Abstract
Summary: The symbiosis between cnidarians (e.g., corals or sea anemones) and intracellular dinoflagellate algae of the genus Symbiodinium is of immense ecological importance. In particular, this symbiosis promotes the growth and survival of reef corals in nutrient-poor tropical waters; indeed, coral reefs could not exist without this symbiosis. However, our fundamental understanding of the cnidarian-dinoflagellate symbiosis and of its links to coral calcification remains poor. Here we review what we currently know about the cell biology of cnidarian-dinoflagellate symbiosis. In doing so, we aim to refocus attention on fundamental cellular aspects that have been somewhat neglected since the early to mid-1980s, when a more ecological approach began to dominate. We review the four major processes that we believe underlie the various phases of establishment and persistence in the cnidarian/coral-dinoflagellate symbiosis: (i) recognition and phagocytosis, (ii) regulation of host-symbiont biomass, (iii) metabolic exchange and nutrient trafficking, and (iv) calcification. Where appropriate, we draw upon examples from a range of cnidarian-alga symbioses, including the symbiosis between green Hydra and its intracellular chlorophyte symbiont, which has considerable potential to inform our understanding of the cnidarian-dinoflagellate symbiosis. Ultimately, we provide a comprehensive overview of the history of the field, its current status, and where it should be going in the future.
INTRODUCTION
Symbiosis, the living together of two or more organisms in a close, protracted relationship, ranges from mutualism, where both partners benefit from the association, to parasitism, where one partner benefits and the other suffers. Moreover, symbioses can shift along a continuum between these extremes, with, for example, some mutualisms becoming parasitic under certain environmental conditions (363).
Symbioses between invertebrates and photosynthetic partners are abundant in the marine environment, with the best known being the mutualism between members of the phylum Cnidaria (e.g., hard and soft corals, sea anemones, jellyfish, and hydrocorals) and dinoflagellate algae of the genus Symbiodinium (commonly referred to as zooxanthellae). These dinoflagellates typically reside within the cells of the host cnidarian's gastrodermis (i.e., the innermost tissue layer that borders the gastrovascular cavity), where they are bound by a membrane complex consisting of a series of membranes of algal origin plus an outermost host-derived membrane (184, 389); this entire entity is referred to as the symbiosome. The dinoflagellates can be acquired by maternal inheritance (79) or, more commonly, anew with each generation from the surrounding seawater (12) when they must invade their host and form a functional partnership in order to persist.
The cnidarian-dinoflagellate symbiosis is found across temperate and subtropical latitudes (see, e.g., references 252 and 410), but has particular ecological significance on tropical coral reefs. Here, the photosynthetic products supplied by the dinoflagellate symbionts support host coral metabolism, growth, reproduction, and survival (74, 268) in a habitat that is relatively lacking in exogenous supplies of food. Furthermore, these dinoflagellates promote the conservation and recycling of essential nutrients (206, 391), thus facilitating survival in the nutrient-poor waters that characterize many coral reefs, and enhance rates of coral skeletogenesis (129, 138), thus enabling the net accretion of the coral reef framework in the face of biological and mechanical erosion. In return for these various benefits, the dinoflagellates have access to nutrients in the coral's waste products, a stable position in the water column for accessing downwelling light, and increased protection from grazers.
The importance of this symbiosis to the success of coral reefs is profound. The appearance of coral reefs in the Triassic is thought to be a direct consequence of the evolution of the coral-dinoflagellate symbiosis (275), while the loss of the dinoflagellate symbionts and/or their photosynthetic pigments from corals (bleaching) in response to environmental stress can ultimately lead to the death of the coral and destruction of the reef (163, 402). Coral bleaching is of particular concern given that the frequency and severity of mass bleaching episodes are increasing as Earth's oceans warm up. Furthermore, other global environmental problems, such as ocean acidification, and the more localized impacts of sedimentation and nutrient pollution all have the potential to disrupt the coral-dinoflagellate symbiosis and so accelerate the loss of coral reefs. Alongside other impacts on reefs such as coral disease, destructive fishing practices, and nutrient-enhanced growth of benthic algae, these impacts have been projected to cause massive loss of reef systems and coral diversity during the 21st century (164, 165). In recent years, even relatively low-impact regions such as the Pacific Ocean have seen declines of about 2% per year in coral cover (38).
Despite the projected loss of coral reefs and the dire socioeconomic consequences associated with this loss (165), our fundamental understanding of the cnidarian-dinoflagellate symbiosis that underlies the ecological success of reefs remains poor. This is especially true compared to those terrestrial symbioses that have direct relevance to human health and productivity, for example, plant–nitrogen-fixing microbe mutualisms (see, e.g., references 72 and 334) or parasitic human-protozoan infections such as toxoplasmosis (see, e.g., references 34 and 181). We have recently highlighted the importance of molecular and cellular studies for deepening our understanding of the physiological mechanisms underlying coral-dinoflagellate symbiosis and calcification (404) and have argued for the application of a model systems approach to these studies (403). A greater understanding of the cell biology of cnidarian-dinoflagellate symbiosis is essential if we are to fully understand the mechanisms by which they are impacted by stress and whether or how corals and other symbiotic cnidarians might survive climate change and other environmental perturbations.
Here we will review in detail what is currently known, and indeed what is not, about the cell biology of cnidarian-dinoflagellate symbiosis, including its links to coral calcification. In doing so, we aim to refocus attention on fundamental cellular aspects that have been somewhat neglected since the early to mid-1980s, when a more ecological approach began to dominate. In particular, the 1990s and early 2000s saw an explosion of research concerning the diversity and ecological distribution of the symbiotic dinoflagellates and their photophysiological responses to environmental stress, driven not only by concerns over climate change but also by the development of readily accessible molecular identification techniques (54, 193, 318) and chlorophyll fluorescence methodologies (180, 295, 395). While unquestionably important, this research has not furthered our understanding of the host-symbiont interplay that controls the initiation and stability of the symbiosis or calcium carbonate deposition. Crucially, it has also meant that the critical mass of researchers needed to make substantial progress in these areas has been slow to build.
As a result of this research history, a number of key questions remain unresolved, which relate to the six phases (Fig. 1) of symbiosis establishment and persistence: (i) initial host-symbiont contact, (ii) symbiont engulfment, (iii) dynamic intracellular sorting of the symbionts, (iv) proliferation of the symbionts within the host tissues, (v) dynamic stability, and (vi) dysfunction and breakdown. For example, how do the host and symbiont recognize each other during and after phagocytosis? How is symbiont proliferation controlled, and are symbiont and host cell division coordinated? How is the translocation of photosynthetic products from the symbiont to the host controlled, and what are these translocated compounds? By what mechanism do the photosynthetic symbionts promote coral skeletogenesis and so enable the building of the coral reef framework? By addressing such gaps in our knowledge, we will be better placed to understand how environmental stresses such as global warming and ocean acidification induce dysfunction. The cell biology of bleaching itself is not a focus of this review, as it has been reviewed recently elsewhere (402).
Fig 1.
The six phases of symbiosis establishment and persistence in cnidarian-algal symbiosis. 1, initial surface contact between the algal symbiont and cnidarian host cell; 2, symbiont engulfment by the host cell; 3, dynamic sorting of the symbionts (now enclosed by a membrane of host origin), leading either to rejection of the symbiont (dashed arrow) or acceptance; 4, proliferation of the symbiont via cell division within the host tissues; 5, dynamic stability, where the symbiont population is maintained at a steady density; and 6, symbiosis dysfunction and breakdown (for example, in response to environmental stress). For simplicity, not all the possible cellular events are represented here; for more detailed descriptions of these events, see the relevant sections in the text.
Here we review the four major processes that we believe underlie these various phases of establishment and persistence in cnidarian/coral-dinoflagellate symbiosis: (i) recognition and phagocytosis, (ii) regulation of host-symbiont biomass, (iii) metabolic exchange and nutrient trafficking, and (iv) calcification. All of these likely involve host-symbiont signaling and signal transduction. This distinction between phase and process is important, because while the phases are distinct, the processes most likely overlap in time and may be involved in more than one phase. Where appropriate we will draw upon examples from a range of cnidarian-alga symbioses. In particular, we will refer not only to corals but also to sea anemones, close relatives of corals that are used widely as model organisms in the study of cnidarian-dinoflagellate symbiosis (403). We will also refer to the symbiosis between the freshwater hydroid Hydra viridis (also known as H. viridissima or green Hydra) and its intracellular chlorophyte symbiont belonging to the genus Chlorella (190). This symbiosis is relatively well studied and has the potential to substantially inform our understanding of the cnidarian-dinoflagellate symbiosis, especially with regard to host-symbiont recognition and phagocytosis. Ultimately, we aim to give a comprehensive overview of the history of the field, its current status, and where it should be going in the future. Furthermore, we aim to identify key questions that will benefit from closer collaboration between coral reef researchers and cell biologists.
RECOGNITION AND PHAGOCYTOSIS
The initial establishment of endosymbioses between host cnidarians and dinoflagellate endosymbionts can be subdivided into several phases: initial host-symbiont contact, symbiont invasion or colonization (sometimes referred to as infection), and host intracellular sorting of symbionts. Underlying this series of events are interconnected functional processes that are central to the successful establishment of the symbiosis. The first is host-symbiont recognition, a cascade of interpartner signaling events that controls and regulates the onset of symbiosis and also likely participates in regulation of stability and breakdown. The second process is the dynamic remodeling of the host cytoskeleton and membrane, resulting in engulfment of the symbiont by phagocytosis followed by stable persistence of symbionts in symbiosomes. This section will (i) start by providing perspective on how the pioneering investigators of cnidarian-alga symbiosis shaped the current questions in symbiosis establishment, (ii) describe how the modern fields of genomics and host-microbe interactions are revolutionizing the approaches to the study of establishment, and (iii) review the information to date on mechanisms governing the establishment of cnidarian-dinoflagellate symbiosis while pointing out areas for future work.
Establishment of symbiosis is one of the original areas of study in the cell biology of animal-alga symbiosis (182, 263). Several groups in the 1970s and 1980s performed studies on recognition, particularly in the green Hydra but also in the Paramecium-Chlorella symbiosis. Work on green Hydra proceeded relatively quickly because of its short generation time and amenability to laboratory culturing (described in reference 262) but also in part because of the architecture of the symbiont-containing host phagocytes and the ability to study macerated symbiotic host cells with relative ease. Furthermore, the process of symbiont invasion and host phagocytosis occurs at the apical end of the cell, followed by a “sorting phase” that involves migration of symbionts to the base of the cell, where they seem to be protected from digestion.
Studies of green Hydra formed the foundation for subsequent studies of symbiosis establishment in cnidarian-dinoflagellate symbiosis. Questions that were posed in this model remain, to this day, highly relevant and still largely unanswered. These include the following. (i) What molecules are used to signal between host and symbiont during symbiosis onset (178, 235, 237, 291)? (ii) When during initial contact and symbiont invasion of hosts does signaling occur (178, 236, 262, 263)? (iii) What are the cytoskeletal and membrane dynamics of symbiont engulfment and, ultimately, persistence in the symbiosome (234, 236)? (iv) Does the host mount an immune response that is modulated by the invading symbiont (167, 263)? (v) How do symbionts avoid being destroyed by the host (167, 262)?
Modern Fields of Genomics and Host-Microbe Interactions Have Caused a Paradigm Shift in the Study of Symbiosis Establishment
The fast-growing number of cnidarian genomic resources has been a “game changer” in the study of symbiosis establishment. The publishing of the nonsymbiotic cnidarian genome sequences for the anemone Nematostella vectensis (292) and the hydroid Hydra magnipapillata (46) and of the symbiotic cnidarian genome sequence for the scleractinian coral Acropora digitifera (328), along with numerous other genomic and transcriptomic resources for other cnidarians (see www.Compagen.org for a complete list), has resulted in rich data sets to mine for information on symbiosis establishment. From this burgeoning genomic information it is clear that ancestral or early-diverged metazoans such as cnidarians have genomic complexity that rivals that of higher vertebrates (243, 292). In contrast, traditional model invertebrates, the ecdysozoan nematodes and flies, have derived genomes that have much less complexity. Cnidarian genomic complexity is highly relevant to the study of symbiosis establishment. For example, with information in hand that cnidarians share gene repertoires for innate immunity (35, 100, 241, 324) and membrane trafficking (47–49) with vertebrates, hypothesis-driven questions on cnidarian symbioses can be posed based on other, better-studied animal-microbe interactions (327, 403).
Furthermore, it is now well recognized that negative and beneficial interactions share many of the same host-microbe signaling pathways and cellular responses, including host innate immune responses to invading microbes and symbiont mechanisms of invasion (156, 309, 327). It is useful, therefore, to understand the mechanisms of recognition, microbe engulfment, and persistence (Fig. 2) (see Appendix 1) in well-studied higher-animal systems and use this information as a scaffold for the patchy knowledge of symbiosis establishment in cnidarian-dinoflagellate symbiosis.
Fig 2.
Host-microbe signaling during microbial invasion and host phagocytosis. Animal innate immunity acts to detect and manage microbial invaders, both negative and positive. Whatever the quality of the interaction, the host needs to recognize the presence of the microbe and then launch downstream effector pathways to either destroy negative invaders or foster the growth of mutualistic ones. There are many excellent reviews of host-microbe signaling that cover these events in great detail (173, 233, 377). For direct detection of microbes, hosts express a dizzying array of proteins, termed pattern recognition receptors (PRRs) (Table 1), either secreted or on cell surfaces that recognize signature microbial compounds termed microbe-associated molecular patterns (MAMPs). (PRRs depicted: C3R, complement 3 receptor; Nods, nucleotide-binding oligomerization domain proteins; SRs, scavenger receptors; TLRs, toll-like receptors). MAMPs are a variety of sugar, protein, lipid, and nucleic acid compounds that are essential to microbial survival and often unique to certain microbe groups. They include lipopolysaccharide (LPS), peptidoglycan (PG), glycans, and glycosylphosphatidylinositol (GPI) anchors. Host cells can also detect the presence of microbes indirectly through the process of opsonization that can amplify a host response. Invading microbes become coated with secreted host compounds or opsonins, such as complement protein (C3) or immunoglobulins (in the case of vertebrates). Like MAMPs, opsonins then bind PRRs on host cell surfaces. The binding of PRRs to MAMPs or opsonins starts a signaling cascade, often involving the activation of the master immunity regulator nuclear factor κB (NF-κB), which then launches a large array of host responses. In the case of invading pathogens, these responses can include phagocytosis of the microbe, an inflammatory response, antimicrobial killing mechanisms, and initiation of host cell apoptosis or autophagy. The three processes shown within the box are described in more detail in Appendix 1.
Investigators of other endosymbiotic mutualisms describe the establishment of symbiosis as “the winnowing,” comprised of a complex series of steps, all of which are necessary but none of which is sufficient alone to result in a stable, specific symbiosis (66, 279). These steps span a range from interpartner molecular communication that occurs immediately upon contact to much longer-term microbial competition for the host niche. If we look back 30 years, we can see that researchers in the green Hydra field reached a similar conclusion (178, 220). McAuley and Smith (220) stated: “Recognition of suitable algae is unlikely to involve identification of a single algal character by (host) cells. The establishment of the symbiosis may depend upon a number of algal properties and interaction within the host cell.” It is the beginning of this winnowing process that is covered in this section.
Mechanisms of Symbiosis Establishment
Recognition.
It is worthwhile to briefly clarify the definitions of recognition and specificity, two terms that permeate the literature and often occur together. Recognition is molecular signaling that takes place between the host cnidarian and algae destined to be symbionts, most often during the onset of the association. Specificity is the taxonomic range of partners with which an organism associates (92). The focus here will be on recognition, i.e., relatively early events in the winnowing process that can contribute to the formation of a specific partnership. Other reviews are devoted to the topic of specificity in cnidarian-dinoflagellate associations and include coverage of studies that describe successional ecological competition for the host intracellular niche that occurs downstream of the initial recognition events (16, 17). It is important to emphasize here that the temporal window of recognition events in relation to engulfment and subsequent persistence of symbionts in host tissues, described below, is unclear. For example microbe-associated molecular pattern (MAMP)-pattern recognition receptor (PRR) interactions (Fig. 2) might be transient, occurring at the beginning of the interaction, or they might also be required for the maintenance of a stable association.
(i) Studies of recognition in green Hydra.
The best-described area of recognition in cnidarian-alga symbiosis, both in green Hydra- and in cnidarian-Symbiodinium associations, is MAMP-PRR signaling early during the onset of symbiosis. In the green Hydra field there was debate surrounding the topic of early recognition. Some studies showed that infection success could be greatly decreased by altering the algal cell surface. This included preincubating Chlorella with antibodies raised against whole intact Chlorella cells (291) or preincubating Chlorella or hosts with the lectin concanavalin A (ConA), which binds to α-mannose/α-glucose glycans (237). This led to the hypothesis that MAMP-PRR signaling via algal glycan-host lectin interactions was playing a role in infection (237). However, Jolley and Smith (178) repeated these experiments and also incubated algae in trypsin to remove putative residues coating the algae. In all cases, no lectin or enzyme treatment resulted in decreased infection success, leading the authors to conclude that initial algal engulfment was nonspecific and lacked any recognition mechanisms.
Two other studies showed that strains of Chlorella that released large amounts of maltose, the chief photosynthesis-derived metabolite translocated to the host from the symbiont in green Hydra, were more successful at colonizing host Hydra than those releasing little or no maltose (167, 220). Interestingly, those releasing little or no maltose were not sorted correctly by the host cell. Whereas high-maltose-releasing algae were phagocytosed at the apical end and transported to the base of the cell, where they are protected from digestion, low-maltose-releasing algae remained at the top of the cell and were subsequently attacked by host lysosomes. Similarly, high-maltose algae that were photoinhibited by incubation in 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) also failed to be sorted correctly, staying at the apical end of the cell, where they were destroyed (220). Together, these data suggest that maltose plays a dual role in the association: as a MAMP signaling to the host and as a translocated metabolite that participates in nutritional exchange in the mutualism (255).
(ii) MAMP-PRR signaling in cnidarian-dinoflagellate associations.
Glycan-lectin signaling is a common MAMP-PRR interaction in animal innate immune repertoires and an active area of research in cnidarian-dinoflagellate recognition. In higher animals, host lectins, either secreted (e.g., collectins and ficolins) or membrane associated (e.g., C-type lectins), bind glycans on invaders and then activate the complement pathway through the binding of specific serine proteases (MASPs) (233). This section will first summarize the evidence for glycans on Symbiodinium surfaces and lectins in host genomes and then discuss the functional evidence for glycan-lectin signaling during symbiosis establishment.
Symbiodinium cells, both in culture and in hospite, have been shown to exude glycoconjugates (205, 212–214). These substances were shown to have both carbohydrate and protein components via protein- and carbohydrate-specific staining of PAGE profiles (205, 212, 213). Profiles differed between algal cell types in culture (213), and antibodies raised against exudates from cultured S. microadriaticum (a clade A alga from the scyphozoan Cassiopeia xamachana) did not cross-react with exudates from other algal clades, indicating that the glycoprotein makeup and possibly the surface glycome (the entire population of glycans on the algal cell surface) varied between strains or clades (214). Immunolocalization experiments with symbiotic C. xamachana using the antiglycoconjugate antibody showed labeling surrounding S. microadriaticum, suggesting that these glycoproteins are exuded in hospite (214).
The makeup of the Symbiodinium cell surface glycome is the subject of two other recent studies (208, 418). Fluorescent lectin probes, together with confocal microscopy and flow cytometry, were used to identify glycan residues on Symbiodinium cell surfaces of both a freshly isolated alga (type C1f) from the host coral Fungia scutaria (418) and several cultured types (in clades A, B, D, E, and F) (208). Lectin-binding patterns varied dramatically in quality and intensity between different Symbiodinium types, suggesting a complex surface glycome that varies between types. N-Acetyl and mannose residues emerged as prominent and consistent glycans between groups (208). Both are well-characterized MAMPs that bind mannose-binding lectins and ficolins, respectively (233). The lectin ConA, which binds α-mannose and other residues, displayed significant labeling in all strains. We will return to this result in discussions of mannose-binding lectin homologs and functional studies with ConA below. Together these studies will point toward mannose as a strong candidate for a MAMP that could participate in the winnowing process. However, whether or not differing glycan profiles between Symbiodinium types play a role in conferring host-symbiont specificity (Nod factors in Rhizobium-leguminous plant symbioses are an example of this [66]) remains an open question.
Cnidarian genomic resources are helping with the identification of cnidarian lectins that could participate in MAMP-PRR interactions during symbiosis establishment. For example, 67 predicted C-type lectins (Table 1) with a total of 92 C-type lectin domains (CTLDs) have been identified in the genome of N. vectensis (419). This gives a glimpse of the diversity of lectins that could be functioning in cnidarian-dinoflagellate signaling. To date, three lectin types have been characterized in symbiotic corals and octocorals. An expressed sequence tag (EST) study of the coral Montastraea faveolata identified a homolog to tachylectin, which is known to bind pathogens in horseshoe crabs (324). A d-galactose-binding lectin, SLL-2, was purified from the octocoral Sinularia lochomodes, sequenced, and found by immunolocalization to occur surrounding symbiotic dinoflagellates in the gastrodermis (174, 175). Another galactose-binding lectin, CecL, from the coral Ctenactis echinata has also recently been described (176). Two lectins with CTLDs specific for mannose have been identified from corals: millectin from Acropora millepora (191, 192) and PdC lectin from Pocillopora damicornis (385). Both sequences contain signal peptides, suggesting that they are secreted, and like SLL-2, both localized around symbionts in gastrodermal cells. PdC lectin expression decreased in response to elevated-temperature stress, perhaps indicating that it functions during the healthy symbiosis and that this function is disrupted with stress and bleaching.
Table 1.
Pattern recognition receptors in animals that have homologs in cnidarians and that could play a role in cnidarian-dinoflagellate recognitiona
| PRRb | Descriptionc | MAMPs recognized |
|---|---|---|
| TLRs | Large group of transmembrane proteins (10 paralogs in humans); extracellular domain, set of LRRs; intracellular domain, TIR domain launches signal transduction pathway to activate NF-κB | LRRs bind a large variety of MAMPs, including glycans, LPS, PG, double-stranded RNA, flagellin, GPI anchors, unmethylated CpG DNA |
| Lectins | Secreted forms, e.g., MBL; membrane anchored forms, e.g., diverse C-type lectins | Glycans on glycolipids or glycoproteins |
| Scavenger receptors | Diverse, multidomain, cell surface glycoproteins; referred to as molecular flypaper due to their ability to bind a diversity of MAMPs; some mediate phagocytosis | Broad range, including LPS, double-stranded RNA, polyanionic ligands |
| Complement receptors | Bind complement in phagocytes, leading to phagocytosis activation | Complement and microbes opsonized with complement |
| NODs | Like TLRs, presence of numerous LRRs; presence of 1 or more CARD domains, thereby activating downstream pathways via CARD-CARD interactions; large family (as many as 30 in humans); evolutionarily ancient (plants to humans) | Recognize intracellular MAMPs, including LPS |
In addition to descriptions of MAMPs and PRRs in cnidarian-dinoflagellate symbioses, there is evidence that MAMP-PRR interactions function in recognition. Purified millectin bound to a variety of marine pathogenic bacteria as well as several Symbiodinium types in vitro (191). There is therefore potential for host millectin-symbiont mannose glycan signaling in hospite. In vitro experiments adding SLL-2 to three types of cultured Symbiodinium resulted in cessation of algal motility and morphological transformation from the biflagellated to the sessile coccoid form within 24 h (188). This same cessation of motility occurred with a variety of other nonsymbiotic phytoplankton. The loss of motility is significant because the nonmotile coccoid phase is typical of Symbiodinium when in the symbiotic state (described in more detail below in discussions of the Symbiodinium cell cycle). Taken together, these findings suggest that there is a generalized MAMP-PRR reaction that affects the phenotype of the invading alga.
Several studies directly address the role of glycan-lectin interactions during symbiosis establishment. In a study with the anemone Aiptasia pulchella (205) and another with larvae of the coral F. scutaria (418), Symbiodinium cell surfaces were altered by enzymatic removal of glycans and by glycan masking via the addition of one of a variety of lectins before the dinoflagellate was inoculated into aposymbiotic hosts. In both studies, glycan removal significantly decreased infection success, measured in A. pulchella by quantifying algal cells per tentacle and in F. scutaria larvae by quantifying both the percentage of larvae infected and the density of algae in larvae. In the A. pulchella study, incubations with 4 different lectins, including ConA, all significantly decreased infection success. In F. scutaria larvae, ConA and Jac (which binds galactose residues) both significantly decreased infection success. Infection success could be partially rescued by addition of the ConA-specific inhibiting sugar α-methyl mannopyranoside. A recent study of onset of symbiosis in juveniles of the coral Acropora tenuis measured infection success of two different Symbiodinium types, C1 and D, after alteration of the glycome by enzymatic digestion. The highest infection rates were achieved by C1 with an altered glycome, suggesting that removal of glycans slowed recognition events and winnowing and resulted in an unnatural superinfection (23). Taken together, these three studies provide direct evidence that glycan-lectin signaling, perhaps mannose-mannose-binding lectin signaling in particular, plays a role in symbiosis establishment and could be a first step in the winnowing process.
It is now clear that cnidarians possess many of the innate immunity PRRs besides lectins that are present in vertebrates (100, 186, 194, 241). Several that could be important in cnidarian-dinoflagellate interpartner recognition are listed and described in Table 1. Examination of the functional significance of PRRs in defense is just beginning (11, 35), and there are only two studies that hint at roles in symbiosis recognition. One study characterizing a complement (C3) homolog in the coral A. millepora localized C3 near the resident symbionts, suggesting that C3 could be opsonizing the symbionts, thereby acting in interpartner communication (191). In a functional genomics microarray study examining genes that were up- and downregulated in the symbiotic state in the sea anemone Anthopleura elegantissima, Rodriguez-Lanetty and coworkers identified a homolog to a scavenger receptor B/CD36 (Table 1; Fig. 2) that was upregulated in symbiotic animals (316). Scavenger receptors are common PRRs in phagocytic cells and are sometimes called “molecular fly paper” due to their ability to bind a diversity of MAMPs. Upregulation of a scavenger receptor in symbiosis could indicate that it functions in communication between partners.
Algal entry into hosts.
Perhaps surprisingly, the process of algal entry into hosts has received the least amount of attention of any stage of symbiosis establishment. Overall there is general agreement throughout studies of both the green Hydra and cnidarian-dinoflagellate symbioses that phagocytosis is the prevalent mode of entry. However, whether or not phagocytosis is selective or differential, for example, between food particles and algae or between different algal strains, is less clear. Green Hydra studies describe differential rates of uptake and differently shaped engulfing phagosomes (phagocytic profiles), visualized by scanning electron microscopy (SEM), in hosts when engulfing latex spheres versus heat-killed versus intact freshly isolated Chlorella (234) and when latex spheres were coated with anions and cations (235). However, others found no evidence that phagocytosis is specific to particle type or is anything more than a general feeding phenomenon (178, 236). These authors attributed the differential profiles to a confounding factor of contaminating host material that was present with freshly isolated algae.
