of [14C]polyethylene glycol-4000 (PEG-4000); (Perkin Elmer, Covina, CA) diluted with DMEM/K12 medium was added into the inner chamber. Medium aliquots were harvested from the inner and outer chamber at 15 and 30 min and measured by a scintillation counter. Permeability was calculated as described elsewhere (91).
Statistical analysis.
All results are expressed as mean ± standard deviation (SD). Student's t test or 2-way analysis of variance (ANOVA) for multiple testing was applied when applicable. A P value of <0.05 was considered significant.
RESULTS
DSG2 expression in human tissues.
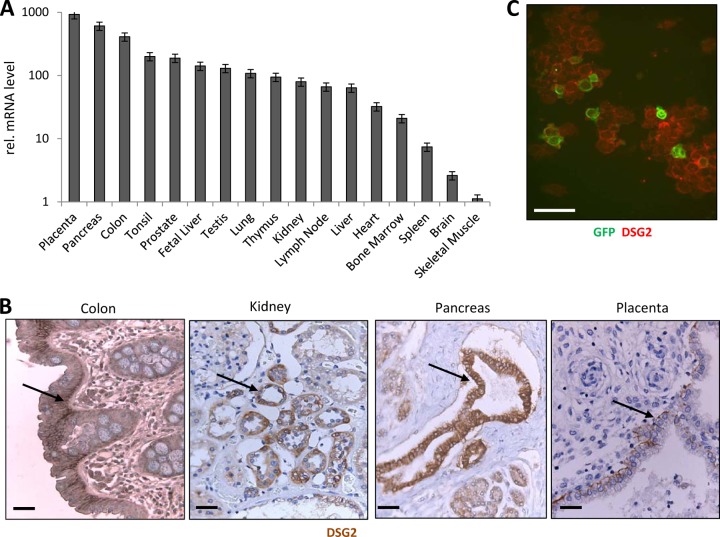
Our goal was to create hDSG2-transgenic mice that can serve as a model to study HAdV-B3, -B7, -B11, and -B14 tropism and, potentially, virus-associated pathology subsequent to various routes of infection. In order to function as a predictive model for infection in humans, these mice should express human DSG2 in a pattern and at a level comparable to humans. To allow for a direct comparison, we performed a side-by-side comparison of hDSG2 mRNA expression and immunoreactivity in tissues of transgenic mice and humans. For analysis of DSG2 mRNA in human tissues, we employed quantitative PCR (qPCR) on commercially available multitissue cDNA panels. Figure 1A shows DSG2 mRNA levels in tissues expressed relative to the level in skeletal muscle, i.e., the tissue that shows the lowest DSG2 mRNA level. The data on DSG2 mRNA isolated from whole tissues have to be interpreted in the context of DSG2 immunohistological analyses of the corresponding tissue sections (Fig. 1B). As expected, high DSG2 mRNA levels were found in the GI and kidney/urinary tracts, where DSG2 is localized to the columnar epithelium of the intestinal mucosa or the kidney tubules, respectively. The high levels of DSG2 mRNA in the pancreas can be explained by the presence of DSG2 in pancreatic duct epithelial cells (Fig. 1B). DSG2 is also readily detectable in intrahepatic bile duct epithelium or ductules in the testis (data not shown). It is also not surprising to see high DSG2 mRNA levels in the placenta, where DSG2 is localized to the trophoblastic cells (Fig. 1B). High DSG2 mRNA levels in lymphatic organs are in agreement with published data (63). Interestingly, we also found high DSG2 mRNA levels in human bone marrow. Specifically, DSG2 expression was detected on nearly 100% of human CD34+ hematopoietic stem cells (Fig. 1C; see Fig. S1A in the supplemental material). As we have a long-standing interest in gene transfer into stem cells, this finding prompted us to test whether the CD34+ cells can be infected ex vivo with Ad3-GFP. Exposure of CD34+ cells to Ad3-GFP at an MOI of 150 PFU/cell resulted in a transduction of 49.2% of cells as analyzed by flow cytometry for GFP (see Fig. S1B in the supplemental material). Considering the presence of DSG2 on hematopoietic stem cells, we also performed flow cytometry studies on peripheral blood cells. In agreement with earlier findings (88), we detected DSG2 on 21.5 ± 5% of platelets (marked by CD41). Five percent of CD45+ leukocytes were positive for DSG2. As discussed before, DSG2 can potentially be shed into the blood circulation (42). We therefore measured the concentration of DSG2 in human serum by ELISA. We found 632 ± 180 ng/ml DSG2 in human serum (n = 5). Western blot analysis confirmed that shed DSG2 represents the extracellular domain of DSG2 or fragments thereof (data not shown).
Fig 1.
DSG2 expression in human tissues. (A) DSG2 mRNA expression. qRT-PCR was performed on MTC cDNA panels from Clontech. These panels are fully normalized to the mRNA expression levels of four housekeeping genes (α-tubulin, β-actin, GAPDH, and phospholipase A2). Shown are relative DSG2 mRNA levels. DSG2 PCR signal intensity of different tissues was compared to that of skeletal muscle (the tissue with the lowest DSG2 expression level). n = 3 (technical replicates). (B) DSG2 immunohistochemistry of human tissue paraffin sections. DSG2 appears in brown. Sections are counterstained with hematoxylin. Representative sections are shown. Scale bars, 20 μm. DSG2 signals in epithelial layers are marked by arrows. (C) Immunofluorescence analysis of GFP (green) and DSG2 (red) of human CD34+ cells. Scale bar, 20 μm.
DSG2 expression in monkeys.
Next, we assessed whether nonhuman primates can serve as a potential model for HAdV-B3 infection. We demonstrated that unlike rodent DSG2, monkey DSG2 is utilized by Ad3 for infection (Fig. 2A and B). African green monkey (Vero) cells can be efficiently transduced by Ad3-GFP, and this transduction is monkey DSG2 mediated. Monkey DSG2-specific siRNA-mediated knockdown of monkey DSG2 expression decreases the transduction efficiency of Ad3-GFP but not that of the CD46-interacting Ad5/35-GFP or CAR-interacting Ad5-GFP vector (Fig. 2A). The hDSG2 ligand JO-1 specifically and efficiently blocked Ad3-GFP infection of Vero cells (Fig. 2B) and increased transepithelial permeability in polarized Vero cell monolayers (data not shown).
Fig 2.
Ad3 transduction of monkey cells and DSG2 expression in tissues of Macaca fascicularis. (A and B) Transduction studies in green monkey Vero cells. (A) siRNA knockdown of monkey DSG2 expression. Vero cells were transfected with monkey DSG2-siRNA or control siRNA and, 48 h later, infected with Ad3-GFP, Ad5/35-GFP, or Ad5-GFP vector. GFP expression was analyzed 18 h after infection. (B) Vero cells were incubated at increasing concentrations of the DSG2 ligands JO-1 or Ad3 penton-dodecahedra (PtDd) for 1 h and then infected with 100 PFU/cell of Ad3-GFP or Ad5-GFP virus. Shown is the percentage of GFP-expressing cells measured 18 h after infection. (C) Monkey DSG2 mRNA expression. Total RNA was isolated and reverse transcribed, and qRT-PCR was performed. Shown are relative DSG2 mRNA levels. ΔCT values were normalized for GAPDH expression, and the log eΔCT was used to compare DSG2 mRNA levels between tissues, in which the value for skeletal muscle was taken as 1. n = 3 (technical replicates). (D) Monkey DSG2 immunofluorescence analysis of macaque tissue. The colon section is also costained for E-cadherin. Representative sections are shown. Scale bar, 20 μm.
qRT-PCR for monkey DSG2 mRNA was performed on total RNA isolated from Macaca fascicularis tissues. Both frozen and paraffin sections were analyzed for DSG2 immunoreactivity. Overall, the pattern of mRNA expression was similar to that seen in humans (Fig. 2C). The use of frozen tissues sections enabled us to perform costaining of monkey DSG2 and the epithelial junction protein E-cadherin (Fig. 2D). Selected images show the localization of DSG2 in epithelial junctions of the GI and respiratory tracts, as well as in intrahepatic bile ducts and prostatic acini. In addition, we demonstrated the presence of DSG2 in lung and cornea epithelium (see Fig. S2A in the supplemental material) and in salivary glands, pancreatic ducts, and dermal sweat glands (see Fig. S2B in the supplemental material). DSG2 was also detected in intercalated discs of myocardiac tissue (see Fig. S2C in the supplemental material). In the brain, DSG2 was observed in the choroid plexus (see Fig. S2D in the supplemental material) but not in endothelial junctions forming the blood-brain barrier (Fig. S2D, right panel). In agreement with human studies, we found DSG2 expression on ∼80% of macaque bone marrow cells and on 27.7 ± 6% of platelets. The fraction of DSG2+ leukocytes (CD45+) was 25.9 ± 8% and therefore higher than in humans (data not shown).
Overall, our analyses of monkey tissue suggest that nonhuman primates express DSG2 in a pattern similar to that of humans and therefore may be a useful model for infection studies with DSG2-targeting Ads.
