Abstract
Trithorax group (TrxG) proteins antagonize Polycomb silencing and are required for maintenance of transcriptionally active states. We previously showed that the Drosophila melanogaster acetyltransferase CREB-binding protein (CBP) acetylates histone H3 lysine 27 (H3K27ac), thereby directly blocking its trimethylation (H3K27me3) by Polycomb repressive complex 2 (PRC2) in Polycomb target genes. Here, we show that H3K27ac levels also depend on other TrxG proteins, including the histone H3K27-specific demethylase UTX and the chromatin-remodeling ATPase Brahma (BRM). We show that UTX and BRM are physically associated with CBP in vivo and that UTX, BRM, and CBP colocalize genome-wide on Polycomb response elements (PREs) and on many active Polycomb target genes marked by H3K27ac. UTX and BRM bind directly to conserved zinc fingers of CBP, suggesting that their individual activities are functionally coupled in vivo. The bromodomain-containing C terminus of BRM binds to the CBP PHD finger, enhances PHD binding to histone H3, and enhances in vitro acetylation of H3K27 by recombinant CBP. brm mutations and knockdown of UTX by RNA interference (RNAi) reduce H3K27ac levels and increase H3K27me3 levels. We propose that direct binding of UTX and BRM to CBP and their modulation of H3K27ac play an important role in antagonizing Polycomb silencing.
INTRODUCTION
Polycomb group (PcG) and trithorax group (TrxG) proteins are epigenetic regulators of gene expression. Together, they carry out a variety of activities that alter local chromatin structure to promote and maintain, respectively, silent and active transcriptional states. Some [e.g., TRX, ASH1, and E(Z)] catalyze posttranslational modifications (PTMs) of specific histone residues. Others (e.g., ATPases BRM and KIS) possess ATP-dependent chromatin remodeling activities that can alter local nucleosome spacing and density, while others carry out functions that are less well understood.
Polycomb silencing is perhaps the best characterized example of epigenetic silencing. It involves trimethylation of histone H3 lysine 27 (H3K27me3) by E(Z), the catalytic methyltransferase subunit of Polycomb repressive complex 2 (PRC2), and specific binding of the H3K27me3 mark by PC, a key subunit of PRC1. Polycomb silencing is antagonized by activities associated with TrxG proteins, a diverse group of chromatin regulators that are required for stable long-term maintenance of transcriptionally active states. Recently acetylation of histone H3 lysine 27 (H3K27ac) has been identified as another posttranslational modification that is highly correlated with transcriptionally active genes (11, 23, 56). Although the specific role that H3K27ac plays in promoting transcription is not yet known, we previously showed that this modification also plays a central role in antagonizing Polycomb silencing (49). At Polycomb target genes, H3K27ac occurs at some of the same H3K27 sites that are alternatively trimethylated by PRC2 and, when present, directly blocks their trimethylation (42, 49), since acetyl- and methyl-lysine modifications are mutually exclusive.
We previously showed that the acetyltransferase CREB-binding protein (CBP) is responsible for the bulk of the H3K27 acetylation in Drosophila (49) and that this activity is conserved in the human CBP ortholog CBP/p300 (37, 49). Overexpression of CBP or knockdown of core PRC2 subunits reduces bulk H3K27me3 and increases bulk H3K27ac levels, while CBP knockdown or E(Z) overexpression has reciprocal effects on these marks (49). Thus, CBP and PRC2 are key components of an acetyl-methyl regulatory switch for maintenance, respectively, of transcriptionally active and repressed chromatin states of Polycomb target genes. Consistent with its antagonistic effect on H3K27me3 levels, moderate overexpression of CBP also enhances the weak dominant impaired-silencing phenotypes of adult Pc3 heterozygotes (49). Mutations in CBP and trithorax (trx), the founding member of the TrxG, also have very similar effects on Polycomb target gene expression in the early embryo (38). In CBP mutants, the spatially restricted expression of the homeotic gene Ubx is initiated normally but fails to be maintained once Polycomb silencing begins, during germ band elongation (38). Thus, CBP, like other TrxG proteins, is required to antagonize/prevent Polycomb silencing and maintain robust expression of Polycomb target genes in cells where they are initially activated (16, 38). The central role of H3K27 acetylation in directly inhibiting Polycomb silencing by preventing H3K27 trimethylation suggested the possibility that some other TrxG proteins may also antagonize Polycomb silencing in part by modulating H3K27 acetylation.
The large CBP protein contains multiple conserved domains, including its histone acetyltranferase (HAT) domain, a bromodomain (BrD), a CREB-binding (or KIX) domain, and three zinc fingers (ZF1 to ZF3), the second of which is a PHD-type C4HC3 zinc finger. This PHD finger is an integral part of the CBP HAT domain and is required for its HAT activity in vivo (7, 22), although its specific function is unknown. Mutations in the PHD finger of human CBP that abrogate HAT activity are associated with Rubinstein-Taybi syndrome (21). These conserved domains mediate interactions between CBP and a large number of other proteins, including many transcription factors (9, 17). However, no “core” CBP complex has been purified, suggesting that most CBP interactions are transient, conditional, or stabilized on chromatin.
In this study, we present evidence that the Drosophila TrxG proteins UTX and BRM are physically associated with CBP and are required for normal levels of H3K27ac in vivo.
UTX is the sole Drosophila ortholog (45) of the mammalian H3K27-specific demethylases UTX, UTY, and JmjD3 (2, 25, 28). Mutations in the Utx gene cause homeotic phenotypes similar to those of other TrxG mutants (18), consistent with a role in antagonizing Polycomb silencing. Drosophila BRM is the catalytic subunit of two related ATP-dependent chromatin remodeling complexes (33, 36, 47). It is the sole Drosophila ortholog of yeast SWI2/SNF2 and the two closely related mammalian proteins, BRM and BRG1, which are alternative catalytic subunits of the corresponding mammalian SWI/SNF complexes (54, 55). SWI/SNF family complexes are required for robust transcriptional activation of many genes and also repression of some (30). Consistent with their direct physical interactions, we show that chromatin sites with the highest occupancy of UTX, BRM, and CBP coincide with each other and with high levels of H3K27ac on many genes genome-wide. We also show that the conserved tetratricopeptide repeat (TPR) motifs in the N terminus of UTX bind directly to the CBP ZF1, suggesting that H3K27 demethylation and acetylation reactions may be coupled. We find that the BrD-containing C terminus of BRM binds directly to the CBP PHD finger. It also enhances histone H3 binding to the CBP PHD finger and enhances H3K27 acetylation by CBP in vitro. H3K27ac levels are reduced in vivo in brm mutants and by UTX RNA interference (RNAi). We propose that the binding of the BRM BrD to the CBP PHD finger plays a role in antagonizing Polycomb silencing by enhancing CBP acetylation of H3K27. Similarly, direct binding of UTX to CBP promotes efficient coupling of the sequential H3K27 demethylation and acetylation reactions required for switching from silent to active chromatin states and to reverse adventitious H3K27 deacetylation and methylation, thereby promoting high-fidelity maintenance of active chromatin states at Polycomb target genes.
MATERIALS AND METHODS
Constructs and antibodies.