In cnidarian-dinoflagellate symbioses, phagocytic profiles of host gastrodermal cells engulfing Symbiodinium spp. have been described for C. xamachana scyphistomae (56) and for larvae of F. scutaria (325). In experiments measuring rates of algal uptake, competition with inert carmine particles resulted in a decreased infection rate, suggesting that phagocytosis was nonspecific (56). In contrast, confocal imaging of infection in F. scutaria larvae revealed evidence of phagocytosis as a selective process (317). Larvae were challenged with both Symbiodinium type C1f freshly isolated from adult F. scutaria and type C31 from cooccurring Montipora capitata, which cannot successfully colonize F. scutaria larvae (401). Rates of incorporation of algae into gastrodermal cells from the gastrovascular cavity were lower for Symbiodinium C31 than for C1f. In addition, larvae demonstrated spatial selectivity, whereby C1f dinoflagellates were taken up preferentially around the equator of the larva compared to latex spheres and C31, which were taken up throughout the gastrodermis.
Both studies detailed above and all of the green Hydra work that examined algal engulfment used experimental manipulation to enhance infection: injecting algae into polyps in the case of C. xamachana and green Hydra and inducing a feeding response by the addition of food in the case of F. scutaria larvae. Very few studies have examined algal uptake under conditions that more closely approximate natural uptake from the surrounding environment. Hirose and coworkers (158) described dinoflagellate acquisition in juvenile polyps of Acropora spp. after coincubation with freshly isolated dinoflagellates. After several days of coincubation, dinoflagellates were still free in the gastrovascular cavity and associated with host cellular debris. After this, some dinoflagellates appeared to be trapped by cytoplasmic processes and/or elongate cilia extending from host gastrodermal cells before later appearing in host gastrodermal cells. In a study of F. scutaria embryonic development, Marlow and Martindale (215) found symbionts in the endoderm and ectoderm prior to the formation of a mouth. This indicates that there are modes of invasion other than phagocytosis of algae after entry into the gastrovascular cavity. Mechanisms of symbiont sequestration into germ cells or developing embryos that occurs in cnidarian species that undergo vertical symbiont transmission is also very poorly understood (24, 79). Symbiodinium acquisition by or invasion of cnidarian hosts is an obvious area that needs substantial further study.
Arrest of phagosomal maturation.
The question of how invading algae manage to avoid intracellular attack in host phagocytes has been of interest for decades. Simply put, how is a phagosome, destined to destroy its occupant, converted to a symbiosome that tolerates algae and allows them to persist? The problem was first addressed with the ciliate Paramecium bursaria, which is colonized by a species of Chlorella. Both pioneering (182, 183) and recent work (reviewed in reference 187) found evidence that Chlorella inhibits phagosome-lysosome fusion. Similar examinations describing phagosome-lysosome fusion followed with green Hydra and with the jellyfish C. xamachana, which harbors S. microadriaticum. Aposymbiotic H. viridis fed healthy Chlorella showed the lysosomal markers acid phosphatase, ferritin, and thorium surrounding the phagocytosed heat-killed algae or other particles but showed no label around healthy algae (167, 280). Likewise, in C. xamachana, Fitt and Trench (110) found that both ferritin and acid phosphatase colocalized to symbiosomes with heat-killed ingested S. microadriaticum, while symbiosomes containing healthy dinoflagellates remained free of either marker.
Studies of the anemone A. pulchella have generated further evidence, by tracking the location of Rab GTPases, that phagosomal maturation and endosomal trafficking (described in Appendix 1) are altered by the presence of Symbiodinium cells in phagosomes (see Appendix 1). First, cnidarian orthologs to human Rab5 and -7 were sequenced and shown in heterologous systems to be present in the same endosomal locations as their corresponding orthologs in vertebrates: Rab5 and Rab7 in early and late endosomes, respectively (47, 48). In immunofluorescence examinations of A. pulchella gastrodermal cell macerates, anti-human Rab5 appeared around healthy newly ingested and already-established Symbiodinium but was absent from around heat-killed or DCMU-treated newly ingested Symbiodinium. Conversely, anti-human Rab7 localized around heat-killed or DCMU-treated newly ingested Symbiodinium but was absent from untreated newly infected or already-established Symbiodinium. These studies suggest that the Symbiodinium cell somehow arrests phagosomal maturation in the early phagosome stage, as evidenced by the presence of Rab5 and absence of Rab7.
Subsequent work by this group has continued along these same lines to investigate the roles of Rab11 and -4 (49, 168), two controllers of endosomal membrane recycling (323). Symbiosomes containing healthy Symbiodinium were Rab11 negative and Rab4 positive. Rab4 was found to be immediately recruited to all early phagosomes but was retained only in those containing healthy symbionts, suggesting that Rab4 is essential to the generation of the symbiosome.
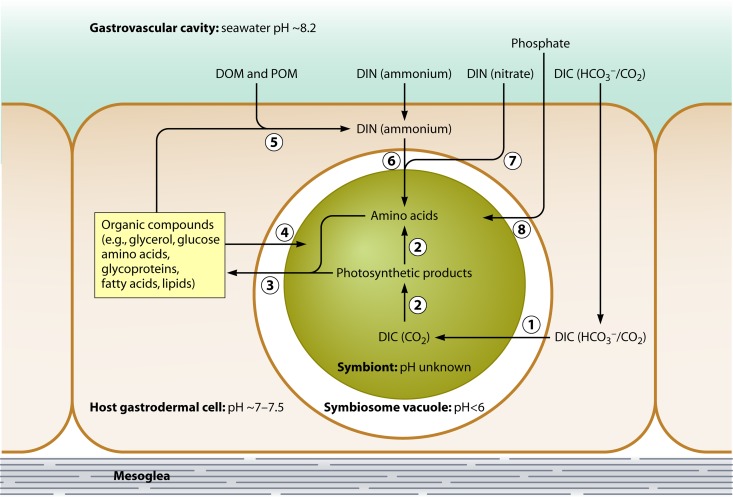
Venn and coworkers have recently measured the intracellular pH of macerated host gastrodermal cells with resident symbionts from the coral Stylophora pistillata using the pH-sensitive fluorophore SNARF-1 and confocal imaging (383; see below for more details). They estimated that the pH of the symbiosome is <6.0, a level consistent with the luminal pH of late phagosomes (see Appendix 1). Taken together these investigations indicate that, like Leishmania spp., Mycobacterium tuberculosis, and others (some are listed in Table A1 in Appendix 1), Symbiodinium survives in symbiosomes in part by manipulating endosomal trafficking. Studies examining the specific effectors of this arrest are a fundamental next step.
Evidence of apoptosis and autophagy during recognition.
Following engulfment, there is a complex suite of downstream cellular responses to an invading microbe (Fig. 2; see Appendix 1). To date, this is an area of symbiosis establishment that has received relatively little attention. Dunn and Weis (101) found evidence that apoptosis plays a role in postphagocytic sorting processes. As described above, larvae of F. scutaria were challenged with both Symbiodinium C1f from F. scutaria and Symbiodinium C31 from M. capitata, which cannot colonize F. scutaria. Larvae challenged with C31 showed high caspase activity in the gastrodermis, measured by quantifying a caspase-specific fluorophore with confocal microscopy, compared to those with C1f symbionts. This activity was inhibited with the addition of caspase inhibitors. These data suggest that the host mounts an innate immune response against incompatible Symbiodinium. To expand on the winnowing process, this indicates that Symbiodinium C31 cells make it past the glycan-lectin stage but not past a subsequent step which in turn sets off the apoptotic response by the host.
The phenomenon of autophagy, the cellular process of removal and degradation of organelles, cytoplasmic contents, and microbial invaders (202, 203), is a microbial control mechanism that is yet to be investigated in cnidarian-dinoflagellate symbiosis recognition. Autophagy is of interest because of its links to other membrane trafficking pathways and to apoptosis. Furthermore, there is some evidence that it plays an active role in the elimination of symbionts during the bleaching response and could therefore also function in recognition (93, 99). Characterization of this highly conserved process in cnidarians and its potential involvement in recognition could be a fruitful area for future research.
Evidence of cell signaling processes in recognition.
Two of the most highly upregulated genes in symbiotic anemones encode sym32, a protein described first in Anthopleura elegantissima (311, 326, 399) and more recently in Anemonia viridis (122), and calumenin (122). Ganot and coworkers (122) have developed a testable model for the role that these two proteins play in recognition and tolerance of symbionts. sym32 is a member of the fasciclin 1 family of cell adhesion and signaling proteins. A previous study localized sym32 to symbiosomes (326) and hypothesized that it serves an interpartner signaling function. Calumenin is a Ca2+-binding EF hand protein that participates in critical posttranslational γ-carboxylation of proteins, including fasciclin I orthologs in mammals (68). The model proposes that the presence of symbionts signals calumenin to promote γ-carboxylation maturation of sym32 on symbiosomes. How sym32 participates in cell signaling or recognition interactions between host and symbiont is a topic for future investigation.
In summary, the processes of recognition and phagocytosis are areas of cnidarian-dinoflagellate symbiosis cell biology under active investigation. Rapidly increasing genomic resources will continue to provide crucial information about both the innate immune repertoire and the genes involved in phagocytosis in the host. Complementary genomic resources for the dinoflagellate are far behind, in large part because the genome sizes of dinoflagellates are equal to or greater than the size of the human genome. Investment in these resources is important for the elucidation of pathways that symbionts use to induce host tolerance to invasion. The continuing development of techniques such as transformation and reverse genetics (97, 417) will allow for direct description of the functional mechanisms that underlie the establishment of symbiosis.
REGULATION OF HOST-SYMBIONT BIOMASS: SYMBIONT EXPULSION, DEGRADATION, AND CELL CYCLE CONTROL
Once established, the long-term persistence and stability of a symbiosis requires coordination between host cell growth and proliferation of the symbiont population, so that both occur in dynamic equilibrium. Without this coordination, the symbiont population might be diluted and hence be less effective at supporting the host's metabolic needs, or the symbionts might overgrow the host's tissues (277, 359). To date, there have been numerous descriptive studies of the processes that regulate host-symbiont biomass (see below), yet the cellular mechanisms that underlie this regulation are largely unknown.
Diagrammatic depictions of symbiont-containing host cells discussed in this review are shown in Fig. 3. The gastrodermal cells of symbiotic corals and other anthozoans are about 10 by 25 μm in size and harbor 1 or 2 Symbiodinium cells of about 10 μm in diameter (124, 179, 265, 274). Muscatine and coworkers (274) were the first and to date the only researchers to study this cell-specific density (CSD) in detail, measuring an average CSD of 1.54 (range, 1.11 to 2.19) and a maximum CSD of 12 dinoflagellate cells per host cell in macerated preparations of 33 different species of Anthozoa (under nonstressful conditions both in the field and in culture), including 19 species of reef-building coral. The CSD of anthozoans is very different from that seen in some other invertebrates, however, with larger host cells tending to house greater numbers of symbionts. For instance, in the field, hydranth cells of the marine hydroid Myrionema amboinense (30 by 50 μm in size) typically harbor 1 to 3 Symbiodinium cells (113), while the often disc-shaped host cells in the tentacles (50 to 70 μm diameter) may contain 10 to 50 symbionts (R. K. Trench, personal communication, cited in references 113 and 274). Similarly, in culture, cells of the green Hydra (20 by 100 μm in size) contain 15 to 20 Chlorella cells (219, 265), while the protozoan Paramecium bursaria (50 by 150 μm) contains as many as 300 Chlorella cells (265). However, while the volume of the host cell may partly determine its carrying capacity (113, 218, 265, 274), it is important to note that the symbiont density can be regulated at a constant level (i.e., steady state) below this upper limit. For example, chronic nutrient enrichment increased the CSDs of 11 coral species by an average of 21.2% (274).
Fig 3.
Diagrammatic representation of individual cnidarian host gastrodermal cells with resident symbionts. Each symbiont is surrounded by a symbiosome membrane complex, depicted as a single black line. Note the dramatically different host cell sizes, with the hydroids green Hydra and Myrionema sp. having very large cells compared to the typical anthozoan cell. The larger hydroid cells harbor more symbionts per cell than the anthozoan. Chlorella cells are elliptoid in shape. Symbiodinium cells are spherical in shape and approximately 10 μm in diameter. Reports of Chlorella cell size vary, and so they are depicted here as approximately 5 to 8 μm in diameter, which is within the range described in many studies.
The growth rate of the symbiotic dinoflagellates is potentially much higher than that of the host, as demonstrated by the more rapid growth of Symbiodinium cells when in culture than in hospite. Typically, the frequency of dinoflagellate mitosis (mitotic index [MI]) in hospite, as determined by the appearance of doublet cells, is <5%, though it has been measured as >10% in some species; these values translate to doubling times that commonly exceed 10 days and may even exceed 70 days (see, e.g., references 75, 159, 179, 268, 412, and 414). In contrast, when in culture, doubling times are commonly 2 to 5 days (see, e.g., references 45, 89, 111, and 207). Research has focused on the control of symbiont cell division and proliferation but has largely ignored complementary studies on these processes in the host.
Three potential mechanisms have been suggested for the regulation of symbiont numbers in alga-invertebrate symbioses (265) and are depicted in Fig. 4: (i) expulsion of excess symbionts, (ii) degradation of excess symbionts by host cells, and (iii) inhibition of symbiont cell growth and division. The green Hydra symbiosis has been reasonably well studied with regard to premitotic cell cycle control and host-symbiont coordination; the postmitotic processes of degradation and expulsion have received less attention. In contrast, there have been surprisingly few attempts to elucidate these various mechanisms in the cnidarian-dinoflagellate symbiosis.
Fig 4.
Potential mechanisms for the regulation of host-symbiont biomass in cnidarian-alga symbiosis. 1, expulsion of symbiont cells in either detached whole host cells or pinched-off portions of host cells (i.e., aposomes). 2, expulsion of symbiont cells either via active exocytosis or as a result of host cell apoptosis. 3, intracellular degradation of the symbiont, as a result of programmed cell death of the symbiont, reengagement of the phagosomal maturation process in the host, or autophagic digestion of the symbiont by the host cell. 4, control of progression through the symbiont cell cycle by the host. G0, G1, S, G2, and M are the phases of the eukaryotic cell cycle (see Appendix 2), with G1 often being the longest phase and M always being the shortest (as generalized in the schematic). The host may render the intracellular environment unfavorable or signal to the symbiont in such a way that the cell cycle does not, for example, pass through the G1/S checkpoint; in this case, the cell could enter the G0 resting state. 5, control of host cell proliferation by the symbiont.
Expulsion and Degradation of Excess Symbionts
Both expulsion and active degradation of supernumerary symbionts require the host to detect these symbionts and reduce their density back to the steady-state level (113). Expulsion seems to be a common feature of cnidarian-dinoflagellate symbiosis, but there is relatively little information about the degradation of healthy symbionts in this association. In comparison, in green Hydra both expulsion and intracellular degradation were for many years considered unimportant, except under environmental stress (see, e.g., references 91, 217, 277, and 341). However, there is now good evidence that expulsion (109) (see below), if not degradation (but see reference 96), might play an important role.
Symbiont expulsion.
The extrusion of pellets containing symbiotic dinoflagellates was reported in the early 1970s for a range of coral, sea anemone, and zoanthid species (307, 338, 339, 372). Steele (339) was the first to consider the significance of these pellets in detail. He conducted light microscopic examinations of pellets released by 11 tropical sea anemone species, noting the life history stage of the dinoflagellates and their state of health, and found that these pellets consisted largely of algal debris held together by mucus but also contained “a very small number” of intact symbiont cells. Steele concluded that the expulsion of these pellets must be a common occurrence and that they help to control the symbiont population by removing degraded and, to a lesser extent, healthy symbiont cells; the expulsion of healthy symbionts (with the potential to infect new hosts) has more recently been supported by assessments of their photosynthetic health (29, 295).
A wide range of expulsion rates has now been reported, suggesting that the importance of this regulatory mechanism varies between symbioses. Very low rates of symbiont expulsion (∼0.1 to 1.0% of the symbiont standing stock per day) have been reported for a range of tropical cnidarians, including the scleractinian corals Stylophora pistillata, Seriatopora hystrix, Pocillopora damicornis, and Acropora formosa (159, 160, 179, 271, 346), the soft corals Xenia macrospiculata and Heteroxenia fuscescens, and the fire coral Millepora dichotoma (159). These expulsion rates are not constant throughout the day, however, with no clear diel pattern across all species (159, 179, 346). The lowest rates of ∼0.1%, observed in various Red Sea corals, equated to just 4% of the rate at which cells were added to the symbiont population (159). This, together with the relative constancy of expulsion under steady environmental conditions (332) and the stimulation of expulsion only under conditions of stress (123, 159, 160, 332, 345), led Smith and Muscatine (332) to propose that expulsion is associated with host cell turnover, consistent with observations of symbiotic dinoflagellates being expelled within intact host cells (123, 179).
A more dominant role for expulsion has been suggested elsewhere. Expulsion was suggested as the primary process by which the symbiont density is maintained in the temperate sea anemone Anthopleura elegantissima, given that expulsion can exceed daily growth of the symbionts at irradiances of less than full sunlight (232), while in the temperate Atlantic coral Astrangia poculata, relatively high rates of expulsion allow the coral to sometimes persist in a stable, near-nonsymbiotic state (86). Similarly, Baghdasarian and Muscatine (15) concluded that expulsion is one of the primary regulators of the symbiont density in some (though not all) tropical cnidarian-dinoflagellate symbioses. These authors measured a daily expulsion rate of 4.6% in Aiptasia pulchella under steady-state conditions, with those symbionts undergoing division (as determined by 3H incorporation) being preferentially expelled; they also observed preferential expulsion in the coral Pocillopora damicornis but not in the corals Montipora verrucosa (= Montipora capitata), Porites compressa, and Fungia scutaria.
Despite these various observations, the cellular mechanisms that lead to symbiont expulsion as a part of host-symbiont biomass homeostasis remain completely unexplored in the cnidarian-dinoflagellate symbiosis. In contrast, symbiont loss as a part of the coral bleaching process has been examined and reviewed extensively elsewhere (201, 402), with processes shown to cause symbiont expulsion including exocytosis of symbionts, host cell apoptosis, and host cell detachment. These same mechanisms could be at play in a more modulated fashion, as a part of symbiont population maintenance. Indeed, a recent histological study of the green Hydra symbiosis showed that immediately following feeding under normal conditions, the symbiotic algae migrate from their usual basal location to the apex of the cell and are then expelled into the gastrovascular cavity via either “pinching off” of aposomes or active exocytosis (109). Establishing the role that such mechanisms play in the cnidarian-dinoflagellate symbiosis is an obvious area for future research.
Symbiont degradation.
Degraded symbiotic dinoflagellates tend to show a loss of circular symmetry, uneven and relatively dark coloration, an accumulation of unidentified droplets and starch-like globules, and a loss of cellular integrity (e.g., cell wall damage) before degenerating into smaller fragments that contain “accumulation bodies,” unpacked thylakoids, and starch (113, 364, 372). Degradation appears to be an intracellular process, regulated by the activities of the host's gastrodermal cells. Symbiont degradation by a coral (Astrangia danae) was first reported by Boschma (36), who noted dinoflagellate cells in various stages of degradation in the gastrovascular cavity of the host animal. Degraded symbionts and cellular debris were subsequently observed to form a substantial component of the pellets extruded by various tropical sea anemones (339, 340), and moribund or dead dinoflagellate cells have now been observed in the tissues of numerous species of reef corals (364–366) and other anthozoans (340, 372), as well as the hydroid Myrionema amboinense (112, 113). In the coral Stylophora pistillata, the frequency of degrading symbionts was found to be 5 to 6 times and 30 times greater in the digestive cells of the mesenteries than in those of the connecting sheet and tentacles, respectively (364). This finding supported the previous suggestion of Trench (372) and Steele and Goreau (340) that the symbiont cells are expelled into the gastrovascular cavity and then incorporated into the mesenteries by phagocytosis, where they undergo degradation; the unassimilated cell fragments are then released back into the gastrovascular cavity before being expelled into the ambient environment as a pellet. However, Trench (372) described the degradation of only senescent cells in the zoanthid Zoanthus sociatus, whereas Titlyanov et al. (364) also observed the degradation of apparently healthy symbionts.
Titlyanov and coworkers (364) estimated that, in eight different coral species, 1 to 6% of symbionts per day are in a state of degradation, a value that is notably similar to the symbiont division rate. The same authors reported a diel periodicity in the appearance of degrading symbionts, with a peak in the middle of the night and the whole degradation process lasting for about 6 h. A nighttime peak was similarly reported for M. amboinense, which in the morning was surrounded by expelled pellets composed largely of “unhealthy” (i.e., asymmetrical and dark) dinoflagellate cells (113); this peak equated to ∼1 unhealthy symbiont per host digestive cell out of an average of 2.67 symbionts per cell. Titlyanov et al. (364) considered this degradative process as “digestion,” based on four criteria: (i) its occurrence inside the host's gastrodermal cells; (ii) the observation of a diel rhythm of degradation; (iii) the absence of nuclei, proteinaceous structures, lipid droplets, and chlorophyll c in the expelled cell debris; and (iv) intensified degradation during periods of host starvation. Older or “weaker” symbionts were hypothesized to be more susceptible to lysosomal attack in the hydroid M. amboinense (112); in contrast, Titlyanov et al. (364) did not observe this mechanism in reef-building corals.
As with the process of expulsion, the mechanisms of symbiont degradation have been examined only in the context of stress and bleaching and not as part of a homeostatic mechanism. Processes that could result in degradation of symbionts include programmed cell death of the symbiont (98), a reengagement of the phagosomal maturation process in the host (see Appendix 1), and autophagic digestion of the symbiont by the host cell. Indeed, the observation of increased symbiont degradation during starvation is consistent with an onset of autophagy, which is commonly induced by nutrient limitation (see, e.g., reference 33). Moreover, Dunn and coworkers (99) found significantly increased bleaching in samples of A. pallida incubated at ambient temperatures in the presence of rapamycin, a promoter of autophagy. This suggests that autophagy could play a role in the regulation of the symbiont population even in the absence of stress. We are still a long way, though, from resolving the mechanisms underlying symbiont degradation and their relative importance in the regulation of the cnidarian-dinoflagellate symbiosis.
Regulation of Symbiont and Host Cell Growth and Proliferation
Understanding how the host might regulate the growth and proliferation of its symbionts requires an appreciation of the eukaryotic cell cycle (cell division cycle) and the specific “checkpoints” at which the control system could act (see Appendix 2). Our knowledge of how the eukaryotic cell cycle is controlled gained momentum only in the late 1980s (1), so it is perhaps not surprising that we still know very little about how this control is enforced in the cnidarian-alga symbiosis. Nevertheless, there have been numerous relevant studies of symbiont growth and division patterns, especially in the green Hydra symbiosis, and many of the patterns reported in these studies are consistent with our current knowledge of cell cycle control in other eukaryotic systems.
Studies of green Hydra.
When green Hydra is starved, the symbiont size slowly increases until the cells accumulate at the size typical for division (6 to 9 μm in diameter), yet the frequency of division is reduced substantially (91, 221, 225 [but see reference 286]). Furthermore, in the dark, the symbiont cells divide at a smaller size than in the light (222), suggesting that in the light they grow beyond their critical size for division. Taken together, these observations indicate that algal proliferation in the green Hydra symbiosis is regulated, with potential restrictions on the cell cycle that limit progression through interphase and into the M phase.
Four different mechanisms for the control of the symbiont cell cycle have been suggested for the green Hydra symbiosis: (i) pH-stimulated release of photosynthate (maltose) to the host and concomitant reduction in symbiont growth (91, 223); (ii) host-regulated supply of a metabolite(s), a so-called “division factor” (219, 221, 222, 225, 265); (iii) host-regulated supply of inorganic nutrients to the symbiotic algae (31, 219, 223, 265, 266, 277, 299, 301); and (iv) production of a density-dependent inhibitor by the algae themselves (219, 265). With the exception of self-inhibition by the algae, these mechanisms have been explored experimentally.
Isolated Chlorella cells have been shown to release maltose in response to acidification, with maximal release at about pH 4.5 (43, 239, 258). Moreover, Douglas and Smith (91) demonstrated that those Chlorella strains that release large amounts of maltose do not grow at low pH in vitro, whereas those strains that release less maltose are able to do so. Together, these observations provide a mechanism by which a small, temporary shift in the pH of the symbiosome vacuole (i.e., the space between the symbiont and host) could modify the amount of photosynthate released to the host. This diversion of carbon could then cause carbon starvation and so hinder progression through the cell cycle either directly (91) or indirectly, by reducing the capacity for nitrogen assimilation and protein synthesis (90, 223, 229, 231, 300, 304). Direct evidence for the operation of such a mechanism in hospite is, however, lacking; indeed, immunocytochemical analysis of the symbiosome vacuole in green Hydra did not support the view that it is acidic (296).
As the algal symbionts continue to divide and increase in density, albeit slowly, in the digestive cells of starved green Hydra (91, 221) and excised and unfed regenerating heads and peduncles (217, 219), it was speculated that symbiont division may be limited (except during periods of host cell division) by restricted access to a pool or pools of metabolites (a “division factor”) derived from host digestion (221, 222, 228). However, the identity of this putative division factor has never been explored in detail.
In contrast, inorganic nutrients are well known to influence the symbiont division rate in green Hydra. In one study, the addition of a complex mixture of nutrients even caused the symbionts to overgrow their host (266). This suggests that the host might regulate symbiont growth by controlling the supply of inorganic nutrients arising directly from host digestion. In particular, while there appears to be little capacity for the host to withhold phosphate (411) and sulfate (60), there is evidence that the control of nitrogen delivery (especially excretory ammonium) to the symbionts is important (224–226, 299–304). Indeed, McAuley and Muscatine (225) showed that when Hydra is starved, the Chlorella cells can slowly progress through S phase of the cell cycle, but feeding is required before mitosis can occur and the cell cycle is completed; this observation suggests arrest of the cell cycle at the G2/M checkpoint (Appendix 2), but how the host controls this is unknown. Enzymatic assays showed that Hydra has the potential to assimilate ammonium and withhold it from its symbionts (299). This ultimately led to a model in which the host maintains a low concentration of ammonium in the symbiosome vacuole except for when the algal symbionts divide; this flux of ammonium not only would provide nitrogen for amino acid synthesis but also would increase the pH of the symbiosome vacuole, thus reducing the rate of maltose release and leaving more carbon for symbiont growth (300, 305). Support for this model was provided by Rees (303), who reported rates of uptake of the ammonium analog methylammonium by freshly isolated Chlorella cells that were consistent with a low-ammonium environment in the symbiosome vacuole. However, whether this proposed mechanism actually operates in hospite has yet to be confirmed, while we still cannot be certain how it relates to the other mechanisms discussed, which could be interrelated.