Generation and characterization of human DSG2 transgenic mice.
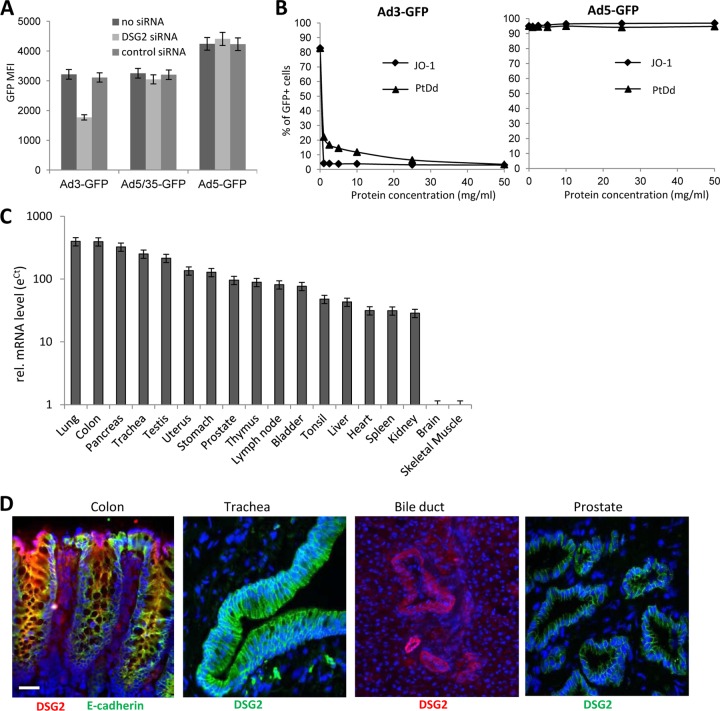
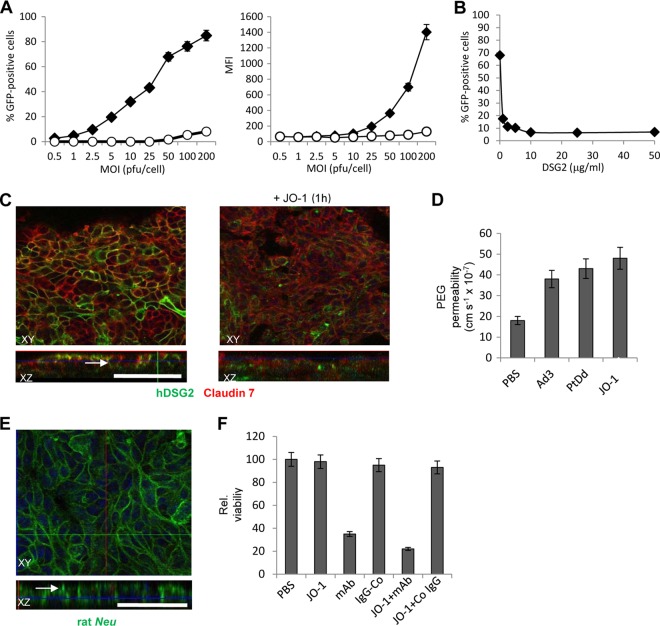
Clearly, a small-animal model would greatly facilitate studying DSG2-interacting HAdVs, as well as their derivatives, e.g., JO-1. To test whether human DSG2 can interact with cell junction proteins and regulate epithelial junctions in mouse cells, we created a mouse mammary cancer (MMC) cell line (49), which ectopically expressed human DSG2 after lentivirus transduction. We called this cell line MMC-hDSG2. Ectopic hDSG2 expression conferred infectibility by Ad3-GFP (Fig. 3A), whereby Ad3-GFP transduction could be blocked by recombinant soluble hDSG2 (Fig. 3B). For further studies, MMC-hDSG2 cells were cultured under conditions that allowed for cell polarization. Studies of these cells were initiated once the transepithelial resistance was constant. Exposure of such MMC-hDSG2 cells to the hDSG2-ligand, JO-1, resulted in the partial removal of hDSG2 from epithelial junctions and in a partial dissolution of the junctions (Fig. 3C). The finding that Ad3, PtDd, or JO-1 treatment of polarized MMC-hDSG2 cells cultured in transwell chambers increased the transepithelial transport of [14C]PEG (molecular weight [MW], 4,000) (Fig. 3D) supports the opening of junctions by JO-1. In control MMC cells (without hDSG2), Ad3 incubation did not increase the transepithelial transport compared to PBS-treated cells (data not shown). The effect of JO-1 on MMC-hDSG2 cells is comparable to that seen on human cells (5, 19, 87).
Fig 3.
Function of human DSG2 in mouse mammary carcinoma cells expressing human DSG2 after lentivirus gene transfer (MMC-hDSG2 cells). (A) Transduction of MMC cells (empty circles) and MMC-hDSG2 cells (black diamonds) by Ad3-GFP at increasing MOIs. The percentage of GFP-expressing cells and mean fluorescence intensity (MFI) were analyzed 18 h after infection. n = 3. (B) Competition of Ad3-GFP infection by recombinant, soluble hDSG2. MMC-hDSG2 cells were incubated with increasing concentrations of hDSG2 for 60 min before being infected with Ad3-GFP vectors at an MOI of 100 PFU/cell for 60 min, after which the viruses were removed and new medium was added. GFP fluorescence was measured 18 h later. n = 3. Shown are average values. The standard deviation was less than 10% for all samples. (C) Confocal immunofluorescence microscopy of MMC-hDSG2 cells before and 12 h after exposure to 0.5 μg/ml of JO-1. Scale bar, 40 μm. The white arrow in the XZ panel indicates an area with junction-localized DSG2. (D) 14C-PEG-4000 diffusion through monolayers of MMC-hDSG2 cells at 30 min after adding inactivated Ad3 virions (Ad3) (2 × 108 particles/ml), Ad3 penton-dodecahedra (PtDd) (0.5 μg/ml), or JO-1 (0.5 μg/ml). n = 5. (E) Confocal immunofluorescence microscopy of MMC-hDSG2 cells. Rat Neu (green) appears to be trapped in junctions (see white arrow). (F) JO-1 enhances killing of MMC-hDSG2 cells by the Neu-specific monoclonal antibody 7.16.4-MAb. MMC-hDSG2 cells were incubated with 0.5 μg/ml JO-1 or PBS followed by 7.16.4-MAb (mAb) or control antibody (IgG-Co) 12 h later. Viability was measured 4 days later. Viability of PBS treated cells was taken as 100%, n = 5. P < 0.05 for mAb versus JO-1+MAb.
MMC cells express rat Neu (49). As seen in human breast cancer samples, rat Neu in MMC-hDSG2 cells appears to be trapped in the epithelial junctions (Fig. 3E). JO-1 incubation of MMC-hDSG2 cells increases complement-dependent killing by a rat Neu-specific monoclonal antibody (7.16.4-MAb) (17, 38), most likely as a result of improved access to Neu (Fig. 3F). These data suggest that human DSG2 in transgenic mice functions as a junction protein in a way similar to that in human cells.
To confer tissue-specific human DSG2 expression in transgenic mice, we used the complete (∼90-kb) human DSG2 locus, including its endogenous regulatory elements (Fig. 4A). The locus was released from a bacterial artificial chromosome by NotI digestion, and the corresponding fragment was gel purified and injected into the pronuclei of 40 mouse embryos (strain B6C3, i.e., C57BL/6 × C3H). Fourteen pups were born, from which two (females) were found to be positive for human DSG2 by genotyping of tail DNA. Expression of human DSG2 in skin epidermis was confirmed by immunohistochemistry on tail and ear sections. One of the transgenic mice (no. 187) developed normally; the other animal (no. 189) was significantly smaller and blind (as evidenced by the presence of cataracts) and had apparent defects in skin elasticity (see Fig. S3A in the supplemental material). Tail skin fibroblasts from both animals were cultured and metaphase chromosomes were subjected to DNA fluorescence in situ hybridization using the human DSG2 locus as a probe (Fig. 4B; see Fig. S3B in the supplemental material). For mouse no. 187, we found the hDSG2 signal associated with one of the 40 total chromosomes, suggesting one integration site (Fig. 4B). Mouse no. 189 displayed at least two signals on different chromosomes. Our future analyses focused on founder no. 187, as animal no. 189 did not produce offspring. We employed an inverse PCR approach on genomic DNA isolated from fibroblasts to map the transgene/mouse genome junctions in founder no. 187 (the offspring of which we called hDSG2-transgenic mice). The transgene was inserted into position 81491601 of mouse chromosome 16 (Fig. 4C). The insertion site is located within the second intron of the neural cell adhesion molecule 2 (NCAM2) gene. Previous studies have shown that knockout of this gene has no critical side effects (7). These studies also detected the presence of a head-to-tail concatemer. Consistent with this, we measured two transgene copies per genome by hDSG2 qPCR on genomic DNA using a standard curve based on the human DSG2 BAC.
Fig 4.
