Abstract
The identification of peripheral biomarkers for neurodegenerative diseases is required to improve the accuracy of clinical diagnosis and monitor both disease progression and response to treatments. The data reviewed in this paper suggest that, in neurodegenerative disease, cytokines are links between peripheral immune system and nervous system dysfunction.
Keywords: Alzheimer’s Disease, Parkinson’s Disease, neuroinflammation, neurodegeneration, cytokines, chemokines
Introduction
Alzheimer disease (AD) is an age-dependent neurodegenerative disorder that results in a progressive loss of cognitive abilities. It is characterized by the accumulation of the amyloid-β (Aβ) peptide into amyloid plaques in the extracellular brain parenchyma as well as by intracellular neurofibrillary tangles primarily comprised of hyperphosphorylated tau. By contrast, the age-dependent neurodegenerative disorder of Parkinson’s disease (PD) is characterised by tremor, rigidity, and slowness of movements, and is associated with progressive neuronal loss of the substantia nigra (SN) and other brain structures. Although clinically and pathologically different, there is ample evidence of chronic inflammatory reactions in the brain of both diseases. Neuroinflammatory responses in the brain involve different cells of the immune system (e.g., macrophages, mast cells, T and B lymphocytes, dendritic cells), resident cells of the CNS (e.g., microglia, astrocytes, neurons), many protein components (e.g., complement, adhesion molecules, chemokines, cytokines) and cytotoxic substances (e.g., reactive oxygen and nitrogen species).
Elevated levels of proinflammatory cytokines, such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and the colony-stimulating factor, have been demonstrated in the brain, cerebrospinal fluid (CSF) as well as basal ganglia of PD patients. Moreover, these cytokines have, likewise, been determined to be able to promote Aβ accumulation, and thus play an important role in the amplification of detrimental mechanisms likely involved in progression of AD.
Soluble molecules or cytokines, released by immunocompetent cells, may be responsible for the bidirectional communication between cells of the nervous and immune systems. One hypothesis is that inflammation starts within the central nervous system (CNS), where several inflammatory products are formed and are quickly removed into the blood stream. On the other hand, it has been also proposed that inflammation develops at first in the periphery, and then will contribute to brain damage and finally neurodegeneration.
The identification of biomarkers for neurodegenerative diseases, epitomized by PD and AD, is required to improve the accuracy of clinical diagnosis and monitor both disease progression and response to treatments.
Alzheimer’s Disease
AD afflicts mostly people 65 years or older. The clinical hallmarks are progressive impairments in memory, judgment, decision-making, orientation to physical surroundings, and language. AD’s pathological characteristics are the presence of senile plaques and neurofibrillary tangles (NFT) in the brain of patients, together with synapse loss and a deficit of presynaptic markers of the cholinergic system in brain regions involved in cognition and mood, such as the hippocampus, entorhinal cortex, basal forebrain, and the frontal and parietal lobes [1, 2]. Although cerebral senile plaques can develop with age and may be seen in the brain of elderly people without cognitive decline, the presence of a large plaque number is usually associated with AD. Thus, a diagnosis of AD can be confirmed after death in patients with cognitive decline, in the event that the brain shows larger numbers of neuritic senile plaques and neurofibrillary tangles than expected from normal aging. AD has a heterogeneous etiology with a large percentage, termed sporadic AD, arising from unknown causes and a smaller fraction of early onset familial AD (FAD) caused by mutations in one of several genes, such as the amyloid-β precursor protein (APP) and presenilins (PS1, PS2) [1, 3]. Senile plaques comprise, in large part, of aberrant aggregates of amyloid-β (Aβ) peptide whereas NFTs largely consist of a hyperphosphorylated form of the microtubular protein, tau [1,3]. Aβ in senile plaques is the product of cleavage of a much larger protein, APP, by a series of protease activities, termed α-, β- and γ-secretases [1,3]. APP processing by β- and γ-secretases appears to be responsible for generation of Aβ that can self-aggregate to form both soluble toxic aggregates of Aβ, such as the dodecameric (56 kDa) form and Aβ-derived diffusible ligands (ADDLs), in addition to insoluble toxic fibrils that accumulate in the senile plaques [2]. A commonality of the described mutations is that they, albeit through different routes, increase production of Aβ and, in particular, the longer more hydrophobic Aβ1–42 form. In addition to targeting and impairing synapses [2], most investigators believe that Aβ can induce an intracerebral inflammatory response that leads to the production of inflammatory cytokines, deposition of complement, and the generation of free radicals that individually or collectively can lead to neuron death [4]. Over the last decade it has become clear that chronic neuroinflammation represents a further constant feature of AD [5]. Indeed, neuroinflammation, long considered to be peripheral to the disease, is being increasingly accepted as being associated with the onset of this neurodegenerative disorder [6–8]. As its peripheral counterpart, neuroinflammation is widely regarded as a double-edged sword: it can exert both neuroprotective and neurotoxic functions. Beneficial or harmful expression of the local immune response in the damaged CNS depends on how microglia interpret the threat, and that a well-regulated T-cell-mediated response enables microglia to alleviate rather than exacerbate stressful situations in the CNS [9,10]. The mechanisms that switch between neuroprotection and neurotoxicity are, thus far, poorly understood.
Parkinson’s Disease
PD is the second most common neurodegenerativedisorder, after AD, with a prevalence of approximately 0.5 to 1 percent among persons 65 to 69 yearsof age, rising to 1 to 3 percent among those of 80 years ageand older. It is characterized, clinically, by parkinsonism (resting tremor, bradykinesia, rigidity, and postural instability) [11,12], and pathologically by the loss of neurons in the SN and elsewhere in association with the presence of ubiquinated protein deposits in the cytoplasm ofneurons and thread-like proteinaceous inclusionswithin neurites (Lewy neurites) [12–15]. Sporadic PD accounts for >90% of the incidence of the disease,with the remaining fraction of mostly familial PD that is suspected to be linked to mutations in several recently identified genes such as parkin, alpha-synuclein (SNCA), synphilin-1 (SNCAIP), dardarin (LRRK2) and glucocerebrosidase (GBA) with specific mutations associated with Parkinson’s disease [12–18]. The neuronal degeneration leads to the loss of dopaminergic (DA) terminals in the striatum and in other basal ganglia and cortical brain regions. It has recently been suggested that a glial reaction and inflammatory processes may also participate in the cascade of events leading to neuronal degeneration [19,20]. Although diagnosis is made clinically, other disorders with prominent symptoms and signs of parkinsonism, such as postencephalitic, drug-induced, and arteriosclerotic parkinsonism, may cause confusion with PD until a diagnosis is confirmed by autopsy [21]. The primary mediators of neuroinflammatory responses in PD are activated microglia. Potential activators include environmental toxicants, endogenous substances, as well as aggregated α-synuclein. α-Synuclein may activate microglia via a direct interaction or indirectly through release of neuronal-derived soluble factors. α-Synuclein-mediated microglial activation has been recently demonstrated in rat primary cultures, human microglia cultures and a monocytic cell line, THP-1, following treatment with exogenous α-synuclein [22].
Cytokines: mediators in the central nervous system
Interleukins serve as important inflammatory molecules in many organs and tissues. Their function was initially studied in macrophages, blood leukocytes and other bone marrow origin cells from which they are produced and released as cell messengers [23]. Classified as a cytokine, these molecules facilitate communication between cells within the peripheral immune system, but are now recognized to act also as mediators between the immune system and other organ and tissue systems. Cytokines act in an autocrine (self), paracrine (local) and endocrine (distant) fashion to affect, amongst numerous processes, inflammation. In general, during the process of peripheral inflammation, cytokines released by activated macrophages play a role in immunomodulation, either by activating other inflammatory cells or by inhibiting their function, to thereby establish a balance between these apparently diverse influences. A failure in coordinated activity of cytokines, inflammatory cells, and other inflammatory triggers leads to inflammatory disorders. The biological activity of cytokines is extremely complex as it concomitantly depends on several factors, such as the target cell type and state of activation, the local cytokine concentration, receptor type and interaction with other cytokine mediators. Due to these factors, the precise effect of cytokines on specific tissue types may be difficult to predict. The concept of cytokine’s affect on non-immune cells and tissues is of particular importance when discussing the consequences of inflammation within the AD brain [24].
Chemokines, or chemoattractant cytokines, are small (8–10 kilodaltons), structurally-related, endogenous seven transmembrane G-protein coupled receptor (GPCR) ligands that regulate cell trafficking and the directional migration of leukocytes. Chemokines are divided into four subgroups based on their pattern of cysteine residues in conserved locations towards their amino terminus that are key to forming their three-dimensional shape as follows: C, CC, CXC and CX3C. Chemokines are most clearly implicated in diseases involving leukocyte modulation but the role of chemokines and chemokine receptors (CCR) is becoming more apparent in a vast number of conditions, including inflammation. The essence of chemokine-mediated signalling is the ability to selectively activate a specific subset of a cell population in order to elicit a targeted and specialized immune response. The relationship between chemokines and their receptors is complex, in that individual chemokines can often bind to several different receptors and a single chemokine receptor can be activated by multiple chemokines [25].