As first suggested by Pardy (283, 284), the host has the potential to regulate symbiont division so that it is closely linked to the division of its own cells. When green Hydra is fed, the diel pattern of host cell division becomes synchronized, with a peak MI of about 2% that occurs 10 to 12 h after feeding (219, 266). Symbiont division closely matches this synchronous pattern (219), though the synchrony of host and symbiont division can be disrupted by starvation (221). A different pattern is seen in excised regenerating peduncles, where first the algae and then the host cells divide (217, 219), causing an initial increase and then a decrease in the symbiont density (285). In this case it was suggested that nondividing host cells with a full complement of symbionts inhibit algal division but that in recently divided host cells with fewer symbionts, inhibition is partially or wholly removed (217). This same density-dependent relationship has been suggested as the mechanism by which symbionts partitioned randomly and unevenly between host daughter cells at division ultimately attain similar densities in each digestive cell (227, 228). Competition between symbionts for their host cell's pools of inorganic nutrients or a “division factor” is consistent with this density dependence (228).
Studies of cnidarian-dinoflagellate symbiosis. (i) Resource limitation.
There are parallels between the regulatory mechanisms suggested for the green Hydra symbiosis and those that operate in the cnidarian-dinoflagellate symbiosis, most obviously with respect to resource limitation, i.e., the restricted supply of inorganic nutrients and the loss of photosynthetic carbon to the host. Symbiotic dinoflagellates are typically nitrogen limited in hospite, as evidenced by their enhanced growth rate, population density, photosynthetic performance, and nitrogen status when supplied with an exogenous source of particulate or dissolved nitrogen (see, e.g., references 61, 64, 80, 161, 189, 230, 245, 250, 269, and 332). Similarly, there is evidence for phosphorus limitation, based on phosphatase activities (171, 172) (see below) and phosphate fluxes (249), and for carbon limitation, based on the enhancement of cell-specific photosynthesis by the addition of dissolved inorganic carbon (Ci) (200, 398) or when the in hospite density of symbionts is experimentally reduced (78). These limitations could explain the rapid growth of the symbiotic dinoflagellates upon release into the surrounding environment (232, 349) (but see reference 15) and during the early stages of symbiosis establishment, when the dinoflagellates can grow as rapidly as they do in culture (26, 322, 371). Moreover, the respiratory rate of Symbiodinium cells is higher in culture than when the dinoflagellates are freshly isolated from their host, providing further evidence that symbiont metabolism is inhibited when in hospite (134).
Given this evidence, alongside the host's capacity to regulate the delivery of carbon, nitrogen, and phosphorus to the symbiont (see below), it seems reasonable to suppose that, as in the green Hydra symbiosis, inorganic nutrients and/or metabolites play an important role in the control of symbiont proliferation in the cnidarian-dinoflagellate symbiosis. Indeed, Rahav et al. (294) calculated that in the coral Stylophora pistillata, unrestricted access to the host's waste ammonium would still only be enough to support about one-third of the maximal symbiont growth rate seen in culture. In addition, the leakage of photosynthetic carbon compounds to the host, perhaps as a result of a stimulatory “host release factor” (HRF) (see below), would further hinder the symbionts from achieving a state of balanced growth (106, 332); this unproven mechanism would be analogous to the influence of low pH in the green Hydra symbiosis.
(ii) Progression of the symbiont cell cycle.
Despite the potential importance of nutrients, we know very little about the symbiont cell cycle and how nutrients and other factors act on this cycle to restrict symbiont population growth. The study by Smith and Muscatine (332) is therefore especially important and will be discussed in detail here. Using Aiptasia pulchella and its dinoflagellate symbiont Symbiodinium pulchrorum (clade B), these authors tested whether the host actively regulates nutrient supply to the symbionts, and hence symbiont growth, or whether nutrient limitation is simply a consequence of the host's nutritional status and the ambient environment. They subjected anemones to a range of nutritional treatments (starved or fed and with or without dissolved inorganic nutrients) and then measured the symbiont MI and population growth rate. Starvation caused reduced growth rates and chlorophyll content and an increase in the cellular C/N ratio. The C/N ratio decreased rapidly, however, when the anemones were provided with dissolved inorganic nutrients (ammonium plus phosphate), indicating a return to nitrogen sufficiency. In contrast, the same degree of recovery was not seen with respect to the MI and population growth, suggesting an uncoupling of the nutrient-induced changes in the symbionts' cellular biomass and their rate of division. This finding is very significant, as it implies that symbiont growth is limited by some factor other than the availability of inorganic nutrients.
Smith and Muscatine's study went even further, by using flow cytometry to analyze dinoflagellate cell size versus DNA content and so characterize the kinetics of the cell cycle under different nutritional regimens. This showed that the G1 phase of the S. pulchrorum cell cycle is much longer in hospite than it is in culture, whereas the S, G2, and M phases are shorter and less impacted by the symbiotic state. This intriguing finding suggests that the host somehow delays the symbiont's progress through the G1 phase; indeed, the symbiont may even enter the G0 resting state for a time, with the host regulating the symbiont's emergence from this state (see Appendix 2). Nutrient limitation is one potential explanation for this, yet these same authors found that the duration of the G1 phase was only marginally less under nutrient enrichment, indicating that the progression of the cell cycle is restricted by a mechanism other than the withholding of nutrients. This mechanism remains elusive, but Smith and Muscatine found that feeding of the host with particulate food (brine shrimp) after a period of starvation did stimulate a full recovery of the symbiont MI and population density and substantially shortened the duration of the G1 phase. This was interpreted as evidence that while nutrient supply influences the cellular biomass, composition, and physiology of the dinoflagellate symbionts, progression through the cell division cycle is linked to cellular growth of the host, which is also enhanced by particulate feeding. A similar conclusion was reached by Fitt and Cook (114) for the hydroid Myrionema amboinense, in which symbiont division was maintained perpetually by feeding particulate food at least twice per day but not by the addition of dissolved inorganic nutrients, which at most supported only one round of symbiont division. Of note, this potential coupling between host and symbiont cellular growth is similar to that described above for the green Hydra symbiosis, though again the mechanism that coordinates this relationship is unknown.
One further point worth considering is the recent demonstration that the intracellular pH of the host (coral and sea anemone) cell is acidic (383) (see below for further details). Oscillations in the intracellular pH (pHi) of eukaryotes are known to influence progression through the cell cycle in many species, with a low pHi being typical of resting states and internal alkalization promoting cell cycle progression (e.g., from G0 to G1 to S phase) (see, e.g., references 10, 152, and 210). It is therefore plausible that symbiont proliferation is controlled by the pH of the host cell, should this in turn influence the pHi of the symbiont; this would be a fascinating area for future study.
(iii) Circadian rhythmicity of the symbiont cell cycle.
Light is also important for the division of symbiotic dinoflagellates, presumably because carbon skeletons arising from photosynthesis are needed for the assimilation of dissolved inorganic nutrients and to support the metabolic demands of the cell cycle. For example, when M. amboinense was collected at the end of the night, when the symbiont MI was at its peak, and incubated in either continuous light or continuous dark, the symbionts completed cytokinesis at their normal rate only when in the light (113). Fitt (113) likewise demonstrated that the symbiont MI in starved M. amboinense recovered to normal only when the hydroids were both fed and maintained in the light; darkness repressed symbiont division greatly.
The light/dark period has been shown to encourage progression from the G1 phase to the S phase, and then to the G2 and M phases, in a cultured clade B Symbiodinium sp. from the reef coral Euphyllia glabrescens (393). A period of darkness was required for these cells to undergo cytokinesis (in contrast to the situation in M. amboinense described above) and so return from the G2/M phase to G1; prolonged periods of light delayed cytokinesis and generated undivided cells with multiple copies of chromosomes. Blue light was found to be particularly important, as only this and white light successfully entrained the cell cycle. As both blue and red wavelengths are within the action spectrum of Symbiodinium photosynthesis, Wang and coworkers (393) interpreted this specificity for blue light as evidence for a direct effect on cell cycle progression rather than just an indirect effect on photosynthesis. This interpretation was consistent with the previous finding of a cryptochrome blue-light receptor involved in the circadian control of the cell cycle of a free-living dinoflagellate species, Karenia brevis (37). A further interesting point raised by Wang et al. (393) was that a green fluorescent protein found in the coral's tissues dissipates blue light and, when added to a Symbiodinium culture, inhibits the progression through the G1 phase to S phase to G2/M phase but does not inhibit cytokinesis. This raises the possibility that manipulation of the light regimen by the host cell directly controls symbiont growth. We await subsequent studies to elucidate this mechanism further; for example, Wang et al. (393) presented preliminary evidence for a regulatory role of the secondary messenger cyclic AMP. Furthermore, we need to ascertain whether these light-driven processes operate when the dinoflagellates are in hospite and how they interact with the influence of feeding discussed above, as well as establish their generality across a range of cnidarian-dinoflagellate associations.
These infrequent but detailed explorations of cell cycle progression and the impacts of food, nutrients, and light provide some basis for the phasing of symbiont division seen in a number of cnidarian-dinoflagellate symbioses. As mentioned above, symbiotic dinoflagellates often show a division peak either at dawn or at the start of the light period, which in culture can be followed by a motile stage that is repressed in hospite (111, 420); this motile gymnodinioid stage therefore coincides with the G1 phase of the cell cycle. In hospite, phased division with a peak at dawn has been reported in a range of corals, sea anemones, and hydroids (62, 112, 113, 161, 162, 331, 332), while a nighttime peak was observed in the jellyfish Mastigias sp. (412). Fitt and Cook (114) demonstrated that the dawn peak of symbiont division in the hydroid M. amboinense occurred irrespective of whether the hydroid was in the field or laboratory, or starved or fed, though the size of the peak was dramatically lower under host starvation or nitrogen limitation. Asynchronous division has also been reported to occur in a number of marine cnidarians (75, 159, 342, 412, 414). Fitt (113) speculated that the lack of synchrony in at least some of these cases could have resulted from high concentrations of dissolved inorganic nutrients in the experimental holding tanks, as elevated nutrient levels may dampen out the division peak (162).
(iv) Host cell division.
For the symbiosis to persist in a steady state, the proliferation of the host's cells must keep pace with that of its symbionts (or vice versa). A positive correlation between host and symbiont growth is evident in fast-growing host species, ontogenetic stages, and body/colony regions (414). More precisely, synchronized division of the symbionts must be preceded or followed by an appropriate division of host cells. In contrast to the case for the symbionts, however, we know virtually nothing about the frequency, sites, and periodicity of host cell division in cnidarian-dinoflagellate symbiosis or about whether and how this is coordinated with symbiont division. Gladfelter (132) was the first to study the temporal pattern of mitosis in coral cells, by applying the fluorescent nuclear stain DAPI (4′,6′-diamidino-2-phenylindole), and reported that both gastrodermal and calicoblastic ectodermal cells of Acropora cervicornis exhibit phased division, with a peak MI of about 2% at midnight and a minimum of 0.5% at midday. She suggested that this periodicity was related to the periodicity of feeding and/or photosynthesis in the field. However, there has been just one attempt to compare the patterns of host cell and symbiont division in a cnidarian-dinoflagellate symbiosis, when Fitt (113) used the hydroid M. amboinense as his model system. This important study revealed that the frequency of host digestive cell division in the field, once again measured with DAPI, peaked in the night at 2.4% but was <1% for much of the day. In comparison, dinoflagellate division peaked at dawn at 16.6% but was nearly zero from the early afternoon to evening. Therefore, host and symbiont divisions in this symbiosis peak several hours apart, with host cell division peaking prior to symbiont division. Moreover, based on a series of laboratory experiments where the feeding and light regimens were manipulated, Fitt (113) concluded that host cell division peaks at 12 to 24 h after feeding but that the symbiont cell cycle is set a further 12 to 24 h later (i.e., 24 to 36 h after feeding); a similar offset was seen in cultured green Hydra (221).
Evidence for manipulation of host cell proliferation by symbionts was uncovered in a functional genomics study that compared symbiotic and aposymbiotic specimens of the anemone A. elegantissima (316). Rodriguez-Lanetty and coworkers identified sphingosine 1-phosphate phosphatase (SPPase) as being differentially expressed in the symbiotic state. The sphingosine rheostat, which includes the sphingolipids sphingosine (Sph), formed by the action of SPPase, and sphingosine 1-phosphate (S1P), formed by sphingosine kinase, is a key homeostatic regulatory pathway known to function in cell fate and immunity in animals (336). S1P promotes cell survival and proliferation, while Sph pushes a cell toward apoptosis and cell death. Certain microbes can alter the rheostat in mammals (211, 314), and new evidence suggests that Symbiodinium may modulate it in cnidarians (85). Empirical investigation of the rheostat in the anemone Aiptasia pallida showed that treatment with exogenous S1P shielded anemones from temperature-induced bleaching whereas treatment with exogenous Sph resulted in severe bleaching. These data suggest that the rheostat may play a regulatory role in decisions to tolerate symbionts by surviving and proliferating or to remove symbionts by committing host cell suicide.
Clearly, we are still a long way from understanding the control and synchrony of host and symbiont division in the cnidarian-dinoflagellate symbiosis and the mechanisms by which the symbionts proliferate through the host's cells. We recommend a reassessment of the pioneering 1980s studies of the green Hydra symbiosis, as well as the limited studies of the cnidarian-dinoflagellate symbiosis, in light of our current knowledge of the eukaryotic cell cycle (see Appendix 2). Indeed, the genetic and biochemical approaches applied widely, for example, in the study of the yeast cell cycle provide an ideal starting point for future studies of the cnidarian-dinoflagellate symbiosis, especially if applied to a model system such as the symbiotic sea anemone Aiptasia sp (403). Moreover, we potentially have much to learn from far better studied terrestrial symbioses such as the Rhizobium-legume symbiosis, where the host plant is known to control bacteroid differentiation by means of peptide signaling molecules (238, 382). Such a cross-systems approach will be invaluable as we attempt to understand the complexities and interrelationships of the host and symbiont cell cycles in the cnidarian-dinoflagellate symbiosis.
METABOLIC EXCHANGE AND NUTRIENT TRAFFICKING
Metabolic exchange is central to the ecological success of the coral-dinoflagellate symbiosis (270) and may also play a role in host-symbiont recognition and specificity (see above). As a consequence, it is perhaps the most widely studied physiological aspect of cnidarian-dinoflagellate symbiosis, yet there are still huge gaps in our knowledge with respect to the synthesis and identity of the various metabolic compounds and the mechanisms that control their exchange across the host-symbiont interface. These issues will be reviewed here and are summarized in Fig. 5.
Fig 5.
Schematic summary of nutritional interactions in the cnidarian-dinoflagellate symbiosis. 1, dissolved inorganic carbon (DIC) uptake. DIC is acquired either as bicarbonate (HCO3−) from the surrounding seawater or as CO2 from the seawater or host metabolism/calcification. In the case of HCO3−, it must be converted to CO2 prior to photosynthesis by the dinoflagellate symbiont. 2, photosynthesis. CO2 is photosynthetically fixed through the Calvin-Benson cycle (i.e., the C3 pathway), with the dinoflagellate ultimately synthesizing a range of organic compounds, including amino acids. 3, translocation. A portion of the photosynthetic products are translocated to the host cell. 4, reverse translocation. Organic compounds are likely translocated from the host to the symbiont; these compounds could arise from host metabolism or be in the same forms as those originally translocated by the symbiont. 5, host metabolism. Translocated compounds are used, alongside dissolved organic matter (DOM) and particulate organic matter (POM) taken up from seawater, to support host metabolism. The catabolism of nitrogenous compounds ultimately leads to the generation of ammonium waste that can be assimilated by the symbiont. 6, ammonium assimilation. Excretory and seawater ammonium can be assimilated by both the host cell (pathway not shown) and the symbiont, with translocated organic compounds providing carbon skeletons necessary for host assimilation. The assimilation of excretory ammonium back into amino acids by the dinoflagellate symbiont completes the process of “nitrogen recycling” by the symbiosis. 7, nitrate assimilation. Nitrate is taken up from the seawater, but only the symbiont can convert it to ammonium for subsequent assimilation into amino acids. 8, phosphate assimilation. Phosphate is likewise taken up from seawater and can be assimilated by the dinoflagellate symbiont. Note that uptake of nutrients can also occur from the ambient seawater via the epidermis (not illustrated), but for simplicity these pathways are not shown.
Inorganic Carbon Acquisition and Fixation
Photosynthesis is the primary feature of the cnidarian-dinoflagellate symbiosis. Photosynthetically fixed carbon is translocated to the host, where it supports respiration, growth, and reproduction, or is released to the seawater (e.g., as coral mucus) (270). Inorganic carbon (Ci) for photosynthesis may originate from (i) host and symbiont respiration, i.e., as CO2 (154), (ii) skeletogenesis in corals, where CO2 is produced as a by-product of calcification (394), or (iii) the ambient seawater (118, 119). Most attention has focused on the ambient seawater as a source of Ci and in particular on the uptake of Ci by the coral and its delivery to the dinoflagellate symbiont (3). At a typical pH of 8.2, most Ci in seawater is in the form of bicarbonate (HCO3−; ∼2.2 mM) as opposed to CO2 (∼30 μM). This means that marine phototrophs either must have RubisCO with a high affinity for CO2 or a carbon-concentrating mechanism (CCM) that enables them to utilize the extensive pool of ambient HCO3−. However, the situation for symbiotic dinoflagellates is rather different than that for free-living algae, as they do not have direct access to the ambient seawater and instead must take up Ci from their host's cells. This means that the cnidarian host must have a CCM and transport pathway that facilitate delivery of external Ci to the dinoflagellate cell, a very different scenario than in nonsymbiotic animals, where transport pathways have instead evolved to rid the organism of Ci (3, 198).
The first evidence for the presence of the enzyme carbonic anhydrase (CA), which catalyzes the interconversion of HCO3− and CO2, in a symbiotic cnidarian was provided by Isa and Yamazato (169), but this CA was involved in calcification. The potential role of CA in the delivery of Ci to symbiotic dinoflagellates was not explored more fully until several years later. Weis and coworkers (396) detected CA activity in 22 species of symbiotic Cnidaria, including various hard and soft corals, sea anemones, zoanthids, hydrozoans, and jellyfish. CA activity was 2 to 3 times greater in the host animal's tissues than in the symbiotic dinoflagellates, and the importance of CA to photosynthesis was evidenced by (i) ∼29-fold-higher CA activity in species that harbored symbiotic dinoflagellates than in those that did not, (ii) more CA in the symbiont-rich tentacles of the sea anemone Condylactis gigantea than in the column, which lacks symbiotic dinoflagellates, (iii) relatively low CA activity in shade-adapted and deeper-water corals, and (iv) a 56 to 85% reduction in photosynthetic carbon assimilation upon treatment with an inhibitor of CA. Similarly, reinfection of aposymbiotic (i.e., symbiont-free) individuals of the sea anemone Aiptasia pulchella with symbiotic dinoflagellates induced a 2.5-fold increase in CA activity in the host's tissues (397). Weis and Reynolds (400) subsequently used an anti-human CA immunoprobe and semiquantitative PCR to quantify increased amounts of the enzyme and its transcript, respectively, in the temperate sea anemone Anthopleura elegantissima when symbiotic versus aposymbiotic, and in the process they provided the first direct evidence of a host gene whose expression is influenced by its symbiotic dinoflagellates; these authors also, for the first time, reported a full-length CA cDNA sequence from a symbiotic cnidarian. Most recently, by employing a genomic approach, Ganot et al. (122) have confirmed that CA expression is under the control of the symbiotic state.
While there is now strong evidence for CA activity in cnidarian-dinoflagellate symbioses, we are still lacking a comprehensive knowledge of the CA isoforms involved and their exact locations. However, immunolocalization and enzyme activity assays indicate the presence of CA around the periphery of the dinoflagellate cell, in close proximity to the symbiosome vacuole or membrane complex, and on the plasma membrane and in the cytoplasm of both the ectodermal and gastrodermal cells (8, 25, 28, 118, 398). This positioning of the CA likely provides some clues as to the carbon transport pathways involved. It was initially thought that HCO3− is actively taken up via HCO3− transporters, largely across the coral ectodermis (8, 25). However, more recent evidence (118) suggests that an H+-ATPase acts to secrete H+ from the ectodermal cells into the surrounding seawater, where it promotes the formation of carbonic acid which is then dehydrated into CO2 by a membrane-bound CA, thus facilitating its passive diffusion into the cnidarian cell. The CO2 is then hydrated back into HCO3− by a cytosolic CA, thereby trapping it in the host's tissues. Despite this evidence for active transepithelial absorption of Ci, we do not know the mechanism by which the intracellular HCO3− is transported from the ectodermis to the symbiotic dinoflagellates located in the gastrodermal cells. Whatever the mechanism, the transported HCO3− must be dehydrated once more to CO2 so that it can be utilized for photosynthesis. Recently, Bertucci et al. (27) demonstrated the presence of an H+-ATPase localized within the dinoflagellate symbiont and specifically expressed in hospite. This enzyme is thought to be associated with the symbiont plasma membrane and to maintain an acidic pH within the symbiosome vacuole that facilitates the dehydration of HCO3− (8, 383), in addition to CA associated with the symbiosome membrane complex or vacuole or the dinoflagellate cell itself (8, 398). Symbiotic dinoflagellates freshly isolated from the coral Galaxea fascicularis can take up both CO2 and HCO3− indiscriminately (134); the uptake of HCO3− is mediated by an Na+-dependent carrier (8). These results indicate the presence of an active Ci uptake system and an intracellular CCM.
Further evidence for this comes from the higher concentration of Ci than is possible by passive diffusion alone, measured either in symbiotic dinoflagellates isolated from the giant clam Tridacna gigas (197) or in the Stylophora pistillata holobiont (119). CA is perhaps associated with the thylakoids and/or the large chloroplast pyrenoid that is so conspicuous in Symbiodinium spp.; the potential role of the pyrenoid in the localized elevation of CO2 is supported by the immunolocalization of RubisCO within the pyrenoid of the dinoflagellate symbionts of giant clams (197).
Once taken up by the dinoflagellate symbiont, CO2 is fixed through the Calvin-Benson cycle (i.e., the “C3” pathway), though in one type of Symbiodinium at least (S. kawagutii) there is also evidence for enzymes that are more typical of the β-carboxylation or “C4” pathway (for a more detailed review, see references 373, 374, and 376). The Calvin-Benson cycle here involves a form II RubisCO (408, 409) which, like other form II RubisCO isoforms, is relatively inefficient in comparison to the form I RubisCO found in other eukaryotic photosynthetic organisms. However, it has been hypothesized that this inefficiency could be overcome by the localized activity of intracellular CCMs, especially the CCM situated adjacent to the pyrenoid (13, 197). The form II RubisCO is nucleus encoded and expressed as a polyprotein, though studies of its kinetics have been hampered by a failure to purify the enzyme in an active form. Several genes coding for other enzymes of the Calvin-Benson cycle have been identified, with the most abundant being glyceraldehyde 3-phosphate dehydrogenase (D. Yellowlees, personal communication), though none of these have been isolated and studied. Far more research is needed to fully elucidate the biochemical pathways and enzymology associated with photosynthetic carbon fixation in cnidarian-dinoflagellate symbiosis.
Translocation of Photosynthetic Products
The translocation of photosynthetically fixed carbon (photosynthate) from the symbiont to host is probably the best-known feature of the cnidarian-dinoflagellate symbiosis. Muscatine and Hand (254) were the first to demonstrate photosynthate translocation in a symbiotic cnidarian when they incubated the temperate sea anemone A. elegantissima in the light with Na214CO3 for 1 to 5 weeks and then visualized the presence of radiolabeled organic carbon in sections of the anemone's tissues via autoradiography. In a number of ways, this seminal work kick-started the field of cnidarian-dinoflagellate symbiosis, with numerous researchers focusing on the following four pivotal questions over the subsequent 30 years or so. (i) How much photosynthetically fixed carbon is translocated to the host? (ii) In what forms is the photosynthetically fixed carbon translocated? (iii) What stimulates and controls this translocation? (iv) How important is this translocated carbon to the nutrition and survival of the host cnidarian? The fourth, more ecological of these questions is beyond the scope of this review. Rather, we will review in detail what we currently know in answer to the first three questions, as they each relate to transport across the host-symbiont interface.
How much photosynthetically fixed carbon is translocated?
Quantification of the amount of photosynthetically fixed carbon translocated from the symbiont to host must take account of both the amount of carbon fixed in photosynthesis and the percentage of this production that is transported out of the symbiont cell. This quantification has largely relied on one of two methods, each with its own drawbacks. Indeed, it would be fair to say that carbon translocation has never been quantified conclusively. The earliest and most widely employed method involves labeling with [14C]bicarbonate in the light and measurement of the proportion of label found in the host's tissues. Using this method, translocation has been measured as anywhere between 5% and 60% of photosynthetically fixed carbon for a wide range of coral- and other cnidarian-dinoflagellate symbioses (51, 76, 107, 128, 166, 259, 268, 337, 351, 370, 386). Moreover, visual examination of autoradiographs from the temperate sea anemone Anemonia sulcata (= Anemonia viridis) suggested that >60% of photosynthate was translocated (360). The use of 14C has its limitations, however, especially because translocated products might be rapidly metabolized, thus liberating radiolabel from the system as 14CO2 and leading to an underestimation of the percent translocation. Alternatively, 14CO2 could be refixed by photosynthesis, thus confounding any translocation measurements, while there are also potential problems associated with the 14C-to-12C ratio disequilibrium (20, 21, 270, 333).
Given the questions surrounding the use of 14C labeling, Muscatine et al. (267) introduced an alternative method for the quantification of translocation that was based on the assumption that all photosynthetic carbon not used for respiration or growth by the dinoflagellate symbionts is passed to the host. This approach therefore requires knowledge of the net photosynthetic production (i.e., the gross photosynthetic carbon fixation less the carbon used for dinoflagellate respiration) and the amount of carbon used for dinoflagellate cell growth, both of which rely on a combination of direct and indirect measures that are explained in detail elsewhere (see, e.g., references 75, 267, 268, and 342). Typically, this so-called “growth rate method” estimates that >90%, and sometimes as much as 99%, of photosynthetically fixed carbon is translocated to the host under well-lit conditions (74, 75, 81, 268, 342). Once again, however, this technique and the translocation rates estimated are questionable, for three key reasons: (i) no allowance is made for carbon storage by the dinoflagellate cell, which undoubtedly occurs, largely via starch accumulation (251); (ii) symbiont cell growth is estimated indirectly only, based on the percentage of dinoflagellates in a state of division (the mitotic index [MI]) and the duration of symbiont cytokinesis, which is often assumed as 11 h based on a derived estimate from just one symbiont type from the jellyfish Mastigias sp. (267, 412 [but see reference 232]); and (iii) no method has yet been developed for the accurate measurement of symbiont (and indeed host) respiration in the intact symbiosis, where the nutritional interplay between the two partners has stimulatory effects on cell physiology (both photosynthesis and respiration) that are not replicated when the partners are studied in isolation (102, 154, 330).