Characterization of hDSG2-transgenic mice. (A) The bacterial artificial chromosome (BAC) (Invitrogen CTD-223319) used for generation of transgenic mice contains an 89.17-kb fragment of human chromosome 18 from position 29054153 to 29143320. The fragment contains the 24-kb DSG2 promoter and 5′untranslated regions (UTR), the 50.6-kb DSG2 gene, and 14.5 kb of the 3′UTR. (B) Analysis of mouse strain 187. Fluorescence in situ hybridization for hDSG2 on fibroblasts from hDSG2-transgenic mice. Cultures of tail fibroblasts were treated with colcemid, and metaphase chromosomes were hybridized with the hDSG2 BAC labeled with Cy3. Positive signals appear as green “snake eyes” (see arrow). (C) Integration site analysis of mouse strain 187. Genomic DNA from tail fibroblasts was subjected to inverse PCR analysis, and transgene/chromosome junctions were sequenced. The human DSG2 locus is inserted as a head-to-tail tandem into mouse chromosome 16 position 81491601 without chromosomal rearrangements. The insertion site is within the second intron of the mouse Ncam2 (neural cell adhesion molecule 2) gene. The presence of two transgene copies per diploid genome was confirmed by qPCR for the human DSG2 gene.
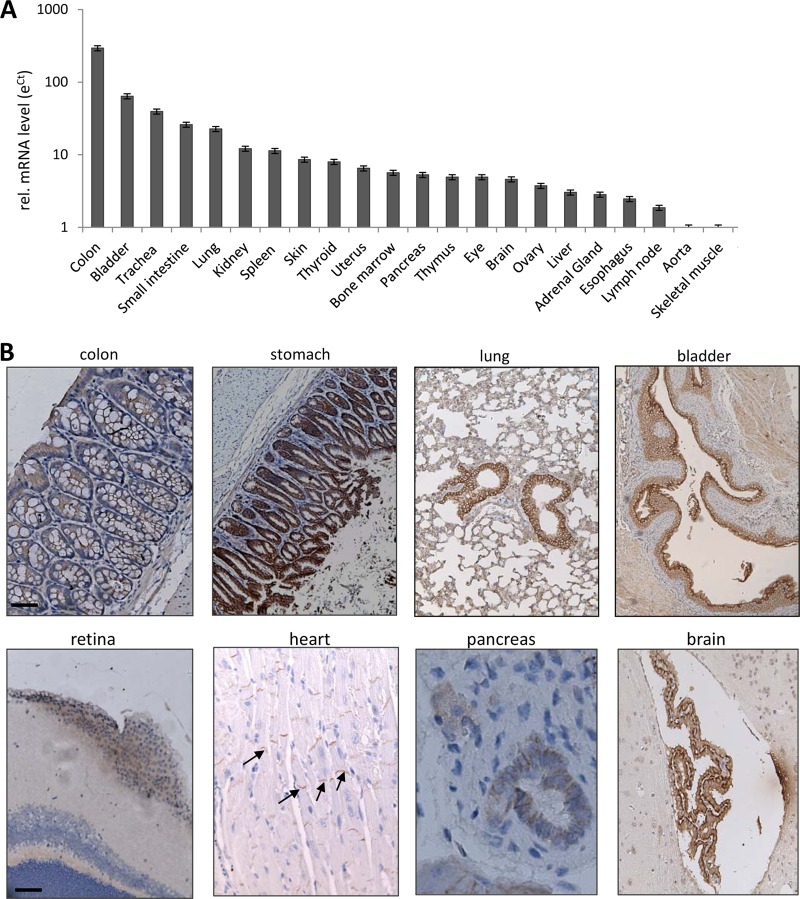
Human DSG2 mRNA in tissues of hDSG2-transgenic mice was measured by qRT-PCR, and mRNA levels were compared to hDSG2 mRNA levels in skeletal muscle, again, as in the human and Macaca fascicularis samples, the tissue with the lowest hDSG2 expression. Both the distribution and level of hDSG2 mRNA expression in transgenic mice were similar to those in humans and nonhuman primates (Fig. 5A). In immunohistochemistry analysis, marked hDSG2 signal expression was seen in the GI, respiratory, and urinary tract epithelium. hDSG2 was found in the intercalated discs of the myocardium, in pancreatic ducts, and in the choroid plexus of the brain (Fig. 5B). Flow cytometry studies of blood cells from hDSG2-transgenic mice revealed that 11.6 ± 1.8% platelets were hDSG2 positive; 46 ± 12% of leukocytes stained positive for hDSG2 (see Fig. S4 in the supplemental material). The concentration of hDSG2 in serum of hDSG2-transgenic mice was 1,230 ± 190 ng/ml. hDSG2 was not detectable in serum and on blood cells of nontransgenic littermates. In summary, these data demonstrate that hDSG2-transgenic mice are an adequate model to study HAdV-B3 tropism. Notably, biodistribution of mouse DSG2 has not been studied so far, in part because of the lack of mouse DSG2-specific antibodies.
Fig 5.
hDSG2 expression in tissues of hDSG2-transgenic mice. (A) hDSG2 mRNA expression. Total RNA was isolated and reverse transcribed, and qRT-PCR was performed. Shown are relative hDSG2 mRNA levels. The eΔCT value of skeletal muscle was taken as 1. n = 3 (different animals). Notably, hDSG2 mRNA background signals in nontransgenic littermates were below the levels in skeletal muscle of hDSG2-transgenic mice. (B) hDSG2 immunohistochemistry analysis of paraffin sections. hDSG2 appears in brown. Sections are counterstained with hematoxylin. Representative sections are shown. Scale bars, 20 μm. Examples of hDSG2 staining in intercalated discs in the myocardium are labeled by arrows.
hDSG2-dependent Ad3 infection of airway epithelium after intranasal application of Ad3-GFP.
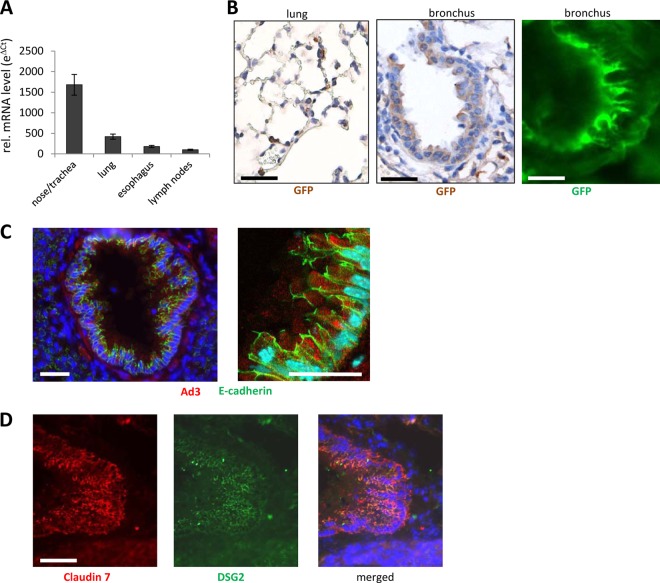
In the majority of HAdV-B3 infections, the virus enters the body through the mouth or nose. We therefore studied GFP expression after intranasal application of Ad3-GFP into hDSG2-transgenic mice and nontransgenic littermates. Major tissues were analyzed 3 days after Ad3-GFP administration for GFP mRNA by qRT-PCR and GFP immunoreactivity. (Due to high autofluorescence in many tissues, it is difficult to assess transduction based on GFP fluorescence). No GFP expression was detected in tissues of nontransgenic littermates. In hDSG2-transgenic mice, GFP mRNA signals were found in the respiratory tract and lung, as well as in the esophagus and bronchial lymph nodes (Fig. 6A). mRNA data were confirmed by GFP staining of tissue sections. GFP was found in epithelial cells of bronchi and bronchioli as well as in alveolar epithelial cells (Fig. 6B). When counted, approximately 25% of alveolar cells and 58% of bronchial epithelial cells were GFP positive. GFP signals were also found in the mucosal epithelium cells of the esophagus, indicating that some of the intranasally applied virus had been swallowed (data not shown). To consolidate these findings, we harvested lung tissue 2 h after intranasal Ad3-GFP application and stained viral particles using antibodies against the Ad3 fiber knob. At this time point, Ad3 particles were readily detectable in airway epithelial cells of hDSG2-transgenic mice (Fig. 6C). hDSG2 immunoreactivity in relation to the junction marker claudin 7 in airway epithelial cells is shown in Fig. 6D. No colocalization of Ad3 signals with markers for leukocytes, macrophages, or endothelial cells was found (see Fig. S5 in the supplemental material).
Fig 6.