Inflammation and neurodegeneration
Microglia are immunocompetent cells that take up residence in the developing brain as fetal macrophages. They constitute some 20% of the total glial cell population within the brain and are the first responsive element to brain damage or insult. The microglia are central to the inflammatory response that has been documented in chronic neurodegenerative diseases, such as AD, prion disease and PD. In chronic neurodegenerative disease, the microglia become activated which induces specific morphological changes relative to their resting state. In the resting state, microglial cells generally possess long branching processes and a relatively small cell body. These branches are responsive to small changes in physiological conditions and, unlike activated microglia that take on a large, amoeboid shape, quiescent ones are unable to phagocytose cells and display little or no immunomolecules. Hence, they are extremely poor antigen presenters, but function to maintain a constant level of available microglia to detect and fight infection, while maintaining an immunologically silent environment. By contrast, in the activated state they up regulate a number of cell surface or cytoplasmic antigens, become able to phagocytose foreign materials and display the resulting immunomolecules for T-cell activation. Phagocytic microglia are capable of migrating to sites of injury, engulfing material, and secreting pro-inflammatory factors to promote the proliferation of additional cells to do the same. In normal circumstances, they also interact with astrocytes and neural cells to deal with an infection or challenge with minimal damage to healthy brain cells.
As microglia become activated in a slowly evolving chronic neurodegenerative disease, the cytokine profile in the brain may be switched from an anti-inflammatory profile to a pro-inflammatory one by systemic inflammation. Microglial activation has been associated with neurodegeneration via the production of a variety of proinflammatory and neurotoxic factors, including TNF-α, IL-1β, eicosanoids, nitric oxide (NO), and superoxide (O2−). Whereas there is limited evidence regarding the synthesis and secretion of cytokines from astrocyte or microglial cells under normal healthy homeostatic conditions, these same cells are important sources of cytokines in pathological states [26]. The function and secreted products of all these molecules are diverse, and likely regulate the intensity and duration of an immune response. In addition, microglia play an important role in orchestrating the behaviour of other immune cells that enter the brain via their secretion of both cytokines and chemokines, key regulatory molecules that are dramatically up-regulated during CNS inflammation.
Godoy et al. [27] have reported that microglial cells produce increased mRNA levels of pro-inflammatory cytokinessuch as IL-1β within the SN during neurodegeneration, but translation of these transcripts was notobserved [28]. These and other observations prompted the hypothesis that these ‘atypical’ microglial cells are ‘primed’ cells. Hypothetically, these ‘primed’ microglia cells could be stimulated to adopt a potent neurotoxic pro-inflammatory phenotype by a second stimulus and exacerbate neurodegeneration [29,30]. Investigators have determined that a second, pro-inflammatory stimulus in the degenerating SN switches ‘primed’ microglia into a pro-inflammatory phenotype, which leads to increased neurodegenerationand earlier motor symptoms in a Parkinson’s disease model. It is widely reported that numerous substances involved in the promotion of inflammatory processes are present in the CNS of patients with neurodegenerative disease. Likewise, it is well established that local inflammatory processes in the brain contribute to the damage caused by acute brain injuryand the progression of neurodegenerative disease. In contrast, the role of systemic inflammatory processes has been poorly investigated, although growing evidence suggests that the incidence of, and response to, acute brain injury and the progression of several neurodegenerative diseases are modulated by systemic inflammatory events [31,32]. It is known that systemic inflammatory mediators can induce the synthesis of cytokines within the brain, suggesting that this second stimulus could have its genesis in the periphery [33].
Systemic inflammation in chronic neurodegeneration
Recent evidence suggests that systemic inflammation can induce behavioural changes and may induce local inflammation in the diseased brain, leading to exaggerated synthesis of inflammatory mediators in the brain. Inflammatory mediators pass from the blood to the brain via macrophage populations associated with the brain, the perivascular macrophages and microglia. Such interactions suggest that a systemic challenge that promotes a systemic inflammatory response, may contribute to the outcome or progression of a chronic neurodegenerative disease [34]. Indeed, the classical slow induction of dopaminergic marker depletion in rodent brain following an acute systemic administration of lipopolysaccharides (LPS), a major component of the outer membrane of gram-negative bacteria, supports this contention and has provided a useful animal model of PD.
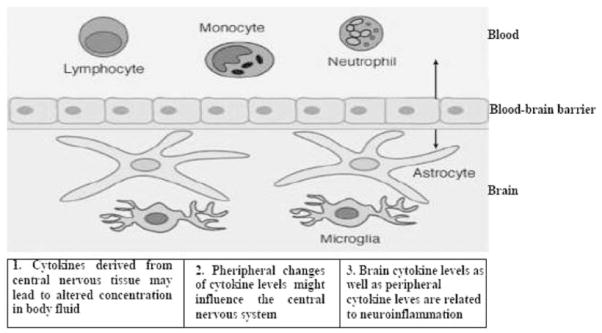
A bidirectional communication occurs between cells of the nervous and immune systems, the origin for this communication is the release of soluble molecules or cytokines, and the status of the blood-brain barrier’s permeability to them. The source of the circulating cytokines found in plasma has yet to be fully elucidated. They may be produced by blood or endothelial cells or, alternatively, come from the brain or, indeed, both compartments (Fig. 1). Experimental data show that an acute inflammatory stimulus (such as IL-1 or an endotoxin) delivered in the brain efficiently induces inflammatory cytokine production in the periphery [35,36]. Thus, it is apparent that the CNS is not only able to produce cytokines [37], but it can also contribute to the pool of peripheral cytokines. Recent reports indicate that a pro-inflammatory event in the periphery can induce chronic, self-propagating neuroinflammation in the brain, and that systemic cytokines are critical for CNS effects in response to peripheral immune activation. Qin et al. [38] showed that the entry of proinflammatory factors, such as TNF-α, to the brain induced the activation of microglia and subsequent production of more inflammatory factors, which may then cause neuronal death. This has clinical implications and, additionally, provides a link between peripheral inflammation and neuroinflammation. Support of the hypothesis that peripheral inflammation may amplify the neuroinflammation contributing to PD pathogenesis and that its inhibition may hence slow disease progress can be argued from epidemiologic findings indicating that prior long-term nonsteroidal antiinflammatory drug (NSAID) use was associated with a lower PD risk [39]. Most NSAIDs, including ibuprofen, flurbiprofen, and indomethacin, have poor brain entry consequent to their high restrictive plasma protein binding and/or their existence in an ionized form that has limited blood-brain barrier permeability; hence, their favorable brain actions must be mediated systemically rather than centrally, as brain concentrations of these agents are sub-therapeutic and only 1–2% of concomitant plasma levels.
Figure 1.
Relations between peripheral cytokines and the pathological process in neuroinflammation and neurodegenerative disease.
Stimulation of immunocompetent cells and hyper-production of cytokines are considered to play a key role in the development and progression of PD [40]. Abnormalities in peripheral immune function, such as a deviation in a T lymphocyte subset, have been reported by several authors in neurodegenerative diseases [34, 41, 42]. It is established that inflammation in peripheral tissues induces the synthesis of cytokines that communicate with the brain by inducing activation of macrophages. Within the CNS, these then play a central role in the transduction of signals from the blood to the brain and in the synthesis of inflammatory mediators, which include many that are found peripherally. Recent studies indicate that peripheral proinflammatory factors may be transferred through specific receptors to the brain, where they induce the synthesis of additional proinflammatory factors, creating a persistent and self-perpetuating neuroinflammation [38]. The brain can receive information from the immune system and respond to immune-derived signals via the cytokine network. In large part because of such actions, the immune system has recently begun to be referred to as “the second brain” [43]. Such aspects account for the inherent complexity of the immune network, and dissection of these will hopefully form a focus of future work to aid in their elucidation.
Cytokines in AD
Experimental evidence indicates that inflammation plays a major role in neurodegeneration and that the cytokine IL-1 is a pivotal mediator. IL-1 is expressed rapidly in response to neuronal injury, predominantly by microglia, and elevated levels of endogenous or exogenous IL-1 markedly exacerbate injury. IL-1 over-expression in Alzheimer brain is directly related to plaque progression and tangle formation, and IL-1 induces excessive synthesis, translation and processing of neuronal APP in addition to the synthesis of most of the known plaque associated proteins [44–47]. IL-1 has been reported to co-localizes immunohistochemically to neuritic plaques, which are requisite neuropathologicalfeatures of AD. A polymorphism in the IL-1α gene may induce an over-expression of IL-1α,a finding reported to be associated with inflammatory diseases [44]. IL-1 may also impact AD pathopharmacology via its ability to influence neuronal expression and activity of acetylcholinesterase (AChE) [48]. In a similar manner, systemically administered AChE inhibitors have been reported to significantly attenuate the production of IL-1β in the hippocampus and blood [49].