Full-length cDNA clones of UTX (LD02225) and BRM isoform C/D (RE61274; 1,634 residues) were obtained from the Drosophila Genomics Resource Center (DGRC). Plasmid constructs for expression of glutathione S-transferase (GST) fusion and six-histidine (H6)-tagged proteins (CBP, BRM, and UTX) in Escherichia coli were generated by inserting PCR fragments (cut by NdeI and NsiI or NheI and SalI) into modified pGEX-2T and pET-11 (cut by NdeI and NsiI) (50) or pET-28b (cut by NheI and XhoI). A PCR fragment containing the full-length UTX gene was inserted into pGEX-2T at the BamHI and EcoRI sites. Constructs for expression of GST-UTX(1-262), UTX(1-597), UTX(1-705), and UTXΔ(79-363) were generated from pGEX-UTX by removing the C-terminal-encoding SacI-EcoRI, XhoI-EcoRI, and BglII-EcoRI fragments and the internal NdeI-NdeI fragment of the UTX gene and then end-filling to produce blunt ends and recircularizing. Recombinant UTX(420-633) and BRM(1417-1634) were expressed in BL-21 cells by IPTG (isopropyl-β-d-thiogalactopyranoside) induction and were purified by H6 tag on Ni-nitrilotriacetic acid (NTA) beads (Qiagen) as antigens to raise antibodies in rabbit and guinea pig. We confirmed the specificity of these antibodies by Western blotting on S2 cells after RNAi knockdowns of Utx and brm. Anti-CBP antibodies and various antihistone antibodies were described previously (49).
In vitro HAT assay.
Recombinant GST-CBP(1603-2678), containing the HAT domain, was induced in E. coli for 2 h at 30°C by addition of 0.2 mM IPTG and 50 μM ZnCl2 when optical density at 595 nm (OD595) was 0.4. HAT assays were done as previously described (49) using purified GST-CBP(1603-2678) (0.1 to 0.5 μg) or human p300 in acetylation buffer containing 0.2 mM acetyl coenzyme A (acetyl-CoA) and 4 μg of recombinant histone H3 and H4.
RNAi knockdown in Drosophila S2 cells.
S2 cells were treated for 8 days with a brm double-stranded RNA (dsRNA) (654 bp of 3′ coding region) and 6 days with two Utx dsRNAs (coding region of bp 235 to 1089 and bp 1258 to 1899) or E(z) dsRNAs as previously described (53). Fresh dsRNA (15 μg/ml) and media were added every other day.
RNAi knockdown and overexpression of UTX in flies.
The GAL4-inducible UTX RNAi transgene line was obtained from the Japan National Institute of Genetics Fly Stock Center. To knock down UTX in flies, the UTX RNAi strain 5640R-2 was crossed to a tubulin-Gal4 driver at 25°C. To overexpress UTX in flies, UAS-Utx transgenic lines were generated by cloning a 3.4-kb EcoRI-KpnI fragment that contains the entire UTX coding sequence from a full-length UTX cDNA clone (LD02225) into the plasmid pUAST (7a). Transgenic fly lines were generated by P-element-mediated transformations of yw strains in BestGene Inc. (Chino Hills, CA) using standard methods. At least 8 independent transgenic lines were recovered. Two independent lines, UAS-UTX 6F and UAS-UTX 4M, with insertions on chromosomes 2 and 3, respectively, were used for further analyses. All the Gal4 drivers were obtained from Bloomington Stock Center.
Embryo staining.
Df(3L)BSC831 deletes 12,188 bp and removes all but the first 80 brm codons. It also deletes 3 of 4 inositol monophosphatase genes (CG17026, CG17029, CG17028) within the third intron of brm, as well as the 3′-adjacent CG10516 and 5′ end of CG5931 (K. Cook, personal communication to FlyBase). Embryos were collected from brm2/TM3 (ftz-lacZ), Df(3L)BSC831/TM3 (ftz-lacZ) and w; TM3 (ftz-lacZ)/TM6B (control) files, fixed by standard methods, and immunostained with the following primary antibodies: rabbit anti-H3K27ac (Abcam; 1:500 dilution), anti-H3K27me3 (Millipore; 1:500), anti-CBP (1:300), and anti-β-galactosidase (anti-β-Gal) (Sigma; 1:700) antibodies. Secondary antibodies used were goat anti-rabbit antibody—Alexa Fluor 488, goat anti-mouse antibody—Alexa Fluor 594, and goat anti-guinea pig antibody—Alexa Fluor 633 (Molecular Probes). Homozygous mutant embryos, identified by the absence of ftz-lacZ expression. Images were collected using a Leica TCS-SP2 confocal microscope and analyzed using Leica software.
Other methods.
Nuclear extracts were prepared from embryos (0 to 16 h) with buffer containing 110 mM KCl and 250 mM NaCl and were fractionated on a Superose 6 column (10/300 mm) as previously described (51). Immunoprecipitation (IP) using mixed protein A and G beads and GST pulldown assay were performed as previously described (50) with 4 washes by 0.6 ml of 50 mM HEPES buffer containing 0.3 M NaCl, 0.2% NP-40, and 5% glycerol and with one wash by the buffer containing 0.1 M NaCl. To prevent DNA-dependent protein associations, we treated nuclear extracts with ethidium bromide to remove DNA prior to IP and GST pulldown (24, 52). Chemiluminescent signals from Western blots were quantified using a GE Typhoon Trio system (mean of two measurements). Preparation of histones from embryos and adult flies and chromatin immunoprecipitation coupled with DNA microarray (ChIP-chip) assays using the Drosophila RNAi screening center (DRSC) or DGRC S2 cells were previously described (49). ChIP-chip datasets are available upon request.
RESULTS
UTX and BRM are physically associated with CBP in vivo.
Switching Polycomb target genes from a transcriptionally silent to an active state requires demethylation of preexisting H3K27me3 by UTX as a prerequisite to H3K27 acetylation by CBP. However, it is unclear how UTX activity is regulated and coordinated with CBP and other TrxG proteins that antagonize Polycomb silencing. We hypothesized that UTX and CBP might be physically associated to enable efficient coupling of these sequential reactions. BRG1 was reported to be physically associated with human CBP and p300 in tumor cells (34). However, CBP was not found stably associated with the previously purified human (54, 55) or Drosophila BRM-containing complexes (33, 36).
To determine whether UTX and BRM are physically associated with CBP in Drosophila, we generated anti-BRM and anti-UTX antibodies (Fig. 1A) and carried out immunoprecipitation (IP) from embryo nuclear extracts (NE). As shown in Fig. 1B, both UTX and BRM but not E(Z) were coimmunoprecipitated with anti-CBP antibodies (lane 3), suggesting an association of CBP with UTX and BRM in vivo. Similarly, CBP and BRM were coimmunoprecipitated with anti-UTX antibodies (lane 4), and CBP and UTX were coimmunoprecipitated with anti-BRM antibodies (lane 5) but not with anti-PHO or anti-SU(Z)12 antibodies (lanes 6 and 7), indicating a specific association of CBP, UTX, and BRM.
Fig 1.