These various knowledge gaps severely hamper the quantification of carbon utilization and flux within the cnidarian-dinoflagellate symbiosis and hence our understanding of the supposed mutualistic nature of the association. However, a very recent, though currently expensive, methodological development may offer a solution: multi-isotope imaging mass spectrometry (MIMS). This method allows for the high-resolution tracking and quantification of molecules labeled simultaneously with a range of stable isotopes (e.g., 13C or 15N) or radioactive isotopes (e.g., 14C) (195, 196, 405). The locality of these isotopes can be imaged by nanoautography and the amount of an isotopic tracer present at the subcellular level accurately quantified by comparing the experimental isotopic ratio (e.g., 13C to 12C) with the natural abundance ratio. This method could in fact prove to be a very powerful tool in the study of cnidarian-dinoflagellate symbioses, given its capacity not only to quantify nutritional fluxes but also to visualize the fates of numerous metabolic compounds within the symbiosis.
In what forms is photosynthetically fixed carbon translocated?
Not only are we uncertain about the amount of photosynthetic carbon translocated to the host, but we also lack comprehensive and reliable data about the identity of the compounds released. Muscatine (257) isolated symbiotic dinoflagellates from corals as well as giant clams, incubated them in homogenates of their host tissue in the presence of [14C]bicarbonate, and then identified both the intracellular and released products by two-dimensional (2-D) radiochromatography; he had previously developed this technique for identifying the photosynthetic products released in the green Hydra symbiosis (255). Analysis of the medium revealed that the photosynthate liberated by the dinoflagellates was largely (>90%) in the form of glycerol, with glucose and a ninhydrin-positive compound also being released. In contrast, glucose was the major photosynthetic product identified in intracellular extracts of the dinoflagellates, with other intracellular products being various photosynthetic intermediates and lipid. By applying this method, glycerol has subsequently been identified as the primary form (24.8 to 95.0%) in which photosynthetically fixed carbon is released in a number of different cnidarian-dinoflagellate symbioses, with other reported compounds including glucose, the amino acid alanine, and organic acids such as fumarate, succinate, malate, citrate, and glycolate (351, 370–372, 386). In addition to these various compounds, microscopic observation of isolated dinoflagellates from the coral Stylophora pistillata and the sea anemone Condylactis gigantea led to reports of fat droplets being released from the cells, suggesting that lipids are also translocated (185, 287). Muscatine et al. (272) later showed that these “blebs” stained positively with a DNA-specific fluorochrome and were in fact host cell nuclei, though this should not be taken as evidence for a lack of lipid translocation in hospite (see below).
A persistent problem, however, is that the isolated dinoflagellates do not necessarily behave in the same manner as they do in the intact symbiosis (see, e.g., reference 351). Furthermore, identifying the mobile compounds in hospite is problematic given the intracellular nature of the symbiotic dinoflagellates and the likelihood that translocated carbon is metabolized rapidly by the host. Nevertheless, some information has been gleaned from (i) patterns of incorporation of 14C-labeled compounds into the host animal's tissues (19, 20, 32, 259, 369), (ii) biochemical and/or microscopic analysis of cnidarian tissues in different symbiotic states and under different irradiance regimes (50, 130, 153, 209, 392), as well as more targeted proteomic and ultrastructural analysis of “lipid bodies” isolated from symbiotic coral cells (288), (iii) identification of compounds in the host's tissues that are specifically synthesized by the symbionts (282), (iv) measurement of respiratory quotients (127), and, very recently, (v) analysis of the host genome (328). Together, these various approaches again point to glycerol, amino acids (including seven essential amino acids [392]), and various lipids as major components of the translocated material. However, none of these approaches is direct and hence conclusive with regard to the identity of the mobile compounds. Lewis and Smith (204) applied the somewhat more direct “inhibition technique,” originally developed for the study of the lichen symbiosis (94), to a range of corals and other cnidarians. This method involves the addition of potential translocated compounds to the medium surrounding a 14C-labeled symbiosis, which thereby saturate all sites of uptake in the host and cause any corresponding 14C-labeled compounds that are released by the symbionts to leak out of the symbiosis altogether. Lewis and Smith again concluded that glycerol, glucose, and alanine were the dominant forms in which carbon was translocated; they did not consider lipids.
More recently, Whitehead and Douglas (407) have used “metabolite” comparison: to elucidate the organic compounds translocated in the sea anemone A. viridis. This method compares 14C-labeled compounds in the host's tissues between treatments with [14C]bicarbonate in the light (i.e., photosynthesizing conditions) and when exogenous 14C-labeled substrates are provided in the absence of light. When the two treatments produce comparable labeling patterns in the host's tissues, then the exogenous compounds are thought to be similar to those translocated. This study suggested that glucose, succinate, and fumarate are all translocated, but crucially, there was no evidence for glycerol translocation. This finding was a major departure from previous reports and, interestingly, matched the situation in the intercellular giant clam-Symbiodinium symbiosis, where both temporal biochemical assays and radiotracer studies have suggested that glucose but not glycerol is translocated into the clam hemolymph (170, 306), while glycerol is released when the dinoflagellate symbionts are isolated and incubated in a host tissue homogenate (170). These studies highlight that there is still some uncertainty around the forms in which photosynthetic carbon is translocated to the host, with different symbioses not necessarily translocating the same compounds and isolated Symbiodinium cells responding differently to those in hospite, due to either the physiological impacts of isolation from the host (135) or the fact that homogenized host tissue is not representative of the environment to which symbiotic algae are exposed when inside their host. In addition, our steadily expanding knowledge of the Symbiodinium cell surface has revealed various glycoproteins that are likely exuded by the cell and hence form part of the translocated material (208, 212, 418) (see above). Such compounds are also exuded in Symbiodinium cultures (213), and their proteinaceous nature has enabled their transfer from symbiont to host to be confirmed in hospite (in the jellyfish Cassiopeia xamachana) via immunohistochemistry (214).
Currently, therefore, after several decades of research, we are still uncertain about the major compounds translocated and know little of the more minor compounds released. We also know virtually nothing about “reverse translocation” of carbon compounds, the transfer of metabolites from host to symbiont, though it is known that isolated symbionts can take up a range of organic and inorganic molecules from the surrounding medium (39, 40, 82, 89, 151, 344). The uptake of 35S by symbiotic dinoflagellates from food ingested by Aiptasia sp. suggests that the reverse translocation of organic compounds does happen (58, 343), though the transfer of inorganic sulfate rather than organic sulfurous compounds cannot be excluded (343). Similar evidence via the supply of 35S-, 3H-, and 14C-labeled food also exists for the green Hydra symbiosis (59, 256, 362). Importantly, however, two recent methodological advances should rapidly increase our knowledge of the metabolites translocated from the symbiont to host and vice versa.
(i) Identification of nutrient transporters at the host-symbiont interface.
Our increasing capacity to characterize the symbiosome membrane complex, the interface between the host and symbiont through which all translocated metabolites must pass, will yield valuable information about the identity of membrane transporters and hence the compounds transferred. Rands et al. (297) used enzyme cytochemistry to identify ATPase activity associated with both the animal- and dinoflagellate-derived components of the symbiosome membrane complex of the sea anemone A. viridis, providing evidence for the active control of metabolite transfer. Much more recently, though, the symbiosome membrane complex has been successfully isolated via mechanical disruption of the symbiosis and purification of symbiosomal membranes by continuous sucrose gradient centrifugation; this complex can then visualized with confocal and transmission electron microscopy and its purity assessed with Western blot analysis (184, 289, 368). Isolation of sufficient symbiosomal membrane material potentially allows for identification of membrane transporters, as well as other components of the membrane, by proteomic analysis or the application of heterologous expression techniques (see, e.g., reference 276). We are still in the very early stages of symbiosome characterization, but Peng and coworkers (289) recently subjected biotinylated symbiosome proteins from Aiptasia pulchella to 2-D SDS-PAGE and mass spectrometry, identifying a total of 17 proteins belonging to a range of functional groups, including transporters. These included the ABC transporter, which belongs to a family of proteins that is responsible for the ATP-dependent transport of a wide range of molecules, including long-chain fatty acids, lipids, enzymes, peptides, and nitrate, and the chaperone protein disulfide isomerase (PDI), which is involved in the transport of triglycerides, cholesteryl esters, and phosphatidylcholines. The next few years will hopefully see further attempts to identify symbiosome-associated transporters across a range of cnidarian-dinoflagellate symbioses and to elucidate their function.
(ii) Metabolite profiling.
One major route forward will be the application of metabolite profiling, a metabolomic approach that is becoming commonplace in terrestrial plant biology and microbiology (reviewed in references 136, 320, and 347). The method involves the unbiased, quantitative profiling of metabolites by a range of techniques, such as gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis-mass spectrometry (CE-MS), Fourier transform infrared mass spectrometry (FT-IR), and/or nuclear magnetic resonance (NMR); enzymatic determinations may be performed for those metabolites that cannot be detected accurately by these more cutting-edge techniques. The resulting metabolite data sets can be used to statistically model metabolic networks, which can be improved further by the incorporation of functional data concerning the regulatory and kinetic properties of the associated enzymes. Ultimately, metabolomic data can be integrated with transcriptomic and genomic data (328) to provide a comprehensive account of an organism's metabolic pathways.
Symbiosis-dependent metabolism in terrestrial plant-fungus symbioses has begun to be elucidated by comparison of metabolite profiles of plants with and without their fungal partners and under a range of nutrient conditions (298, 321), and we strongly recommend that a similar approach be applied to cnidarian-alga symbiosis (see also reference 137). Indeed, together with an improved knowledge of the host-symbiont interface and the use of methods such as MIMS (see above), comparative metabolite profiling has the potential to greatly enhance our knowledge of symbiosis-dependent metabolism and photosynthate translocation from symbiont to host.
What stimulates and controls the translocation of photosynthetically fixed carbon?
The control of photosynthetic carbon translocation is one of the most controversial aspects of cnidarian-dinoflagellate symbiosis research. One hypothesis is that the host could limit symbiont growth, either by restricting the supply of nutrients or by actively “blocking” symbiont mitosis (see the previous section), thus generating a surplus of photosynthetic carbon for release (105, 267). It is important to note, however, that nutrient limitation may cause carbon storage rather than release in symbiotic dinoflagellates (78, 251), as it also does in free-living microalgae (375).
An alternative hypothesis has arisen from the observation that when isolated symbiotic dinoflagellates are incubated in homogenized host tissue and labeled with [14C]bicarbonate in the light, they release a substantial proportion of their photosynthetically fixed carbon (sometimes >50%) to the surrounding medium; these same dinoflagellates typically release <5% when incubated in seawater. In most cases an elevation in the cellular photosynthetic rate is also seen. Muscatine (257) was the first to observe this phenomenon (for corals and giant clams), and it has since been confirmed for a wide range of cnidarian-dinoflagellate symbioses (77, 139, 157, 260, 268, 351, 371) as well as a number of other alga-invertebrate symbioses (120, 261). Interestingly, however, no such activity is evident for the green Hydra symbiosis, where pH is instead thought to regulate translocation (43, 91). The identity of the chemical signal(s) responsible remains unknown, but there have been a number of attempts to characterize this so-called “host release factor” (HRF). It was originally suggested that HRF is proteinaceous due to its heat-labile properties (260, 351) and is >10 kDa in size (351). However, it has become increasingly apparent that HRF differs in different host species with respect to its thermal stability, host-symbiont specificity, and molecular weight (65, 125, 140, 157, 244, 260, 351), highlighting the dangers of generalizing across species. It has also become increasingly apparent that different cell signaling molecules may be responsible for the increased percent release of photosynthate and the control of photosynthetic performance (65, 141, 144, 390 [but see references 125 and 126), further complicating matters. Gates and coworkers (125, 126) suggested that HRF in the coral Pocillopora damicornis and the sea anemone A. pulchella consists of a suite of free amino acids (FAAs), as a synthetic HRF consisting of these FAAs both induced the release of photosynthetically fixed carbon and enhanced the rate of photosynthesis; it was proposed that these FAAs optimize the symbiont's intracellular environment, thus enabling it to fully realize its photosynthetic potential (30, 126). Evidence also suggested that mycosporine-like amino acids (MAAs) could be involved, as the active fraction (<4 kDa) had an absorbance peak at 320 nm and partially purified MAAs from other marine organisms enhanced the percentage of photosynthate released by dinoflagellates isolated from A. pulchella; MAAs did not elevate the photosynthetic rate, however, at least at the concentrations used (125).
These interesting findings stimulated a number of follow-up studies in the late 1990s, but these in turn raised considerable doubt about the universal role of FAAs as the HRF. For example, Wang and Douglas (390) suggested that the nonprotein amino acid taurine may be largely responsible for the increased translocation rate of dinoflagellates freshly isolated from A. pulchella, yet Withers et al. (415) could not replicate this response with dinoflagellates from the coral Plesiastrea versipora. Indeed, Withers and coworkers concluded that FAAs are not responsible at all for HRF activity in extracts of P. versipora, though the HRF of this coral appears to be of a correspondingly low molecular mass (<1 kDa) (140, 143). Similarly, Cook and Davy (65) found that the response (both percent release and photosynthetic rate) of symbiotic dinoflagellates to a <3-kDa extract of the coral Montastraea annularis was dose dependent but that there was no such relationship between the HRF response and the concentration of ninhydrin-positive FAAs present in tissue extracts; they did not measure taurine. These authors also found no effect of the amino acids glycine and glutamic acid on either photosynthate release or photosynthesis, though alanine did have an effect on the percent release only, particularly at high concentrations.
Outside this debate, only one research group (Hinde and coworkers, University of Sydney) has continued to focus intensively on the identity and mode of action of the HRF, focusing in particular on the coral Plesiastrea versipora (139–144, 157, 312, 313). To date, they have isolated two water-soluble signaling molecules of 1,000 kDa or less, one that stimulates photosynthate release but not the photosynthetic rate (HRF) and one that partially inhibits photosynthesis and hence was named “photosynthesis-inhibiting factor” (PIF). Homogenized P. versipora tissues induce similar stimulatory and inhibitory effects in dinoflagellates from a number of other cnidarians (142, 351), suggesting that the HRF and PIF in this coral might be “typical,” though photosynthetic inhibition does not occur in all cases (141, 142). Unfortunately, identification of the HRF and PIF has been severely hampered by their thermal lability and loss of activity when salt is removed during analysis (144), though the comparable effects of several synthetic compounds, such as clotrimazole, suggest that the HRF and PIF might act as calmodulin antagonists (144, 313). It is thought that the HRF from P. versipora stimulates translocation by either increasing glycerol synthesis or diverting it or other photosynthetic intermediates away from the symbiont's triglyceride stores (139). Moreover, the operation of both the HRF and PIF is unaffected by the presence of the symbiosome membrane complex around the isolated dinoflagellates (142).
Perhaps the biggest problem with all HRF studies is that they are conducted in vitro, with isolated dinoflagellates incubated in homogenized host tissue that is by no means representative of the environment in the intact symbiosis. Indeed, it is conceivable that HRF is an experimental artifact. This was suggested by different translocation patterns in response to host (A. pallida) starvation when the symbionts were either exposed in vitro to HRF or retained in hospite (77, 78). We therefore remain a long way from understanding the control of photosynthate release in cnidarian-dinoflagellate symbiosis. Hopefully, a better knowledge of the nutrient transporters associated with the symbiosome might provide some insight into their control, while knowledge of the host genome may facilitate the identification of candidate signal molecules whose function can be resolved by the likes of chemical genomics and reverse genetics (e.g., RNA interference), methods that are becoming widespread in more advanced fields such as plant science (reviewed in reference 347).
Fluxes of Nitrogen and Phosphorus
Given the abundance of coral-dinoflagellate symbioses in nutrient-poor tropical seas, their ability to take up, conserve, and recycle nitrogen has received reasonable attention over the past few decades. Nitrogen can be acquired as ammonium arising from the host's catabolic processes (41, 294) or from the ambient seawater as dissolved inorganic nitrogen (i.e., ammonium and nitrate) (14, 146, 147, 264), dissolved organic nitrogen (e.g., FAAs and urea) (7, 148, 149, 361), or particulate organic nitrogen (e.g., plankton) (see, e.g., references 63, 80, and 108). Both the cnidarian and dinoflagellate partners can assimilate ammonium (42, 95, 146, 206), but it has been suggested that the dinoflagellate is the primary site of this assimilation (146). The host utilizes photosynthetic products from the symbionts to provide the carbon skeletons necessary for ammonium assimilation (242, 391). Enzymatic assays and HPLC-analysis of internal FAA pools have demonstrated that in the cnidarian host, ammonium assimilation occurs largely by the NADP-glutamate dehydrogenase (NADP-GDH) pathway; this catalyzes the conversion of ammonium to the amino acid glutamate (242, 315), a precursor of other amino acids. Ammonium assimilation in the dinoflagellate symbionts is instead by the glutamine synthetase/glutamine 2-oxoglutarate amido transferase (GS/GOGAT) pathway, but the end product is again glutamate (315, 350, 413). Only the dinoflagellate partner can convert nitrate to ammonium for subsequent assimilation into amino acids. Indeed, when the coral Stylophora pistillata was labeled with [15N]nitrate, the label was incorporated predominantly into the dinoflagellate partner (147). Similarly, Tanaka et al. (358) demonstrated [15N]nitrate assimilation in the coral Acropora pulchra, with more rapid labeling in the symbiotic dinoflagellates than in the host. The enzymes necessary for the conversion of nitrate to ammonium are nitrate and nitrite reductases, which have been detected on one occasion in symbiotic dinoflagellates (70); more recently, the gene sequence for the former of these enzymes has also been identified in Symbiodinium type C3 from the coral Acropora aspera (199).
Discussion of the physiological role of intracellular nitrogenous compounds is beyond the scope of this review, as is the physiology underlying nitrogen conservation (for an excellent discussion of this concept, see reference 391). Rather, we will focus on nitrogen recycling across the host-symbiont interface. This involves assimilation of waste ammonium by the dinoflagellate symbionts that is, ultimately, translocated back to the host as either free amino acids (351, 370, 392) or proteinaceous glycoconjugates (213, 214); these compounds are eventually catabolized to ammonium again. The diversity of these translocated nitrogenous organic compounds and the control of their release were discussed above, but we still need to consider the mechanism of their transport. As with carbon transport, proteomic analysis of symbiosome membranes has revealed the presence of putative transporters (289) (see above), but far more detailed analysis of the symbiosome proteome is still needed to resolve their role, if any, in nitrogen transport both into and out of the symbiosome. Of additional interest is the pH of the symbiosome vacuole, as this will influence the form in which inorganic nitrogen (and indeed carbon and phosphorus) is made available to the symbiont (242). In particular, at more acidic pH levels, ammonia (NH3) becomes less available in favor of charged ammonium ions (NH4+), which instead of being freely permeant to biological membranes must move across them by carrier-mediated transport; nitrate remains as NO3− irrespective of pH. Consequently, if as predicted from vacuoles in other systems (242), the symbiosome vacuole is acidic, then ammonia must largely be carried across the symbiosome membrane complex and into the dinoflagellate cell.
Despite the importance of the symbiosome pH for our understanding of host-symbiont nutrient trafficking, there have been few attempts to measure this parameter. Rands et al. (296) were the first to study pH in an alga-invertebrate endosymbiosis (green Hydra), when they used immunocytochemical analysis of a weak base. This same method was subsequently applied to a cnidarian-dinoflagellate symbiosis involving the sea anemone A. viridis (297), and it was concluded that “the symbiosomal pH almost certainly exceeds 5.7.” While far from definitive, this was the first evidence that the pH of the symbiosome vacuole could be acidic, if not excessively so. The advent of confocal microscopy has provided much greater opportunities for studying intracellular pH, and in an important recent study, Venn and coworkers (383) measured intracellular pH in A. viridis and the coral Stylophora pistillata, in both the light and dark, with the probe carboxy SNARF-1. They revealed that the pH in the host cells was mildly acidic and was more acidic in the dark than in the light (e.g., pH 7.13 versus 7.41, respectively, in S. pistillata). Crucially, though, ratiometric imaging of the region between the dinoflagellate cell and host cytoplasm suggested a pH of <6.0, in both the light and dark. As pointed out by Venn et al., however, greater resolution is needed to determine if this region of low pH coincides directly with the symbiosome vacuole; further information could also be garnered by applying this technique to isolated symbiosomes (184, 289, 368).
Relatively little is known about the flux of phosphorus in cnidarian-dinoflagellate symbioses. Symbiotic cnidarians can acquire both dissolved inorganic phosphorus (as phosphate [PO43−]) and organic phosphorus (e.g., as zooplankton) from seawater. Phosphate uptake has long been thought to be brought about by the dinoflagellate partner, as aposymbiotic hosts (249) and nonsymbiotic species of reef corals (290) are not able to take up PO43− from seawater, while the isolated dinoflagellate cell is able to do so (84). Moreover, the rate of phosphate uptake increases in the light (83, 133). However, a recent analysis of uptake kinetics in the coral S. pistillata and its isolated symbionts suggests that both the animal and dinoflagellate partners must possess phosphate transporters (133). Indeed given that phosphate is a negatively charged ion at both seawater and physiological pHs, uptake must be by active transport, and carrier-mediated transport is needed to move phosphate against the concentration gradient from the submicromolar concentrations typical of seawater into the more phosphate-rich cell cytoplasm (172). Evidence for carrier-mediated phosphate transport comes from the isolation of two acid phosphatases (named phosphatases P-1 and P-2) from the dinoflagellate symbionts of the coral Acropora formosa, one of which (P-2) was also isolated from a culture of Symbiodinium kawagutii (171). These authors reported a high activity of phosphatase P-1 with polyphosphate, suggesting that this enzyme might mobilize this intracellular phosphate storage compound, while they suggested that the most likely role for phosphatase P-2 is the hydrolysis of phosphate esters external to the plasmalemma. The latter phosphatase is therefore particularly interesting, as it could provide a mechanism by which phosphate esters translocated from the host cell cytoplasm into the symbiosome act as a source of inorganic phosphate, which can then be actively taken up by the symbiont. Additional support for this mechanism was provided by Rands et al. (297), who detected phosphatase activity, via enzyme cytochemistry, on the symbiosome membrane complex of A. viridis; this phosphatase had substrate specificity for β-glycerophosphate and α-naphthylphosphate. There is therefore good evidence that phosphate delivery across the host-symbiont interface can be controlled. Indeed, the capacity to limit phosphate supply could explain why when freshly isolated symbionts from A. formosa were incubated at phosphate concentrations similar to those of the host cell cytoplasm (2 mM) rather than the symbiosome vacuole, their phosphatase activity became suppressed (171). Once again, however, more details of the transporters present at the host-symbiont interface and the pH of the symbiosome lumen will clarify the transport and flux control of this nutrient.
It is therefore clear that the passage of carbon and nutrients, such as nitrogen and phosphorus, across the host-symbiont interface has the potential to be tightly controlled. This evidence opposes the diffusion-depletion model of nutrient supply, where nutrients diffuse along a concentration gradient from the host cell cytoplasm into the symbiont cell in response to the symbiont's nutrient demands (83, 84). Indeed, it suggests that the host might actively cause the nutrient limitation of the symbionts that is seen in many of these associations (see, e.g., references 63, 64, 171, 172, and 398), thus providing a mechanism by which symbiont population growth can be regulated (see above).
CALCIFICATION WITHIN A SYMBIOTIC ORGANISM
As early as 1931, Yonge found that the association between symbiotic dinoflagellates and their host is indispensable for coral growth (421). As this effect is inhibited in the light by DCMU, a specific inhibitor of the Hill reaction (18), it is clear that “light-enhanced calcification” (LEC) and hence skeleton formation are mediated by symbiont photosynthesis (378). However, the exact links between the two partners via the process of LEC is still a matter of debate (see reviews in references 5, 129, 216, 352, and 357).
Coral Calcification
Calcification is a major feature of corals, as this process promotes skeleton formation and is hence the foundation of the world's largest bioconstruction, coral reefs. In spite of this importance, the mechanism of calcification, or more broadly biomineralization, is poorly understood, and it is only slightly better known in other animals (for an update on biomineralization, see reference 22). In corals, the skeleton is external and covered by the animal's tissues (hence the name “hard” or “stony” corals). It is made of calcium carbonate (CaCO3) crystallized in the form of aragonite. The study of coral calcification started at the same time as the study of coral biology and then followed the evolution of techniques, from descriptive microscopy (73) to physiology (138), molecular biology (116, 422, 423), and genomics (145). This study has been greatly improved by the development of biological models such as microcolonies (353, 354) and coral larvae (145), but the lack of an appropriate cellular model (88 [but see references 87 and 155]), as well as poor knowledge of the coral genome, continues to hinder progress. The purpose of this section is to review what is known about the role of the dinoflagellate symbionts in the calcification process at the cellular level. For more extensive information on calcification at the organismal or ecosystem level, the reader is referred to recent reviews (5, 6, 55, 129, 357).
The Calcification Process
The skeleton is entirely covered by a single layer of thin epithelial cells, called the calicoblastic epithelium or, more recently, the calicodermis (121), which is firmly attached to the skeleton by hemidesmosome-like extensions of the desmocytes (“anchoring cells”) (273). These cells are formed during settlement of coral larvae by rapid replacement of aboral ectodermal cells (53, 379, 381). They are devoid of symbiotic dinoflagellates. The calicoblastic cell plays a major role in coral calcification. It controls the ionic composition of the medium in which calcification takes place, especially the extracellular calcifying medium (ECM) between the apical membrane of the calicoblastic cells and the skeleton, and regulates the growth of the biomineral. To achieve these goals, the calicoblastic cell uses an organic framework, called the organic matrix (OM) (reviewed in reference 356), and ion carriers (Fig. 6). Coral calcification is an extracellular process (177) that occurs within the ECM. It has been suggested that this medium is a colloidal gel matrix secreted by the calicoblastic cells (52, 71, 177); however, the physicochemical characteristics of this medium are still a matter of debate (for reviews, see references 6 and 357). Nevertheless, it must be alkaline for calcification to proceed. To test this, Al-Horani and coworkers (2) directly measured the pH and calcium concentration in the ECM with an invasive microelectrode, and they recorded an alkaline pH ranging from 8.13 at night to 9.29 during the day. Recently, Venn et al. (384) used a noninvasive method to perform live tissue imaging when they applied a pH-sensitive fluorescent dye. They found that the calicodermis increased the pH of the ECM by 0.2 and 0.5 pH unit relative to the surrounding seawater pH in the dark and light, respectively. Consequently, by shifting the equation HCO3− → CO32− + H+ to the right, this increases the saturation state of aragonite (Ωarag) from 11 in the dark to about 20 in the light, enhancing the rate of calcification.
Fig 6.
Schematic drawing of the major proteins identified within calicoblastic cells. Calicoblastic cells form an epithelium (calicodermis) whose apical membrane (AM) is attached to the coral skeleton by desmocytes via a hemidesmosome adhesion complex. Their basal pole is close to the mesoglea, a sheet of extracellular matrix proteins. Various transport proteins within calicoblastic cells have been identified or suggested on the basis of pharmacological evidence: ATPases (Na+ pump and Ca2+ pump), antiporters (Na+/Ca2+ antiporter and bicarbonate carrier) and channel proteins (Ca2+ channel). The presence of numerous mitochondria within calicoblastic cells both energizes ion transport and supplies metabolic CO2 as a source of carbon for calcification. The presence of carbonic anhydrase within the extracellular calcifying medium (between the calicodermis and skeleton) facilitates the chemical equilibrium between the different carbon species. In addition, calicoblastic cells synthesize and secrete via vesicles a mixture of macromolecules, called the organic matrix (OM), which acts as an organic framework. Calcium ions may reach this medium both by a paracellular pathway through septate junctions and by transcellular transport, aided by the large surface area of the basal lateral membrane (BLM) of the calicoblastic cells. The symbiont cell may enhance calcification either by altering the physicochemical composition of extracellular fluids by either absorbing CO2 or releasing O2 (1) or by supplying organic compounds to the calicoblastic cells, such as precursors for skeletal organic matrix synthesis or high-energy molecules (2) (see text for more details).