The pro-inflammatory cytokine, TNF-α, frequently exhibits synergistic effects with IL-1 during the inflammatory process. Several studies have quantified soluble TNF-α in AD brain tissue and CSF and, have found a decrease, an increase and no change compared to non-AD cases, as recently reviewed by Tweedie at al. [50]. Similar variability has been reported using mRNA analyses of AD brain tissue [51]. Luterman and colleagues [52] reported no detectable expression of TNF-α in elderly patients with variable clinical dementia ratings. By contrast, Paganelli et al. [53] reported elevated serum TNF-α in subjects with dementia that appeared to be disease-stage dependent. Specifically, the level of serum TNF-α was significantly lower in mild moderate AD compared to severe AD and vascular dementia, suggesting a difference in the cytokine profile at different stages of AD as well as between other types of dementia. Whereas interleukin-6 (IL-6) has been reported up regulated in AD brains, other studies have reported no significant increase in IL-6 in CSF, and despite these variable results, IL-6, together with IL-1β and TNF-α, is considered one of major mediators of the inflammatory response in AD [54]. The clinical consequence of a CNS dysregulation in this cytokine’s balance (e.g., high levels of pro-inflammatory cytokines and low levels or activity of anti-inflammatory cytokines) can lead to cytokine production and synergistic cytokine actions. Thus, both cytokine-cytokine interactions and cytokine interactions with existing AD pathology may play critical roles in AD neuroinflammation. Transforming growth factor (TGF)-β1 has been detected in AD plaques, and prominent immunostaining for TGF-β2 has been observed in reactive astrocytes, ramified microglia, as well as in a portion of tangle-bearing neurons in AD cases [55]. In contrast to these proinflammatory and amyloidogenic properties, TGF-β1 and TGF-β2 may serve to protect against neuronal cell damage in the brain parenchyma [56,57], and hence their presence may be associated with an alternative role.
Peripheral levels of cytokines in AD
Circulating cytokines have a short half-life, and may reach high concentrations at sites of release, but much lower concentrations in the blood, or they may bind to molecules that do not permit their detection by current immunological methodology. These and other caveats may accounts for the contradictory results of several studies that have measured circulating cytokines in AD or PD (Tab.1). Thus, effects on peripheral cells, and potentially following the disease by analysing such cells is based on the hypothesis that both AD and PD can be considered as systemic diseases that, in spite archetypical damage in brain that results from a greater sensitivity to injury in postmitotic neuronal cells, the disease also affects other cells and tissues in the body (Fig. 2).
Table 1.
Summary of Peripheral Cytokines in AD and PD Patients
| Marker | AD | PD | ||
|---|---|---|---|---|
| Levela | References | Levela | References | |
| IL-1β | + | [60–61, 69–70, 73, 74] | + | [96] |
| − | [99] | |||
| IL-2 | + | [60] | + | [100–102] |
| − | [98] | |||
| IL-6 | + | [41, 60, 61, 63, 77, 78] | + | [97] |
| IL-8 | = | [63] | + | [96] |
| = | [65, 67] | − | [99] | |
| IL-4 | − | [76, 79] | ||
| IL-12 | − | [80–82] | ||
| = | [41] | |||
| IL-15 | + | [100–102] | ||
| IL-16 | + | [83] | ||
| IL-18 | + | [60, 83, 86, 88] | ||
| = | [85] | |||
| IFNγ | = | [41, 64] | + | [96] |
| TGFβ | + | [83, 86, 89–90] | ||
| TNFα | + | [60, 64, 68–74] | + | [93, 96] |
| − | [70] | − | [99] | |
| = | [64, 65] | |||
+: Increased levels were observed compared to healthy controls −: Decreased levels were observed compared to healthy controls =: No different levels were observed compared to healthy controls.
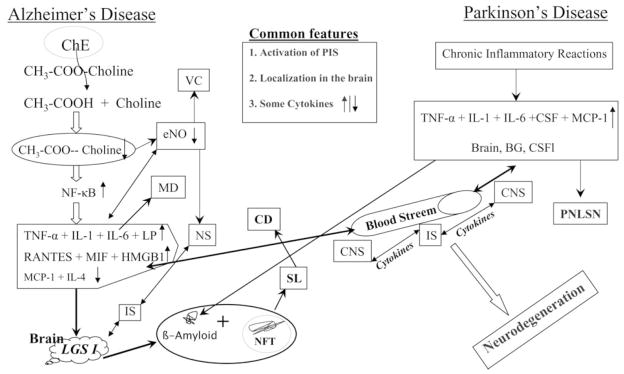
Figure 2.
Major common features for chemo- and cytokines in Alzheimer’s disease and Parkinson’s disease.
A peripheral immune alteration in AD patients might be triggered by a brain immune activation, given the existence of an inflammatory signalling pathway between the brain and periphery [58]. Recently, intracerebroventricular injection of Aβ1–42 in mice has been reported to generate an IL-6 elevation in not only brain but also plasma, demonstrating that centrally administered Aβ1–42 effectively induces a systemic IL-6 response [59], alternatively, circulating Aβ may directly affect immune function.
Results of studies evaluating markers of systemic inflammation in AD patients are conflicting as both increased [41, 60–63, 68–70] and normal [64–67] plasma levels of pro-inflammatory cytokines, such as IL-1β, TNF-α and IL-6, have been reported [41, 61, 66, 67, 73–76]. Higher TNF-α levels in AD than healthy controls, were not related with disease onset (early versus late), disease severity (mild versus moderate), sex (male versus female) or APOE status (epsilon4 carriers versus non-carriers) [71].
High plasma levels of IL-6 have been associated with an inferior cognitive performance at baseline and with a greater risk of cognitive decline over a 2-year follow-up period [77]. An elevation in circulating IL-6 is consistent with its enhanced synthesis by blood mononuclear cells [78], which may additionally imply a problem with immunoregulation in AD. Systematically elevated IL-6 may permeate across the blood-brain barrier and, after reaching the brain, may cause or contribute to the neuropathology of AD [41]. IL-4 induced by Th2 cells has been reported to be reduced in AD patients compared to control subjects, and expression and secretion were significantly elevated when the AD patients were treated with an AChE inhibitor [79]. IL-12 is considered important in the early inflammatory response, acting to induce IFNγ production from T and NK cells, which then enhances macrophage activity. Whereas no significant difference was observed in severe AD-patients compared to non-demented age matched controls, an increase in IL-12 concentration was apparent in mild AD patients and progressively decreased in AD-moderate patients. It is hence possible that during the early AD phase, the modest increase in the levels of IL-12 may be a part of the activated Th1 response, reflecting reactivity against Aβ and other components co-localized with plaques. A positive correlation between IL-12 and mini-mental state examination (MMSE) scores has been reported in mild AD patients [80–82]. An elevation in IL-16 levels in early phases of the disease may contribute to chemo-attraction of pathogenic CD4 lymphocytes to cross the blood brain barrier [83]. Such data lends support for IL-16 playing a potentially crucial role in the regulation of communication between the brain and the immune system, leading to the activation of humoral pathways, and in a subsequent highly modulated peripheral inflammatory response [84]. Moreover, IL-16 could potentially enhance the cytotoxic activity of immune cells contributing to the inflammatory response by triggering the release of further pro-inflammatory cytokines.
An association between IL-18 and AD is currently unclear and remains to be elucidated, but convergent results suggest that this cytokine is of interest in AD. Although no differences in circulating IL-18 levels have been determined between AD patients and controls, a significantly increased production of IL-18 was obtained from stimulated PBMCs of AD patients. This was particularly striking in AD subjects carrying the C/C genotype at the -607 position of the IL-18 gene promoter who demonstrated a high risk of developing AD. In addition, a significant correlation has been observed between IL-18 production and cognitive decline in AD subjects. Elevated IL-18 plasma levels correlated with the severity indexes in patients with AD. On the whole, these recent data are suggestive of a role of IL-18 in the pathogenic processes that lead to dementia and AD, and again are supportive of a hypothesis of the occurrence of immune dysfunction; in this case, potentially mediated by the helper T cell response [85–88]. The precise role of TGF-β in AD pathogenesis remains interesting but unclear, as the increased levels could potentially drive the disease or may, conversely, reflect a defense mechanism to lower soluble forms of Aβ in the brain parenchyma. These dual effects of TGF-β are not mutually exclusive, and may be dependent on disease severity [89–91].
Assuming that the brain’s pathological events are replicated in some manner in the periphery, it would seem rational to propose a link between the cytokine profiles of the brain and systemic circulation. Aiming to determine the presence of detectable circulating markers, a large number of studies have analyzed peripheral inflammatory indices, including cytokines and related molecules in blood (serum, plasma or cell cultures) from AD patients. At present, data is proving interesting but definitive conclusions cannot yet be drawn.