UTX and BRM are associated with Drosophila CBP in vivo. (A) Specificity of anti-BRM and anti-UTX antibodies. Whole S2 cell extracts (5 × 105 cells in left and 2.5 ×105 cells in right two panels) were separated by SDS-PAGE and visualized by Coomassie staining or Western blotting with anti-BRM and anti-UTX antibodies as indicated. Arrows indicate BRM and UTX bands that disappeared upon S2 cell treatment by Brm and Utx dsRNAs (see Fig. 7B and data not shown). (B) Immunoblots of proteins after IP from embryo nuclear extracts (NE) with guinea pig anti-CBP, anti-UTX, anti-BRM, anti-PHO, and anti-SU(Z)12 antibodies (lanes 3 to 7). Preimmune serum in lane 2 serves as a negative control. NEs were treated with ethidium bromide prior to IP. (C) Fractionation of NE on a Superose 6 size exclusion column. Fraction numbers and sizes are indicated at top. Void volume ends with fraction 6. Note that UTX but not BRM or CBP was also detected in smaller-size fractions (fractions 18 to 20, ∼150 kDa). PRC2, identified by the distribution of its SU(Z)12 subunit, peaks at fraction 15 (670 kDa). (D) IP by anti-CBP antibodies from fraction 11 in panel C. An asterisk indicates IgG heavy chain.
To further confirm the association of UTX and BRM with CBP in vivo, we fractionated NE on a size exclusion column (Fig. 1C). UTX, BRM, and CBP have broad distributions. UTX (fractions 8 to 11) and BRM (fractions 8 to 13) cofractionated with CBP in fractions 9 to 11, suggesting that a portion of UTX and BRM may be physically associated with CBP. We carried out IP from fraction 11 with anti-CBP antibodies. Both UTX and BRM were coimmunoprecipitated with anti-CBP antibodies (Fig. 1D, top two panels, lane 3). The specificity of the co-IP is indicated by the absence of the abundant CAF1 subunits p105 and p75 in CBP immunoprecipitates, which are present in some of the same fractions as CBP (fractions 10 to 11) (Fig. 1D, bottom two panels, lane 3). After IP with anti-CBP antibodies, substantial amounts of UTX and BRM remain in the unbound fraction (Fig. 1D, lane 5) but less than in the control IP with preimmune serum (lane 4). The partial cofractionation (Fig. 1C) and co-IP of UTX and BRM with CBP from fraction 11 (Fig. 1D) suggests that only a portion of the total UTX and BRM may be stably associated with CBP.
The mammalian chromatin remodeler CHD7 has also been reported to be physically associated with p300 (40) and BRG1 (4). We examined whether the Drosophila CHD7 homolog KIS, another TrxG protein (13), is associated with CBP, BRM, and UTX. We found that KIS was coimmunoprecipitated with anti-CBP antibodies (data available upon request), raising the possibility that KIS directly influences H3K27 acetylation by CBP at some locations, accounting for the elevated H3K27me3 level previously observed on polytene chromosomes in KIS mutants (46). Further investigation will be required to resolve this.
UTX, BRM, and CBP colocalize with H3K27ac genome-wide.
The physical association of UTX and BRM with CBP may reflect coordinate action of these proteins on their target genes in vivo. To investigate this possibility, we examined the genome-wide distribution of these TrxG proteins in S2 cells by ChIP-chip to determine the extent of their coenrichment on active genes marked with H3K27ac. (CHIP-chip datasets are available upon request.) As previously shown, the distributions of H3K27ac and H3K27me3 are mutually exclusive on many genes in DGRC S2 cells and the Sg4 derivative of S2 cells (42, 49). The presence in one cell line of H3K27me3 on genes that are instead marked by H3K27ac in the other line indicates that they are alternative modifications on many Polycomb target genes. We confirmed this observation in the DRSC S2 cell line. Extensive domains of H3K27me3 cover the entire regions comprising the two homeotic gene clusters, the Antennapedia and bithorax complexes (ANT-C and BX-C). Each of these broad domains contains no H3K27ac but is flanked by H3K27ac-marked regions (Fig. 2A and data available upon request). As previously shown (41), PC is strongly enriched at all identified Polycomb response elements (PREs) in the ANT-C and BX-C and is also broadly distributed over the regions adjacent to PREs, although at a lower level than at the PREs (Fig. 2A). In these large H3K27me3 domains, relatively weak UTX, BRM, and CBP peaks are present on some PREs, suggesting that these silent genes in the ANT-C and BX-C may be poised for activation or have a greater potential to be activated. The BX-C gene Abd-B, which is transcriptionally active in Sg4 cells and has strong H3K27ac signals across the entire gene (42), is repressed in S2 cells, where it is marked with strong H3K27me3 and PC signals (Fig. 2A). Conversely, the putative Polycomb target genes GATAe, CG17631, and pnr on chromosome 3R, which were previously shown to be marked by H3K27me3 and PC binding in Sg4 cells (41), are enriched with H3K27ac in S2 cells (Fig. 2B), suggesting that Polycomb silencing of these genes has been prevented by H3K27 acetylation in S2 cells. Within this H3K27ac domain (Fig. 2B), UTX, BRM, and CBP are also enriched at the transcription start site (TSS) of pnr, with a 2-fold-higher ChIP/Input ratio than at the PREs of the repressed BX-C. Notably, enrichment of UTX and BRM on these active genes (Fig. 2B) is also accompanied by spreading of these two proteins broadly around their peaks with H3K27ac. In contrast, weak PC signals (with a ChIP/Input ratio of 12, approximately one-third of the value of its strong peaks at BX-C PREs) are also present at two sharp peaks (Fig. 2B, top, asterisk) with no spreading. Similarly, the putative Polycomb target gene pnt (on chromosome 3R) is marked by strong UTX, BRM, CBP, and H3K27ac signals extending from 5′ of the TSS well into the transcribed region (data available upon request). In Sg4 cells, a broad H3K27me3 domain encompasses the Polycomb target gene cut (ct) locus, and PcG proteins are enriched at its presumptive PREs (43). In contrast, in S2 cells the H3K27me3 domain is restricted to the 5′ regulatory region of ct, while a broad domain of H3K27ac extends from its 5′ regulatory region through the entire transcribed region of ct (data available upon request). This is accompanied by enrichment of BRM, UTX, and CBP within the H3K27ac domain and on the presumptive PREs (data available upon request). Similarly, UTX, BRM, CBP, and H3K27ac are enriched in a broad omain encompassing the promoter-proximal 5′ regulatory region and 5′ transcribed region of the apoptosis-promoting rpr gene (data available upon request). This large H3K27ac domain is flanked by H3K27me3 domains (data available upon request) (29), suggesting that UTX, BRM, and CBP may act together to facilitate deposition and maintenance of H3K27ac and to prevent H3K27 trimethylation by PRC2.
Fig 2.