Relationships between Symbiosis and Calcification
Light-enhanced calcification.
Two groups of hypotheses, not mutually exclusive, have been proposed to explain the stimulation of calcification by the presence of the dinoflagellate symbionts.
(i) Alteration of the inorganic chemistry.
Symbiotic dinoflagellates may stimulate calcification by either absorbing CO2 or releasing O2. In this case, the process of CO2 transport from the seawater (117) or photosynthetic CO2 fixation by the dinoflagellates (138) may indirectly modify the inorganic carbon chemistry within the gastrovascular cavity and/or the ECM by altering the pH and/or Ci content, thus driving calcification by the precipitation of CaCO3. This hypothesis is supported by several observations showing changes in the pH within the gastrovascular cavity (2) or ECM (384). It is also supported by the results of Moya and coworkers (248), who showed that the expression of a secreted or calicodermis membrane-bound CA is twice as high in the dark as in the light, suggesting a link between the daily light cycle and Ci equilibrium. However, none of these observations confirm a direct link between the presence of symbionts and the enhancement of calcification, and all attempts to mimic the effects of symbionts on the LEC have failed (but see reference 57). It has also been hypothesized that symbiotic dinoflagellates may absorb inhibitors of calcification, such as phosphates (329), but this hypothesis has not been confirmed.
(ii) Production of organic molecules by symbiotic dinoflagellates.
Organic molecules involved in calcification may be biochemical compounds used to provide energy for calcification (44, 69), nitrogen compounds which can modify the buffering capacity of the ECM (69), or essential precursors for OM synthesis (see above and references 130, 275, and 388). Most of these hypotheses are based on differences between symbiotic and nonsymbiotic corals (130, 275) or experiments using inhibitors (4, 18), and an experimental stimulation of calcification in the dark by adding organic compounds (e.g., amino acids, glycerol, glucose, ATP, or succinate) has been observed in only very few instances, and even then it did not reach the rate seen in the light (18, 57, 69, 380). As calicoblastic cells have been identified as the unique site of OM synthesis (293) and ion transport, we can therefore hypothesize that photosynthetic products are secreted into the gastrovascular cavity by symbiont-containing gastrodermal cells situated toward the oral end of the coral polyp (i.e., not in the immediate vicinity of the calicodermis, where symbionts are absent [355]) and then transferred to the calicoblastic cells via the aboral gastrodermal cells. No steps of this proposed pathway have yet been described, but its total duration must be about 25 min, the time necessary to switch between dark and light rates of calcification (246).
Despite a physiological link, there are no obvious morphological or cellular adaptations linking photosynthesis and calcification, except that the coral skeleton may act as a solar energy collector, enhancing the light field in the coral's tissues by light scattering (104). However, this is probably not particular to symbiotic corals.
Light regulation of skeletal morphology.
While the morphology of the skeleton is genetically controlled, light, through the symbiotic relationship, can also modify it to some extent. These changes, known as phenotypic plasticity, can act both on the general morphology of the coral colony and at the level of skeletal microstructure and density (reviewed in references 357 and 367). These changes are adaptive, as they allow corals to optimize light capture by the symbiotic dinoflagellates. One can hypothesize that light may change the genetically controlled morphology by altering ion-pumping mechanisms and/or increasing OM synthesis and secretion toward the extracellular calcifying medium. However, the underlying mechanism is totally unknown. The diffusion of internal regulators such as the coral bone morphogenetic protein (coral BMP2/4) described in the calcifying epithelium (424) may be part of this mechanism.
Even if it is clear that the dinoflagellate symbionts are responsible for the LEC process, their exact role is still a matter of debate, and their action is probably mediated not by a single parameter but by several operating at the same time. The recent development of molecular methods such as transcriptomic analyses (145, 240, 310) will likely provide insight into the process by which the dinoflagellate symbionts enhance calcification, though it should be noted that the mechanism is metabolic rather than genetic, as the genes coding for proteins involved in coral calcification are not differentially expressed between light and dark conditions, while expression of photosynthetic genes is enhanced in light (247). Complete elucidation of this mechanism will therefore require the development of cell physiology and functional genomic (i.e., gene knockdown) approaches, while a comparative approach with nonsymbiotic corals will help to elucidate the mechanisms by which symbiotic dinoflagellates control calcification.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
It will be evident that, despite several decades of research, there are huge gaps in our knowledge of the cnidarian-dinoflagellate symbiosis and hence our understanding of how reef corals function. Indeed, even aspects of this symbiosis that are often considered well known, such as the amount and types of photosynthetic carbon compounds passed from the symbiont to host, are in fact highly debatable. This lack of fundamental knowledge is a serious concern given the global threats that currently face coral reefs. We therefore hope that this review has a number of important outcomes. First, we hope to draw attention to the often-forgotten historical literature on the cnidarian-dinoflagellate symbiosis, as well as the green Hydra symbiosis, that has considerable potential to inform future studies of the cnidarian-dinoflagellate symbiosis. Second, we hope that by reminding coral reef and symbiosis researchers of the extensive literature available (or indeed introducing them to some of it) and by suggesting key avenues and approaches for future research, we will stimulate both new and established researchers to tackle these often challenging problems. Last, but by no means least, we hope that this review will encourage cell biologists who have not previously considered the coral reef field to apply their skills to this hugely important topic. By doing so, cell biologists may develop novel research avenues of relevance to their existing programs. For example, cnidarian-dinoflagellate symbioses can (i) provide insight into the evolution of negative as well as positive intracellular host-microbe interactions, given the ancestral phylogenetic placement of cnidarians and the close alignment of dinoflagellates with apicomplexan parasites, (ii) provide information on intimate interorganismal genetic interactions that occur, whereby microbes can modulate animal innate immune cascades, metabolism, and other critical host cellular pathways, and (iii) serve as a model for vertebrate phagocytic cells that engulf particles as large as or larger than themselves, requiring dramatic membrane trafficking and recycling events. Most crucially, however, cell biologists can contribute to the field of cnidarian-dinoflagellate symbiosis in a number of important ways, as outlined below (see also reference 403).
A significant investment in high-throughput, systems biology approaches to the study of Symbiodinium is of highest priority. With this investment will come a full understanding of the repertoire of dinoflagellate effector molecules that result in host-symbiont specificity, promote invasion, modulate host immune responses, and allow for nutrient exchange. At present there are two EST studies (199, 387) on two Symbiodinium strains and one proteomic study comparing cultured and symbiotic Symbiodinium strains (348). Systems-level information is critical for generating hypotheses for targeted empirical studies at the cellular level. Suggested approaches that would benefit from the input of cell biologists include (i) genome and transcriptome sequencing, (ii) expression and proteomic profiling of Symbiodinium in culture and in hospite during different phases of symbiosis and under different environmental conditions or perturbations, and (iii) glycomic analysis of the algal cell surface in different phases of the symbiosis. At the time of this article going to press, there are at least two high-throughput sequencing efforts under way for Symbiodinium spp., in Saudi Arabia (C. R. Voolstra and coworkers, unpublished data) and Japan (N. Satoh, C. Shinzato, and E. Shoguchi, unpublished data).
Other high-priority projects to which cell biologists could make significant contributions include the following. (i) Detailed cellular investigation of the dynamics of membrane trafficking in symbiotic gastrodermal cells. Imaging techniques such as fluorescence recovery after photobleaching (FRAP) (308), which follows the dynamic diffusion and movement of subcellular compartments and macromolecules, could be used in conjunction with numerous membrane trafficking probes to capture the dynamics of postphagocytic recognition events in the gastrodermal cell. (ii) Elucidation of the mechanisms of regulation and coordination of host and symbiont cell division. Comparative genomic approaches and the biochemical characterization of potential signaling molecules could be informative in this regard. (iii) Characterization of metabolites transferred between partners during different phases of the symbiosis and with different host-symbiont combinations. Modern chromatographic and mass spectrometric techniques offer exciting prospects for such research, as does comparative metabolic profiling, and some significant results are already being achieved with such approaches (M. Burriesci and J. R. Pringle, personal communication). (iv) Comparative proteomic analysis of the host-symbiont interface during different phases of symbiosis, to map the transition from phagosome to symbiosome and characterize the nutrient transporters associated with the symbiosome membrane complex. (v) Clarification of the mechanism by which symbiotic dinoflagellates enhance coral calcification in the light, through the application of cell physiology techniques and functional genomics. Study of the biology of the calicoblastic cell as a model for transepithelial transport and organic matrix secretion would also be valuable.
It is therefore clear that cell biologists with expertise in any of the “omics” (genomics, transcriptomics, proteomics, or metabolomics), as well as the likes of eukaryotic cell cycle control or parasite immunology, have the potential to contribute enormously to our understanding of the cnidarian-dinoflagellate symbiosis and coral biology and hence the future management of the world's seriously threatened coral reefs.
ACKNOWLEDGMENTS
D.A. thanks his colleagues for stimulating discussions and particularly Sylvie Tambutté and Didier Zoccola for their critical reading of an early version of the coral calcification section. We also thank Joanne Davy for editorial assistance, John Pringle for his encouragement and helpful discussions during the preparation of this review, and two anonymous reviewers for their thorough and insightful comments.
A visit by S.K.D. to Oregon State University, when much of this review was written, was funded by a research and study leave grant from Victoria University of Wellington. The Government of the Principality of Monaco funded work performed at the Centre Scientifique de Monaco. The idea of constructing this review was spawned from discussions during an NSF-funded (to V.M.W. and John Pringle; OISE:0605814) international workshop aimed at promoting the study of coral cell biology.
Biographies

Simon K. Davy is Associate Professor and Deputy Head of the School of Biological Sciences, Victoria University of Wellington (VUW). He obtained his Ph.D. in 1994 from the University of Wales, Bangor, in the area of temperate cnidarian-dinoflagellate symbiosis. After this, he held postdoctoral fellowships at the Harbor Branch Oceanographic Institution and the University of Sydney, where he pursued his interest in the nutritional physiology of alga-invertebrate symbioses. From 1999 to 2003 he held a faculty position at the University of Plymouth, after which he moved to VUW. His research group focuses on molecular, cellular, physiological, and ecological aspects of cnidarian-dinoflagellate symbiosis, as well as coral disease. He has strong interests in host-symbiont signaling, carbon and nitrogen metabolism, and coral bleaching physiology. He has authored >45 publications, including the textbook The Biology of Coral Reefs. He has supervised two postdoctoral fellows, 20 Ph.D. students, 27 Master's students, and numerous undergraduates. For more details of his research activities, see http://www.victoria.ac.nz/sbs/research/marine-biology-research/mar-sym-coral-reef-bio/default.aspx.

Denis Allemand is Professor and Scientific Director of the Centre Scientifique de Monaco (Principality of Monaco). He obtained his Ph.D. in pharmacological sciences from the University of Montpellier II in 1986. He was the head of the symbiosis team at the University of Nice-Sophia Antipolis until September 2007. His main scientific interest is the study of anthozoan physiology (reef-building corals, sea anemones, and gorgonians), with particular emphasis on both biomineralization and symbiosis. He is interested in the mechanism of formation of coral skeleton and more particularly in the physiology of skeletogenesis (ion transport and characterization and the role of organic matrix) and the interaction with symbiosis (light-enhanced calcification). He is also interested in the mutual adaptation of both partners (animal host and dinoflagellate symbionts) of the cnidarian symbiosis. He has published more than 120 peer-reviewed papers and supervised 12 Ph.D. students. He sits on the advisory boards of a number of different scientific institutions. For more details of his research activities, see www.centrescientifique.mc.

Virginia M. Weis is Professor and Chair of the Department of Zoology at Oregon State University (OSU). She obtained her Ph.D. in 1990 in biology from the University of California at Los Angeles in the area of coral-dinoflagellate symbiosis. She performed two postdoctoral fellowships, one at the University of Southern California studying squid-bacterium symbioses and the other at Stanford University examining sea anemone-dinoflagellate symbiosis. Since 1996 she has been at OSU, where her group has been interested in cellular and molecular mechanisms underlying the onset, maintenance, and breakdown of coral-dinoflagellate symbiosis. Her work has included investigations of inorganic carbon transport mechanisms, innate immunity, oxidative stress, and apoptosis. Her studies have employed biochemical, cellular, molecular, proteomic, and genomic approaches. She has authored over 55 publications and supervised 9 Ph.D. students, 7 postdoctoral fellows, and dozens of undergraduates. For more details of her research activities, see http://weis.science.oregonstate.edu/.
APPENDIX 1
The Endocytic Pathway and Phagocytosis
Phagocytosis is best understood for the professional phagocytes of the vertebrates, such as macrophages and neutrophils, which participate in immunity by actively seeking out, engulfing, and destroying microbial invaders. There are many reviews on this large topic, including those that discuss microbial invaders' strategies for subverting host defenses (115, 150). Phagocytosis of large particles, including microbes, is a highly choreographed two-step process of particle internalization followed by phagosome maturation. Engulfment is triggered either by direct signaling between host and invader, such as the MAMP-PRR interactions described in Fig. 2, or through indirect mediation by host opsonins that target them for engulfment. Actin remodeling at the site of engulfment is triggered to allow for the formation and extension of pseudopods. Fusion of numerous endocytic vesicles at the site of the growing phagosome enables enough membrane to be generated to surround the entire particle.
Once fully pinched off from the plasma membrane, the compartment is referred to as an early phagosome and phagosomal maturation begins. Complex membrane trafficking, mediated by numerous signaling molecules, including most notably the family of Rab GTPases (323), results in remodeling, recycling, sorting, and swapping of membrane protein and lipid components throughout phagosome maturation. The early phagosome can be identified in particular by a slightly acidic luminal pH, ranging from pH 6.1 to 6.5, and the presence of Rab5, which conducts and controls events on the early phagosome, and phosphatidylinositol-3-phosphate [PI(3)P] kinase, which makes the early phagosomal lipid PI(3)P. PI(3)P is important in anchoring effector proteins in the membrane which act as bridges to fuse incoming vesicles to the phagosome. Early phagosomes cannot fuse with lysosomes, as they lack the correct membrane proteins that enable fusogenic activity. Early phagosomes transition into late phagosomes through complex membrane remodeling and sometimes transport through the cell.
The late phagosome has a more acidic luminal pH of 5.5 to 6.0, achieved by the docking of proton-pumping ATPases on membrane. In late phagosomes, PI(3)P content has decreased, with a concomitant appearance of the unique lipid lysobisphosphatidic acid (LBPA) in luminal vesicles and lysosome-associated marker proteins (LAMPs). Rab5 has been replaced by Rab7, which mediates trafficking between late phagosomes and lysosomes, including through the effector protein Rab-interacting lysosomal protein (RILP), which promotes fusion of the late phagosome with lysosomes, resulting in the generation of the phagolysosome. The phagolysosome is a microbicidal environment with a battery of microbial killing mechanisms that includes a highly acidic pH of 4.5, NAPDH oxidase and nitric oxide synthase which generate toxic reactive oxygen and nitrogen species, antimicrobial peptides, and numerous degradative protein, carbohydrate, and lipid hydrolases released from the lysosome.
Pathogenic and parasitic microbes have evolved a multitude of ways to battle host phagocyte defenses. Some have even evolved sophisticated mechanisms to coopt portions of both the MAMP-PRR signaling pathways and phagocytic pathways to survive and thrive as intracellular parasites. Again, there is a rich literature describing these mechanisms; however, just a few examples are given in Table A1 to provide perspective on the range of mechanisms that dinoflagellates could be using to persist in symbiosomes.
Table A1.
Strategies employed by both prokaryotic and eukaryotic pathogens and parasites during invasion of vertebrate phagocytesa
| Strategy employed by invading microbe | Microbe(s) | Mechanism |
|---|---|---|
| Escapes phagosome | Trypanosoma cruzi, Listeria monocytogenes | Escapes phagosome by making a pore |
| Arrests phagosome maturation | Legionella pneumophila | Alters lipid and protein sorting |
| Salmonella enterica, Leishmania donovani, Mycobacterium tuberculosis | Halts or slows vesicle trafficking | |
| M. tuberculosis | Arrests progression of early phagosome by preventing production of PI(3)P and causing persistence of Rab5 | |
| S. enterica, Plasmodium falciparum, Leishmania spp. | Prevents host oxidative burst | |
| M. tuberculosis | Blocks lysosomal fusion machinery | |
| L. donovani | Reduces fusogenic nature of phagosome by addition of own lipoglycan to phagosome | |
| Tolerates environment of phagolysosome | Coxiella burnetii, Leishmania mexicana | Adapted to acidic environment of pH 4.8 |
APPENDIX 2
The Eukaryotic Cell Cycle and Its Control
The eukaryotic cell cycle has been highly conserved during evolution, and hence lessons learned from model systems, such as yeast (see, e.g., references 67, 335, and 406), can inform our knowledge of the cell cycles of both cnidarians and their algal symbionts and how these cell cycles might be coordinated so that a stable symbiosis is maintained. The eukaryotic cell cycle is highly complex and still not completely understood, for example, in terms of the specific regulatory interactions that control cell cycle transcription. The cell cycle will be summarized only briefly here; it is discussed in detail elsewhere (see, e.g., references 1, 103, 253, 278, 281, and 416).
The cell cycle consists of two major phases: (i) the S (synthesis) phase, during which DNA replication occurs, and (ii) the M (mitosis) phase, during which both mitosis (nuclear division) and then cytokinesis (cytoplasmic division) lead to cell division. Two further phases, known as gap (G) phases, have generally been inserted between the S and M phases: G1 between the completion of the M phase and start of the S phase and G2 between the end of the S phase and start of the M phase. Collectively, G1, S, and G2 are known as interphase. These gap phases provide additional time for cell growth and environmental assessment (see below) prior to any investment in the S and M phases. In addition, cells in G1 may enter a nonproliferative, resting state known as G0 and remain there for prolonged periods or even indefinitely. The cell cycle always occurs in the same ordered fashion and is irreversible.
The timing and orderly progression of the cell cycle are regulated by a control system, on which either inhibitory or stimulatory intra- or extracellular signals can act. Key to this control system in eukaryotes is a family of cyclin-dependent protein kinases (Cdks). These enzymes bind to proteins known as cyclins, specific for the different stages of the cell cycle, thus producing cyclin-Cdk complexes (i.e., G1-Cdk, G1/S-Gdk, S-Gdk, or M-Cdk). These cyclin-Cdk complexes activate target proteins via phosphorylation and hence trigger the corresponding portion of the cell cycle; their inactivation occurs by a range of processes, including cyclin proteolysis, decreased cyclin gene transcription, and the actions of inhibitors. The oscillatory activation and inhibition of cyclin-Cdk complexes are essential for controlling the periodic events of the cell cycle and may (except in early embryos, where transcription does not occur) operate in tandem with a separate, oscillatory transcriptional network.
The cell cycle may be further regulated by a series of “checkpoints,” where specialized proteins assess cell cycle progression and halt it if there is a problem with previous events (e.g., incomplete DNA replication or faulty spindle assembly), if there is cell damage, or if the extracellular environment is unfavorable. Moreover, these checkpoints provide an opportunity for extracellular signals (e.g., mitogens, which promote progression from G1 to S phase) to influence the cell cycle. The most significant checkpoints are typically the G1/S, G2/M and M checkpoints. It is not unreasonable to suggest that one or more of these checkpoints could play an important role in the control and coordination of the host and symbiont cell cycles in cnidarian-alga symbiosis. This is especially the case for the G1/S checkpoint, which is particularly important for assessing whether the environment and extracellular signals are favorable for replication.