Cytokines in PD
Epidemiological evidence suggests an association between neuroinflammation and PD with a previous study reporting that inflammatory gene cytokine polymorphisms of TNFα and IL-1β increase the risk of PD [92]. Higher levels of TNF-α, the interleukins, IL-1β, IL-2, IL-4, IL-6, as well as of TGF-α,-β1 and -β2 have been detected in the brain parenchyma or CSF of PD patients [44,93]. In addition, elevated levels of inflammatory cytokines, including IL-1β and IL-6, as well as of TGF-α and epidermal growth factor (EGF) have been reported in striatal dopaminergic neurons, and highlight the participation of immuno-competent cells and hyper-production of cytokines in PD [94,95].
Peripheral levels of cytokines in PD
PBMCs deriving from patients with PD have been reported to induce an altered (often reduced) production of TNF-α as well as IL-1α and -1βcomparedto healthy controls, and their monocyte/macrophages induce less TNF-α[50]. This picture remains confusing as Reale et al. [96] observed a higher expression and production of IL-1β from PBMCs isolated from PD subjects compared to controls. Production of IFNγ and IL-2 from LPS-stimulated PBMCs from PD patients have been determined to be significantly lower than controls, and a lack of difference in IL-10 production has been described. A significant negative correlation has been noted in IL-6, TNF-α, IL-1β and IL-1α levels produced by both LPS-stimulated and unstimulated PBMCs from PD patients versus their Hoehn Yahr disability score, signifying that impaired cytokine production may progress with advancing disease. Changes in cytokine production on the rate of PD progression reveal a relationship between clinical manifestations of the disease and plasma cytokine content, and reflect a systemic immune hyper-reactivity that likely contributes to the development of higher mental disorders [96–99]. Although an elevated brain level of IL-6 has been considered evidence of neuroinflammation, its specific roles in PD pathogenesis remains to be elucidated. In animal models, both protective and detrimental actions have been described for IL-6 toward the dopaminergic system. A moderate elevation in IL-6 observed in blood may originate from the brain and reflect a local rather than a chronic systemic inflammatory process. Some studies have reported an elevation in the circulating levels of IL-15 and IL-2 in patients with advanced stages of PD [100–102], whereas Kluter et al. [98], when studying mitogen-stimulated PBMCs from patients with PD, noted a significant decline in IL-2, with levels of IFNγ, IL-6 and plasma soluble interleukin-2 receptor (sIL-2R) being no different from healthy controls. In a nutshell, PBMCs from PD subjects generally show a lower capacity to produce IL-2 and a higher capacity to produce TNF-α. Albeit that the present review has not revealed a general pattern of detectable inflammatory biomarkers in the peripheral blood of PD patients, it supports the hypothesis of the existence of a systemic immune abnormality during the pathological cascade of PD, and thereby provides a basis for further investigation.
Chemokines and relationship to AD and PD
Consequent to their induction or upregulation during CNS pathologies, members of the chemokine family can be used as biological markers [103]. Chemokines have been purported to play a germane role in the pathogenesis of inflammatory processes that occur during AD development based on their chemotactic activity on brain phagocytes. Observations from animal studies indicate that chemokines and their receptors are associated with neuritic plaques, supporting an inflammatory hypothesis for AD [104, 105]. It is plausible that plaque-associated chemokine production plays a function in the recruitment and accumulation of astrocytes and microglia to senile plaques. The participation of chemokines in AD and PD is substantiated by alterations in their concentration in both CSF and serum [106]. There is growing support that chemokines and their receptors are upregulated in resident CNS cells in AD brain, and that chemokines may contribute to plaque-associated inflammation and neurodegeneration [107]. Histological studies have associated both interleukin-8 receptor (IL-8R) and monocyte chemotactic protein-1 (MCP-1) (also known as CC chemokine ligand (CCL)-2) with AD pathology. Furthermore, the elevated expression of the chemokine receptors CCR3 and CCR5 on reactive microglia, together with the presence of macrophage inflammatory protein-1β (MIP-1β) in reactive astrocytes in AD brain [108], suggests the involvement of these β-chemokine receptors and their ligands in AD pathogenesis.
An Aβ-induced upregulation of MCP-1 has been demonstrated in several studies. Meda et al [109] showed that Aβ and IFNγ, synergistically, induced the production of MCP-1 in cultured human monocytes and in murine microglial cells. Aβ-induced production of MCP-1 by human THP-1 monocytes has been confirmed in other cell culture studies [110–113]. In addition, production of MCP-1 has also been demonstrated to occur in Aβ-stimulated astrocytes and oligodendrocytes. Other chemokines are thought to play a role in AD pathogenesis based on in vitro studies. MIP-1α and β expression in human monocytes and human microglia increased with Aβ stimulation, as did production of CCL5 (also known as RANTES) in astrocytes and oligodendrocytes. Whereas relatively few studies have examined the expression of chemokine receptors in AD, an increase in the expression of CCR3 and CCR5 has been noted on reactive microglia, and CXCR2 is expressed in neuritic plaques [107,108].
A peripheral increase of chemokines may, likewise, represent a crucial step in the pathogenesis and progression of AD. In this regard, clinical studies have demonstrated that MCP-1 levels are elevated in serum from subjects with Mild Cognitive Impairment (MCI) and mild to moderate AD, whereas they decline during AD progression, signifying that MCP-1 upregulation may be an early event in AD pathogenesis, preceding the clinical onset of the disease. This view is supported by expression rates determined in PBMC-based studies that parallel serum MCP-1 levels in MCI and the various stages of AD. The modifications described to occur in brain may additionally activate blood macrophages, which share common functions with microglial cells within the nervous system, to produce MCP-1, and thereby induce a slight increase in its serum level. Likewise, other peripheral cells, such as monocytes and vascular endothelium, similar to microglia and macrophages, both produce and secrete a wide range of cytokines and chemokines, and hence it is difficult to truly define whether changes in potential biomarkers like MCP-1 originate from brain or the periphery [114]. Alternatively, given the potential neuroprotective effect of MCP-1, its early rise could be related to an attempt of glial cells to eliminate Aβ and plaques, to forestall or block ongoing neuronal death. In this scenario, as this immunological response fails to re-establish the normal brain environment and neurons begin to fail and die, chemokine production would gradually become down regulated and decline. In the face of this process occurring chiefly within the brain, it would be expected to also influence peripheral cells and thereby account for the observed decline in MCP-1 production with disease progression. Further corroboration of a generalized decrease of MCP-1 production with disease severity derives from an observed negative correlation between disease duration and MCP-1 levels, in spite of a reported age-dependent rise in MCP-1 levels [115].
Amongst the chemokine family, the CXC3-chemokine, fractalkine, shows unusual properties as it exists as a membrane-bound and soluble protein. Both fractalkine and its receptor, CX(3)CR1, are predominantly found in the central nervous system, and are constitutively expressed in neurons, microglia and astrocytes. Soluble fractalkine can function as a pro-inflammatory chemoattractant that activates receptive inflammatory cells, particularly T cells and monocytes [116], whereas the cell-bound chemokine promotes strong adhesion of leukocytes to activated endothelial cells, where it is primarily expressed. Plasma levels of soluble fractalkine have been reported to be significantly elevated in patients with MCI and AD, in comparison to healthy age-matched controls. In addition, subjects with MCI possessed higher levels of plasma soluble fractalkine than those with AD. Previous studies have explored the relationship between blood levels of soluble fractalkine and CNS inflammatory diseases; however, the results have been largely contradictory. As fractalkine mRNA has not been found in the peripheral blood, a release of soluble fractalkine from endothelial cells may provide the source of fractalkine within the peripheral circulation. Soluble fractalkine has been proposed as a pro-inflammatory chemoattractant for T cells, NK cells, and monocytes during the early stage of AD. Hence, it was initially assumed that the presence of elevated plasma soluble fractalkine was linked to the induction of the inflammatory process within the AD brain. Nonetheless, emerging evidence points towards likely anti-inflammatory and neuroprotective roles for soluble fractalkine to function as an intrinsic inhibitor against neurotoxicity by suppressing activated microglia, and thereby providing a better potential explanation for its elevated plasma level in MCI [117]. During an inflammatory process leading to neuronal dysfunction and eventual death or microvascular disruption in AD, proteolytic release of soluble fractalkine from neurons and endothelial cells may function to shield the brain from insult, a response that gradually fades during disease progression. Such a scenario is in accord with previous findings on altered levels of serum MCP-1, which may likewise have a neuroprotective effect, and increases during the progression from MCI to early AD, but not during the progression from mild to severe AD [118].