UTX and BRM colocalize with CBP on many genes marked by H3K27ac. (A and B) ChIP-chip plots across the BX-C (Ubx, abd-A, and Abd-B in panel A) marked with H3K27me3 and genes (He189B, srp, GATAe, and pnr in panel B) marked with H3K27ac in DRSC S2 cells. Antibodies used in ChIP are indicated on the left of each plot. Vertical lines below each plot indicate the regions where binding is enriched (ChIP/Input ratio of ≥2). Arrows at TSS of Abd-B and srp indicate direction of transcription. (C) Heat map illustrating the extent of overlap of CBP, UTX, BRM, H3K27ac, H3K27me3, and PC signals across chr3R. The 5 kb on each side of peak midpoints is shown. ChIP/Input signals are scaled to a range of −3 to +3. Note that H3K27ac signals overlap with CBP, UTX, and BRM in clusters 1 and 4, where H3K27me3 is absent. (D) Aggregate plots (average value at peak midpoint and 5 kb on each side) of CBP, UTX, BRM, H3K27ac, H3K27me3, and PC following clustering shown in panel C.
To visualize the extent of genome-wide overlap of UTX, BRM, CBP, H3K27ac, H3K27me3, and PC signals, we generated a heat map (Fig. 2C) and aggregate plots (Fig. 2D) from chromosome 3R for the 10-kb intervals centered on peak signals. This resulted in 4 distinct clusters. Cluster 1 contains the strongest BRM, UTX, and CBP signals and is enriched for H3K27ac. A striking feature of this cluster is the extensive spreading of the H3K27ac, BRM, UTX, and CBP signals around their peaks. Cluster 1 also contains weak PC signals. The active genes in Fig. 2B are found in this cluster. Cluster 4 also contains high H3K27ac but somewhat weaker BRM, UTX, and CBP signals than cluster 1. In contrast to cluster 1, these cluster 4 signals are distributed in much sharper peaks. Both clusters 1 and 4 suggest that UTX, BRM, and CBP may act together in acetylation of H3K27 genome-wide. Cluster 2 contains the strongest H3K27me3 and PC signals (with extensive spreading around their peaks) and weak UTX signals. The repressed BX-C and ANT-C are found in this cluster. Cluster 3 contains sharp peaks of H3K27me3and weak UTX signals. The relatively weak UTX signals in clusters 2 and 3 are accompanied by H3K27me3. A ChIP-PCR analysis of UTX binding to the human HOX genes reported similar weak UTX signals on some HOX genes marked by H3K27me3 domains (25).
In light of the co-IP of UTX, BRM, and CBP, the cooccurrence of strong UTX, BRM, and CBP signals with H3K27ac suggests that UTX, BRM, and CBP act together on chromatin to maintain acetylation of H3K27 and antagonize Polycomb silencing by restricting the formation of H3K27me3 domains. To understand how they might do this, below we further explored the interactions between CBP, BRM, and UTX.
UTX and BRM interact with the first zinc finger and PHD finger of CBP.
CBP binds directly to many proteins, mostly via its conserved domains (17). To map the regions of CBP responsible for its physical association with BRM and UTX, we first used a set of GST-CBP fusion proteins spanning most of CBP (Fig. 3C, lanes a to d) to pull down proteins from NE.
Fig 3.
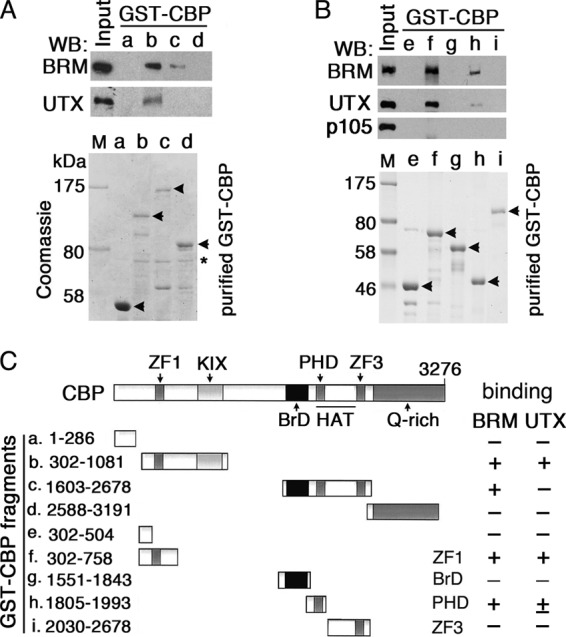
UTX and BRM bind to zinc fingers of CBP. (A and B) Immunoblots of UTX and BRM recovered in GST-CBP pulldown assays from ethidium bromide-treated NE (top) and Coomassie staining of purified GST-CBP fusion proteins, indicated by arrowheads (bottom). Some degraded forms of GST-CBP proteins are present. A nonspecific band is indicated by an asterisk in panel A. (C) Schematic summary of GST-CBP fragments tested and results of UTX and BRM pulldowns from NE. Conserved domains in CBP include three zinc fingers (ZF1, residues 509 to 594; PHD finger or ZF2, 1858 to 1932; and ZF3, 2397 to 2470), KIX domain (940 to 1012), BrD (1699 to 1806), and HAT (1858 to 2378).
Endogenous UTX and BRM were pulled down by GST-CBP(302-1081) (Fig. 3A, lane b), which contains ZF1 and the KIX domain, and not by GST-CBP(1-286) or CBP(2588-3191) (Fig. 3A, lanes a and d). Endogenous BRM was also pulled down by GST-CBP(1603-2678) (Fig. 3A, lane c), which contains the BrD, HAT domain (including the PHD finger), and ZF3. To further map these interactions, five additional GST-CBP fusion proteins (see Fig. 3C, lanes e to i) were used in pulldown assays. GST-CBP(302-758), a KIX domain deletion from GST-CBP(302-1081), was still able to pull down UTX and BRM (Fig. 3B, lane f). The PHD finger of CBP (Fig. 3B, lane h) independently pulled down BRM and only weakly pulled down UTX, but the BrD- and ZF3-containing regions of CBP (Fig. 3B, lanes g and i) did not. All GST-CBP fragments and in vitro binding results are summarized in Fig. 3C. In summary, both in vitro and in vivo results demonstrate interactions of CBP with BRM and UTX, suggesting that UTX and BRM activities are coupled to CBP HAT activity.
The first two TPR motifs of UTX bind directly to CBP.
This is the first observation to our knowledge that UTX and CBP are physically associated. To map the region of UTX responsible for its physical association with CBP, we used a set of GST-UTX fusion proteins to pull down proteins from NE. Besides its C-terminal JmjC catalytic domain, UTX also contains five conserved TPR motifs near its N terminus (45). Endogenous CBP was pulled down by GST-UTX(1-262), which contains the first two of the five TPR motifs (Fig. 4A, lane 3), as well as by two larger fragments containing these two TPR motifs (Fig. 4A, lanes 4 and 5). A full-length UTX fusion containing a deletion of this region, GST-UTXΔ(79-363), failed to pull down CBP (Fig. 4A, lane 6). Neither BRM nor SU(Z)12 were pulled down by any GST-UTX fragments, suggesting that UTX interacts with CBP but not with BRM via its first two TPR motifs.
Fig 4.