REFERENCES
- 1. Alberts B, et al. 2008. Molecular biology of the cell, 4th ed Garland Science, New York, NY [Google Scholar]
- 2. Al-Horani F, Al-Moghrabi SM, de Beer D. 2003. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: active internal carbon cycle. J. Exp. Mar. Biol. Ecol. 288:1–15 [Google Scholar]
- 3. Allemand D, Furla P, Bénazet-Tambutté S. 1998. Mechanisms of carbon acquisition for endosymbiont photosynthesis in Anthozoa. Can. J. Bot. 76:925–941 [Google Scholar]
- 4. Allemand D, Tambutté E, Girard JP, Jaubert J. 1998. Organic matrix synthesis in the scleractinian coral Stylophora pistillata: role in biomineralization and potential target of the organotin tributyltin. J. Exp. Biol. 201:2001–2009 [DOI] [PubMed] [Google Scholar]
- 5. Allemand D, et al. 2004. Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C. R. Palevol. 3:453–467 [Google Scholar]
- 6. Allemand D, Tambutté E, Zoccola D, Tambutté S. 2011. Coral calcification, cells to reefs, p 119–150 In Dubinsky Z, Stambler N. (ed), Coral reefs: an ecosystem in transition. Springer, Berlin, Germany [Google Scholar]
- 7. Al-Moghrabi S, Allemand D, Jaubert J. 1993. Valine uptake by the scleractinian coral Galaxea fascicularis: characterisation and effect of light and nutritional status. J. Comp. Physiol. 163:355–362 [Google Scholar]
- 8. Al-Moghrabi S, Goiran C, Allemand D, Speziale N, Jaubert J. 1996. Inorganic carbon uptake for photosynthesis by the symbiotic coral-dinoflagellate association. II. Mechanisms for bicarbonate uptake. J. Exp. Mar. Biol. Ecol. 199:227–248 [Google Scholar]
- 9. Amer AO, Swanson MS. 2002. A phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 5:56–61 [DOI] [PubMed] [Google Scholar]
- 10. Anand S, Prasad R. 1989. Rise in intracellular pH is concurrent with start progression of Saccharomyces cerevisiae. J. Gen. Microbiol. 135:2173–2179 [DOI] [PubMed] [Google Scholar]
- 11. Augustin R, Fraune S, Bosch TC. 2010. How Hydra senses and destroys microbes. Semin. Immunol. 22:54–58 [DOI] [PubMed] [Google Scholar]
- 12. Babcock RC, et al. 1986. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90:379–394 [Google Scholar]
- 13. Badger MR, et al. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 76:1052–1071 [Google Scholar]
- 14. Badgley BD, Lipschultz F, Sebens KP. 2006. Nitrate uptake by the reef coral Diploria strigosa: effects of concentration, water flow, and irradiance. Mar. Biol. 149:327–338 [Google Scholar]
- 15. Baghdasarian G, Muscatine L. 2000. Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol. Bull. 199:278–286 [DOI] [PubMed] [Google Scholar]
- 16. Baird A, Cumbo V, Leggat W, Rodriguez-Lanetty M. 2007. Fidelity and flexibility in coral symbioses. Mar. Ecol. Prog. Ser. 347:307–309 [Google Scholar]
- 17. Baker AC. 2003. Flexibility and specificity in coral-algal symbiosis: diversity, ecology and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34:661–689 [Google Scholar]
- 18. Barnes DJ. 1985. The effect of photosynthetic and respiratory inhibitors upon calcification in the staghorn coral, Acropora formosa, p 161–166 In Proceedings of the 5th International Coral Reef Symposium, vol. 6 ReefBase, Penang, Malaysia [Google Scholar]
- 19. Battey JF, Patton JS. 1984. A reevaluation of the role of glycerol in carbon translocation in zooxanthellae-coelenterate symbiosis. Mar. Biol. 79:27–38 [Google Scholar]
- 20. Battey JF, Patton JS. 1987. Glycerol translocation in Condylactis gigantea. Mar. Biol. 95:37–46 [Google Scholar]
- 21. Battey JF. 1992. Carbon metabolism in zooxanthellae-coelenterate symbioses, p 154–187 In Reisser W. (ed), Algae and symbioses. Biopress Ltd., Bristol, United Kingdom [Google Scholar]
- 22. Bauerlein E. 2007. Handbook of biomineralization. The biology of biominerals structure formation. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 23. Bay LK, et al. 2011. Infection dynamics vary between Symbiodinium types and cell surface treatments during establishment of endosymbiosis with coral larvae. Diversity 3:356–374 [Google Scholar]
- 24. Benayahu Y, Achituv Y. 1989. Metamorphosis of an octocoral primary polyp and its infection by algal symbiosis. Symbiosis 7:159–169 [Google Scholar]
- 25. Bénazet-Tambutté S, Allemand D, Jaubert J. 1996. Inorganic carbon supply to symbiont photosynthesis of the sea anemone Anemonia viridis: role of the oral epithelial layers. Symbiosis 20:199–217 [Google Scholar]
- 26. Berner T, Baghdasarian G, Muscatine L. 1993. Repopulation of a sea anemone with symbiotic dinoflagellates—analysis by in vivo fluorescence. J. Exp. Mar. Biol. Ecol. 170:145–158 [Google Scholar]
- 27. Bertucci A, Tambutté E, Tambutté S, Allemand D, Zoccola D. 2010. Symbiosis-dependent gene expression in coral-dinoflagellate association: cloning and characterization of a P-type H+-ATPase gene. Proc. R. Soc. Lond. B 277:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertucci A, Tambutté S, Supuran CT, Allemand D, Zoccola D. 2011. A new carbonic anhydrase in the coral Stylophora pistillata. Mar. Biotech. 13:992–1002 [DOI] [PubMed] [Google Scholar]
- 29. Bhagooli R, Hidaka M. 2004. Release of zooxanthellae with intact photosynthetic activity by the coral Galaxea fascicularis in response to high temperature stress. Mar. Biol. 145:329–337 [Google Scholar]
- 30. Biel K, Gates R, Muscatine L. 2007. Effects of free amino acids on the photosynthetic carbon metabolism of symbiotic dinoflagellates. Russ. J. Plant Physiol. 54:171–183 [Google Scholar]
- 31. Blank RJ, Muscatine L. 1987. How do combinations of nutrients cause symbiotic Chlorella to overgrow Hydra? Symbiosis 3:123–134 [Google Scholar]
- 32. Blanquet RS, Nevenzel JC, Benson AA. 1979. Acetate incorporation into the lipids of the anemone Anthopleura elegantissima and its associated zooxanthellae. Mar. Biol. 54:185–194 [Google Scholar]
- 33. Bodemann BO, et al. 2011. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144:253–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boothroyd JC, Dubremetz JF. 2008. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 6:79–88 [DOI] [PubMed] [Google Scholar]
- 35. Bosch TC, et al. 2009. Uncovering the evolutionary history of innate immunity: the simple metazoan Hydra uses epithelial cells for host defence. Dev. Comp. Immunol. 33:559–569 [DOI] [PubMed] [Google Scholar]
- 36. Boschma H. 1925. On the feeding reactions and digestion in the coral polyp Astrangia danae, with notes on its symbiosis with zooxanthellae. Biol. Bull. 49:407–439 [Google Scholar]
- 37. Brunelle SA, Hazard ES, Sotka EE, Van Dolah FM. 2007. Characterization of a dinoflagellate cryptochrome blue-light receptor with a possible role in circadian control of the cell cycle. J. Phycol. 43:509–518 [Google Scholar]
- 38. Bruno JF, Selig ER. 2007. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One 2:e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carroll S, Blanquet RS. 1984. Alanine uptake by isolated zooxanthellae of the mangrove jellyfish, Cassiopea xamachana. I. Transport mechanisms and utilization. Biol. Bull. 166:409–418 [Google Scholar]
- 40. Carroll S, Blanquet RS. 1984. Alanine uptake by isolated zooxanthellae of the mangrove jellyfish, Cassiopea xamachana. II. Inhibition by host homogenate fraction. Biol. Bull. 166:419–426 [Google Scholar]
- 41. Cates N, Mclaughlin JJA. 1976. Differences of ammonia metabolism in symbiotic and aposymbiotic Condylactis and Cassiopea spp. J. Exp. Mar. Biol. Ecol. 21:1–5 [Google Scholar]
- 42. Catmull J, Yellowlees D, Miller DJ. 1987. NADP+-dependent glutamate dehydrogenase from Acropora formosa: purification and properties. Mar. Biol. 95:559–563 [Google Scholar]
- 43. Cernichiari E, Muscatine L, Smith DC. 1969. Maltose excretion by symbiotic algae of Hydra viridis Proc. R. Soc. Lond. B 173:557–576 [Google Scholar]
- 44. Chalker BE, Taylor DL. 1975. Light-enhanced calcification, and the role of oxidative phosphorylation in calcification of the coral Acropora cervicornis. Proc. R. Soc. Lond. B 190:323–331 [DOI] [PubMed] [Google Scholar]
- 45. Chang SS, Prezelin BB, Trench RK. 1983. Mechanisms of photoadaptation in 3 strains of the symbiotic dinoflagellate Symbiodinium microadriaticum. Mar. Biol. 76:219–229 [Google Scholar]
- 46. Chapman JA, et al. 2010. The dynamic genome of Hydra. Nature 464:592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen M-C, Cheng Y-M, Sung P-J, Kuo C-E, Fang L-S. 2003. Molecular identification of Rab7 (ApRab7) in Aiptasia pulchella and its exclusion from phagosomes harboring zooxanthellae. Biochem. Biophys. Res. Commun. 308:586–595 [DOI] [PubMed] [Google Scholar]
- 48. Chen M-C, Cheng Y-M, Hong M-C, Fang L-S. 2004. Molecular cloning of Rab5 (ApRab5) in Aiptasia pulchella and its retention in phagosomes harboring live zooxanthellae. Biochem. Biophys. Res. Commun. 324:1024–1033 [DOI] [PubMed] [Google Scholar]
- 49. Chen M-C, et al. 2005. ApRab11, a cnidarian homologue of the recycling regulatory protein Rab11, is involved in the establishment and maintenance of the Aiptasia-Symbiodinium endosymbiosis. Biochem. Biophys. Res. Commun. 338:1607–1616 [DOI] [PubMed] [Google Scholar]
- 50. Chen W-NU, et al. 16 December 2011. Diel rhythmicity of lipid-body formation in a coral-Symbiodinium endosymbiosis. Coral Reefs doi10.1007/s00338-011-0868-6 [Google Scholar]
- 51. Clayton WS, Lasker HR. 1984. Host feeding regime and zooxanthellal photosynthesis in the anemone Aiptasia pallida (Verrill). Biol. Bull. 167:590–600 [DOI] [PubMed] [Google Scholar]
- 52. Clode PL, Marshall AT. 2002. Low temperature FESEM of the calcifying interface of a scleractinian coral. Tissue Cell 34:187–198 [DOI] [PubMed] [Google Scholar]
- 53. Clode PL, Marshall AT. 2004. Calcium localisation by X-ray microanalysis and fluorescence microscopy in larvae of zooxanthellate and azooxanthellate corals. Tissue Cell 36:379–390 [DOI] [PubMed] [Google Scholar]
- 54. Coffroth MA, Santos SR. 2005. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 156:19–34 [DOI] [PubMed] [Google Scholar]
- 55. Cohen AL, McConnaughey TA. 2003. Geochemical perspectives on coral mineralization. Rev. Mineral. Geochem. 54:151–187 [Google Scholar]
- 56. Colley NJ, Trench RK. 1983. Selectivity in phagocytosis and persistence of symbiotic algae by the scyphistoma stage of the jellyfish Cassiopeia xamachana. Proc. R. Soc. Lond. B 219:61–82 [DOI] [PubMed] [Google Scholar]
- 57. Colombo-Pallotta MF, Rodríguez-Román A, Iglesias-Prieto R. 2010. Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29:899–907 [Google Scholar]
- 58. Cook CB. 1971. Transfer of 35S-labeled material from food ingested by Aiptasia sp. to its endosymbiotic zooxanthellae, p 218–224 In Lenhoff HM, Muscatine L, Davis LV. (ed), Experimental coelenterate biology. University of Hawaii Press, Honolulu, HI [Google Scholar]
- 59. Cook CB. 1972. Benefit to symbiotic zoochlorellae from feeding by green Hydra Biol. Bull. 142:236–242 [Google Scholar]
- 60. Cook CB. 1980. Sulfur metabolism in the green hydra symbiosis: the incorporation of sulfate-sulfur by symbiotic and aposymbiotic Hydra viridis, p 249–257 In Schwemmler W, Schenk HEA. (ed), Endocytobiology. Walter de Gruyter, Berlin, Germany [Google Scholar]
- 61. Cook CB, D'Elia CF. 1987. Are natural populations of zooxanthellae ever nutrient-limited? Symbiosis 4:199–211 [Google Scholar]
- 62. Cook CB, D'Elia CF, Muller-Parker G. 1988. Host feeding and nutrient sufficiency for zooxanthellae in the sea anemone Aiptasia pallida. Mar. Biol. 98:253–262 [Google Scholar]
- 63. Cook CB, Muller-Parker G, D'Elia CF. 1992. Ammonium enhancement of dark carbon fixation and nitrogen limitation in symbiotic zooxanthellae—effects of feeding and starvation of the sea anemone Aiptasia pallida. Limnol. Oceanogr. 37:131–139 [Google Scholar]
- 64. Cook CB, Muller-Parker G, Orlandini CD. 1994. Ammonium enhancement of dark carbon fixation and nitrogen limitation in zooxanthellae symbiotic with the reef corals Madracis mirabilis and Montastrea annularis. Mar. Biol. 118:157–165 [Google Scholar]
- 65. Cook CB, Davy SK. 2001. Are free amino acids responsible for the ‘host factor’ effects on symbiotic zooxanthellae in extracts of host tissue? Hydrobiologia 461:71–78 [Google Scholar]
- 66. Cooper JE. 2007. Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J. Appl. Microbiol. 103:1355–1365 [DOI] [PubMed] [Google Scholar]
- 67. Costanzo M, et al. 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117:899–913 [DOI] [PubMed] [Google Scholar]
- 68. Coutu DL, et al. 2008. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J. Biol. Chem. 283:17991–18001 [DOI] [PubMed] [Google Scholar]
- 69. Crossland CJ, Barnes DJ. 1974. The role of metabolic nitrogen in coral calcification. Mar. Biol. 28:325–332 [Google Scholar]
- 70. Crossland CJ, Barnes DJ. 1977. Nitrate assimilation enzymes from 2 hard corals, Acropora acuminata and Goniastrea autralensis. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 57:151–157 [Google Scholar]
- 71. Cuif J-P, Dauphin Y. 2005. The Environment Recording Unit in coral skeletons—a synthesis of structural and chemical evidences for a biochemically driven, stepping-growth process in fibres. Biogeosciences 2:61–73 [Google Scholar]
- 72. Cullimore J, Denarie J. 2003. How legumes select their sweet talking symbionts. Science 302:575–578 [DOI] [PubMed] [Google Scholar]
- 73. Dana JD. 1846. Structure and classification of zoophytes. Lea and Blanchard, Philadelphia, PA [Google Scholar]
- 74. Davies PS. 1991. Effect of daylight variations on the energy budgets of shallow-water corals. Mar. Biol. 108:137–144 [Google Scholar]
- 75. Davy SK, Lucas IAN, Turner JR. 1996. Carbon budgets in temperate anthozoan-dinoflagellate symbioses. Mar. Biol. 126:773–783 [Google Scholar]
- 76. Davy SK, Turner JR, Lucas IAN. 1997. The nature of temperate anthozoan-dinoflagellate symbioses, p 1307–1312 In Proceedings of the 8th International Coral Reef Symposium, vol. 2 ReefBase, Penang, Malaysia [Google Scholar]
- 77. Davy SK, Cook CB. 2001. The influence of “host release factor” on carbon release by zooxanthellae isolated from fed and starved Aiptasia pallida (Verrill). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129:487–494 [DOI] [PubMed] [Google Scholar]
- 78. Davy SK, Cook CB. 2001. The relationship between nutritional status and carbon flux in the zooxanthellate sea anemone Aiptasia pallida. Mar. Biol. 139:999–1005 [Google Scholar]
- 79. Davy SK, Turner JR. 2003. Early development and acquisition of zooxanthellae in the temperate symbiotic sea anemone Anthopleura ballii (Cocks). Biol. Bull. 205:66–72 [DOI] [PubMed] [Google Scholar]
- 80. Davy SK, Withers KJT, Hinde R. 2006. Effects of host nutritional status and seasonality on the nitrogen status of zooxanthellae in the temperate coral Plesiastrea versipora (Lamarck). J. Exp. Mar. Biol. Ecol. 335:256–265 [Google Scholar]
- 81. Day RJ. 1994. Algal symbiosis in Bunodeopsis—sea anemones with auxiliary structures. Biol. Bull. 186:182–194 [DOI] [PubMed] [Google Scholar]
- 82. Deane EM, O'Brien RW. 1981. Uptake of phosphate by symbiotic and free-living dinoflagellates. Arch. Microbiol. 128:307–310 [Google Scholar]
- 83. D'Elia CF. 1977. Uptake and release of dissolved phosphorus by reef corals. Limnol. Oceanogr. 22:301–315 [Google Scholar]
- 84. D'Elia CF, Domotor SL, Webb KL. 1983. Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar. Biol. 75:157–167 [Google Scholar]
- 85. Detournay O, Weis VM. 2011. Role of the sphingosine rheostat in the regulation of cnidarian-dinoflagellate symbioses. Biol. Bull. 221:261–269 [DOI] [PubMed] [Google Scholar]
- 86. Dimond J, Carrington E. 2008. Symbiosis regulation in a facultatively symbiotic temperate coral: zooxanthellae division and expulsion. Coral Reefs 27:601–604 [Google Scholar]
- 87. Domart-Coulon I, Tambutté S, Tambutté E, Allemand D. 2004. Short term viability of soft tissue detached from the skeleton of reef-building corals. J. Exp. Mar. Biol. Ecol. 309:199–217 [Google Scholar]
- 88. Domart-Coulon IJ, Elbert DC, Scully EP, Calimlim PS, Ostrander GK. 2001. Aragonite crystallization in primary cell cultures of multicellular isolates from a hard coral, Pocillopora damicornis. Proc. Natl. Acad. Sci. U. S. A. 98:11885–11890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Domotor SL, D'Elia CF. 1984. Nutrient uptake kinetics and growth of zooxanthellae maintained in laboratory culture. Mar. Biol. 80:93–101 [Google Scholar]
- 90. Dorling M, McAuley PJ, Hodge H. 1997. Effect of pH on growth and carbon metabolism of maltose-releasing Chlorella (Chlorophyta). Eur. J. Phycol. 32:19–24 [Google Scholar]
- 91. Douglas AE, Smith DC. 1984. The green Hydra symbiosis. 8. Mechanisms in symbiont regulation. Proc. R. Soc. Lond. B 221:291–319 [Google Scholar]
- 92. Douglas AE. 2010. The symbiotic habit. Princeton University Press, Princeton, NJ [Google Scholar]
- 93. Downs CA, et al. 2009. Symbiophagy as a cellular mechanism for coral bleaching. Autophagy 5:211–216 [DOI] [PubMed] [Google Scholar]
- 94. Drew EA, Smith DC. 1967. Studies in the physiology of lichens. VIII. Movement of glucose from alga to fungus during photosynthesis in the thallus of Peltigera polydactyla. New Phytol. 66:389–400 [Google Scholar]
- 95. Dudler N, Miller DJ. 1988. Characterization of 2 glutamate-dehydrogenases from the symbiotic microalga Symbiodinium microadriaticum isolated from the coral Acropora formosa. Mar. Biol. 97:427–430 [Google Scholar]
- 96. Dunn K. 1987. Growth of endosymbiotic algae in the green hydra, Hydra viridissima. J. Cell Sci. 88:571–578 [Google Scholar]
- 97. Dunn SR, Phillips WS, Green DR, Weis VM. 2007. Knockdown of actin and caspase gene expression by RNA interference in the symbiotic anemone Aiptasia pallida. Biol. Bull. 212:250–258 [DOI] [PubMed] [Google Scholar]
- 98. Dunn SR, Bythell JC, Le Tissier MDA, Burnett WJ, Thomason JC. 2002. Programmed cell death and cell necrosis activity during hyperthermic stress-induced bleaching of the symbiotic sea anemone Aiptasia sp. J. Exp. Mar. Biol. Ecol. 272:29–53 [Google Scholar]
- 99. Dunn SR, Schnitzler CE, Weis VM. 2007. Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: every which way you lose. Proc. R. Soc. Lond. B 274:3079–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dunn SR. 2009. Immunorecogntion and immunoreceptors in the Cnidaria. Invertebrate Surviv. J. 6:7–14 [Google Scholar]
- 101. Dunn SR, Weis VM. 2009. Apoptosis as a post-phagocytic winnowing mechanism in a coral-dinoflagellate mutualism. Environ. Microbiol. 11:268–276 [DOI] [PubMed] [Google Scholar]
- 102. Edmunds PJ, Davies PS. 1988. Post-illumination stimulation of respiration rate in the coral Porites porites. Coral Reefs 7:7–9 [Google Scholar]
- 103. Elledge SJ. 1996. Cell cycle checkpoints: preventing an identity crisis. Science 274:1664–1672 [DOI] [PubMed] [Google Scholar]
- 104. Enriquez S, Méndez ER, Iglesias-Prieto R. 2005. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol. Oceanogr. 50:1025–1032 [Google Scholar]
- 105. Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. 1984. Light and the bioenergetics of a symbiotic coral. Bioscience 34:705–709 [Google Scholar]
- 106. Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L. 1993. Population control in symbiotic corals. Bioscience 43:606–611 [Google Scholar]
- 107. Farrant PA, Borowitzka MA, Hinde R, King RJ. 1987. Nutrition of the temperate Australian soft coral Capnella gaboensis. II. The role of zooxanthellae and feeding. Mar. Biol. 95:575–581 [Google Scholar]
- 108. Ferrier-Pagès C, Allemand D, Gattuso JP, Jaubert J, Rassoulzadegan R. 1998. Microheterotrophy in the zooxanthellate coral Stylophora pistillata: effects of light and ciliate density. Limnol. Oceanogr. 43:1639–1648 [Google Scholar]
- 109. Fishman Y, Zlotkin E, Sher D. 2008. Expulsion of symbiotic algae during feeding by the green hydra—a mechanism for regulating symbiont density? PLoS One 3:e2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fitt WK, Trench RK. 1983. Endocytosis of the symbiotic dinoflagellate Symbiodinium microadriaticum Freudenthal by endodermal cells of the scyphistomae of Cassiopeia xamachana and the resistance of the algae to host digestion. J. Cell Sci. 64:195–212 [DOI] [PubMed] [Google Scholar]
- 111. Fitt WK, Trench RK. 1983. The relation of diel patterns of cell division to diel patterns of motility in the symbiotic dinoflagellate Symbiodinium microadriaticum (Freudenthal) in culture. New Phytol. 94:421–432 [Google Scholar]
- 112. Fitt WK, Cook CB. 1990. Some effects of host feeding on growth of zooxanthellae in the marine hydroid Myrionema ambionense in the laboratory and in nature, p 281–284 In Narclon P. (ed), Endocytobiology IV. INRA, Paris, France [Google Scholar]
- 113. Fitt WK. 2000. Cellular growth of host and symbiont in a cnidarian-zooxanthellar symbiosis. Biol. Bull. 198:110–120 [DOI] [PubMed] [Google Scholar]
- 114. Fitt WK, Cook CB. 2001. The effects of feeding or addition of dissolved inorganic nutrients in maintaining the symbiosis between dinoflagellates and a tropical marine cnidarian. Mar. Biol. 139:507–517 [Google Scholar]
- 115. Flannagan RS, Cosio G, Grinstein S. 2009. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7:355–366 [DOI] [PubMed] [Google Scholar]
- 116. Fukuda I, et al. 2003. Molecular cloning of a cDNA encoding a soluble protein in the coral exoskeleton. Biochem. Biophys. Res. Commun. 304:11–17 [DOI] [PubMed] [Google Scholar]
- 117. Furla P, Bénazet-Tambutté S, Jaubert J, Allemand D. 1998. Functional polarity of the tentacle of the sea anemone Anemonia viridis: role in inorganic carbon acquisition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 274:R303–R310 [DOI] [PubMed] [Google Scholar]
- 118. Furla P, Allemand D, Orsenigo MN. 2000. Involvement of H+-ATPase and carbonic anhydrase in inorganic carbon uptake for endosymbiont photosynthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278:R870–R881 [DOI] [PubMed] [Google Scholar]
- 119. Furla P, Galgani I, Durand I, Allemand D. 2000. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203:3445–3457 [DOI] [PubMed] [Google Scholar]
- 120. Gallop A. 1974. Evidence for the presence of a ‘factor’ in Elysia viridis which stimulates photosynthate release from its symbiotic chloroplasts. New Phytol. 73:1111–1117 [Google Scholar]
- 121. Galloway SB, et al. 2007. Coral Disease and Health Workshop: Coral Histopathology Workshop II. National Oceanic and Atmospheric Administration, Silver Spring, MD [Google Scholar]
- 122. Ganot P, et al. 2011. Adaptations to endosymbiosis in a cnidarian-dinoflagellate association: differential gene expression and specific gene duplications. PLoS Genet. 7:e1002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gates RD, Baghdasarian G, Muscatine L. 1992. Temperature stress causes host-cell detachment in symbiotic cnidarians—implications for coral bleaching. Biol. Bull. 182:324–332 [DOI] [PubMed] [Google Scholar]
- 124. Gates RD, Muscatine L. 1992. Three methods for isolating viable anthozoan endoderm cells with their intracellular symbiotic dinoflagellates. Coral Reefs 11:143–145 [Google Scholar]
- 125. Gates RD, Hoegh-Guldberg O, McFall-Ngai MJ, Bil KY, Muscatine L. 1995. Free amino acids exhibit anthozoan “host factor” activity: they induce the release of photosynthate from symbiotic dinoflagellates in vitro. Proc. Natl. Acad. Sci. U. S. A. 92:7430–7434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gates RD, Bil KY, Muscatine L. 1999. The influence of an anthozoan “host factor” on the physiology of a symbiotic dinoflagellate. J. Exp. Mar. Biol. Ecol. 232:241–259 [Google Scholar]
- 127. Gattuso J-P, Jaubert J. 1990. Effect of light on oxygen and carbon dioxide fluxes and on metabolic quotients measured in situ in a zooxanthellate coral. Limnol. Oceanogr. 35:1796–1804 [Google Scholar]
- 128. Gattuso J-P, Yellowlees D, Lesser M. 1993. Depth-dependent and light-dependent variation of carbon partitioning and utilization in the zooxanthellate scleractinian coral Stylophora pistillata. Mar. Ecol. Prog. Ser. 92:267–276 [Google Scholar]
- 129. Gattuso J-P, Allemand D, Frankignoulle M. 1999. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am. Zool. 39:160–183 [Google Scholar]
- 130. Gautret P, Cuif J-P, Freiwald A. 1997. Composition of soluble mineralizing matrices in azooxanthellate and non-zooxanthellate scleractinian corals: biochemical assessment of photosynthetic metabolism through the study of a skeletal feature. Facies 36:189–194 [Google Scholar]
- 131. Reference deleted.