The chemokine, IL-8 (also known as CXCL8), has been reported to be highly expressed in the CSF of patients with AD, compared to controls, although serum levels proved to be no different [119]. Its elevated CSF concentration negatively correlated to MMSE scores in AD patients [119]. Like other chemokines, the meaning of an increase in IL-8 in AD is difficult to gauge. Clearly the source for an elevated CSF IL-8 level, in the absence of a serum change, is the brain. A potential involvement in compensative and reparative mechanisms has been suggested, as IL-8 has been described to promote neuronal survival of hippocampal neuronal cultures [120]. Hence, the inverse correlation determined in AD between IL-8 levels and MMSE scores may represent an attempt to limit neuronal damage in patients as the disease progresses [119]. In a recent cross-sectional study of community dwelling elderly subjects in which the relationship between a variety of chemokines and cognitive performance was assessed by a variety of domains, increased serum concentrations of IL-8 were associated with poor performance in the memory and speed domains as well as in motor function [121]. CSF chemokine levels were not assessed in this latter study, and the results suggest an association between circulating IL-8 concentrations and cognitive dysfunction in the elderly; however, the basis of this association remains to be clarified.
The peripheral T cells of AD patients have been reported to over express MIP-1α, which binds to CCR5 on brain endothelial cells to promote the ability of T cells to cross the tight junctions that comprise the blood-brain barrier. This implies that a high level of MIP-1α is associated with augmented entry into brain, which may account for an increased occurrence of T cells in the brain of patients with AD, compared with age-matched controls [122, 123]. Chemokines are involved not only in AD pathogenesis but also in a wide variety of neurodegenerative disorders, including PD. Basal in addition to LPS-induced levels of MCP-1, RANTES, MIP-1α and IL-8 in PBMCs have been determined to be significantly higher in PD patients than in healthy controls, and the levels of these chemokines were significantly correlated with PD stage, as determined by Hoehn and Yahr staging of PD and the Unified Parkinson’s Disease Rating Scale (UPDRS) [95]. Serum RANTES levels have been reported increased in the serum of patients with PD, compared with healthy subjects. A likely recruitment of activated monocytes, macrophages and T lymphocytes to sites of inflammation within the brain of PD patients may occur through interaction with RANTES receptor CCR5 to potentially exacerbate injury, and the systemic presence of RANTES in PD may reflect both a central and/or peripheral source of activation. Different studies have examined whether or not DNA-polymorphisms at the genes encoding chemokines, MCP-1 (−2518 A/G) and RANTES (−403 A/G), and their receptors, CCR5 (Delta32) and CCR2 (V64I), were associated with the risk and/or clinical outcome of AD and PD, and have concluded that these four DNA polymorphisms did not contribute to the risk of either PD or AD [124]. Nishimura et al., instead, found that MCP-1 (−2518 A/G) genotype affected the age-at-onset of subjects afflicted by PD [125].
General concepts and future directions of AD, PD and cytokine relations
Diagnostic implications
Clearly, the longitudinal sampling and analysis for potential abnormal expression and production of cytokines from the CNS cannot readily be accomplished in living humans, and the possibility of post-mortem RNA degradation and protein modification may confound the interpretation of data obtained from neuropathological materials. This challenge may be circumvented by sampling and analysis of peripheral tissues, in light of growing evidence of systemic abnormalities in key cellular functions in affected individuals with neurodegenerative disease, including alterations in APP metabolism, post-translational protein modifications (e.g., Aβ and α-synuclein), antioxidant defences and acute phase reactants [30,31]. In this regard, the use PBMCs or alike blood components may offer specific advantages as (i) such cells can be readily obtained by simple venous sampling, (ii) the CNS is known to communicate with the immune system through multiple molecular, hormonal and neurotransmitter mechanisms, (iii) there are multiple commonalities between specific immune and neural cells, including aberrant APP expression, altered levels of antioxidant enzymes, occurrence of oxidative damage to DNA, RNA and protein, a deregulated cytokine secretion and elevated rates of apoptosis in conditions such as AD [128–130] and (iv) PBMCs have been previously utilized in the diagnosis and prognosis of other neurological diseases [131,132]. The notion of exploiting PBMCs as a “window” into the CNS has been proposed by Percy et al., [133] in their comprehensive review of the peripheral manifestations of AD. Whether or not the future holds the promise of a diagnostic based on cytokine/chemokine research remains to be elucidated. By sampling, measuring and profiling peripheral levels of these as well as other markers of immune response, one may be able to rationally move towards the development of a diagnostic, whether used alone or in combination with other biomarkers like tau, Aβ or α-synuclein, as well as more clearly define the role of these proteins in AD, PD and similar diseases involving neuroinflammation.
Treatment implications
Clearly an exciting and driving desire behind much current research is the potential of a new treatment. By controlling and tempering the inflammatory reaction to Aβ and/or other reactive aberrant disease-associated proteins, one could potentially diminish further protein build up, reduce neuronal dysfunction, and improve outcome. As an example among potentially many, specific inflammatory modulators in the brain, such as a TNF-α synthesis inhibitors or selective receptor antagonists [50], may perturb the vicious self-propagating cycle of events initiated by the aberrant over expression of this protein to inappropriately induce apoptotic pathways in neurons, and thereby provide a theoretical future direction in the field of AD or PD therapy.
IL-4 is an effective adjuvant for Aβ immunotherapy and drives an attenuated Th2 response to immunization with Aβ, inducing moderate antibody titres [134]. Spleens from Aβ1–42-immunized mice have demonstrated a decreased IL-12 receptor beta chain expression, an elevated secretion of IL-4 and IL-10, and a decrease in IFNγ levels [135], and IL-4, IL-10, and TGF-β1, but not IL-13 and IL-27 have been reported to enhance degradation of fibrillar Aβ, whereas proinflammatory cytokines suppress Aβ degradation [136]. Well-tolerated and selective inhibition of the biological activities of chemokine receptors could thus be of therapeutic value for select neurodegenerative disorders. In recent years, non-peptide antagonists of chemokine receptors have been disclosed and assessed in relevant pharmacological models. Some of these inhibitors have now entered clinical trials, and results are awaited with interest.
Conclusion
Although interpretation of the results of many AD and PD studies have been somewhat controversial and confounded by limitations in sampling; in general, all demonstrated an activation of the peripheral immune status that probably was linked to an inflammatory condition present in brain. Interestingly, the patterns of the systemic inflammatory response observed in PD and AD share similarities, although they are not identical. Examples amongst many are that IL-1β and RANTES are increased in both AD and PD, whereas MCP-1 levels are increased in PD but not in AD, when compared with healthy age-matched controls. Such apparent differences between the results of AD and PD subjects may reflect the different immunological features triggered by PD and AD, as well as the different vulnerable target brain areas and neurons that define these diseases.
Certainly the most important limitation of the majority of research reviewed is the acknowledged cross-sectional design of the studies, from which it is not possible to define any ‘cause-effect relationship’. In particular, such studies cannot distinguish whether or not high cytokines levels might contribute to the pathogenesis of dementia or represent a protective response. The two are not mutually exclusive, and could be time-, location- and concentration-dependent. Moreover, dementia might lead to high cytokines levels, or other as yet unknown factors might contribute to both cognitive impairment and systemic inflammation. Although the precise molecular and cellular relationship between neurodegeneration and inflammation remains ambiguous, there is strong evidence that cytokines may induce activation of signalling pathways that lead to further inflammation and neuronal injury, and both pharmacological and immunological tools are now available to affirm or refute this. An interesting further question is whether or not systemic inflammation influences the progression of a chronic neurodegenerative disease, or is simply a consequence of tissue degeneration. In order to account for these different scenarios, two not mutually exclusive possibilities have been proposed. On one hand, the overproduction of brain cytokines might contribute to the pool of peripheral cytokines by a spill over from CNS [137], whereas on the other, peripheral cytokines might impact brain functions by crossing the blood brain barrier and directly interacting with CNS targets [138].
In the end, however, the greatest potential to aid the development of mechanistic-driven treatments will likely derive from identifying the crucial factors responsible for the detrimental neuroinflammatory responses that occur in neurodegenerative diseases. In this regard, promising results for neurological disease treatment may derive by targeting cytokines and chemokines in the development of antagonists and synthesis inhibitors.
Acknowledgments
The authors thank members of their respective laboratory for advice and encouragement. This work was supported in part by the University “Gabriele D’Annunzio”, Chieti-Pescara, Italy, and the Intramural Research Program of the National Institute on Aging, NIH, Baltimore, USA.