The TPR motifs of UTX and the C-terminal BrD-containing region of BRM bind directly to CBP. (A) Immunoblots of proteins pulled down from NE by GST-UTX (top). Purified GST-UTX fragments were stained with Coomassie (bottom). (B) Immunoblots of purified H6-tagged UTX fragments pulled down by GST-CBP fragments (left). UTX fragments were detected by anti-Penta-His antibodies (Qiagen). Coomassie staining of the two purified H6-tagged UTX fragments is at the right. (C) Schematic summary of recombinant UTX fragments tested for binding to CBP. UTX (total of 1,136 residues) contains five TPR motifs (115 to 216 and 307 to 402) and a JmjC catalytic domain (870 to 978). Note that UTX(1-262) and UTX(79-363) both contain the first two TPR motifs, strongly suggesting that they are sufficient for CBP binding. (D) GST-CBP fragments e to i (see Fig. 3C) were used to pull down purified BRM as in panel B. Input (left lane) is 10% of the purified BRM proteins used in the assay. (E) Schematic summary of recombinant BRM fragments tested for binding to GST-CBP in vitro. Conserved domains in Drosophila BRM include the ATPase domain (735 to 1364) and a BrD (1421 to 1526).
UTX has also been found associated with the purified mammalian MLL2 and MLL3 complexes (10, 20, 28) and the homologous Drosophila trithorax-related (TRR) complex (32). Interestingly, we found that GST-UTX(1-262), the minimal UTX fragment that pulls down CBP, does not pull down TRR from NE. However, GST-UTX(1-597) and GST-UTX(1-705), both of which contain all five TPR motifs, does pull down TRR (Fig. 4A). This indicates that CBP and the TRR complex interact specifically with different parts of the TPR-containing region of UTX.
To determine whether the above GST pulldowns from NE reflect direct UTX-CBP interactions and to map the UTX region mediating CBP binding, we tested whether the GST-CBP fusion proteins could pull down purified recombinant UTX fragments. We found that UTX(79-363), containing the first four conserved TPR motifs, binds weakly to CBP ZF1 but not to the CBP PHD finger (Fig. 4B, top, lanes f and h), while UTX(420-633), a nonconserved region, binds to neither (Fig. 4B, bottom). Figure 4C summarizes the results of these pulldown experiments. We conclude that the first two TPRs of UTX bind directly to CBP ZF1. The weak pulldown of endogenous UTX from nuclear extracts by the CBP PHD finger [GST-CBP(1805-1993)] (Fig. 3B, lane h) was not recapitulated with recombinant UTX proteins, indicating that it probably reflects an indirect interaction in vivo.
The BrD-containing C terminus of BRM binds directly to the CBP PHD finger.
BRM contains two highly conserved domains, both located in its C-terminal half [BRM(748-1634)]: an ATPase domain, required for its chromatin remodeling activity, and a BrD, the founding member of this large family of acetyl-lysine binding domains (57). We found that recombinant BRM(748-1634) binds directly to the CBP PHD finger (Fig. 4D, top, lane h). To map the CBP-binding region of BRM more precisely, we examined six additional subfragments of BRM(748-1634) (Fig. 4D, bottom 6 panels). Removing the BrD and C terminus while retaining the ATPase domain [BRM(748-1379)] abrogates CBP binding (second panel), indicating that residues C-terminal to the ATPase domain are required for CBP binding. Consistent with this, purified BRM(1417-1634), which contains just the BrD and the 108 adjacent C-terminal residues, binds to the CBP ZF1 and PHD finger (Fig. 4D, lanes f and h in third panel), while the BRM C terminus without the BrD [BRM(1519-1634)] binds to neither (fourth panel). Partial truncation of the BrD [BRM(1461-1634)] also abolishes CBP binding (fifth panel), indicating that the BRM BrD is required for binding to the CPB PHD finger. However, the BRM BrD alone [BRM(1417-1533)] also failed to bind to CBP (second panel from bottom), indicating that the BRM BrD is required but not sufficient for CBP binding in vitro. Finally, deletion of the C-terminal 56 residues from BRM(1417-1634) [BRM(1417-1578)] does not affect its binding to CBP (Fig. 4D, bottom, lanes f and h). Thus, the BrD and the adjacent 52 residues constitute the minimal BRM region that binds to the CBP ZF1 and PHD finger in vitro. The only obvious noteworthy feature of the BRM C terminus is two clusters of acidic residues that are conserved in metazoan BRM homologs. CBP ZF1 did not pull down the large BRM(748-1634) protein (Fig. 4D, lane f in top panel), possibly due to inappropriate folding of recombinant BRM. Figure 4E summarizes the results of these pulldown experiments. We conclude that BRM(1417-1578) binds directly to both ZF1 and the PHD finger of CBP.
BRM enhances histone H3 binding to CBP.
Mammalian BRG1 has been reported to bind to the globular domain of histone H3 (27). To test whether the BRM C terminus also binds unmodified histone H3, we performed a GST pulldown assay using purified GST-H3 and BRM(1417-1634). As shown in Fig. 5A, BRM(1417-1634) did not bind to unmodified GST-H3(1-46) (lane 3) but bound specifically to GST-H3(full length) (top, lane 4). In contrast, neither BRM(1417-1578), which is missing the 56 C-terminal residues, nor BRM(1461-1634), which is missing part of the BrD, was pulled down in a significant amount by GST-H3 (lane 4 in 2nd and 3rd panels). Thus, we conclude that BRM(1417-1634), containing an intact BrD and adjacent C terminus, is the minimal BRM fragment that is sufficient to bind H3, probably via the globular domain of unmodified H3.
Fig 5.

BRM binds to histone H3 and enhances H3 binding to CBP PHD finger. (A) BRM binds to unmodified full-length H3. Three BRM fragments (see Coomassie staining in Fig. 6D) were tested for binding to GST-H3 proteins (lanes 3 and 4). Lane 1 shows 15% of BRM proteins used in GST pulldown. Coomassie staining of purified GST, GST-H3 N-terminal tail, and GST-H3(full length) from E. coli is at the bottom. (B) Cooperative interactions among GST-CBP-PHD, BRM, and histone H3. Western blotting of BRM(1417-1634) and histone H3 in GST-CBP PHD pulldown in the presence/absence of BRM (total 1 μg or ∼0.16 mM) and core histones (1 to 3 μg indicated on the top of each lane) were performed. Lane 1 shows 15% of total proteins used in the assay. The ratios of pulldown BRM signals to lane 1 (set as 100%) are listed below each lane.
Mammalian p300 has been reported to bind directly to unmodified H3 and H4 tails (3). The region containing the BrD and PHD finger of p300 was also reported to bind to nucleosomes containing highly acetylated histones (39). Using GST pulldown assays, we examined whether the same minimal CBP PHD construct that pulls down BRM(1417-1634) (Fig. 4D) also pulls down histone H3 from calf thymus core histones. We also examined whether both BRM and core histones can simultaneously bind to the CBP PHD finger. As shown in Fig. 5B, GST-CBP PHD pulled down histone H3 in the absence of BRM (bottom panel, lane 7) but pulled down an increasing amount of histone H3 in the presence of BRM(1417-1634) (compare lane 5 to lane 7). Similarly, while GST-CBP PHD pulled down BRM in the absence of histones (top, lane 3), it pulled down an increasing amount of BRM in the presence of increasing amounts of histones (1 to 3 μg) (lanes 4 to 6). Thus, we conclude that BRM enhances histone H3 binding to CBP and that the binding of CBP and BRM to each other is enhanced by their mutual binding to H3.