GFP expression after intranasal application of Ad3-GFP into hDSG2-transgenic mice. hDSG2-transgenic mice or nontransgenic littermates were intranasally injected with 5 × 108 PFU of Ad3-GFP. Tissues were harvested 3 days later. (A) GFP mRNA expression. Total RNA was isolated and reverse transcribed, and qRT-PCR was performed. Shown are relative GFP mRNA levels. ΔCT values were normalized for GAPDH expression, and eΔCT was used to compare GFP mRNA levels between tissues. Only tissues with clearly detectable GFP levels (i.e., levels above those in mock-transduced animals) are shown. (B) GFP immunoreactivity analysis of paraffin sections of tissues harvested at day 3 after Ad3-GFP application. Left and middle panels: GFP appears in brown. Sections are counterstained with hematoxylin. Right panel: GFP was visualized with a FITC-conjugated antibody and appears in green. (C) Confocal immunofluorescence analysis of airway tract sections from hDSG2-positive mice 2 h after intranasal Ad3-GFP injection. Ad3 appears in red, E-cadherin in green. Shown is a bronchus. (D) Immunofluorescence analysis of airway tract sections from hDSG2-positive mice 2 h after intranasal Ad3-GFP application. The junction protein claudin 7 appears in red, hDSG2 in green. Scale bars, 20 μm.
The finding that Ad3-GFP transduces lung epithelial cells after intranasal administration elucidates how Ad3 enters the human body. While HAdV-B3 replicates in human cells and eventually enters the blood circulation, murine cells do not support de novo production of HAdVs and therefore no viremia is expected in hDSG2-transgenic mice to which Ad3-GFP was applied intranasally. In support of this, qPCR for viral genomes performed on serum at day 3 and day 14 after viral application did not detect circulating Ad3-GFP.
hDSG2-dependent Ad3 infection of intestinal epithelium after intravenous injection of Ad3-GFP.
To simulate viremia, we injected Ad3-GFP intravenously at different doses into hDSG2-transgenic and nontransgenic mice. Three days after injection, GFP in vivo imaging was performed (Fig. 7A). Although this technique has low sensitivity and resolution, significantly more GFP signals (based on radiant efficiency), particularly in the abdomen, can be observed in Ad3-GFP-injected hDSG2-transgenic mice than in nontransgenic mice, indicating hDSG2-dependent transduction by Ad3-GFP (P < 0.01). Analysis of mRNA revealed GFP expression in the colon and small intestine of hDSG2-transgenic mice but not in nontransgenic littermates (Fig. 7B). There was no GFP mRNA expression detected in the stomach or esophagus. GFP mRNA was detected in the spleen at about 100-fold-lower levels than those found in the colon. hDSG2-transgenic and nontransgenic littermates had comparable rates of GFP mRNA expression, indicating hDSG2-independent Ad3-GFP transduction in the spleen.
Fig 7.
GFP expression after intravenous injection of Ad3-GFP. (A to C) hDSG2-transgenic mice (hDSG2-pos) or nontransgenic littermates (hDSG2-neg) were intravenously injected with 1 × 109 or 2 × 109 PFU of Ad3-GFP. (A) Three days later, GFP fluorescence was analyzed by in vivo imaging using an IVIS Spectrum machine (Xenogen Corporation/Caliper Life Sciences, Inc.). The numbers below the images show the radiant efficiency in the circled region of interest (n = 3). Note that background signals from skin areas without fur (tail, feet, nose) are common in GFP in vivo imaging. (B) Tissues (colon, bladder, trachea, small intestine, lung, kidney, spleen, skin, thyroid, uterus, bone marrow, pancreas, thymus, eye, brain, ovary, liver, adrenal gland, esophagus, lymph node, aorta, heart, and skeletal muscle) were harvested. For mice injected with 2 × 109 Ad3-GFP, GFP mRNA, in comparison to GAPDH mRNA, was measured by qRT-PCR (three hDSG2-pos and three hDSG2-neg animals). Detectable GFP RNA was only found in the colon and small intestine of hDSG2-positive mice and in the spleen of both hDSG2-neg and hDSG2-pos mice. (C) Sections of all the tissues listed above were stained for GFP. Positive staining (marked by arrows) was found in epithelial cells of the GI tract in hDSG2-pos mice but not in hDSG2-neg mice. Representative sections are shown. Scale bars, 20 μm.
Immunohistochemical analysis of GI tract tissue sections showed GFP-positive cells in the mucosal epithelium of the small intestine and colon of hDSG2-transgenic mice (Fig. 7C). In the spleen, sparse GFP-positive cells were found in the marginal zone of the germinal centers (see Fig. S6 in the supplemental material). When counted in 20 independent tissue sections, 95 ± 18 and 4.2 ± 0.8 GFP-positive cells per cm2 tissue were present in the colon and spleen, respectively.
Considering the presence of hDSG2 on platelets, we performed transmission electron microscopy (TEM) analysis of a platelet fraction in hDSG2-transgenic mice collected 1 h after Ad3-GFP injection. TEM images clearly show electron-dense icosahedral Ad particles bound to platelets (see Fig. S7 in the supplemental material). Association of Ad3 with blood cells might have implications in viremic spread.
Interaction of Ad3-GFP with intestinal epithelial cells.
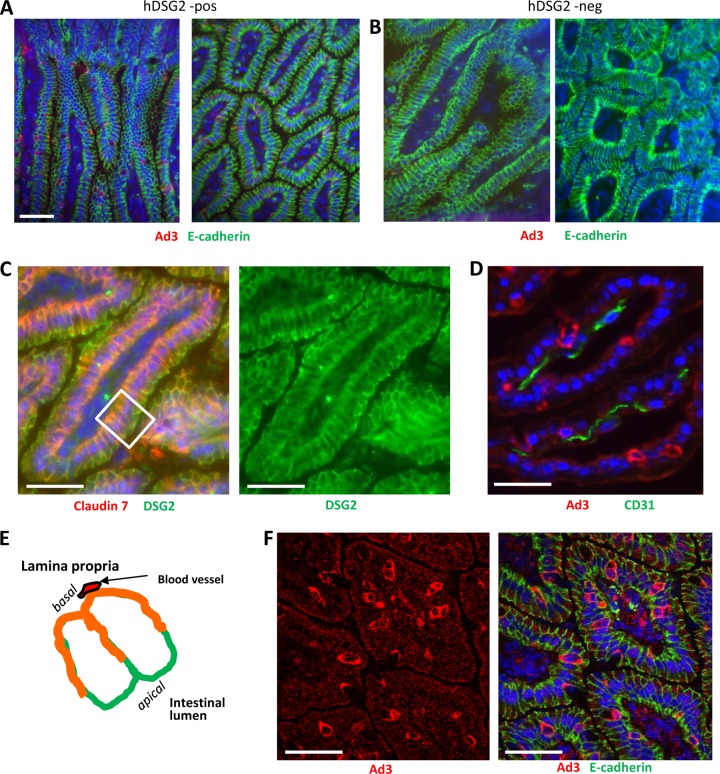
At 2 h after injection, Ad3-GFP particles were detectable by immunofluorescence microscopy in the epithelial cells of the colon and intestinal mucosa in all hDSG2-transgenic mice injected with Ad3-GFP (n = 5) (Fig. 8A). Ad3 signals were absent in nontransgenic mice that received Ad3-GFP intravenously (n = 5) (Fig. 8B). hDSG2 dependence of Ad3-GFP transduction is supported by the fact that intravenous preinjection of recombinant hDSG2 protein 15 min before Ad3-GFP injection reduced about 3-fold Ad3 signals in intestinal epithelial cells (see Fig. S8 in the supplemental material). An important question is how intravenously injected Ad3 reaches hDSG2 in polarized epithelial cells. On intestinal sections, hDSG2 is found on both apical and basolateral sides of polarized epithelial cells (Fig. 8C and D). This could imply that hDSG2 is accessible to intravenously injected Ad3, which reaches the epithelial cells from the basal side (see Fig. 8E). It also appears that the fast turnover of epithelial cells facilitates Ad3-GFP transduction, for example by transiently liberating hDSG2 trapped in adherens junctions and making it accessible to virus. About one-fifth of Ad3-positive signals were found in pairs of cells (Fig. 8F). Clearly, further studies, including electron microscopy studies, are required to investigate the details of Ad3-hDSG2 interaction in vivo.
Fig 8.
Uptake of Ad3-GFP by intestinal epithelial cells after intravenous injection. Ad3-GFP (1 × 109 PFU/mouse) was injected into the tail vein of hDSG2-transgenic mice (hDSG2-pos) and nontransgenic littermates (hDSG2 neg). Two hours later, intestines were harvested and analyzed. (A and B) Intestine sections of hDSG2-positive (A) and hDSG2-negative (B) mice stained for Ad3 fiber knob (red) and E-cadherin. (C) Localization of hDSG2 (green) with regard to epithelial junctions. Junctions are marked by claudin 7 (red). Overlapping colors appear in orange. (D) Staining for Ad3-GFP (red) and blood vessels (marked by the endothelial cell marker CD31) (green). The scale bars are 20 μm. (E) Schematic representation of an intestinal villus (transverse section). Shown are two epithelial cells. The basal side faces the lamina propria, containing blood vessels. The apical side faces the intestinal lumen. hDSG2 is localized on all lateral membranes. Membrane areas that contain the adherens junction protein claudin 7 appear in orange. (F) Intestine section from hDSG2-positive mice injected with Ad3-GFP. Ad3-GFP appears in red; E-cadherin appears in green.