Abbreviation
- IL-
interleukin-
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- CNS
Central nervous system
- TNF-α
tumour necrosis factor-α
- CSF
cerebrospinal fluid
- NFT
neurofibrillary tangles
- DA
dopaminergic
- SN
substantia nigra
- GPCR
G-protein coupled receptor
- βAPP
β-amyloid precursor protein
- AChE
acetylcholinesterase
- TGF
Transforming Growth Factor
- LOAD
late-onset Alzheimer’s disease
- MCI
Mild Cognitive Impairment
- PBMC
peripheral blood mononuclear cells
- MMSE
mini-mental state examination
References
- 1.Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2002;1(1):1–31. doi: 10.1385/NMM:1:1:1. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192(1):106–13. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sambamurti K, Suram A, Venugopal C, Prakasam A, Zhou Y, Lahiri DK, Greig NH. A partial failure of membrane protein turnover may cause Alzheimer’s disease: a new hypothesis. Curr Alzheimer Res. 2006;3(1):81–90. doi: 10.2174/156720506775697142. [DOI] [PubMed] [Google Scholar]
- 4.Steinkan L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008;118(11):3557–63. doi: 10.1172/JCI36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- 6.Rojo LE, Fernández JA, Maccioni AA, Jimenez JM, Maccioni RB. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res. 2008;39(1):1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide-induced neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–145. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 8.Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm. 2004 doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated β-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-γ and IL-4 render them protective. Molecular and Cellular Neuroscience. 2005;29 (3):381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M, London A, Shechter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience. 2009;158(3):1133–42. doi: 10.1016/j.neuroscience.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 12.Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci. 2009;30(1):41–47. doi: 10.1016/j.tips.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Pollanen MS, Dickson DW, Bergeron C. Pathology and biology of the Lewy body. J Neuropathol Exp Neurol. 1993;52:183–191. doi: 10.1097/00005072-199305000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y. Lewy bodies are ubiquitinated: a light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl) 1988;75:345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- 15.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 16.de Silva HR, Khan NL, Wood NW. The genetics of Parkinson’s disease. Curr Opin Genet Dev. 2000;10:292–298. doi: 10.1016/s0959-437x(00)00082-4. [DOI] [PubMed] [Google Scholar]
- 17.De Marco EV, Annesi G, Tarantino P, Rocca FE, Provenzano G, Civitelli D, Ciro Candiano IC, Annesi F, Carrideo S, Condino F, Nicoletti G, Messina D, Novellino F, Morelli M, Quattrone A. Glucocerebrosidase gene mutations are associated with Parkinson’s disease in southern Italy. Mov Disord. 2008;23(3):460–463. doi: 10.1002/mds.21892. [DOI] [PubMed] [Google Scholar]
- 18.Passey S. Gene mutation detected in Parkinson’s disease. The Lancet Neurology. 2005;4 (3):142–143. doi: 10.1016/s1474-4422(05)01003-3. [DOI] [PubMed] [Google Scholar]
- 19.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hald A, Van Beek J, Lotharius J. Inflammation in Parkinson’s disease: causative or epiphenomenal? Subcell Biochem. 2007;42:249–279. [PubMed] [Google Scholar]
- 21.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. alpha-Synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2006;29(5):739–52. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A. The interplay between primary and secondary cytokines. Cytokines involved in the regulation of monocyte recruitment. Drugs. 1997;54(1):15–23. doi: 10.2165/00003495-199700541-00006. [DOI] [PubMed] [Google Scholar]
- 24.Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5(6):529–34. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- 25.Cardona AE, Li M, Liu L, Savarin C, Ransohoff RM. Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J Leukoc Biol. 2008;84(3):587–94. doi: 10.1189/jlb.1107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–18. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 27.Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain. 2008;131(7):1880–94. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Depino AM, Earl C, Kaczmarczyk E, Ferrari C, Besedovsky H, del Rey A, Pitossi FJ, Oertel WH. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson’s disease. Eur J Neurosci. 2003;18(10):2731–42. doi: 10.1111/j.1460-9568.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–12. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22(12):1399–419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- 32.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18(5):407–13. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Pitossi F, del Rey A, Kabiersch A, Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res. 1997;48:287–98. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Guerreiro R, Santana I, Bras JM, Santiago M, Santiago B, Paiva A, Oliveira C. Peripheral Inflammatory Cytokines as Biomarkers in Alzheimer’s Disease and Mild Cognitive Impairment. Neurodegenerative Dis. 2007;4:406–412. doi: 10.1159/000107700. [DOI] [PubMed] [Google Scholar]
- 35.De Simoni MG, Sironi M, De Luigi A, Manfridi A, Mantovani A, Ghezzi P. Intracerebroventricular injection of interleukin 1 induces high circulating levels of interleukin 6. J Exp Med. 1990;171:1773–8. doi: 10.1084/jem.171.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero LI, Kakucska I, Lechan RM, Reichlin S. Interleukin-6 (IL-6) is secreted from the brain after intracerebroventricular injection of IL-1 beta in rats. Am J Physiol. 1996;270:R518–24. doi: 10.1152/ajpregu.1996.270.3.R518. [DOI] [PubMed] [Google Scholar]
- 37.Lahiri DK, Chen D, Vivien D, Ge YW, Greig NH, Rogers JT. Role of cytokines in the gene expression of amyloid beta-protein precursor: identification of a 5′-UTR-binding nuclear factor and its implications in Alzheimer’s disease. J Alzheimers Dis. 2003;5(2):81–90. doi: 10.3233/jad-2003-5203. [DOI] [PubMed] [Google Scholar]
- 38.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong J, Knapp DJ, Crews FT. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral Inflammatory Biomarkers and Risk of Parkinson’s Disease. Am J Epidemiol. 2008;167(1):90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 40.Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta Neurol Scand. 1999;100:34–41. doi: 10.1111/j.1600-0404.1999.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 41.Singh VK, Guthikonda P. Circulating cytokines in Alzheimer’s Disease. J Psychiat Res. 1997;31:657–660. doi: 10.1016/s0022-3956(97)00023-x. [DOI] [PubMed] [Google Scholar]
- 42.Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y. Increase in peripheral CD4 bright+ CD8 dull+ T cells in Parkinson disease. Arch Neurol. 2001;58:1580–1583. doi: 10.1001/archneur.58.10.1580. [DOI] [PubMed] [Google Scholar]
- 43.Goncharova LB, Tarakano AO. Molecular networks of brain and immunity. Brain Res Rev. 2007;55:155–166. doi: 10.1016/j.brainresrev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Du Y, Dodel RC, Eastwood BJ, Bales KR, Gao F, Lohmuller F, Muller U, Kurz A, Zimmer R, Evans RM, Hake A, Gasser T, Oertel WH, Griffin WST, Paul SM, Farlow MR. Association of an interleukin 1 alpha polymorphism with Alzheimer’s disease. Neurology. 2000;55:480–483. doi: 10.1212/wnl.55.4.480. [DOI] [PubMed] [Google Scholar]
- 45.Griffin WS, Sheng JG, Roberts GW, Mrak RE. IL-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–81. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers JT, Lahiri DK. Metal and inflammatory targets for Alzheimer’s disease. Curr Drug Target. 2004;5(6):535–51. doi: 10.2174/1389450043345272. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, Griffin WS. Neuronal-glial interactions mediated by IL-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–55. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollak Y, Gilboa A, Ben-Menachem O, Ben-Hur T, Soreq H, Yirmiya R. Acetylcholinesterase inhibitors reduce brain and blood interleukin-1beta production. Ann Neurol. 2005;57(5):741–45. doi: 10.1002/ana.20454. [DOI] [PubMed] [Google Scholar]
- 50.Tweedie D, Sambamurti K, Greig NH. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res. 2007;4:378–85. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto M, Horiba M, Buescher J, Huang D, Gendelman H, Ranshoff R, Ikezu T. Overexpression of monocyte chemotactic protein—1/CCL2 in B-amyloid precursor protein transgenic mice show accelerated diffuse β-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luterman J, Haroutunian V, Yemul S, Ho L, Purohit D, Aisen P, Mohs R, Pasinetti GM. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch Neurol. 2000;57:1153–1160. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- 53.Paganelli R, Di Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, Iarlori C, Porreca E, Di Gioacchino M, Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer’s disease patients. Exp Gerontol. 2002;37(2–3):257–63. doi: 10.1016/s0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 54.Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, Gambi D. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp Gerontol. 2005;40(3):165–71. doi: 10.1016/j.exger.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Flanders KC, Lippa CF, Smith TW, Pollen DA, Sporn MB. Altered expression of transforming growth factor-beta in Alzheimer’s disease. Neurology. 1995;45:1561–69. doi: 10.1212/wnl.45.8.1561. [DOI] [PubMed] [Google Scholar]