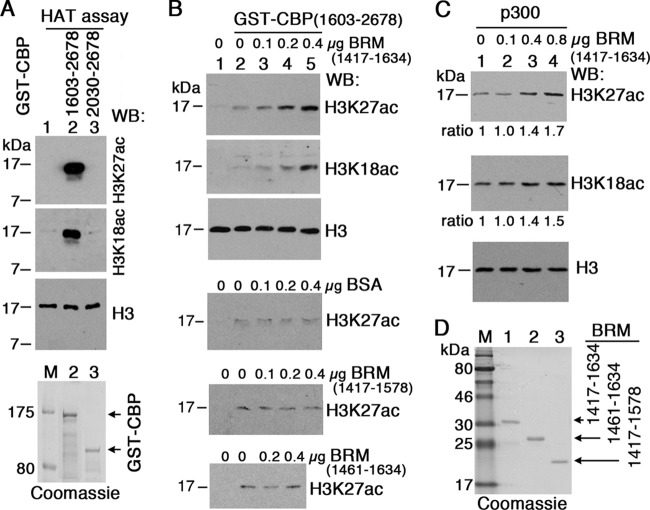
BRM enhances acetylation of H3K27 and H3K18 by CBP in vitro.
Since BRM can enhance histone H3 binding to the CBP PHD finger (Fig. 5B), which is required for CBP HAT activity in vivo, we tested whether the binding of BRM to the PHD finger influences CBP HAT activity. We previously showed that a recombinant Drosophila CBP protein (residues 1476 to 3232) purified from S2 cells possesses HAT activity (49). Smaller recombinant proteins containing the mammalian CBP/p300 HAT purified from E. coli have also been shown to possess HAT activity (5, 35). We purified a similar Drosophila GST-CBP(1603-2678) containing the BrD, PHD finger, HAT domain, and ZF3 from E. coli and confirmed that it acetylates H3K27 and H3K18 on a free histone H3 substrate (Fig. 6A, lane 2) in vitro. Deleting the BrD and PHD finger from GST-CBP(1603-2678) abolished its HAT activity (lane 3).
Fig 6.
CBP HAT activity on histone H3 is enhanced by BRM(1417-1634) in vitro. (A) HAT activity of recombinant GST-CBP proteins on free histone H3. Two purified GST-CBP proteins (Coomassie stained at the bottom) were incubated with recombinant histones H3 and H4 in an in vitro HAT assay (see Materials and Methods). Note that only GST-CBP(1603-2678) in lane 2 possesses HAT activity. Lane 1 is a negative control (no GST-CBP) for the HAT assay. (B and C) Enhancement of CBP and p300 HAT activities by BRM polypeptides added to HAT assays. A total of 0.1, 0.2, and 0.4 μg of BRM(1417-1634) (top in panel B), BRM(1417-1578), BRM(1461-1634) (bottom two panels in panel B), or BSA (middle in panel B) were added to a mixture of purified GST-CBP(1603-2678) and free histones H3 and H4. For p300 HAT assay, 0.1, 0.4, and 0.8 μg of BRM(1421-1638) was added to the HAT reaction (lanes 2 to 4). Western analyses of histone H3, H3K27ac, and H3K18ac were performed with the ECL system (Pierce). Signals in panel C were quantified relative to lane 1. (D) Coomassie staining of the three purified BRM fragments used in panel B.
Next we tested whether BRM could affect acetylation of H3 by CBP. Among three BRM fragments, BRM(1417-1634) binds both histone H3 and CBP, BRM(1461-1634) does not bind CBP (Fig. 4D), and BRM(1417-1578] binds CBP but does not bind histone H3 (Fig. 4D and 5A). The addition of BRM(1417-1634) (0.1, 0.2, or 0.4 μg) to a GST-CBP(1603-2678) HAT assay increased the yield of both H3K27ac and H3K18ac (Fig. 6B, top two panels, lanes 3 to 5), but the addition of bovine serum albumin (BSA) did not (middle panel). Neither BRM(1461-1634) nor BRM(1417-1578) enhanced H3K27 or H3K18 acetylation by CBP HAT (bottom two panels), suggesting that simultaneous interactions among BRM, histone H3, and the PHD finger of CBP are required for enhancement of H3K27 and H3K18 acetylation by BRM. Because the PHD finger and HAT domains of Drosophila CBP and the closely related mammalian CBP and p300 proteins are highly conserved, we also tested whether Drosophila BRM(1417-1634) could also enhance H3K27 and H3K18 acetylation by p300 HAT. In a similar HAT assay, adding 0.4 and 0.8 μg of BRM(1417-1634) to p300 also enhanced acetylation of H3K27 and H3K18 (Fig. 6C, lanes 3 and 4), though less robustly than Drosophila CBP, suggesting that stimulation of H3K27 or H3K18 acetylation by the BRM BrD-containing C terminus has been conserved from Drosophila to humans.
BRM and UTX modulate acetylation and trimethylation of H3K27 in vivo.
Since some TrxG mutants affect H3K27ac and H3K27me3 levels (46, 49) and BRM can enhance CBP HAT activity in vitro (Fig. 6B), we tested whether brm mutants have an effect on H3K27ac and H3K27me3 levels. Embryos homozygous for brm2, a strong hypomorphic or null brm allele (8, 14), and wild-type controls (see Materials and Methods) were costained with anti-CBP, anti-H3K27ac, and anti-H3K37me3 antibodies. While the CBP level appears indistinguishable in brm2 and control embryos (Fig. 7A, columns a and c), the H3K27ac level is substantially decreased compared to control embryos (Fig. 7A, columns b and d), and the H3K37me3 level is concomitantly increased (Fig. 7A, column f). Note that the reduced H3K27ac level is already evident in brm2 mutant embryos by stage 4, not long after global transcriptional activation of the zygotic genome. Consistent with this, the BRM protein level itself is already substantially decreased in homozygous brm2 embryos by stage 4 (Fig. 7A). This suggests that the early decrease in H3K27ac is due in large part to a decrease in the pool of wild-type BRM protein and/or mRNA of maternal origin inherited from their heterozygous brm2/+ mothers. A similar effect on the H3K27ac level was seen in embryos homozygous for Df(3L)BSC831 (a 12-kb brm deletion, which removes all but the 80 N-terminal codons of the brm coding sequence and like brm2 causes late embryonic lethality) (Fig. 7E), strongly suggesting that the altered H3K27ac and H3K27me3 levels in the brm2 mutants are not caused by another mutation in their genetic backgrounds. Thus, we conclude that the reduced H3K27ac and increased H3K27me3 levels observed are a consequence of the perturbation of BRM function. RNAi knockdown of BRM in S2 cells for 8 days, which decreased the BRM level by over 90%, also resulted in reduced H3K27ac and increased H3K27me3 levels (data not shown), although, as expected, the effect is weaker than that caused by RNAi knockdown of CBP, the H3K27 acetyltransferase itself (49). Together, these data indicate that BRM is required for normal levels of H3K27ac in vivo and suggest that BRM directly modulates H3K27 acetylation by CBP through physical interaction with the PHD finger in its HAT domain.
Fig 7.