Pathophysiological link between Ad3 infection and GI tract symptoms.
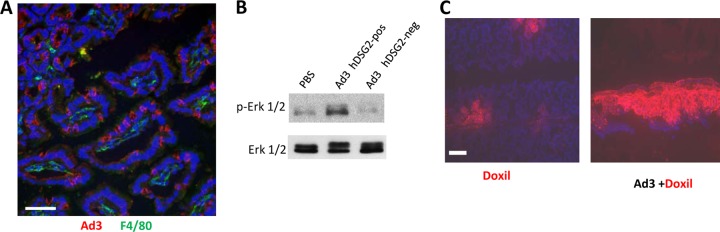
Within 16 h after intravenous Ad3-GFP injection, hDSG2-transgenic mice, but not hDSG2-negative littermates, developed a mild diarrhea which subsided by 36 h after Ad injection. A similar effect had been seen before with the hDSG2 ligand JO-1 (5). We attempted to delineate the mechanism behind the apparent effect of Ad3 on the GI tract. At 2 h postinfection (p.i.), no Ad3 signals were found associated with intestinal macrophages of hDSG2-transgenic mice (Fig. 9A). This suggests that other cells in the mucosa are involved in Ad3 intestinal pathology, including epithelial cells which take up Ad3. We recently reported that Ad3 binding to DSG2 of epithelial cells triggered intracellular signaling, including pathways that are involved in EMT, and eventually led to transient opening of epithelial junctions (88). Among the in vivo features that characterize EMT is the activation of Erk1/2 (MAPK) (81). When intestine lysates were analyzed by Western blotting, we found more phosphorylated Erk1/2 in hDSG2-transgenic mice that received Ad3-GFP (Fig. 9B.) This suggests that Ad3 triggers the opening of junctions in intestinal epithelial cells shortly after intravenous injection. This is indirectly supported by our observation that penetration and dissemination of Doxil, a polyethylene glycol-coated, liposome-encapsulated form of doxorubicin with a diameter of ∼90 nm, is increased upon Ad3-GFP injection (Fig. 9C). Transient changes in intestinal epithelium barrier integrity are also suggested by cytokine analyses. At 2 h after Ad3-GFP injection, levels of key proinflammatory cyto/chemokines interleukin-1α (IL-1α), IL-1β, IL-2, IL-27, MIG/CXCL9, MIP-1α/CCL3, and tumor necrosis alpha (TNF-α) were significantly (P < 0.05) higher in intestine lysates of hDSG2-transgenic mice than in those of hDSG2-negative mice (Fig. 10A). Levels of the anti-inflammatory cytokine IL-10 were lower in hDSG2-transgenic mice. Of particular interest is the elevation of IL-1β, TNF-α, and MIP-1α expression, which is usually activated through Toll-like receptors (TLRs), specifically TLR2 and TLR6. TLR2 and TLR6 are in turn activated in epithelial or dendritic cells/macrophages upon recognition of bacterial or mycoplasma lipoproteins (24). In this context, we found stronger TLR6 signals in intestinal epithelial cells of Ad3-GFP-injected hDSG2-transgenic mice than in control mice (Ad3-GFP-injected hDSG2-negative mice or mock-injected hDSG2 transgenics) (Fig. 10B). These findings support a potential etiological link between Ad3-GFP infection and diarrhea seen shortly after intravenous virus injection.
Fig 9.
Potential rearrangements in epithelial junctions. Ad3-GFP (1 × 109 PFU/mouse) was injected into the tail vein of hDSG2-transgenic mice (hDSG2-pos) and nontransgenic littermates (hDSG2 neg). Two hours later, intestine and colon were harvested and analyzed. (A) Intestine sections of hDSG2-positive mice were stained for Ad3-fiber knob and the macrophage marker F4/80. Representative sections are shown. (B) Western blot analysis of intestine tissues (pooled from three animals per group) of hDSG2-positive and hDSG2-negative mice for Erk1/2 and phosphorylated Erk1/2. (C) Penetration of liposomal doxorubicin/Doxil (diameter, ∼90 nm) in intestinal mucosa. Doxil (3 mg/kg) was intravenously injected alone or 1 h after Ad3-GFP injection (1 × 109 PFU). Tissues were analyzed 2 h after Doxil injection. Doxil was visualized using an anti-PEG antibody and appears in red. Scale bars, 20 μm.
Fig 10.
Activation of cytokines and TLR following intravenous Ad3-GFP. (A and B) Cytokine array. Small intestine and colon samples were combined and lysed. Lysates were analyzed for a set of 40 mouse cytokines and chemokines using the Proteome profiler array kit from R&D Systems. n = 3. (A) Representative image of dot blot from tissue of one mouse per group. The array includes positive controls (“pos.”) provided by the manufacturer. Cyto-/chemokines that significantly differ in their concentrations (P < 0.05) in both groups are highlighted in the upper dot blot. (B) Quantification of signals from all animals (n = 3). The blots were scanned and signal intensity was measured. MIG, CXCL9, MIP-1, CCL3. (Notably, as antibodies specific for each individual cytokine are used in this cytokine array, absolute levels for different cytokines cannot be compared. This implies that the levels of IL-1ra, a protein that inhibits IL-1α and -β activity, might be lower than those of IL-1α and IL-1β.) (C) Immunofluorescence analysis on intestine sections of hDSG2-positive mice for TLR6 and E-cadherin (left panel) and TLR6 and F4/80 (right panel). Scale bar, 20 μm. Representative sections are shown.
DISCUSSION
hDSG2-transgenic mice as a model for the study of Ad infection.
Widely distributed Ad serotypes HAdV-B3 and -B7 as well as newly emerged pathogenic strains of HAdV-B14 utilize DSG2 as a receptor for infection of human cells in vitro (88). HAdV-B3 does not bind to mouse and hamster cells (82, 88) and mouse cells are refractory to HAdV-B3 infection (Fig. 3A). Previous studies by various groups, including our own, have also shown that intravenous injection of HAdV-B3 vectors into wild-type, i.e., non-hDSG2-transgenic, mice did not yield significant transduction of tissues (31, 73). This indicates either that rodent DSG2 is not expressed in a pattern seen in humans or, more likely, that rodent DSG2 is not recognized by HAdV-B3. (The homology of mouse and hDSG2 is 77%.) Ectopic expression of hDSG2 in mouse epithelial cells (MMC-hDSG2) supports efficient transduction by Ad3-GFP. Furthermore, Ad3-hDSG2 interaction in MMC-hDSG2 cells triggered transient opening of epithelial junctions similar to what we observed in human epithelial cells, indicating that hDSG2 can interact with other mouse junction proteins. Taken together, this suggests that hDSG2-transgenic mice would be an adequate model to study (i) the tropism of HAdVs, vectors or proteins that use hDSG2 as a receptor, (ii) antiviral drugs or vaccines that interfere with Ad infection, and (iii) certain downstream effects of Ad3-hDSG2 interaction, including potential toxicity associated with transient epithelial junction opening. As human Ads do not replicate in mice (10), hDSG2-transgenic mice have limitations as a model to study Ad pathology, specifically effects of viral cell lysis and dissemination of de novo-produced virions in infected tissues via the blood circulation. The same limitations apply to nonhuman primates, which, as we show in this study, express DSG2 in a distribution similar to that of humans; however, they do not support human Ad replication (61). While Syrian hamsters (16) or cotton rats (25) can support HAdV-C5 replication to some degree, it remains to be investigated whether hDSG2-transgenic hamsters or rats will be adequate to study HAdV-B3 replication.
To characterize hDSG2-transgenic mice as a model for HAdV infection, we analyzed hDSG2 mRNA and hDSG2 immunoreactivity in direct comparison to humans and macaques. These studies showed similar patterns and levels of DSG2 expression in all three species. As expected, hDSG2 was found in epithelial layers of the GI, respiratory, and genitourinary tracts, as well as in epithelial ducts of major organs, including the pancreas. hDSG2 was also expressed in nonepithelial tissues, such as the heart and lymphoid tissues. We did not detect hDSG2 in endothelial junctions, e.g., in blood vessels of the brain. Of importance is the finding that hDSG2 is present at the surface of hematopoietic stem cells and a significant fraction of platelets and leukocytes. The presence of the Ad3 receptor (receptor X/DSG2) on hematopoietic stem cells is in agreement with our previous studies (82).
The discovery of DSG2 as the HAdV-B3 receptor and the delineation of its tissue distribution create a basis to explain clinical manifestations of HAdV-B3 infections in humans. The presence of hDSG2 in the airway and alveolar epithelium apparently confers Ad3-GFP infection of these tissues and therefore explains the respiratory symptoms of HAdV-B3 infection. We showed that intranasal Ad3-GFP application results in efficient GFP expression in bronchial and alveolar epithelial cells in hDSG2-transgenic mice. It is noteworthy that the initial infection by another serotype that causes respiratory symptoms, HAdV-C5, is relatively ineffective, most likely because the HAdV-C5 receptor, CAR, a tight junction protein, is not as accessible in airway epithelial cells as DSG2 (26, 60, 86). CAR is considered to be mostly involved in lateral HAdV-C5 spread (85, 86).