- 56.Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM. TGF-beta 1 is an organizer of responses to neurodegeneration. J Cell Biochem. 1993;53:314–22. doi: 10.1002/jcb.240530408. [DOI] [PubMed] [Google Scholar]
- 57.Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobio. 1998;54:71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 58.De Simoni MG, Del Bo R, De Luigi A, Simard S, Forloni G. Central endotoxin induces different patterns of interleukin (IL)-1β and IL-6 messanger ribonucleic acid expression and IL-6 secretion in the brain and periphery. Endocrinology. 1995;136:897–902. doi: 10.1210/endo.136.3.7867598. [DOI] [PubMed] [Google Scholar]
- 59.Song DK, Im YB, Jung JS, Cho J, Suh HW, Kim YH. Central beta-amyloid peptide-induced peripheral interleukin-6 responses in mice. J Neurochem. 2001;76:1326–35. doi: 10.1046/j.1471-4159.2001.00121.x. [DOI] [PubMed] [Google Scholar]
- 60.Ozturk C, Ozge A, Yalin OO, Yilmaz IA, Delialioglu N, Yildiz C, Tesdelen B, Kudiaki C. The diagnostic role of serum inflammatory and soluble proteins on dementia subtypes: correlation with cognitive and functional decline. Behav Neurol. 2007;18(4):207–15. doi: 10.1155/2007/432190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 62.De Luigi A, Fragiacomo C, Lucca U, Quadri P, Tettamanti M, De Simoni MG. Inflammatory markers in Alzheimer’s disease and multi-infarct dementia. Mech Ageing Dev. 2001;122(16):1985–95. doi: 10.1016/s0047-6374(01)00313-x. [DOI] [PubMed] [Google Scholar]
- 63.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–40. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solerte SB, Cravello L, Ferrari E, Fioravanti M. Overproduction of IFN-gamma and TNF-alpha from natural killer (NK) cells is associated with abnormal NK reactivity and cognitive derangement in Alzheimer’s disease. Ann N Y Acad Sci. 2000;917:331–40. doi: 10.1111/j.1749-6632.2000.tb05399.x. [DOI] [PubMed] [Google Scholar]
- 65.Lanzrein AS, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1beta, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-alpha, the soluble tumor necrosis factor receptors I and II, and alpha1-antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12(3):215–27. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 66.van Duijn CM, Hofman A, Nagelkerken L. Serum levels of interleukin-6 are not elevated in patients with Alzheimer’s disease. Neurosci Lett. 1990;108:350–54. doi: 10.1016/0304-3940(90)90666-w. [DOI] [PubMed] [Google Scholar]
- 67.Angelis P, Scharf S, Mander A, Vajda F, Christophidis N. Serum interleukin-6 and interleukin-6 soluble receptor in Alzheimer’s disease. Neurosci Lett. 1998;244:106–108. doi: 10.1016/s0304-3940(98)00136-0. [DOI] [PubMed] [Google Scholar]
- 68.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:357–64. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 69.Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. J Psychiatr Res. 2007;41(8):686–93. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 70.De Luigi A, Pizzimenti S, Quadri P, Lucca U, Tettamanti M, Fragiacomo C, De Simoni MG. Peripheral inflammatory response in Alzheimer’s disease and multiinfarct dementia. Neurobiol Dis. 2002;11(2):308–14. doi: 10.1006/nbdi.2002.0556. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez A, Cacabelos R, Sampedro C, Garcia-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disesae. Neurobiology of Aging. 2007;28:533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Reale M, Iarlori C, Gambi F, Feliciani C, Lucci I, Gambi D. The acetylcholinesterase inhibitor, Donepezil, regulates a Th2 bias in Alzheimer’s disease patients. Neuropharmacology. 2006;50(5):606–13. doi: 10.1016/j.neuropharm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: The Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 74.Alvarez XA, Franco A, Fernandez-Novoa L, Ccabelos R. Blood levels of histamine, IL-1β and TNF-α in patients with mild to moderate Alzheimer disease. Mol Chem Neuropathol. 1996;29:237–52. doi: 10.1007/BF02815005. [DOI] [PubMed] [Google Scholar]
- 75.Chao CC, Ala TA, Hu S, Crossley KB, Sherman RE, Peterson PK, Frey WH. Serum cytokine levels in patients with Alzheimer Disease. Clin and Diagn Lab Immunol. 41994:433–436. doi: 10.1128/cdli.1.4.433-436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gambi F, Reale M, Iarlori C, Salone A, Toma L, Paladini C, De Luca G, Feliciani C, Salvatore M, Salerno RM, Theoharides TC, Conti P, Exton M, Gambi D. Alzheimer patients treated with an AchE inhibitor show higher IL-4 and lower IL-1 beta levels and expression in peripheral blood mononuclear cells. J Clin Psychopharmacol. 2004;24(3):314–21. doi: 10.1097/01.jcp.0000125683.74595.2f. [DOI] [PubMed] [Google Scholar]
- 77.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 78.Singh V. Studies of neuroimmune markers in Alzheimer’s disease. Progress in Drug Research. 1994;34:383–393. doi: 10.1007/978-3-0348-7128-0_12. [DOI] [PubMed] [Google Scholar]
- 79.Lugaresi A, Di Iorio A, Iarlori C, Reale M, De Luca G, Sparvieri E, Michetti A, Conti P, Gambi D, Abate G, Paganelli R. IL-4 in vitro production is upregulated in Alzheimer’s disease patients treated with acetylcholinesterase inhibitors. Exp Gerontol. 2004;39(4):653–57. doi: 10.1016/j.exger.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 80.Rentzos M, Paraskevas GP, Kapaki E, Nikolaou C, Zoga M, Rombos A, Tsoutsou A, Vassilopoulos DD. Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. J Neurol Sci. 2006;15, 249(2):110–4. doi: 10.1016/j.jns.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 81.Rota E, Bellone G, Rocca P, Bergamasco B, Emanuelli G, Ferrero P. Creased intrathecal TGF-beta1, but not IL-12, IFN-gamma and IL-10 levels in Alzheimer’s disease patients. Neurol Sci. 2006;27(1):33–39. doi: 10.1007/s10072-006-0562-6. [DOI] [PubMed] [Google Scholar]
- 82.Richartz E, Batra A, Simon P, Wormstall H, Bartels M, Buchkremer G, Schott K. Diminished production of proinflammatory cytokines in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(4):184–88. doi: 10.1159/000083497. [DOI] [PubMed] [Google Scholar]
- 83.Motta M, Imbesi R, Di Rosa M, Stivala F, Malaguarnera L. Altered plasma cytokine levels in Alzheimer’s disease: Correlation with the disease progression. Immunology Letters. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Schwab JM, Nguyen TD, Meyermann R, Schluesener HJ. Human focal cerebral infarctions induce differential lesional interleukin-16 (IL-16) expression confined to infiltrating granulocytes, CD8+ T-lymphocytes and activated microglia/macrophages. J Neuroimmunol. 2001;114:232–41. doi: 10.1016/s0165-5728(00)00433-1. [DOI] [PubMed] [Google Scholar]
- 85.Lindberg C, Chromek M, Ahrengart L, Brauner A, Schultzberg M, Garlind A. Soluble interleukin-1 receptor type II, IL-18 and caspase-1 in mild cognitive impairment and severe Alzheimer’s disease. Neurochemistry International. 2005;46:551–557. doi: 10.1016/j.neuint.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Malaguarnera L, Motta M, Di Rosa M, Anzaldi M, Malaguarnera M. Interleukin-18 and transforming growth factor-beta 1 plasma levels in Alzheimer’s disease and vascular dementia. Neuropathology. 2006;26:307–312. doi: 10.1111/j.1440-1789.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 87.Felderhoff-Mueser U, Schmidt OI, Oberholzer A, Buhrer C, Stahel PF. IL-18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005;28:487–93. doi: 10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 88.Bossu P, Ciaramella A, Salani F, Bizzoni F, Varsi E, Di Iulio F, Giubilei F, Gianni W, Trequattrini A, Moro ML, Bernardini S, Caltagirone C, Spalletta G. Interleukin-18 produced by peripheral blood cells is increased in Alzheimer’s disease and correlates with cognitive impairment. Brain, Behavior, and Immunity. 2008;22:487–492. doi: 10.1016/j.bbi.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Chao CC, Hu S, Frey WH, Ala TA, Tourtellotte WW, Peterson PK. Transforming growth factor beta in Alzheimer’s disease. Clin Diagn Lab Immunol. 1994;1:109–10. doi: 10.1128/cdli.1.1.109-110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zetterberg H, Andreasen N, Blennow K. Increased cerebrospinal fluid levels of transforming growth factor-beta1 in Alzheimer’s disease. Neurosci Lett. 2004;2,367(2):194–96. doi: 10.1016/j.neulet.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 91.Wyss-Coray T, Lin C, Yan F, Yu G, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–18. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 92.Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms increase risk of Parkinson’s disease. Arc Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- 93.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. TNF-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–10. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 94.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 95.Mogi M, Kondo T, Mizuno Y, Nagatsu T. p53 protein, interferon-γ, and NF-κB levels are elevated in the parkinsonian brain. Neuroscience letters. 2007;414:94–97. doi: 10.1016/j.neulet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2008;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Selikhova MV, Kushlinskii NE, Lyubimova NV, Gusev EI. Impaired production of plasma interleukin-6 in patients with parkinson’s disease. Bull Exp Biol and Med. 2002;133:81–83. doi: 10.1023/a:1015120930920. [DOI] [PubMed] [Google Scholar]