BRM and UTX are required for normal H3K27ac and H3K27me3 levels. (A) Control and brm2 mutant embryos coimmunostained with anti-CBP and anti-H3K27ac (columns a and b, c and d) or anti-H3K27me3 (columns e and f) or stained with anti-BRM alone. Note that H3K27me3 is detected in wild-type embryos at stage 13 (column f) but not at stage 4 (embryo indicated by the arrow in top panel of f), when H3K27me3 is barely detectable by Western blotting (49). The bottom two panels show the same embryos as the top two panels at a 4× higher magnification. (B and C) Knockdown of UTX in vivo increases the H3K27me3 level and reduces the H3K27ac level. Immunoblots of proteins (top panels) and histone H3 (bottom panels) from S2 cells after RNAi knockdown of UTX (lanes 2 and 3 in panel A) and E(Z) (lane 4 in panel A) for 6 days and from adult hsp70-Gal4 flies (control) and UTX RNAi line (UTX RNAi/tub-Gal4) (lane 2 in panel B). Quantified H3K27ac signals relative to lane 1 are listed at the bottom. (D) Overexpression of UTX in vivo reduces H3K27me3 and increases H3K27ac. Quantitative Western analysis (49) of histone H3 extracted from adult male flies overexpressing UTX (UAS-UTX 4 M/tub-Gal4 and UAS-UTX 6F/tub-Gal4 in columns 2 and 3) and from wild-type (w1118) and tub-Gal4 control males (columns 1 and 4). Western blots of protein extracts are shown at the top of the left panel (lanes 1 and 4, control; lanes 2 and 3, UTX overexpression). (E) Wild type control and Df(3L)BSC831 embryos (stage 14) coimmunostained as in panel A with anti-CBP and anti-H3K27me3 (top panel) or anti-CBP and anti-H3K27ac (bottom panel).
As expected, knockdown of UTX in S2 cells, by independently targeting two different regions of the Utx coding sequence, resulted in an increase of H3K27me3 (Fig. 7B, lanes 2 and 3), indicating that maintenance of normal H3K27me3 levels requires UTX and further suggesting that this involves a dynamic counterbalance between H3K27 trimethylation by PRC2 and demethylation by UTX. UTX knockdown also caused a concomitant decrease of H3K27ac (lanes 2 and 3, bottom), while the CBP protein level appeared unaffected (panel 3), indicating that UTX is also required indirectly to maintain normal H3K27ac levels, presumably at H3K27 sites that can be alternatively trimethylated by PRC2, i.e., in Polycomb target genes. As we previously reported (49), knockdown of E(Z) has the reciprocal effect, increasing the H3K27ac level (Fig. 7B, lane 4). Depletion of UTX in vivo, using a GAL4-inducible UTX RNAi transgene line, resulted in a similar increase in H3K27me3, as previously reported (19, 45), and also caused a decrease in H3K27ac (Fig. 7C, lane 2, bottom). The level of H3K18ac, a modification also catalyzed by CBP, appears unchanged, indicating that UTX knockdown does not nonspecifically affect histone acetylation or CBP activity. Although it was previously reported that UTX can demethylate mono-, di-, and trimethylated H3K27 in vitro (19), only the H3K27me3 level was noticeably elevated after UTX knockdown in vivo (Fig. 7C, lane 2), consistent with previous reports that H3K27me3 is more sensitive to demethylation than H3K27me2 or H3K27me1 (19, 45). Selective recruitment of UTX to sites containing H3K27me3 might also contribute to its preference for H3K27me3 over H3K27me1/2.
We overexpressed UTX in vivo using two independent GAL4-inducible UAS-Utx transgenic lines. UTX levels increased by over 5-fold, but this did not appear to affect survival to adulthood. Fractionation on a size exclusion column revealed that, in contrast to wild-type adult fly extracts, which contain little monomeric UTX, most of the excess UTX resulting from overexpression is found in monomer form, with only a moderate increase in higher-molecular-weight UTX-associated complexes (data not shown), raising the possibility that limits on UTX complex formation may limit the impact of UTX overexpression. Nevertheless, UTX overexpression resulted in a reciprocal decrease in H3K27me3 and increase in H3K27ac in adult extracts (Fig. 7D, top, columns 2 and 3), with no significant changes in the CBP level (left, top panel) or the H3K4me3 and H3K18ac levels (bottom panels). The increase in H3K27ac caused by UTX overexpression suggests that the sequential UTX and CBP reactions are coupled and that increasing demethylation of H3K27me3 sites is sufficient to promote subsequent acetylation of some of these same sites, i.e., CBP is poised to acetylate them once they have been demethylated.
DISCUSSION
Acetylation of histone H3K27, a strong predictor of active genes (11, 23, 56), has emerged as one of the central mechanisms for antagonizing/reversing Polycomb silencing since it directly prevents trimethylation of H3K27 by PRC2. Interestingly, H3K27ac is present in animals, plants, and fungi, but H3K27me3 and PRC2 homologs are present only in animals and plants, clearly indicating that H3K27ac has functions other than preventing H3K27 methylation. In this study, we provide evidence that the Drosophila TrxG proteins UTX and BRM modulate H3K27 acetylation by CBP. We showed that UTX and BRM are physically associated with CBP in vivo and bind directly to ZF1 and the PHD finger of CBP. Genome-wide ChIP-chip analysis revealed that the chromatin binding sites of UTX, BRM, and CBP coincide on many genes and that strong peaks of all three are highly correlated with the presence of high levels of H3K27ac (cluster 1 in Fig. 2C and D). Importantly, brm mutants and RNAi knockdown of BRM in vivo result in a decrease of H3K27ac and a concomitant increase of H3K27me3. Similarly, knockdown and overexpression of UTX with no change in the CBP level are sufficient to promote, respectively, a decrease and increase in the bulk H3K27ac level. This suggests that coupled H3K27 deacetylation/trimethylation and demethylation/acetylation are dynamically antagonistic. It further suggests that regulating the balance of these opposing activities is likely to play an important role in determining whether active and silent chromatin states will be maintained or switched.
This is the first report that UTX is physically associated with CBP. While functional collaboration between UTX and CBP is required to execute the sequential reactions required to switch Polycomb target genes from silent to active states, it was not obvious that this should require that they be physically associated. The fact that they are suggests that their two reactions are more efficiently coupled. It also suggests that despite the many histone and nonhistone substrates of CBP (48), H3K27 acetylation on Polycomb target genes to prevent their silencing is sufficiently critical to have evolved a UTX-CBP methyl-to-acetyl switching module, perhaps to counter the complementary coupling effect of the physical association of the H3K27 deacetylase RPD3 with PRC2 to create the antagonistic acetyl-to-methyl switch (49, 52). Coupling of the UTX and CBP activities may increase the fidelity of maintenance of active chromatin states of Polycomb target genes by ensuring rapid reversal of H3K27ac deacetylation and methylation by RPD3 and PRC2 that may occur either adventitiously or as part of an ongoing dynamic balance between these antagonistic activities. Such coupling could also increase the efficiency of switching Polycomb target genes from a transcriptionally silent to an active state in response to developmentally programmed signals or other cellular signals, ensuring definitive establishment of the new active transcriptional state.