If the human immune system cannot control Ad infection, de novo-produced virions enter the bloodstream and infect other tissues. At this stage, Ad particles or genomes can be readily detected in serum (41). Our finding that DSG2 is present on platelets and that Ad3-GFP is associated with platelets after intravenous injection provides a potential additional mechanism of viremic spread. To model viremia, we injected Ad3-GFP intravenously in hDSG2-transgenic mice. We found clearly detectable transduction of intestinal and colon epithelial cells. This transduction was hDSG2 dependent. It could be decreased by intravenously preinjected recombinant hDSG2 protein and was absent in mice that did not express hDSG2. This, to our knowledge, is the first demonstration in mice that an intravenously injected Ad uses the receptor that has been identified in in vitro studies. For the other two main Ad attachment receptors, CAR and CD46, studies conducted by our laboratory and others have shown in mice that other mechanisms, such as binding to blood factors and receptor accessibility in tissues, appear to override the in vitro rules of Ad tropism (2, 68, 83). One of these pathways that has been described to mediate liver transduction involves the interaction of Ad5 hexon with coagulation factor X (83). As we did not see detectable hepatic GFP expression in our studies in transgenic or nontransgenic mice, it is unlikely that this pathway has a major impact on in vivo tropism of HAdV-B3. Although surface plasmon resonance studies showed that HAdV-B3 can bind to recombinant human factor X (83), it has not been demonstrated that this mechanism confers HAdV-B3 liver transduction in mice. Moreover, if HAdV-C5 vectors contain short Ad3 fibers and the Ad3 fiber knob, they are unable to transduce hepatocytes after intravenous injection into mice or monkeys (40, 53, 54). This indicates that the length of the fiber shaft can affect interaction between HAdV-C5 and blood factors. Notably, in an earlier study with CD46-transgenic mice, we detected Ad3 genomes in the liver at 6 h after intravenous Ad3 injection (73). In the present study, performed at day 3 after Ad3-GFP injection into DSG2-transgenic mice, we did not find detectable transgene (mRNA and protein) expression in the liver. We speculate that at 6 h after Ad3 injection, viral genomes are present in Kupffer cells, which subsequently die, resulting in the loss of hepatic viral genomes. The latter scenario has been described for Ad5 vectors (72).
The spleen was the other tissue that showed detectable GFP expression after intravenous Ad3-GFP injection. Sparse GFP-positive cells were found in the marginal zone surrounding germinal centers in both hDSG2-positive and hDSG2-negative mice. This implies that transduction of these cells is DSG2 independent. Receptor-independent transduction of splenic cells with the same tissue localization has been reported before, e.g., in baboons and mice that received intravenous injection of Ad5/35 vectors (53, 54).
Notably, while hDSG2 is present on hematopoietic cells, we did not observe GFP-positive cells in the bone marrow of hDSG2-positive mice after intravenous Ad3-GFP injection. We speculate that Ad3-GFP egress from bone marrow blood vessels is inefficient and that bone marrow cells are not accessible to intravenously injected Ad vectors.
GI symptoms associated with Ad3 infection.
Our finding that Ad3-GFP can transduce GI epithelium after intravenous injection could provide a potential etiologic link to acute gastroenteritis that is found in HAdV-B3-infected patients. Although viral gastroenteritis has been routinely associated with “enteric” HAdV serotypes HAdV-F40 and HAdV-F41/G52, our findings in hDSG2-transgenic mice make HAdV-B3, and potentially other hDSG2-interacting HAdVs, a potential cause for acute gastroenteritis. Notably, as the vast majority of adult humans have neutralizing anti-HAdV-B3 and -B7 antibodies (62, 75), GI symptoms might be less pronounced in adults than in children. The following reports support an etiological link between HAdV-hDSG2 infection and gastroenteritis in children. (i) There are independent reports from different Asian countries that HAdV-B3 and -B7 infection in children is associated with GI symptoms, specifically diarrhea. In a 2004-2005 outbreak of HAdV-B3a in Taiwan (338 analyzed samples), 50% of children presented with GI discomfort, in which the symptoms were more severe in younger children (10). In a 2001-2002 HAdV-B3 outbreak in Taiwan (317 samples with adenovirus), 18.9% of children had abdominal pain, 21.8% vomiting, and 25.2% diarrhea (11). Independent studies in China reported that about 20% of children had diarrhea in three independent outbreaks, in a 2007 HAdV-B3 outbreak (97 patients) (56), in a 2004 HAdV-B7 outbreak (127 patients) (47), and in a 2004 HAdV-B3/7 outbreak (709 patients) (76). (ii) Studies of 3,577 fecal specimens from infants and children with acute gastroenteritis in Japan, South Korea, and Vietnam during 1998 to 2001 detected HAdV-B3 and -B7 DNA in up to 30% of samples (45). (iii) After cough and shortness of breath, the main symptoms associated with HAdV-B14p1 infection in a recent community outbreak in Oregon were vomiting and diarrhea (44). (iv) In cancer gene therapy studies, after intravenous infusion of oncolytic Ad3 vectors into cancer patients, diarrhea was seen in 5 out of 25 patients and nausea/vomiting in 13 out of 25 patients (30; Akseli Hemminki, personal communication). (v) HAdV DNA (without serotype specification) was readily detectable in a metagenomic analysis of acute diarrhea (21).
Based on our preliminary studies shown in Fig. 9 and 10, we propose the following possible scenario for the development of transient diarrhea after intravenous Ad3-GFP injection in hDSG2-transgenic mice. Ad3-GFP egress from blood capillaries in the small intestine and colon results in the interaction with hDSG2 on mucosal epithelial cells, which appears to be accessible to Ad3. Binding of Ad3-GFP to hDSG2, in turn, triggers transient opening of epithelial junctions. This might facilitate the influx of intestinal flora or bacterial proteins, which are recognized by TLR6 and trigger the activation proinflammatory cytokines and chemokines in the intestine. Furthermore, in a positive feed-forward mechanism, TLR6 activation can increase transflux through epithelial junctions (18). While it has been reported that TLR pathways can be activated by a variety of “sensor” cells (including dendritic cells and tissue macrophages, monocytes, and epithelial cells) (12), we found TLR6 signals only in epithelial cells of the intestinal mucosa. An alternative mechanism of intestinal injury and disturbed liquid absorption could be based on recognition of Ad3 virus as a danger signal. Previous studies showed that Ad DNA is sensed by TLR9, which in turn triggers the expression of a series of proinflammatory cytokine and chemokines within minutes after Ad uptake (34, 35, 67). Our finding that JO-1, a recombinant protein, also causes transient diarrhea argues against a major impact of the latter pathway. Furthermore, we did not detect TLR9 signals in intestine sections of hDSG2-transgenic mice that received intravenous Ad3-GFP injection. Clearly, further studies in hDSG2-transgenic mice as well as epidemiological studies in humans are required to establish a link between hAdV3 and gastroenteritis.
In this study, we utilized the hDSG2-transgenic mouse model only to study GI tract-related symptoms of Ad3-GFP infection. HAdV-B3 infection has also been associated with myocarditis (77), which could be explained by the presence of hDSG2 in the intercalated discs of the myocardium. Particularly in immune-suppressed patients, infection with hDSG2-interacting Ads can cause hemorrhagic cystitis (9), pancreatitis (3, 36, 55), conjunctivitis (51) and also central nervous system (CNS) diseases (15, 70). While our DSG2 biodistribution studies provided support for a potential etiological link between HAdV-B infection and these symptoms, it would go beyond this study to explore this further.
HAdV-B3 as gene transfer vectors.
Several groups have developed replication-competent and E1-deleted (replication-deficient) HAdV-B3 vectors (31, 71). Furthermore, for more than a decade, Ad5 vectors that possess Ad3 fibers (Ad5/3) (which also infect cells through hDSG2 [87]) have been used for cancer therapy in animal models and humans (43, 57). The application of these vectors for cancer therapy is justified by the fact that hDSG2 is overexpressed in many epithelial cancers (82), including breast cancer (88), gastric cancer (6), squamous cell carcinomas (29), melanoma (64), metastatic prostate cancer (80), and bladder cancer (1) (also see http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.412597). The generation of hDSG2-transgenic mice creates a basis for studying the tropism and potential acute side effects of Ad3- and Ad5/3-based oncolytic vectors in a small-animal model.
A question remains whether soluble DSG2 detected in the serum of humans, monkeys, and hDSG2-transgenic mice affects Ad3 or Ad5/3 vector transduction in vivo. Soluble HAdV receptors present in serum have been reported before. For example, soluble CAR at a concentration of 3 ng/ml was found in malignant pleural effusions of lung cancer patients (4). Reported plasma concentrations of soluble CD46 ranged from 10 to 120 ng/ml (22, 28). Answering the above question requires detailed surface plasmon resonance (SPR) analyses of the association and dissociation constants of Ad3 vectors and soluble DSG2 in the context of whole serum.