- 132. Gladfelter EH. 1983. Spatial and temporal patterns of mitosis in the cells of the axial polyp of the reef coral Acropora cervicornis. Biol. Bull. 165:811–815 [DOI] [PubMed] [Google Scholar]
- 133. Godinot C, Ferrier-Pagès C, Grover R. 2009. Control of phosphate uptake by zooxanthellae and host cells in the scleractinian coral Stylophora pistillata. Limnol. Oceanogr. 54:1627–1633 [Google Scholar]
- 134. Goiran C, Al-Moghrabi S, Allemand D, Jaubert J. 1996. Inorganic carbon uptake for photosynthesis by the symbiotic coral/ dinoflagellate association. I. Photosynthetic performances of symbionts and dependence on sea water bicarbonate. J. Exp. Mar. Biol. Ecol. 199:207–225 [Google Scholar]
- 135. Goiran C, Allemand D, Galgani I. 1997. Transient Na+ stress in symbiotic dinoflagellates after isolation from coral-host cells and subsequent immersion in seawater. Mar. Biol. 129:581–589 [Google Scholar]
- 136. Goodacre R, Vaidyanathan S, Dunn WB, Harrigan GG, Kell DB. 2004. Metabolomics by numbers: acquiring and understanding global metabolite data. Trends Biotechnol. 22:245–252 [DOI] [PubMed] [Google Scholar]
- 137. Gordon BR, Leggat W. 2010. Symbiodinium-invertebrate symbioses and the role of metabolomics. Mar. Drugs 8:2546–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Goreau TF. 1959. The physiology of skeleton formation in corals. I. A method for measuring the rate of calcium deposition by corals under different conditions. Biol. Bull. 116:59–75 [Google Scholar]
- 139. Grant AJ, Rémond M, People J, Hinde R. 1997. Effects of host-tissue homogenate of the scleractinian coral Plesiastrea versipora on glycerol metabolism in isolated symbiotic dinoflagellates. Mar. Biol. 128:665–670 [Google Scholar]
- 140. Grant AJ, Rémond M, Hinde R. 1998. Low molecular-weight factor from Plesiastrea versipora (Scleractinia) that modifies release and glycerol metabolism of isolated symbiotic algae. Mar. Biol. 130:553–557 [Google Scholar]
- 141. Grant AJ, Rémond M, Withers KJT, Hinde R. 2001. Inhibition of algal photosynthesis by a symbiotic coral. Hydrobiologia 461:63–69 [Google Scholar]
- 142. Grant AJ, Trautman DA, Frankland S, Hinde R. 2003. A symbiosome membrane is not required for the actions of two host signalling compounds regulating photosynthesis in symbiotic algae isolated from cnidarians. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 135:337–345 [DOI] [PubMed] [Google Scholar]
- 143. Grant AJ, Rémond M, Starke-Peterkovic T, Hinde R. 2006. A cell signal from the coral Plesiastrea versipora reduces starch synthesis in its symbiotic alga, Symbiodinium sp. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 144:458–463 [DOI] [PubMed] [Google Scholar]
- 144. Grant AJ, Trautman DA, Menz I, Hinde R. 2006. Separation of two cell signalling molecules from a symbiotic sponge that modify algal carbon metabolism. Biochem. Biophys. Res. Commun. 348:92–98 [DOI] [PubMed] [Google Scholar]
- 145. Grasso LC, et al. 2008. Microarray analysis identifies candidate genes for key roles in coral development. BMC Genomics 9:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Grover R, Maguer JF, Reynaud-Vaganay S, Ferrier-Pagès C. 2002. Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol. Oceanogr. 47:782–790 [Google Scholar]
- 147. Grover R, Maguer JF, Allemand D, Ferrier-Pagès C. 2003. Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol. Oceanogr. 48:2266–2274 [Google Scholar]
- 148. Grover R, Maguer JF, Allemand D, Ferrier-Pagès C. 2006. Urea uptake by the scleractinian coral Stylophora pistillata. J. Exp. Mar. Biol. Ecol. 332:216–225 [Google Scholar]
- 149. Grover R, Maguer JF, Allemand D, Ferrier-Pagès C. 2008. Uptake of dissolved free amino acids by the scleractinian coral Stylophora pistillata. J. Exp. Biol. 211:860–865 [DOI] [PubMed] [Google Scholar]
- 150. Gruenberg J, van der Goot FG. 2006. Mechanisms of pathogen entry through the endosomal compartments. Nat. Rev. Mol. Cell Biol. 7:495–504 [DOI] [PubMed] [Google Scholar]
- 151. Gunnersen J, Yellowlees D, Miller DJ. 1988. The ammonium/methylammonium uptake system of Symbiodinium microadriaticum. Mar. Biol. 97:593–596 [Google Scholar]
- 152. Harada K, Fukuda E, Hirohashi N, Chiba K. 2010. Regulation of intracellular pH by p90Rsk-dependent activation of an Na+/H+ exchanger in starfish oocytes. J. Biol. Chem. 285:24044–24054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Harland AD, Fixter LM, Davies PS, Anderson RA. 1991. Distribution of lipids between the zooxanthellae and animal compartment in the symbiotic sea anemone Anemonia viridis: wax esters, triglycerides and fatty acids. Mar. Biol. 110:13–19 [Google Scholar]
- 154. Harland AD, Davies PS. 1995. Symbiont photosynthesis increases both respiration and photosynthesis in the symbiotic sea anemone Anemonia viridis. Mar. Biol. 123:715–722 [Google Scholar]
- 155. Helman Y, Natale F, Sherrell RM, LaVigne M, Falkowski PG. 2008. Extracellular matrix production and calcium carbonate precipitation by corals cells in vitro. Proc. Natl. Acad. Sci. U. S. A. 105:54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hentschel U, Steinert M, Hacker J. 2000. Common molecular mechanisms of symbiosis and pathogenesis. Trends Microbiol. 8:226–231 [DOI] [PubMed] [Google Scholar]
- 157. Hinde R. 1988. Factors produced by symbiotic marine invertebrates which affect translocation between the symbionts, p 311–324 In Scannerini S, Smith DC, Bonfante-Fasolo P, Gianninazzi-Pearson V. (ed), Cell-to-cell signals in plant, animal and microbial symbiosis. Springer-Verlag, Berlin, Germany [Google Scholar]
- 158. Hirose M, Yamamoto H, Nonaka M. 2008. Metamorphosis and acquisition of symbiotic algae in planula larvae and primary polyps of Acropora spp. Coral Reefs 27:247–254 [Google Scholar]
- 159. Hoegh-Guldberg O, McCloskey LR, Muscatine L. 1987. Expulsion of zooxanthellae by symbiotic cnidarians from the Red Sea. Coral Reefs 5:201–204 [Google Scholar]
- 160. Hoegh-Guldberg O, Smith GJ. 1989. The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. J. Exp. Mar. Biol. Ecol. 129:279–303 [Google Scholar]
- 161. Hoegh-Guldberg O, Smith GJ. 1989. Influence of the population density of zooxanthellae and supply of ammonium on the biomass and metabolic characteristics of the reef corals Seriatopora hystrix and Stylophora pistillata. Mar. Ecol. Prog. Ser. 57:173–186 [Google Scholar]
- 162. Hoegh-Guldberg O. 1994. Population dynamics of symbiotic zooxanthellae in the coral Pocillopora damicornis exposed to elevated ammonium [(NH4)2 SO4] concentrations. Pac. Sci. 48:263–272 [Google Scholar]
- 163. Hoegh-Guldberg O. 1999. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Fresh. Res. 50:839–866 [Google Scholar]
- 164. Hoegh-Guldberg O. 2004. Coral reefs in a century of rapid environmental change. Symbiosis 37:1–31 [Google Scholar]
- 165. Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742 [DOI] [PubMed] [Google Scholar]
- 166. Hofmann DK, Kremer BP. 1981. Carbon metabolism and strobilation in Cassiopea andromedea (Cnidaria: Scyphozoa): significance of endosymbiotic dinoflagellates. Mar. Biol. 65:25–33 [Google Scholar]
- 167. Hohman TC, McNeil PL, Muscatine L. 1982. Phagosome-lysosome fusion inhibited by algal symbionts of Hydra viridis. J. Cell Biol. 94:56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hong MC, et al. 2009. Cloning and characterization of ApRab4, a recycling Rab protein of Aiptasia pulchella, and its implication in the symbiosome biogenesis. Mar. Biotechnol. 11:771–785 [DOI] [PubMed] [Google Scholar]
- 169. Isa Y, Yamazato K. 1984. The distribution of carbonic anhydrase in a staghorn coral Acropora hebes (Dana). Galaxea 3:25–36 [Google Scholar]
- 170. Ishikura M, Adachi K, Maruyama T. 1999. Zooxanthellae release glucose in the tissue of a giant clam, Tridacna crocea. Mar. Biol. 133:665–673 [Google Scholar]
- 171. Jackson AE, Miller DJ, Yellowlees D. 1989. Phosphorus metabolism in the coral zooxanthellae symbiosis: characterization and possible roles of 2 acid-phosphatases in the algal symbiont Symbiodinium sp. Proc. R. Soc. Lond. B 238:193–202 [Google Scholar]
- 172. Jackson AE, Yellowlees D. 1990. Phosphate uptake by zooxanthellae isolated from corals. Proc. R. Soc. Lond. B 242:201–204 [Google Scholar]
- 173. Janeway CA, Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216 [DOI] [PubMed] [Google Scholar]
- 174. Jimbo M, et al. 2000. The d-galactose binding lectin of the octocoral Sinularia lochmodes: characterization and possible relationship to the symbiotic dinoflagellates. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 125:227–236 [DOI] [PubMed] [Google Scholar]
- 175. Jimbo M, Koike K, Sakai R, Muramoto K, Kamiya H. 2005. Cloning and characterization of a lectin from the octocoral Sinularia lochmodes. Biochem. Biophys. Res. Commun. 330:157–162 [DOI] [PubMed] [Google Scholar]
- 176. Jimbo M, Yamashita H, Koike K, Sakai R, Kamiya H. 2010. Effects of lectin in the scleractinian coral Ctenactis echinata on symbiotic zooxanthellae. Fish. Sci. 76:355–363 [Google Scholar]
- 177. Johnston IS. 1980. The ultrastructure of skeletogenesis in zooxanthellate corals. Int. Rev. Cytol. 67:171–214 [Google Scholar]
- 178. Jolley E, Smith DC. 1980. The green hydra symbiosis. II. The biology of the establishment of the association. Proc. R. Soc. Lond. B 207:311–333 [Google Scholar]
- 179. Jones RJ, Yellowlees D. 1997. Regulation and control of intracellular algae (= zooxanthellae) in hard corals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:457–468 [Google Scholar]
- 180. Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U. 1998. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 21:1219–1230 [Google Scholar]
- 181. Kafsack BFC, et al. 2009. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323:530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Karakashian MW, Karakashian SJ. 1973. Intracellular digestion and symbiosis in Paramecium bursaria. Exp. Cell Res. 81:111–119 [DOI] [PubMed] [Google Scholar]
- 183. Karakashian SJ, Rudzinska MA. 1981. Inhibition of lysosomal fusion with symbiont-containing vacuoles in Paramecium bursaria. Exp. Cell Res. 131:387–393 [DOI] [PubMed] [Google Scholar]
- 184. Kazandjian A, et al. 2008. Isolation of symbiosomes and the symbiosome membrane complex from the zoanthid Zoanthus robustus. Phycologia 47:294–306 [Google Scholar]
- 185. Kellogg RB, Patton JS. 1983. Lipid droplets, medium of energy exchange in the symbiotic anemone Condylactis gigantea—a model coral polyp. Mar. Biol. 75:137–149 [Google Scholar]
- 186. Kimura A, Sakaguchi E, Nonaka M. 2009. Multi-component complement system of Cnidaria: C3, Bf, and MASP genes expressed in the endodermal tissues of a sea anemone, Nematostella vectensis. Immunobiology 214:165–178 [DOI] [PubMed] [Google Scholar]
- 187. Kodama Y, Fujishima M. 2010. Secondary symbiosis between Paramecium and Chlorella cells. Int. Rev. Cell Mol. Biol. 279:33–77 [DOI] [PubMed] [Google Scholar]
- 188. Koike K, et al. 2004. Octocoral chemical signaling selects and controls dinoflagellate symbionts. Biol. Bull. 207:80–86 [DOI] [PubMed] [Google Scholar]
- 189. Koop K, et al. 2001. ENCORE: the effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar. Poll. Bull. 42:91–120 [DOI] [PubMed] [Google Scholar]
- 190. Kovacevic G, Franjevic D, Jelencic B, Kalafatic M. 2010. Isolation and cultivation of endosymbiotic algae from green hydra and phylogenetic analysis of 18S rDNA sequences. Folia Biol. (Krakow) 58:135–143 [PubMed] [Google Scholar]
- 191. Kvennefors ECE, et al. 2010. Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis. Dev. Comp. Immunol. 34:1219–1229 [DOI] [PubMed] [Google Scholar]
- 192. Kvennefors ECE, Leggat W, Hoegh-Guldberg O, Degnan BM, Barnes AC. 2008. An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts. Dev. Comp. Immunol. 32:1582–1592 [DOI] [PubMed] [Google Scholar]
- 193. LaJeunesse TC. 2001. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J. Phycol. 37:866–880 [Google Scholar]
- 194. Lange C, et al. 2011. Defining the origins of the NOD-like receptor system at the base of animal evolution. Mol. Biol. Evol. 28:1687–1702 [DOI] [PubMed] [Google Scholar]
- 195. Lechene C, et al. 2006. High-resolution quantitative imaging of mammalian and bacterial cells using stable isotope mass spectrometry. J. Biol. 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lechene CP, Luyten Y, McMahon G, Distel DL. 2007. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science 317:1563–1566 [DOI] [PubMed] [Google Scholar]
- 197. Leggat W, Badger MR, Yellowlees D. 1999. Evidence for an inorganic carbon-concentrating mechanism in the symbiotic dinoflagellate Symbiodinium sp. Plant Physiol. 121:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Leggat W, et al. 2002. Dinoflagellate symbioses: strategies and adaptations for the acquisition and fixation of inorganic carbon. Funct. Plant Biol. 29:309–322 [DOI] [PubMed] [Google Scholar]
- 199. Leggat W, Hoegh-Guldberg O, Dove S, Yellowlees D. 2007. Analysis of an EST library from the dinoflagellate (Symbiodinium sp.) symbiont of reef-building corals. J. Phycol. 43:1010–1021 [Google Scholar]
- 200. Lesser MP, Weis VM, Patterson MR, Jokiel PL. 1994. Effects of morphology and water motion on carbon delivery and productivity in the reef coral Pocillopora damicornis (Linnaeus): diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J. Exp. Mar. Biol. Ecol. 178:153–179 [Google Scholar]
- 201. Lesser MP. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 68:253–278 [DOI] [PubMed] [Google Scholar]
- 202. Levine B, Klionsky DJ. 2004. Development by self-digestion: molecular mechanisms and biological function of autophagy. Dev. Cell 6:463–477 [DOI] [PubMed] [Google Scholar]
- 203. Levine B. 2005. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120:159–162 [DOI] [PubMed] [Google Scholar]
- 204. Lewis DH, Smith DC. 1971. Autotrophic nutrition of symbiotic marine coelenterates with special reference to hermatypic corals. I. Movement of photosynthetic products between the symbionts. Proc. R. Soc. Lond. B 178:111–129 [Google Scholar]
- 205. Lin KL, Wang JT, Fang LS. 2000. Participation of glycoproteins on zooxanthellal cell walls in the establishment of a symbiotic relationship with the sea anemone Aiptasia pulchella. Zool. Stud. 39:172–178 [Google Scholar]
- 206. Lipschultz F, Cook CB. 2002. Uptake and assimilation of 15N-ammonium by the symbiotic sea anemones Bartholomea annulata and Aiptasia pallida: conservation versus recycling of nitrogen. Mar. Biol. 140:489–502 [Google Scholar]
- 207. Loeblich AR, Sherley JL. 1979. Observations on the theca of the motile phase of free-living and symbiotic isolates of Zooxanthella microadriatica (Freudenthal) comb nov. J. Mar. Biol. Assoc. UK 59:195–205 [Google Scholar]
- 208. Logan DDK, LaFlamme AC, Weis VM, Davy SK. 2010. Flow cytometric characterizaion of the cell-surface glycans of symbiotic dinoflagellates (Symbiodinium spp.). J. Phycol. 46:525–533 [Google Scholar]
- 209. Luo Y-J, et al. 2009. Ratiometric imaging of gastrodermal lipid bodies in coral-dinoflagellate endosymbiosis. Coral Reefs 28:289–301 [Google Scholar]
- 210. Madshus IH. 1988. Regulation of intracellular pH in eukaryotic cells. Biochem. J. 250:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Malik ZA, et al. 2003. Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J. Immunol. 170:2811–2815 [DOI] [PubMed] [Google Scholar]
- 212. Markell DA, Trench RK, Iglesias-Prieto R. 1992. Macromolecules associated with the cell walls of symbiotic dinoflagellates. Symbiosis 12:19–31 [Google Scholar]
- 213. Markell DA, Trench RK. 1993. Macromolecules exuded by symbiotic dinoflagellates in culture: amino acid and sugar composition. J. Phycol. 29:64–68 [Google Scholar]
- 214. Markell DA, Wood-Charlson EM. 2010. Immunocytochemical evidence that symbiotic algae secrete potential recognition signal molecules in hospite. Mar. Biol. 157:1105–1111 [Google Scholar]
- 215. Marlow HQ, Martindale MQ. 2007. Embryonic development in two species of scleractinian coral embryos: Symbiodinium localization and mode of gastrulation. Evol. Dev. 9:355–367 [DOI] [PubMed] [Google Scholar]
- 216. Marshall AT. 1996. Calcification in hermatypic and ahermatypic corals. Science 271:637–639 [Google Scholar]
- 217. McAuley PJ. 1981. Control of cell division of the intracellular Chlorella symbionts in green hydra. J. Cell Sci. 47:197–206 [DOI] [PubMed] [Google Scholar]
- 218. McAuley PJ. 1981. What determines the population size of the intracellular algal symbionts in the digestive cells of green hydra? Experientia 37:346–347 [Google Scholar]
- 219. McAuley PJ. 1982. Temporal relationships of host cell and algal mitosis in the green hydra symbiosis. J. Cell Sci. 58:423–431 [DOI] [PubMed] [Google Scholar]
- 220. McAuley PJ, Smith DC. 1982. The green hydra symbiosis. V. Stages in the intracellular recognition of algal symbionts by digestive cells. Proc. R. Soc. Lond. B 216:7–23 [Google Scholar]
- 221. McAuley PJ. 1985. The cell cycle of symbiotic Chlorella. I. The relationship between host feeding and algal cell growth and division. J. Cell Sci. 77:225–239 [DOI] [PubMed] [Google Scholar]
- 222. McAuley PJ. 1985. The cell cycle of symbiotic Chlorella. II. The effect of continuous darkness. J. Cell Sci. 77:241–253 [DOI] [PubMed] [Google Scholar]
- 223. McAuley PJ. 1985. Regulation of numbers of symbiotic Chlorella in digestive cells of green hydra. Endocytobiosis Cell Res. 2:179–190 [Google Scholar]
- 224. McAuley PJ. 1986. Uptake of amino acids by cultured and freshly isolated symbiotic Chlorella. New Phytol. 104:415–427 [Google Scholar]
- 225. McAuley PJ, Muscatine L. 1986. The cell cycle of symbiotic Chlorella. IV. DNA content of algae slowly increases during host starvation of green hydra. J. Cell Sci. 85:73–84 [DOI] [PubMed] [Google Scholar]
- 226. McAuley PJ. 1987. Nitrogen limitation and amino acid metabolism of Chlorella symbiotic with green hydra. Planta 171:532–538 [DOI] [PubMed] [Google Scholar]
- 227. McAuley PJ, Darrah PR. 1990. Random partitioning of Chlorella symbionts at digestive cell division in green hydra and its significance in regulation of symbiont numbers, p 265–269 In Narclon P. (ed), Endocytobiology IV. INRA, Paris, France [Google Scholar]
- 228. McAuley PJ, Darrah PR. 1990. Regulation of numbers of symbiotic Chlorella by density-dependent division. Philos. Trans. R. Soc. Lond. B Biol. Sci. 329:55–63 [Google Scholar]
- 229. McAuley PJ. 1992. The effect of maltose release on growth and nitrogen metabolism of symbiotic Chlorella. Br. Phycol. J. 27:417–422 [Google Scholar]
- 230. McAuley PJ, Cook CB. 1994. Effects of host feeding and dissolved ammonium on cell division and nitrogen status of zooxanthellae in the hydroid Myrionema amboinense. Mar. Biol. 121:343–348 [Google Scholar]
- 231. McAuley PJ. 1995. Ammonium metabolism in the green hydra symbiosis. Biol. Bull. 188:210–218 [DOI] [PubMed] [Google Scholar]
- 232. McCloskey LR, Cove TG, Verde EA. 1996. Symbiont expulsion from the anemone Anthopleura elegantissima (Brandt) (Cnidaria; Anthozoa). J. Exp. Mar. Biol. Ecol. 195:173–186 [Google Scholar]
- 233. McGuinness DH, Dehal PK, Pleass RJ. 2003. Pattern recognition molecules and innate immunity to parasites. Trends Parasitol. 19:312–319 [DOI] [PubMed] [Google Scholar]
- 234. McNeil PL. 1981. Mechanisms of nutritive endocytosis. I. Phagocytic versatility and cellular recognition in Chlorohydra digestive cells, a scanning electron microscope study. J. Cell Sci. 49:311–339 [DOI] [PubMed] [Google Scholar]
- 235. McNeil PL, Hohman T, Muscatine L. 1981. Mechanisms of nutrititive endocytosis. II. The effect of charged agents on phagocytic recognition by digestive cells. J. Cell Sci. 52:243–269 [DOI] [PubMed] [Google Scholar]
- 236. McNeil PL, Smith DC. 1982. The green hydra symbiosis. IV. Entry of symbionts into digestive cells. Proc. R. Soc. Lond. B 216:1–6 [Google Scholar]
- 237. Meints RH, Pardy RL. 1980. Quantitative demonstrations of cell surface involvment in a plant-animal symbiosis: lectin inhibition of reassociations. J. Cell Sci. 43:239–251 [DOI] [PubMed] [Google Scholar]
- 238. Mergaert P, et al. 2006. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc. Natl. Acad. Sci. U. S. A. 103:5230–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Mews LK. 1980. The green hydra symbiosis. III. The biotrophic transport of carbohydrate from alga to animal. Proc. R. Soc. Lond. B 209:377–401 [Google Scholar]
- 240. Meyer E, et al. 2009. Sequencing and de novo analysis of a coral larval transcriptome using 454 GSFLx. BMC Genomics 10:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Miller DJ, et al. 2007. The innate immune repertoire in Cnidaria—ancestral complexity and stochastic gene loss. Genome Biol. 8:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Miller DJ, Yellowlees D. 1989. Inorganic nitrogen uptake by symbiotic marine cnidarians—a critical review. Proc. R. Soc. Lond. B 237:109–125 [Google Scholar]
- 243. Miller DJ, Ball EE, Technau U. 2005. Cnidarians and ancestral genetic complexity in the animal kingdom. Trends Genet. 21:536–539 [DOI] [PubMed] [Google Scholar]
- 244. Montaño NE. 1990. Studies on the isolation of host release factors involved in dinoflagellate-marine invertebrate mutualistic symbioses. Griffith University, Brisbane, Australia [Google Scholar]
- 245. Morar SR, Bury SJ, Wilkinson SP, Davy SK. 2011. Sedimentary nitrogen uptake and assimilation in the temperate zooxanthellate sea anemone Anthopleura aureoradiata. J. Exp. Mar. Biol. Ecol. 399:110–119 [Google Scholar]
- 246. Moya A, et al. 2006. Study of calcification during a daily cycle of the coral Stylophora pistillata. Implications for “light-enhanced calcification.” J. Exp. Biol. 209:3413–3419 [DOI] [PubMed] [Google Scholar]
- 247. Moya A, et al. 2008. Cloning and use of a coral 36B4 gene to study the differential expression of coral genes between light and dark conditions. Mar. Biotechnol. 10:653–663 [DOI] [PubMed] [Google Scholar]
- 248. Moya A, et al. 2008. Carbonic anhydrase in the scleractinian coral Stylophora pistillata: characterization, localization, and role in biomineralization. J. Biol. Chem. 283:25475–25484 [DOI] [PubMed] [Google Scholar]
- 249. Muller-Parker G, Cook CB, D'Elia CF. 1990. Feeding affects phosphate fluxes in the symbiotic sea anemone Aiptasia pallida. Mar. Ecol. Prog. Ser. 60:283–290 [Google Scholar]
- 250. Muller-Parker G, McCloskey L, Hoegh-Guldberg O, McAuley PJ. 1994. Effect of ammonium enrichment on animal and algal biomass of the coral Pocillopora damicornis. Pac. Sci. 48:273–283 [Google Scholar]
- 251. Muller-Parker G, Lee KW, Cook CB. 1996. Changes in the ultrastructure of symbiotic zooxanthellae (Symbiodinium sp, Dinophyceae) in fed and starved sea anemones maintained under high and low light. J. Phycol. 32:987–994 [Google Scholar]
- 252. Muller-Parker G, Davy SK. 2001. Temperate and tropical algal-sea anemone symbioses. Invertebr. Biol. 120:104–123 [Google Scholar]
- 253. Murray AW. 2004. Recycling the cell cycle: cyclins revisited. Cell 116:221–234 [DOI] [PubMed] [Google Scholar]
- 254. Muscatine L, Hand C. 1958. Direct evidence for the transfer of materials from symbiotic algae to the tissues of a coelenterate. Proc. Natl. Acad. Sci. U. S. A. 44:1259–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Muscatine L. 1965. Symbiosis of hydra and algae. III. Extracellular products of the algae. Comp. Biochem. Physiol. 16:77–92 [DOI] [PubMed] [Google Scholar]
- 256. Muscatine L, Lenhoff HM. 1965. Symbiosis of hydra and algae. II. Effects of limited food and starvation on growth of symbiotic and aposymbiotic hydra. Biol. Bull. 129:316–328 [Google Scholar]
- 257. Muscatine L. 1967. Glycerol excretion by symbiotic algae from corals and Tridacna and its control by the host. Science 156:516–519 [DOI] [PubMed] [Google Scholar]
- 258. Muscatine L, Karakash SJ, Karakash MW. 1967. Soluble extracellular products of algae symbiotic with a a ciliate, a sponge and a mutant hydra. Comp. Biochem. Physiol. 20:1–6 [Google Scholar]
- 259. Muscatine L, Cernichiari E. 1969. Assimilation of photosynthetic products of zooxanthellae by a reef coral. Biol. Bull. 137:506–523 [DOI] [PubMed] [Google Scholar]
- 260. Muscatine L, Cernichiari E, Pool RR. 1972. Some factors influencing selective release of soluble organic material by zooxanthellae from reef corals. Mar. Biol. 13:298–308 [Google Scholar]
- 261. Muscatine L, Boyle JE, Smith DC. 1974. Symbiosis of the acoel flatworm Convoluta roscoffensis with the alga Platymonas convolutae. Proc. R. Soc. Lond. B 187:221–234 [DOI] [PubMed] [Google Scholar]
- 262. Muscatine L, Cook CB, Pardy RL, Pool RR. 1975. Uptake, recognition and maintenance of symbiotic Chlorella by Hydra viridis. Symp. Soc. Exp. Biol. 29:175–203 [PubMed] [Google Scholar]
- 263. Muscatine L, Pool RR, Trench RK. 1975. Symbiosis of algae and invetebrates: aspects of the symbiont surface and the host-symbiont interface. Trans. Am. Microsc. Soc. 94:450–469 [PubMed] [Google Scholar]
- 264. Muscatine L, D'Elia CF. 1978. Uptake, retention, and release of ammonium by reef corals. Limnol. Oceanogr. 23:725–734 [Google Scholar]
- 265. Muscatine L, Pool RR. 1979. Regulation of numbers of intracellular algae. Proc. R. Soc. Lond. B 204:131–139 [DOI] [PubMed] [Google Scholar]
- 266. Muscatine L, Neckelmann N. 1981. Regulation of numbers of algae in the Hydra-Chlorella symbiosis. Ber. Dtsch. Bot. Ges. 94:571–582 [Google Scholar]
- 267. Muscatine L, Falkowski PG, Dubinsky Z. 1983. Carbon budgets in symbiotic associations, p 649–658 In Schwemmler W, Schenk HEA. (ed), Endocytobiology II. Walter de Gruyter, Berlin, Germany [Google Scholar]
- 268. Muscatine L, Falkowski PG, Porter JW, Dubinsky Z. 1984. Fate of photosynthetic fixed carbon in light-adapted and shade-adapted colonies of the symbiotic coral Stylophora pistillata Proc. R. Soc. Lond. B 222:181–202 [Google Scholar]
- 269. Muscatine L, Falkowski PG, Dubinsky Z, Cook PA, McCloskey LR. 1989. The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc. R. Soc. Lond. B 236:311–324 [Google Scholar]
- 270. Muscatine L. 1990. The role of symbiotic algae in carbon and energy flux in reef corals, p 75–87 In Dubinsky Z. (ed), Coral reefs. Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 271. Muscatine L, Grossman D, Doino J. 1991. Release of symbiotic algae by tropical sea anemones and corals after cold shock. Mar. Ecol. Prog. Ser. 77:233–243 [Google Scholar]
- 272. Muscatine L, Gates RD, Lafontaine I. 1994. Do symbiotic dinoflagellates secrete lipid droplets? Limnol. Oceanogr. 39:925–929 [Google Scholar]
- 273. Muscatine L, TambuttÉ E, Allemand D. 1997. Morphology of coral desmocytes, cells that anchor the calicoblastic epithelium to the skeleton. Coral Reefs 16:205–213 [Google Scholar]
- 274. Muscatine L, et al. 1998. Cell specific density of symbiotic dinoflagellates in tropical anthozoans. Coral Reefs 17:329–337 [Google Scholar]
- 275. Muscatine L, et al. 2005. Stable isotopes (δ13C and δ15N) of organic matrix from coral skeleton. Proc. Natl. Acad. Sci. U. S. A. 102:1525–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Mus-Veteau I. 2002. Heterologous expression and purification systems for structural proteomics of mammalian membrane proteins. Comp. Funct. Genomics 3:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Neckelmann N, Muscatine L. 1983. Regulatory mechanisms maintaining the Hydra-Chlorella symbiosis. Proc. R. Soc. Lond. B 219:193–210 [Google Scholar]
- 278. Nigg EA. 1995. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays 17:471–480 [DOI] [PubMed] [Google Scholar]