- 98.Kluter H, Vieregge P, Stolze H, Kirchner H. Defective production of Interleukin-2 in patients with idiopathic Parkinson’s disease. J Neurol Sci. 1995;133:134–9. doi: 10.1016/0022-510x(95)00180-a. [DOI] [PubMed] [Google Scholar]
- 99.Hasegawa Y, Inagaki T, Sawada M, Suzumura A. Impaired cytokine production by peripheral blood mononuclear cells and monocytes/macrophages in Parkinson’s disease. Acta Neurol Scand. 2000;101:159–164. doi: 10.1034/j.1600-0404.2000.101003159.x. [DOI] [PubMed] [Google Scholar]
- 100.Gangemi S, Basile G, Merendino RA, Epifanio A, Di Pasquale G, Ferlazzo B, Nicita-Mauro V, Morgante L. Effect of levodopa on interleukin-15 and RANTES circulating levels in patients affected by Parkinson’s disease. Mediators of inflammation. 2003;12:251–253. doi: 10.1080/09629350310001599701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, Zoga M, Tsoutsou A, Boufidou F, Kapaki E, Vassilopoulos D. Circulating interleukin-15 and RANTES chemokine in Parkinson’s disease. Acta Neurol Scand. 2007;116(6):374–79. doi: 10.1111/j.1600-0404.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 102.Stypula G, Kunert-Radek J, Stepien H, Zyliñska K, Pawlikowski M. Evaluation of interleukins, ACTH, cortisol and prolactin concentrations in the blood of patients with Parkinson’s disease. Neuroimmunomodulation. 1996;3:131–134. doi: 10.1159/000097237. [DOI] [PubMed] [Google Scholar]
- 103.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics. 2007;4(4):590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158(6):2882–90. [PubMed] [Google Scholar]
- 105.Huang D, Han Y, Rani MR, Glabinski A, Trebst C, Sorensen T, Tani M, Wang J, Chien P, O’Bryan S, Bielecki B, Zhou ZL, Majumder S, Ransohoff RM. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol Rev. 2000;177:52–67. doi: 10.1034/j.1600-065x.2000.17709.x. [DOI] [PubMed] [Google Scholar]
- 106.Teunissen CE, de Vente J, Steinbusch HW, De Bruijn C. Biochemical markers related to Alzheimer’s dementia in serum and cerebrospinal fluid. Neurobiol Aging. 2002;23:485–508. doi: 10.1016/s0197-4580(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 107.Xia MQ, Hyman B. Chemokines/chemokine receptors in the central nervous system and Alzheimer’s disease. J Neurovirol. 1999;5:32–41. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- 108.Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am J Pathol. 1998;153:31–37. doi: 10.1016/s0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meda L, Bernasconi S, Bonaiuto C, Sozzani S, Zhou D, Otvos L, Jr, Mantovani A, Rossi A, Cassatella MA. Beta-amyloid (25–35) peptide and IFN-gamma synergistically induce the production of the chemotactic cytokine MCP-1/JE in monocytes, and and microglial cells. J Immunol. 1996;157:1213–1218. [PubMed] [Google Scholar]
- 110.Szczepanik AM, Funes S, Petko W, Ringheim GE. IL-4, IL-10 and IL-13 modulate A beta(1–42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol. 2001;113(1):49–62. doi: 10.1016/s0165-5728(00)00404-5. [DOI] [PubMed] [Google Scholar]
- 111.Yates SL, Burgess LH, Kocsis-Angle J, Antal JM, Dority MD, Embury PB, Piotrkowski AM, Brunden KR. Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J Neurochem. 2000;74(3):1017–25. doi: 10.1046/j.1471-4159.2000.0741017.x. [DOI] [PubMed] [Google Scholar]
- 112.Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93(1–2):182–93. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 113.Prat E, Baron P, Meda L, Scarpini E, Galimberti D, Ardolino G, Catania A, Scarlato G. The human astrocytoma cell line U373MG produces monocyte chemotactic protein (MCP)-1 upon stimulation with beta-amyloid protein. Neurosci Lett. 2000;283(3):177–80. doi: 10.1016/s0304-3940(00)00966-6. [DOI] [PubMed] [Google Scholar]
- 114.Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16:136–44. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 115.Inadera H, Egashira K, Takemoto M, Ouchi Y, Matsushima K. Increase in circulating levels of monocyte chemoattractant protein-1 with aging. J Interf Cytokine Res. 1999;19:1179–82. doi: 10.1089/107999099313127. [DOI] [PubMed] [Google Scholar]
- 116.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 117.Zujovic V, Benavides J, Vige X, Varter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]
- 118.Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 119.Galimberti D, Schoonenboom N, Scarpini E, Scheltens P. Chemokines in serum and cerebrospinal fluid of Alzheimer’s disease patients. Ann Neurol. 2003;53(4):547–8. doi: 10.1002/ana.10531. [DOI] [PubMed] [Google Scholar]
- 120.Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- 121.Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, Berger K. Association between IL-8 cytokine and cognitive performance in an elderly general population. The MEMO-Study. Neurobiology of Aging. 2008;29:937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 122.Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological disease. JNeuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 123.Man SM, Ma YR, Shang DS, Zhao WD, Li B, Guo DW, Fang WG, Zhu L, Chen Y-H. Peripheral T cells overexpress MIP-1a to enhance its transendothelial migration in Alzheimer’s disease. Neurobiol of Aging. 2007;28:485–496. doi: 10.1016/j.neurobiolaging.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 124.Huerta C, Alvarez V, Mata IF, Coto E, Ribacoba R, Martinez C, Blazquez M, Guisasola LM, Salvador C, Lahoz CH, Pena J. Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer’s and Parkinson’s disease. Neurosci Lett. 2004;370(2–3):151–4. doi: 10.1016/j.neulet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 125.Nishimura M, Kuno S, Mizuta I, Ohta M, Maruyama H, Kaji R, Kawakami H. Influence of monocyte chemoattractant protein 1 gene polymorphism on age at onset of sporadic Parkinson’s disease. Mov Disord. 2003;18(8):953–55. doi: 10.1002/mds.10462. [DOI] [PubMed] [Google Scholar]
- 126.Colciaghi F, Marcello E, Borroni B, Zimmermann M, Caltagirone C, Cattabeni F, Padovani A, Di Luca M. Platelet APP, ADAM10 and BACE alterations in the early stages of Alzheimer disease. Neurology. 2004;62(3):498–501. doi: 10.1212/01.wnl.0000106953.49802.9c. [DOI] [PubMed] [Google Scholar]
- 127.Yu HL, Chertkow HM, Bergman H, Schipper HM. Aberrant profiles of native and oxidized glycoproteins in Alzheimer plasma. Proteomics. 2003;3:2240–48. doi: 10.1002/pmic.200300475. [DOI] [PubMed] [Google Scholar]
- 128.Bergman H, Salman H, Beloosesky Y, Djaldetti M, Bessler H. Are peripheral blood cells from patients with Alzheimer disease more sensitive to apoptotic stimuli? Alzheimer Dis Assoc Disord. 2002;16(3):156–60. doi: 10.1097/00002093-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 129.Li QX, Fuller SJ, Beyreuther K, Masters CL. The amyloid precursor protein of Alzheimer disease in human brain and blood. J Leukoc Biol. 1999;66(4):567–74. doi: 10.1002/jlb.66.4.567. [DOI] [PubMed] [Google Scholar]
- 130.Mecocci P, Polidori MC, Cherubini A, Ingegni T, Mattioli P, Catani M, Rinaldi P, Cecchetti R, Stahl W, Senin U, Beal MF. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch Neurol. 2002;59(5):794–98. doi: 10.1001/archneur.59.5.794. [DOI] [PubMed] [Google Scholar]
- 131.Kalman J, Kitajka K, Pakaski M, Zvara A, Juhasz A, Vincze G, Janka Z, Puskas LG. Gene expression profile analysis of lymphocytes from Alzheimer’s patients. Psychiatr Genet. 2005;15(1):1–6. doi: 10.1097/00041444-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 132.Shan X, Tashiro H, Lin CL. The identification and characterization of oxidized RNAs in Alzheimer’s disease. J Neurosci. 2003;23(12):4913–4921. doi: 10.1523/JNEUROSCI.23-12-04913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Percy ME, Andrews DE, Potter H. Peripheral markers of Alzheimer’s disease. In: Scinto LFM, Daffner KR, editors. Directions from the Alzheimer pathogenic pathway. Totowa, NJ, USA: Human Press; 2000. pp. 191–268. [Google Scholar]
- 134.DaSilva K, Brown ME, Westaway D, McLaurin J. Immunization with amyloid-beta using GM-CSF and IL-4 reduces amyloid burden and alters plaque morphology. Neurobiol Dis. 2006;23(2):433–44. doi: 10.1016/j.nbd.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 135.Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, Mori T, Smeed R, Crawford F, Klein T, Tan J, Mullan M. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer’s beta-amyloid(1–42) J Neuroimmunol. 2002;132(1–2):49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 136.Yamamoto M, Horiba M, Buescher J, Huang D, Gendelman H, Ranshoff R, Ikezu T. Overexpression of monocyte chemotactic protein—1/CCL2 in β-amyloid precursor protein transgenic mice show accelerated diffuse B-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nybo L, Nielsen B, Pedersen BK, Moller K, Secher NH. Interleukin-6 release from the human brain during prolonged exercise. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pollmächer T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines-do they affect human brain functions? Brain Behav Immun. 2002;16(5):525–32. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]