Considering that HAdV-C5-based vectors are inefficient in transduction of airway epithelium (26, 60, 86), our data on Ad3-GFP transduction upon intranasal vector administration make Ad3-based vectors (e.g., Ad3-based helper-dependent vectors) interesting for lung gene therapy (e.g., gene therapy of cystic fibrosis). Furthermore, our finding that Ad3-GFP transduces small intestinal and colon epithelial cells might be relevant for gene therapy approaches of acquired and genetic GI tract diseases, including inflammatory bowel and malabsorption syndromes. Finally, our finding that DSG2 is expressed on hematopoietic stem cells gives a rationale for using Ad3 vectors in hematopoietic stem cell gene therapy.
In summary, this study improves our understanding of the pathology of HAdV-B3 infection. It creates a basis for developing new antiviral drugs or vaccines that can interfere with HAdV-B3 triggered GI symptoms, particularly in children. Finally, our findings have implications for the use of HAdV-B3-derived vectors in cancer therapy and gene therapy for airway and intestinal diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christine Wang and Suzie Pun (University of Washington, Department of Bioengineering) for their help with the in vivo imaging. We thank Mark Greene (University of Pennsylvania) for providing anti-rat neu antibodies. We are grateful to Bryce Sopher for help in preparing BAC DNA. We thank Nelson DiPaolo and Dmitry Shayakhmetov for helpful advice.
The study was supported by NIH grants R01 CA080192 and R01 HLA078836. I.B. is a recipient of a postdoctoral fellowship award from Deutsche Krebshilfe (108988). A.H. is K. Albin Johansson Research Professor of the Foundation for the Finnish Cancer Institute.
Footnotes
Published ahead of print 28 March 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Abbod MF, Hamdy FC, Linkens DA, Catto JW. 2009. Predictive modeling in cancer: where systems biology meets the stock market. Expert Rev. Anticancer Ther. 9:867–870 [DOI] [PubMed] [Google Scholar]
- 2. Arnberg N. 2009. Adenovirus receptors: implications for tropism, treatment and targeting. Rev. Med. Virol. 19:165–178 [DOI] [PubMed] [Google Scholar]
- 3. Bateman CM, Kesson AM, Shaw PJ. 2006. Pancreatitis and adenoviral infection in children after blood and marrow transplantation. Bone Marrow Transplant. 38:807–811 [DOI] [PubMed] [Google Scholar]
- 4. Bernal RM, et al. 2002. Soluble coxsackievirus adenovirus receptor is a putative inhibitor of adenoviral gene transfer in the tumor milieu. Clin. Cancer Res. 8:1915–1923 [PubMed] [Google Scholar]
- 5. Beyer I, et al. 2011. Epithelial junction opener JO-1 improves monoclonal antibody therapy of cancer. Cancer Res 71:7080–7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biedermann K, et al. 2005. Desmoglein 2 is expressed abnormally rather than mutated in familial and sporadic gastric cancer. J. Pathol. 207:199–206 [DOI] [PubMed] [Google Scholar]
- 7. Borisovska M, McGinley MJ, Bensen A, Westbrook GL. 2011. Loss of olfactory cell adhesion molecule reduces the synchrony of mitral cell activity in olfactory glomeruli. J. Physiol. 589:1927–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carr MJ, et al. 2011. Deaths associated with human adenovirus-14p1 infections, Europe, 2009–2010. Emerg. Infect. Dis. 17:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention 1998. Civilian outbreak of adenovirus acute respiratory disease—South Dakota, 1997. MMWR Morb. Mortal. Wkly. Rep. 47:567–570 [PubMed] [Google Scholar]
- 10. Chang SY, et al. 2008. A community-derived outbreak of adenovirus type 3 in children in Taiwan between 2004 and 2005. J. Med. Virol. 80:102–112 [DOI] [PubMed] [Google Scholar]
- 11. Chen HL, et al. 2004. Respiratory adenoviral infections in children: a study of hospitalized cases in southern Taiwan in 2001–2002. J. Trop. Pediatr. 50:279–284 [DOI] [PubMed] [Google Scholar]
- 12. Chen J, et al. 2007. Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G568–G576 [DOI] [PubMed] [Google Scholar]
- 13. Chitaev NA, Troyanovsky SM. 1997. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion. J. Cell Biol. 138:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi EH, et al. 2006. Ten-year analysis of adenovirus type 7 molecular epidemiology in Korea, 1995–2004: implication of fiber diversity. J. Clin. Virol. 35:388–393 [DOI] [PubMed] [Google Scholar]
- 15. de Azevedo JP, et al. 2004. Characterization of species B adenoviruses isolated from fecal specimens taken from poliomyelitis-suspected cases. J. Clin. Virol. 31:248–252 [DOI] [PubMed] [Google Scholar]
- 16. Dhar D, Toth K, Wold WS. 2012. Syrian hamster tumor model to study oncolytic ad5-based vectors. Methods Mol. Biol. 797:53–63 [DOI] [PubMed] [Google Scholar]
- 17. Drebin JA, Link VC, Greene MI. 1988. Monoclonal antibodies specific for the neu oncogene product directly mediate anti-tumor effects in vivo. Oncogene 2:387–394 [PubMed] [Google Scholar]
- 18. Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. 2009. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J. Biol. Chem. 284:22332–22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fender P, Hall K, Schoehn G, Blair GE. 2012. The impact of human adenovirus type 3 dodecahedron on host cells and its potential role in viral infection. J. Virol. doi:10.1128/JVI.07127–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fender P, Ruigrok RW, Gout E, Buffet S, Chroboczek J. 1997. Adenovirus dodecahedron, a new vector for human gene transfer. Nat. Biotechnol. 15:52–56 [DOI] [PubMed] [Google Scholar]
- 21. Finkbeiner SR, et al. 2008. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 4:e1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fogdell-Hahn A, et al. 2005. Co-purification of soluble membrane cofactor protein (CD46) and human herpesvirus 6 variant A genome in serum from multiple sclerosis patients. Virus Res. 110:57–63 [DOI] [PubMed] [Google Scholar]
- 23. Fox JP, et al. 1969. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am. J. Epidemiol. 89:25–50 [DOI] [PubMed] [Google Scholar]
- 24. Frost BL, Jilling T, Caplan MS. 2008. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin. Perinatol. 32:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ginsberg HS, et al. 1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. U. S. A. 88:1651–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Griesenbach U, Alton EW. 2011. Current status and future directions of gene and cell therapy for cystic fibrosis. BioDrugs 25:77–88 [DOI] [PubMed] [Google Scholar]
- 27. Gustafsson DJ, Segerman A, Lindman K, Mei YF, Wadell G. 2006. The Arg279Gln [corrected] substitution in the adenovirus type 11p (Ad11p) fiber knob abolishes EDTA-resistant binding to A549 and CHO-CD46 cells, converting the phenotype to that of Ad7p. J. Virol. 80:1897–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hara T, et al. 1992. Soluble forms of membrane cofactor protein (CD46, MCP) are present in plasma, tears, and seminal fluid in normal subjects. Clin. Exp. Immunol. 89:490–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harada H, Iwatsuki K, Ohtsuka M, Han GW, Kaneko F. 1996. Abnormal desmoglein expression by squamous cell carcinoma cells. Acta Derm. Venereol. 76:417–420 [DOI] [PubMed] [Google Scholar]
- 30. Hemminki O, et al. 2010. Preclinical and clinical data with a fully serotype 3 oncolytic adenovirus Ad3-hTERT-E1A in the treatment of advanced solid tumors. Mol. Ther. 18:S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hemminki O, et al. 2011. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther. 18:288–296 [DOI] [PubMed] [Google Scholar]
- 32. Hong JY, et al. 2001. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin. Infect. Dis. 32:1423–1429 [DOI] [PubMed] [Google Scholar]
- 33. Hsieh WY, Chiu NC, Chi H, Huang FY, Hung CC. 2009. Respiratory adenoviral infections in Taiwanese children: a hospital-based study. J. Microbiol. Immunol. Infect. 42:371–377 [PubMed] [Google Scholar]
- 34. Huang X, Yang Y. 2009. Innate immune recognition of viruses and viral vectors. Hum. Gene Ther. 20:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iacobelli-Martinez M, Nemerow GR. 2007. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 81:1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawakami T, et al. 2002. Acute pancreatitis following hematopoietic stem cell transplantation: prevalence and cause of pancreatic amylasemia. Rinsho Ketsueki. 43:176–182 (In Japanese.) [PubMed] [Google Scholar]
- 37. Kim YJ, et al. 2003. Genome type analysis of adenovirus types 3 and 7 isolated during successive outbreaks of lower respiratory tract infections in children. J. Clin. Microbiol. 41:4594–4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Knutson KL, Almand B, Dang Y, Disis ML. 2004. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 64:1146–1151 [DOI] [PubMed] [Google Scholar]
- 39. Knutson KL, et al. 2006. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J. Immunol. 177:1526–1533 [DOI] [PubMed] [Google Scholar]