- 279. Nyholm SV, McFall-Ngai MJ. 2004. The winnowing: establishing the squid-Vibrio symbiosis. Nat. Rev. Microbiol. 2:632–642 [DOI] [PubMed] [Google Scholar]
- 280. O'Brien TL. 1982. Inhibition of vacuolar membrane fusion by intracellular symbiotic algae in Hydra viridis (Florida strain). J. Exp. Zool. 223:211–218 [DOI] [PubMed] [Google Scholar]
- 281. Orlando DA, et al. 2008. Global control of cell-cycle transcription by coupled CDK and network oscillators. Nature 453:944–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Papina M, Meziane T, van Woesik R. 2003. Symbiotic zooxanthellae provide the host-coral Montipora digitata with polyunsaturated fatty acids. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 135:533–537 [DOI] [PubMed] [Google Scholar]
- 283. Pardy RL. 1974. Regulation of endosymbiotic algae in hydra by digestive cells and tissue growth. Am. Zool. 14:583–588 [Google Scholar]
- 284. Pardy RL. 1974. Some factors affecting growth and distribution of algal endosymbionts of Hydra viridis. Biol. Bull. 147:105–118 [DOI] [PubMed] [Google Scholar]
- 285. Pardy RL, Heacox AE. 1976. Growth of algal symbionts in regenerating hydra. Nature 260:809–810 [DOI] [PubMed] [Google Scholar]
- 286. Pardy RL. 1981. Cell size distribution of green symbionts from Hydra viridis. Cytobios 32:71–77 [Google Scholar]
- 287. Patton JS, Burris JE. 1983. Lipid synthesis and extrusion by freshly isolated zooxanthellae (symbiotic algae). Mar. Biol. 75:131–136 [Google Scholar]
- 288. Peng S-E, et al. 2011. Lipid bodies in coral-dinoflagellate endosymbiosis: proteomic and ultrastructural studies. Proteomics 11:3540–3555 [DOI] [PubMed] [Google Scholar]
- 289. Peng S-E, et al. 2010. Proteomic analysis of symbiosome membranes in Cnidaria-dinoflagellate endosymbiosis. Proteomics 10:1002–1016 [DOI] [PubMed] [Google Scholar]
- 290. Pomeroy LR, Kuenzler EJ. 1969. Phosphorus turnover by coral reef animals, p 474–482 In Nelson DJ, Evans FC. (ed), Symposium on radioecology National Technical Information Service, Springfield, VA [Google Scholar]
- 291. Pool RR. 1979. The role of algal antigenic determinants in the recognition of potential algal symbionts by cells of Chlorohydra. J. Cell Sci. 35:367–379 [DOI] [PubMed] [Google Scholar]
- 292. Putnam NH, et al. 2007. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94 [DOI] [PubMed] [Google Scholar]
- 293. Puverel S, et al. 2005. Antibodies against the organic matrix in scleractinians: a new tool to study coral biomineralization. Coral Reefs 24:149–156 [Google Scholar]
- 294. Rahav O, Dubinsky Z, Achituv Y, Falkowski PG. 1989. Ammonium metabolism in the zooxanthellate coral, Stylophora pistillata. Proc. R. Soc. Lond. B 236:325–337 [Google Scholar]
- 295. Ralph PJ, Gademann R, Larkum AWD. 2001. Zooxanthellae expelled from bleached corals at 33°C are photosynthetically competent. Mar. Ecol. Prog. Ser. 220:163–168 [Google Scholar]
- 296. Rands ML, Douglas AE, Loughman BC, Hawes CR. 1992. The pH of the perisymbiont space in the green hydra-Chlorella symbiosis—an immunocytochemical investigation. Protoplasma 170:90–93 [Google Scholar]
- 297. Rands ML, Loughman BC, Douglas AE. 1993. The symbiotic interface in an alga-invertebrate symbiosis. Proc. R. Soc. Lond. B 253:161–165 [Google Scholar]
- 298. Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA. 2008. Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol. 146:1440–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Rees TAV. 1986. The green hydra symbiosis and ammonium. I. The role of the host in ammonium assimilation and its possible regulatory significance. Proc. R. Soc. Lond. B 229:299–314 [Google Scholar]
- 300. Rees TAV. 1989. The green hydra symbiosis and ammonium. II. Ammonium assimilation and release by freshly isolated symbionts and cultured algae Proc. R. Soc. Lond. B 235:365–382 [Google Scholar]
- 301. Rees TAV, Ellard FM. 1989. Nitrogen conservation and the green hydra symbiosis. Proc. R. Soc. Lond. B 236:203–212 [Google Scholar]
- 302. Rees TAV, Shah N, Stewart GR. 1989. Glutamine synthetase isoforms in the green hydra symbiosis. New Phytol. 111:621–623 [DOI] [PubMed] [Google Scholar]
- 303. Rees TAV. 1990. The green hydra symbiosis and ammonium. IV. Methylammonium uptake by freshly isolated symbionts and cultured algae; evidence for low-ammonium concentration in the perialgal space. Proc. R. Soc. Lond. B 242:255–259 [Google Scholar]
- 304. Rees TAV. 1990. The green hydra symbiosis and ammonium. III. Characteristics of ammonium release by a high maltose-releasing symbiotic strain of Chlorella. Proc. R. Soc. Lond. B 242:249–253 [Google Scholar]
- 305. Rees TAV. 1991. Are symbiotic algae nutrient deficient? Proc. R. Soc. Lond. B 243:227–233 [Google Scholar]
- 306. Rees TAV, Fitt WK, Baillie B, Yellowlees D. 1993. A method for temporal measurement of hemolymph composition in the giant clam symbiosis and its application to glucose and glycerol levels during a diel cycle. Limnol. Oceanogr. 38:213–217 [Google Scholar]
- 307. Reimer AA. 1971. Observations on the relationships between several species of tropical zoanthids (Zoanthidae, Coelenterata) and their zooxanthellae. J. Exp. Mar. Biol. Ecol. 7:207–214 [Google Scholar]
- 308. Reits EA, Neefjes JJ. 2001. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Biol. 3:E145–E147 [DOI] [PubMed] [Google Scholar]
- 309. Relman DA. 2008. ‘Til death do us part’: coming to terms with symbiotic relationships. Nat. Rev. Microbiol. 6:721. [DOI] [PubMed] [Google Scholar]
- 310. Reyes-Bermudez A, Lin ZY, Hayward DC, Miller DJ, Ball EE. 2009. Differential expression of three galaxin-related genes during settlement and metamorphosis in the scleractinian coral Acropora millepora. BMC Evol. Biol. 9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Reynolds WS, Schwarz JA, Weis VM. 2000. Symbiosis-enhanced gene expression in cnidarian-algal associations: cloning and characterization of a cDNA, sym32, encoding a possible cell adhesion protein. Comp. Biochem. Physiol. A 126:33–44 [DOI] [PubMed] [Google Scholar]
- 312. Ritchie RJ, Eltringham K, Hinde R. 1993. Glycerol uptake by zooxanthellae of the temperate hard coral, Plesiastrea versipora (Lamarck). Proc. R. Soc. Lond. B 253:189–195 [Google Scholar]
- 313. Ritchie RJ, Grant AJ, Eltringham K, Hinde R. 1997. Clotrimazole, a model compound for the host release factor of the coral Plesiastrea versipora. Aust. J. Plant Physiol. 24:283–290 [Google Scholar]
- 314. Rivera J, Proia RL, Olivera A. 2008. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8:753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Roberts JM, Fixter LM, Davies PS. 2001. Ammonium metabolism in the symbiotic sea anemone Anemonia viridis. Hydrobiologia 461:25–35 [Google Scholar]
- 316. Rodriguez-Lanetty M, Phillips WS, Weis VM. 2006. Transcriptome analysis of a cnidarian—dinoflagellate mutualism reveals complex modulation of host gene expression. BMC Genomics 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Rodriguez-Lanetty M, Wood-Charlson E, Hollingsworth L, Krupp DA, Weis VM. 2006. Dynamics of infection and localization of dinoflagellate endosymbionts in larvae of the coral Fungia scutaria during the onset of symbiosis. Mar. Biol. 149:713–719 [Google Scholar]
- 318. Rowan R, Powers DA. 1991. A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbioses. Science 251:1348–1351 [DOI] [PubMed] [Google Scholar]
- 319. Sacks D, Sher A. 2002. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 3:1041–1047 [DOI] [PubMed] [Google Scholar]
- 320. Saito K, Matsuda F. 2010. Metabolomics for functional genomics, systems biology, and biotechnology. Annu. Rev. Plant Biol. 61:463–489 [DOI] [PubMed] [Google Scholar]
- 321. Schliemann W, Ammer C, Strack D. 2008. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 69:112–146 [DOI] [PubMed] [Google Scholar]
- 322. Schoenberg DA, Trench RK. 1980. Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. III. Specificity and infectivity of Symbiodinium microadriaticum. Proc. R. Soc. Lond. B 207:445–460 [Google Scholar]
- 323. Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. 2007. Rab GTPases at a glance. J. Cell Sci. 120:3905–3910 [DOI] [PubMed] [Google Scholar]
- 324. Schwarz J, et al. 2008. Coral life history and symbiosis: functional genomic resources for two reef building Caribbean corals, Acropora palmata and Montastraea faveolata. BMC Genomics 9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Schwarz JA, Krupp DA, Weis VM. 1999. Late larval development and onset of symbiosis in the scleractinian coral Fungia scutaria. Biol. Bull. 196:70–79 [DOI] [PubMed] [Google Scholar]
- 326. Schwarz JA, Weis VM. 2003. Immunolocalization of host sym32 and an undescribed protein, p45/48, in the sea anemone-dinoflagellate association Anthopleura elegantissima-Symbiodinium muscatinei. Biol. Bull. 205:339–350 [DOI] [PubMed] [Google Scholar]
- 327. Schwarz JA. 2008. Understanding the intracellular niche in cnidarian-Symbiodinium symbioses: parasites lead the way. Vie Milieu Paris 58:141–151 [Google Scholar]
- 328. Shinzato C, et al. 2011. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476:320–U82 [DOI] [PubMed] [Google Scholar]
- 329. Simkiss K. 1964. Phosphates as crystal poisons of calcification. Biol. Rev. 39:487–505 [DOI] [PubMed] [Google Scholar]
- 330. Smith GJ, Muscatine L. 1986. Carbon budgets and regulation of the population density of symbiotic algae. Endocytobiosis Cell Res. 3:213–238 [Google Scholar]
- 331. Smith GJ, Hoegh-Guldberg O. 1987. Variation in the growth rates of zooxanthellae with coral host colony size and ontogenetic stage is not controlled by changes in the duration of cytokinesis. EOS 68:724 [Google Scholar]
- 332. Smith GJ, Muscatine L. 1999. Cell cycle of symbiotic dinoflagellates: variation in G(1) phase-duration with anemone nutritional status and macronutrient supply in the Aiptasia pulchella-Symbiodinium pulchrorum symbiosis. Mar. Biol. 134:405–418 [Google Scholar]
- 333. Smith REH. 1982. The estimation of phytoplankton production and excretion by carbon-14. Mar. Biol. Lett. 3:325–334 [Google Scholar]
- 334. Soto MJ, Dominguez-Ferreras A, Perez-Mendoza D, Sanjuan J, Olivares J. 2009. Mutualism versus pathogenesis: the give-and-take in plant-bacteria interactions. Cell. Microbiol. 11:381–388 [DOI] [PubMed] [Google Scholar]
- 335. Spellman PT, et al. 1998. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9:3273–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Spiegel S, Milstein S. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4:397–406 [DOI] [PubMed] [Google Scholar]
- 337. Stambler N, Dubinsky Z. 1987. Energy relationships between Anemonia sulcata and its endosymbiotic zooxanthellae. Symbiosis 3:233–248 [Google Scholar]
- 338. Steele RD. 1975. Stages in the life history of a symbiotic zooxanthella in pellets extruded by its host Aiptasia tagetes (Duch. and Mich.) (Coelenterata, Anthozoa). Biol. Bull. 149:590–600 [DOI] [PubMed] [Google Scholar]
- 339. Steele RD. 1977. The significance of zooxanthella-containing pellets extruded by sea anemones. Bull. Mar. Sci. 27:591–594 [Google Scholar]
- 340. Steele RD, Goreau NI. 1977. The breakdown of symbiotic zooxanthellae in the sea anemone Phyllactis (=Oulactis) flosculifera (Actinaria). J. Zool. 181:421–437 [Google Scholar]
- 341. Steele RD, Smith DC. 1981. Factors affecting the reduction of the algal symbiont population in green hydra. J. Zool. 193:201–214 [Google Scholar]
- 342. Steen RG, Muscatine L. 1984. Daily budgets of photosynthetically fixed carbon in symbiotic zoanthids. Biol. Bull. 167:477–487 [DOI] [PubMed] [Google Scholar]
- 343. Steen RG. 1986. Evidence for heterotrophy by zooxanthellae in symbiosis with Aiptasia pulchella. Biol. Bull. 170:267–278 [Google Scholar]
- 344. Steen RG. 1987. Evidence for facultative heterotrophy in cultured zooxanthellae. Mar. Biol. 95:15–23 [Google Scholar]
- 345. Steen RG, Muscatine L. 1987. Low temperature evokes rapid exocytosis of symbiotic algae by a sea anemone. Biol. Bull. 172:246–263 [Google Scholar]
- 346. Stimson J, Kinzie RA. 1991. The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J. Exp. Mar. Biol. Ecol. 153:63–74 [Google Scholar]
- 347. Stitt M, Sulpice R, Keurentjes J. 2010. Metabolic networks: how to identify key components in the regulation of metabolism and growth. Plant Physiol. 152:428–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Stochaj WR, Grossman AR. 1997. Differences in the protein profiles of cultured and endosymbiotic Symbiodinium sp. (Pyrrophyta) from the anemone Aiptasia pallida (Anthozoa). J. Phycol. 33:44–53 [Google Scholar]
- 349. Suharsono Brown BE. 1992. Comparative measurements of mitotic index in zooxanthellae from a symbiotic cnidarian subject to temperature increase. J. Exp. Mar. Biol. Ecol. 158:179–188 [Google Scholar]
- 350. Summons RE, Boag TS, Osmond CB. 1986. The effect of ammonium on photosynthesis and the pathway of ammonium assimilation in Gymnodinium microadriaticum in vitro and in symbiosis with tridacnid clams and corals. Proc. R. Soc. Lond. B 227:147–159 [Google Scholar]
- 351. Sutton DC, Hoegh-Guldberg O. 1990. Host-zooxanthella interactions in 4 temperate marine invertebrate symbioses: assessment of effect of host extracts on symbionts. Biol. Bull. 178:175–186 [DOI] [PubMed] [Google Scholar]
- 352. Suzuki A, Nakamori T, Kayanne H. 1995. The mechanism of production enhancement in coral reef carbonate systems: model empirical results. Sediment. Geol. 99:259–280 [Google Scholar]
- 353. Tambutté E, Allemand D, Bourge I, Gattuso J-P, Jaubert J. 1995. An improved 45Ca protocol for investigating physiological mechanisms in coral calcification. Mar. Biol. 122:453–459 [Google Scholar]
- 354. Tambutté E, Allemand D, Jaubert J. 1995. The Stylophora pistillata microcolony: a model for studying calcium transport process during coral biomineralization. Bull. Inst. Oceanogr. No. Spec. 14:79–87 [Google Scholar]
- 355. Tambutté E, et al. 2007. Observations of the tissue-skeleton interface in the scleractinian coral Stylophora pistillata. Coral Reefs 26:517–529 [Google Scholar]
- 356. Tambutté S, Tambutté E, Zoccola D, Allemand D. 2007. Organic matrix and biomineralization of scleractinian corals, p 243–259 In Bauerlein E. (ed), Handbook of biomineralization, vol. 1. The biology of biominerals structure formation. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 357. Tambutté S, et al. 2011. Coral biomineralization: from the gene to the environment. J. Exp. Mar. Biol. Ecol. 408:58–78 [Google Scholar]
- 358. Tanaka Y, Miyajima T, Koike I, Hayashibara T, Ogawa H. 2006. Translocation and conservation of organic nitrogen within the coral-zooxanthella symbiotic system of Acropora pulchra, as demonstrated by dual isotope-labeling techniques. J. Exp. Mar. Biol. Ecol. 336:110–119 [Google Scholar]
- 359. Taylor CE, Muscatine L, Jefferson DR. 1989. Maintenance and breakdown of the Hydra-Chlorella symbiosis: a computer model. Proc. R. Soc. Lond. B 238:277–289 [Google Scholar]
- 360. Taylor DL. 1969. The nutritional relationship of Anemonia sulcata (Pennant) and its dinoflagellate symbiont. J. Cell Sci. 4:751–762 [DOI] [PubMed] [Google Scholar]
- 361. Taylor DL. 1978. Nutrition of algal-invertebrate symbiosis. II. Effects of exogenous nitrogen sources on growth, photosynthesis and rate of excretion by algal symbionts in vivo and in vitro. Proc. R. Soc. Lond. B 201:401–412 [Google Scholar]
- 362. Thorington G, Margulis L. 1981. Hydra viridis: transfer of metabolites between Hydra and symbiotic algae. Biol. Bull. 160:175–188 [DOI] [PubMed] [Google Scholar]
- 363. Thrall PH, Hochberg ME, Burdon JJ, Bever JD. 2007. Coevolution of symbiotic mutualists and parasites in a community context. Trends Ecol. Evol. 22:120–126 [DOI] [PubMed] [Google Scholar]
- 364. Titlyanov EA, et al. 1996. Degradation of zooxanthellae and regulation of their density in hermatypic corals. Mar. Ecol. Prog. Ser. 139:167–178 [Google Scholar]
- 365. Titlyanov EA, Titlyanova TV, Kalita TL, Yakovleva IM. 2004. Rhythmicity in division and degradation of zooxanthellae in the hermatypic coral Stylophora pistillata. Symbiosis 36:211–224 [Google Scholar]
- 366. Titlyanov EA, Titlyanova TV, Yakovleva IM, Kalita TL. 2006. Rhythmical changes in the division and degradation of symbiotic algae in hermatypic corals. Russ. J. Mar. Biol. 32:12–19 [Google Scholar]
- 367. Todd PA. 2008. Morphological plasticity in scleractinian corals. Biol. Rev. 83:315–337 [DOI] [PubMed] [Google Scholar]
- 368. Trautman DA, Hinde R, Cole L, Grant A, Quinnell R. 2002. Visualisation of the symbiosome membrane surrounding cnidarian algal cells. Symbiosis 32:133–145 [Google Scholar]
- 369. Trench RK. 1971. Physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. I. Assimilation of photosynthetic products of zooxanthellae by two marine coelenterates. Proc. R. Soc. Lond. B 177:225–235 [Google Scholar]
- 370. Trench RK. 1971. Physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. II. Liberation of fixed C14 by zooxanthellae in vitro. Proc. R. Soc. Lond. B 177:237–250 [Google Scholar]
- 371. Trench RK. 1971. Physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. III. Effect of homogenates of host tissues on excretion of photosynthetic products in vitro by zooxanthellae from two marine coelenterates. Proc. R. Soc. Lond. B 177:251–264 [Google Scholar]
- 372. Trench RK. 1974. Nutritional potentials in Zoanthus sociatus (Coelenterata, Anthozoa). Helgolander Wiss. Meeresunters. 26:174–216 [Google Scholar]
- 373. Trench RK. 1993. Microalgal-invertebrate symbioses: a review. Endocytobiosis Cell Res. 9:135–175 [Google Scholar]
- 374. Trench RK, Fisher CR. 1983. Carbon dioxide fixation in Symbiodinium microadriaticum: problems with mechanisms and pathways, p 659–673 In Schenk HEA, Schwemmler W. (ed), Endocytobiology II. Walter de Gruyter & Co, Berlin, Germany [Google Scholar]
- 375. Turpin DH. 1991. Effects of inorganic N availability on algal photosynthesis and carbon metabolism. J. Phycol. 27:14–20 [Google Scholar]
- 376. Tytler EM, Trench RK. 1986. Activities of enzymes in β-carboxylation reactions and of catalase in cell-free preparations from the symbiotic dinoflagellates Symbiodinium spp. from a coral, a clam, a zoanthid and two sea anemones. Proc. R. Soc. Lond. B 228:483–492 [Google Scholar]
- 377. Underhill DM, Ozinsky A. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825–852 [DOI] [PubMed] [Google Scholar]
- 378. Vandermeulen JH, Davis ND, Muscatine L. 1972. The effect of inhibitors of photosynthesis on zooxanthellae in corals and other marine invertebrates. Mar. Biol. 16:185–191 [Google Scholar]
- 379. Vandermeulen JH. 1974. Studies on reef corals. II. Fine structure of planktonic planula larvae of Pocillopora damicornis, with emphasis on the aboral epidermis. Mar. Biol. 27:239–249 [Google Scholar]
- 380. Vandermeulen JH, Muscatine L. 1974. Influence of symbiotic algae on calcification in reef corals: critique and progress report, p 1–19 In Vernberg WB. (ed), Symbiosis in the sea. University of South Carolina Press, Columbia, SC [Google Scholar]
- 381. Vandermeulen JH. 1975. Studies on reef corals. III. Fine structural changes of calcicoblast cells in Pocillopora damicornis during settling and calcification. Mar. Biol. 31:69–77 [Google Scholar]
- 382. Van de Velde W, et al. 2010. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327:1122–1126 [DOI] [PubMed] [Google Scholar]
- 383. Venn AA, et al. 2009. Imaging intracellular pH in a reef coral and symbiotic anemone. Proc. Natl. Acad. Sci. U. S. A. 106:16574–16579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Venn AA, Tambutté E, Holcomb M, Allemand D, Tambutté S. 2011. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS One 6:e20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Vidal-Dupiol J, et al. 2009. Coral bleaching under thermal stress: putative involvement of host/symbiont recognition mechanisms. BMC Physiol. 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. von Holt C, von Holt M. 1968. Transfer of photosynthetic products from zooxanthellae to coelenterate hosts. Comp. Biochem. Physiol. 24:73–81 [DOI] [PubMed] [Google Scholar]
- 387. Voolstra CR, et al. 2009. Evolutionary analysis of orthologous cDNA sequences from cultured and symbiotic dinoflagellate symbionts of reef-building corals (Dinophyceae: Symbiodinium). Comp. Biochem. Physiol. D Genomics Proteomics 4:67–74 [DOI] [PubMed] [Google Scholar]
- 388. Wainwright SA. 1963. Skeletal organization in the coral, Pocillopora damicornis. Q. J. Microsc. Sci. 104:169–183 [Google Scholar]
- 389. Wakefield TS, Farmer MA, Kempf SC. 2000. Revised description of the fine structure of in situ “zooxanthellae” genus Symbiodinium. Biol. Bull. 199:76–84 [DOI] [PubMed] [Google Scholar]
- 390. Wang JT, Douglas AE. 1997. Nutrients, signals, and photosynthate release by symbiotic algae (the impact of taurine on the dinoflagellate alga Symbiodinium from the sea anemone Aiptasia pulchella). Plant Physiol. 114:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Wang JT, Douglas AE. 1998. Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J. Exp. Biol. 201:2445–2453 [DOI] [PubMed] [Google Scholar]
- 392. Wang JT, Douglas AE. 1999. Essential amino acid synthesis and nitrogen recycling in an alga-invertebrate symbiosis. Mar. Biol. 135:219–222 [Google Scholar]
- 393. Wang LH, et al. 2008. Cell cycle propagation is driven by light-dark stimulation in a cultured symbiotic dinoflagellate isolated from corals. Coral Reefs 27:823–835 [Google Scholar]
- 394. Ware JR, Smith SV, Reaka-Kudla ML. 1991. Coral reefs: sources or sinks of atmospheric CO2? Coral Reefs 11:127–130 [Google Scholar]
- 395. Warner ME, Lesser MP, Ralph PJ. 2011. Chlorophyll fluorescence in reef building corals, p 209–222 In Suggett DJ, Borowitzka MA, Prail O. (ed), Chlorophyll a fluorescence in aquatic sciences: methods and applications, 1st ed Springer, Berlin, Germany [Google Scholar]
- 396. Weis VM, Smith GJ, Muscatine L. 1989. A “CO2 supply” mechanism in zooxanthellate cnidarians: role of carbonic anhydrase. Mar. Biol. 100:195–202 [Google Scholar]
- 397. Weis VM. 1991. The induction of carbonic anhydrase in the symbiotic sea anemone Aiptasia pulchella. Biol. Bull. 180:496–504 [DOI] [PubMed] [Google Scholar]
- 398. Weis VM. 1993. Effect of dissolved inorganic carbon concentration on the photosynthesis of the symbiotic sea anemone Aiptasia pulchella Carlgren: role of carbonic anhydrase. J. Exp. Mar. Biol. Ecol. 174:209–225 [Google Scholar]
- 399. Weis VM, Levine RP. 1996. Differential protein profiles reflect the different lifestyles of symbiotic and aposymbiotic Anthopleura elegantissima, a sea anemone from temperate waters. J. Exp. Biol. 199:883–892 [DOI] [PubMed] [Google Scholar]
- 400. Weis VM, Reynolds WS. 1999. Carbonic anhydrase expression and synthesis in the sea anemone Anthopleura elegantissima are enhanced by the presence of dinoflagellate symbionts. Physiol. Biochem. Zool. 72:307–316 [DOI] [PubMed] [Google Scholar]
- 401. Weis VM, Reynolds WS, deBoer MD, Krupp DA. 2001. Host-symbiont specificity during onset of symbiosis between the dinoflagellate Symbiodinium spp. and the planula larvae of the scleractinian coral Fungia scutaria. Coral Reefs 20:301–308 [Google Scholar]
- 402. Weis VM. 2008. Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J. Exp. Biol. 211:3059–3066 [DOI] [PubMed] [Google Scholar]
- 403. Weis VM, Davy SK, Hoegh-Guldberg O, Rodriguez-Lanetty M, Pringle JR. 2008. Cell biology in model systems as the key to understanding corals. Trends Ecol. Evol. 23:369–376 [DOI] [PubMed] [Google Scholar]
- 404. Weis VM, Allemand D. 2009. What determines coral health? Science 324:1153–1155 [DOI] [PubMed] [Google Scholar]
- 405. Weitzman JB. 2006. Imaging with isotopes: high resolution and quantitation. J. Biol. 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. White MA, Riles L, Cohen BA. 2009. A systematic screen for transcriptional regulators of the yeast cell cycle. Genetics 181:435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Whitehead LF, Douglas AE. 2003. Metabolite comparisons and the identity of nutrients translocated from symbiotic algae to an animal host. J. Exp. Biol. 206:3149–3157 [DOI] [PubMed] [Google Scholar]
- 408. Whitney SM, Shaw DC, Yellowlees D. 1995. Evidence that some dinoflagellates contain a ribulose-1,5-bisphosphate carboxylase oxygenase related to that of the α-proteobacteria. Proc. R. Soc. Lond. B 259:271–275 [DOI] [PubMed] [Google Scholar]
- 409. Whitney SM, Yellowlees D. 1995. Preliminary investigations into the structure and activity of ribulose bisphosphate carboxylase from two photosynthetic dinoflagellates. J. Phycol. 31:138–146 [Google Scholar]
- 410. Wicks LC, Sampayo E, Gardner JPA, Davy SK. 2010. Local endemicity and high diversity characterise high-latitude coral-Symbiodinium partnerships. Coral Reefs 29:989–1003 [Google Scholar]
- 411. Wilkerson FP. 1980. Symbionts involved in phosphate uptake by green hydra, p 269–277 In Schwemmler W, Schenk HEA. (ed), Endocytobiology. Walter de Gruyter, Berlin, Germany [Google Scholar]
- 412. Wilkerson FP, Muller-Parker G, Muscatine L. 1983. Temporal patterns of cell-division in natural populations of endosymbiotic algae. Limnol. Oceanogr. 28:1009–1014 [Google Scholar]
- 413. Wilkerson FP, Muscatine L. 1984. Uptake and assimilation of dissolved inorganic nitrogen by a symbiotic sea anemone. Proc. R. Soc. Lond. B 221:71–86 [Google Scholar]
- 414. Wilkerson FP, Kobayashi D, Muscatine L. 1988. Mitotic index and size of symbiotic algae in Caribbean reef corals. Coral Reefs 7:29–36 [Google Scholar]
- 415. Withers KJT, Grant AJ, Hinde R. 1998. Effects of free amino acids on the isolated symbiotic algae of the coral Plesiastrea versipora (Lamarck): absence of a host release factor response. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 120:599–607 [Google Scholar]
- 416. Wittenberg C, Reed SI. 2005. Cell cycle-dependent transcription in yeast: promoters, transcription factors, and transcriptomes. Oncogene 24:2746–2755 [DOI] [PubMed] [Google Scholar]
- 417. Wittlieb J, Khalturin K, Lohmann JU, Anton-Erxleben F, Bosch TCG. 2006. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 103:6208–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Wood-Charlson EM, Hollingsworth LH, Krupp DA, Weis VM. 2006. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 8:1985–1994 [DOI] [PubMed] [Google Scholar]
- 419. Wood-Charlson EM, Weis VM. 2009. The diversity of C-type lectins in the genome of a basal metazoan, Nematostella vectensis. Dev. Comp. Immunol. 33:881–889 [DOI] [PubMed] [Google Scholar]
- 420. Yacobovitch T, Benayahu Y, Weis VM. 2004. Motility of zooxanthellae isolated from the Red Sea soft coral Heteroxenia fuscescens (Cnidaria). J. Exp. Mar. Biol. Ecol. 298:35–48 [Google Scholar]
- 421. Yonge CM. 1931. The significance of the relationship between corals and zooxanthellae. Nature 128:309–311 [Google Scholar]
- 422. Zoccola D, et al. 1999. Cloning of a calcium channel α1 subunit from the reef-building coral, Stylophora pistillata. Gene 227:157–167 [DOI] [PubMed] [Google Scholar]
- 423. Zoccola D, et al. 2004. Molecular cloning and localization of a PMCA P-type calcium ATPase from the coral Stylophora pistillata. Biochim. Biophys. Acta 1663:117–126 [DOI] [PubMed] [Google Scholar]
- 424. Zoccola D, et al. 2009. Specific expression of BMP2/4 ortholog in biomineralizing tissues of corals and action on mouse BMP receptor. Mar. Biotechnol. 11:260–269 [DOI] [PubMed] [Google Scholar]