- 40. Koizumi N, et al. 2003. Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and αv integrin-binding ablation. J. Virol. 77:13062–13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kojaoghlanian T, Flomenberg P, Horwitz MS. 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 13:155–171 [DOI] [PubMed] [Google Scholar]
- 42. Kolegraff K, Nava P, Laur O, Parkos CA, Nusrat A. 2011. Characterization of full-length and proteolytic cleavage fragments of desmoglein-2 in native human colon and colonic epithelial cell lines. Cell Adh. Migr. 5:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koski A, et al. 2010. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 18:1874–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lewis PF, et al. 2009. A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J. Infect. Dis. 199:1427–1434 [DOI] [PubMed] [Google Scholar]
- 45. Li L, et al. 2005. Molecular epidemiology of adenovirus infection among pediatric population with diarrhea in Asia. Microbiol. Immunol. 49:121–128 [DOI] [PubMed] [Google Scholar]
- 46. Lin CH, et al. 2007. A cluster of adenovirus serotype 3 infections in children in northern Taiwan: clinical features and laboratory findings. J. Microbiol. Immunol. Infect. 40:302–309 [PubMed] [Google Scholar]
- 47. Liu X-L, et al. 2004. Molecular epidemiology study of an adenovirus outbreak. J. Prevent. Med. Chin. People's Liberation Army 22:199–200 (In Chinese.) [Google Scholar]
- 48. Louie JK, et al. 2008. Severe pneumonia due to adenovirus serotype 14: a new respiratory threat? Clin. Infect. Dis. 46:421–425 [DOI] [PubMed] [Google Scholar]
- 49. Lu H, Knutson KL, Gad E, Disis ML. 2006. The tumor antigen repertoire identified in tumor-bearing neu transgenic mice predicts human tumor antigens. Cancer Res. 66:9754–9761 [DOI] [PubMed] [Google Scholar]
- 50. Marttila M, et al. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 79:14429–14436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matsui K, et al. 2008. Monitoring of adenovirus from conjunctival scrapings in Japan during 2005–2006. J. Med. Virol. 80:997–1003 [DOI] [PubMed] [Google Scholar]
- 52. Metzgar D, et al. 2007. Abrupt emergence of diverse species B adenoviruses at US military recruit training centers. J. Infect. Dis. 196:1465–1473 [DOI] [PubMed] [Google Scholar]
- 53. Ni S, et al. 2005. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 16:664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ni S, et al. 2006. Evaluation of adenovirus vectors containing serotype 35 fibers for tumor targeting. Cancer Gene Ther. 13:1072–1081 [DOI] [PubMed] [Google Scholar]
- 55. Niemann TH, Trigg ME, Winick N, Penick GD. 1993. Disseminated adenoviral infection presenting as acute pancreatitis. Hum. Pathol. 24:1145–1148 [DOI] [PubMed] [Google Scholar]
- 56. Pan H-M, et al. 2003. A report of adenovirus infection diseases of children in a swimming pool. J. Prevent. Med. Info. 19:237–238 [Google Scholar]
- 57. Pesonen S, et al. 2010. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther. 17:892–904 [DOI] [PubMed] [Google Scholar]
- 58. Pilichou K, et al. 2006. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation 113:1171–1179 [DOI] [PubMed] [Google Scholar]
- 59. Rebelo-de-Andrade H, et al. 2010. Outbreak of acute respiratory infection among infants in Lisbon, Portugal, caused by human adenovirus serotype 3 and a new 7/3 recombinant strain. J. Clin. Microbiol. 48:1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rosewell A, et al. 2011. Efficient transduction of alveoloar type II epithelial cells in non-human primates by helper-dependent adenovirus 5-fibner 35 chimeric vectors. Mol. Ther. 19:S108 [Google Scholar]
- 61. Rowe WP, Hartley JW. 1962. A general review of the adenoviruses. Ann. N. Y. Acad. Sci. 101:466–474 [DOI] [PubMed] [Google Scholar]
- 62. Sakamoto M, et al. 1995. Longitudinal investigation of epidemiologic feature of adenovirus infections in acute respiratory illnesses among children in Yamagata, Japan (1986–1991). Tohoku J. Exp. Med. 175:185–193 [DOI] [PubMed] [Google Scholar]
- 63. Schafer S, Koch PJ, Franke WW. 1994. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp. Cell Res. 211:391–399 [DOI] [PubMed] [Google Scholar]
- 64. Schmitt CJ, et al. 2007. Homo- and heterotypic cell contacts in malignant melanoma cells and desmoglein 2 as a novel solitary surface glycoprotein. J. Investig. Dermatol. 127:2191–2206 [DOI] [PubMed] [Google Scholar]
- 65. Segerman A, Arnberg N, Erikson A, Lindman K, Wadell G. 2003. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J. Virol. 77:1157–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Segerman A, et al. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183–9191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shayakhmetov DM, Di Paolo NC, Mossman KL. 2010. Recognition of virus infection and innate host responses to viral gene therapy vectors. Mol. Ther. 18:1422–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79:7478–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G, Lieber A. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simila S, Jouppila R, Salmi A, Pohjonen R. 1970. Encephaloningitis in children associated with an adenovirus type 7 epidemic. Acta Paediatr. Scand. 59:310–316 [DOI] [PubMed] [Google Scholar]
- 71. Sirena D, Ruzsics Z, Schaffner W, Greber UF, Hemmi S. 2005. The nucleotide sequence and a first generation gene transfer vector of species B human adenovirus serotype 3. Virology 343:283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith JS, Xu Z, Tian J, Stevenson SC, Byrnes AP. 2008. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum. Gene Ther. 19:547–554 [DOI] [PubMed] [Google Scholar]
- 73. Stone D, et al. 2007. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol. Ther. 15:2146–2153 [DOI] [PubMed] [Google Scholar]
- 74. Su YC, Chen BM, Chuang KH, Cheng TL, Roffler SR. 2010. Sensitive quantification of PEGylated compounds by second-generation anti-poly(ethylene glycol) monoclonal antibodies. Bioconjug. Chem. 21:1264–1270 [DOI] [PubMed] [Google Scholar]
- 75. Sumida SM, et al. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174:7179–7185 [DOI] [PubMed] [Google Scholar]
- 76. Tang L, et al. 2004. A study on the epidemiology and pathology of adenovirus outbreaks in four cities in China. Dis. Surveill. 21:285–287 [Google Scholar]
- 77. Treacy A, et al. 2010. First report of sudden death due to myocarditis caused by adenovirus serotype 3. J. Clin. Microbiol. 48:642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Trei JS, et al. 2010. Spread of adenovirus to geographically dispersed military installations, May-October 2007. Emerg. Infect. Dis. 16:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Trinh HV, et al. 2012. Avidity binding of human adenovirus serotypes 3 and 7 to the membrane cofactor CD46 triggers infection. J. Virol. 86:1623–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Trojan L, et al. 2005. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 25:183–191 [PubMed] [Google Scholar]
- 81. Turley EA, Veiseh M, Radisky DC, Bissell MJ. 2008. Mechanisms of disease: epithelial-mesenchymal transition—does cellular plasticity fuel neoplastic progression? Nat. Clin. Pract. Oncol. 5:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tuve S, et al. 2006. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J. Virol. 80:12109–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Waddington SN, et al. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409 [DOI] [PubMed] [Google Scholar]
- 84. Wadell G, Hammarskjold ML, Winberg G, Varsanyi TM, Sundell G. 1980. Genetic variability of adenoviruses. Ann. N. Y. Acad. Sci. 354:16–42 [DOI] [PubMed] [Google Scholar]
- 85. Walters RW, et al. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789–799 [DOI] [PubMed] [Google Scholar]
- 86. Walters RW, et al. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219–10226 [DOI] [PubMed] [Google Scholar]
- 87. Wang H, et al. 2011. Multimerization of adenovirus serotype 3 fiber knob domains is required for efficient binding of virus to desmoglein 2 and subsequent opening of epithelial junctions. J. Virol. 85:6390–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang H, et al. 2011. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 17:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang H, Tuve S, Erdman DD, Lieber A. 2009. Receptor usage of a newly emergent adenovirus type 14. Virology 387:436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yamadera S, Yamashita K, Akatsuka M, Kato N, Inouye S. 1998. Trend of adenovirus type 7 infection, an emerging disease in Japan. A report of the National Epidemiological Surveillance of Infectious Agents in Japan. Jpn. J. Med. Sci. Biol. 51:43–51 [DOI] [PubMed] [Google Scholar]
- 91. Yang Z, Horn M, Wang J, Shen DD, Ho RJ. 2004. Development and characterization of a recombinant madin-darby canine kidney cell line that expresses rat multidrug resistance-associated protein 1 (rMRP1). AAPS J. 6:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yeung R, et al. 2009. Characterization of culture-positive adenovirus serotypes from respiratory specimens in Toronto, Ontario, Canada: September 2007-June 2008. Virol. J. 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.