While BRM is well known for the chromatin remodeling activity associated with its highly conserved ATPase domain, our findings identify another activity of BRM associated with its highly conserved BrD-containing C terminus [BRM(1417-1634)], which binds histone H3, enhances H3 binding to the CBP PHD finger, and enhances acetylation of H3K27 by CBP in vitro (Fig. 6B, top). The latter effect is most likely due to the simultaneous binding of BRM to H3 and the CBP PHD finger, thereby stabilizing the H3 interaction with the CBP HAT domain. However, we cannot rule out the possibility that it may also reflect a direct stimulatory effect of BRM on the intrinsic activity of the CBP HAT domain. In any case, this suggests that the physical association and genome-wide colocalization of CBP and BRM do not simply reflect a spatial and temporal coordination of their separate acetylation and chromatin remodeling activities but also reflect regulatory interactions. The reduced H3K27ac level in brm2 mutants and after RNAi knockdown is consistent with the observed enhancement of CBP HAT activity in vitro. However, at this time we cannot rule out the possibility that the loss of BRM chromatin-remodeling activity in brm2 mutants may also contribute to their reduced H3K27ac level.
The physical association of BRM with CBP and with UTX reported here is consistent with recent reports that BRG1 can be coimmunoprecipitated with human CBP and p300 from tumor cells (34) and with UTX/Jmjd3 in murine EL4 T cells (31). However, the region of human CBP reported to bind BRG1 differs from our findings. It was reported that BRG1 interacts directly with the human CBP fragment containing ZF3, and the proline-rich region of BRG1 is required for this interaction (34). Our results indicate that only ZF1 and the PHD finger of Drosophila CBP bind directly to the BrD-containing C terminus of BRM (Fig. 4D). Since we did not assay the proline-rich region of Drosophila BRM, due to its insolubility, we cannot rule out the possibility that it mediates additional contact(s) with CBP.
CBP was not found in the previously purified Drosophila BRM-containing complexes (33, 36), suggesting that only a portion of BRM is physically associated with CBP or that this association may be stabilized only on chromatin and/or may be regulated by other cellular signals. Consistent with this, there are some sites detected by ChIP-chip that are enriched for BRM and UTX without CBP and there are some column fractions containing BRM and UTX without CBP (Fig. 1C). It is possible that the CBP-BRM association may also serve to recruit BRM to some sites, e.g., recruitment of human BRM or BRG1 to the beta interferon (IFN-β) promoter depends on the prior presence of CBP and leads to subsequent nucleosome remodeling (1).
We found that UTX, BRM, and CBP colocalize not only with H3K27ac (Fig. 2C and D, cluster 1) at many regions, including promoters, transcribed regions, PREs, and other presumed cis-regulatory elements. They are also present, albeit at lower levels, on repressed genes marked by strong H3K27me3 domains (e.g., the ANT-C and BX-C) (Fig. 2A and data not shown). At such sites, the H3K27me3 may be protected from UTX-mediated demethylation by the binding of PC/PRC1 (26). Alternatively, their lower levels at repressed genes may simply result in a dynamic balance of deacetylation/methylation and demethylation/acetylation that overwhelmingly favors the former. It is also possible that UTX and CBP may also be involved in BRM-dependent transcriptional repression at some sites (30). Recent evidence suggests that maintenance of steady-state levels of histone acetylation is highly dynamic (12), and the reciprocal changes in H3K27me3 and H3K27ac levels that occur upon altering UTX or E(Z) levels suggest that maintenance of histone methylation levels may also be highly dynamic. Much remains to be discovered about the factors that regulate the demethylase and acetyltransferase activities of UTX and CBP in different chromatin environments.
The BrDs of yeast SWI2/SNF2 and human BRG1 bind specifically to H3K14ac (44, 58), indicating that this binding specificity has been highly conserved during evolution. BRM(1417-1634) also binds specifically to H3K14ac (data not shown), and the BrD of BRM is required for CBP binding (Fig. 4D) and histone H3 binding (Fig. 5A). The BrD-containing C termini of human and plant BRM have been reported to be functionally important in vivo (6, 15). Surprisingly, the BrD of Drosophila BRM has been reported to be dispensable for viability. A brm transgene containing a deletion of the central 72 residues of the BrD can rescue the late embryonic lethality of brm2 mutants, allowing them to develop into adults (14). Whether the reduced H3K27ac level of these brm2 mutants is also rescued has not been determined. This rescue could indicate that the BRM BrD is functionally redundant or at least not critical for achieving adequate expression of the genes responsible for the inviability of brm2 mutants. The brm2 mutation behaves genetically as a strong hypomorphic or null allele, but the sequence alteration responsible for its phenotype has not been determined and so the precise nature of its functional deficit is unknown.
In summary, we have shown that UTX and BRM interact directly with CBP and modulate H3K27 acetylation. UTX presumably does so indirectly, at Polycomb target genes, by providing demethylated H3K27 substrate for acetylation by CBP. Their direct physical coupling could provide obvious gains in the efficiency of their two sequential reactions and their consequent H3K27ac yield. The interaction between BRM and CBP may similarly couple their activities, but we also present initial evidence to suggest that the binding of the BRM BrD-containing C terminus to H3 and the CBP PHD finger may also directly enhance H3K27 acetylation through its effect on H3 binding by the CBP HAT domain. We expect that additional TrxG proteins will modulate H3K27 acetylation, including KIS and ASH1, which have recently been shown to affect H3K27me3 levels (46). The broad distributions of H3K27me3 over many Polycomb target genes is mirrored by similar broad distributions of H3K27ac when those genes are active, suggesting that in addition to its general genome-wide association with active genes, it may play a more specialized dual role at Polycomb target genes, where it also dynamically antagonizes the encroachment of Polycomb silencing.
ACKNOWLEDGMENTS
We thank the Bloomington Drosophila Stock Center and TRiP (NIH R01GM084947) for fly stocks, the DGRC for Utx and brm cDNA clones, Alexander Mazo for anti-TRR antibody, Dheepa Balasubramanian and Michael Schnetz for technical assistance on ChIP-chip, and Wei Wang for assistance on plasmid construction.
This work was supported by grants from NIH to P.J.H. (R01GM39255) and P.C.S. (R01HD056369, R01HG004722). Microscope images were acquired with equipment purchased with NIH and NCRR shared instrumentation grants (RR-021228-01, RR-017980-01, RR-024536-01).
Footnotes
Published ahead of print 9 April 2012
REFERENCES
- 1. Agalioti T, et al. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell 103: 667– 678 [DOI] [PubMed] [Google Scholar]
- 2. Agger K, et al. 2007. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449: 731– 734 [DOI] [PubMed] [Google Scholar]
- 3. An W, Roeder RG. 2003. Direct association of p300 with unmodified H3 and H4 N termini modulates p300-dependent acetylation and transcription of nucleosomal templates. J. Biol. Chem. 278: 1504– 1510 [DOI] [PubMed] [Google Scholar]
- 4. Bajpai R, et al. 2010. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463: 958– 962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bannister A, Kouzarides T. 1996. The CBP co-activator is a histone acetyltransferase. Nature 384: 641– 643 [DOI] [PubMed] [Google Scholar]
- 6. Biggs J, et al. 2001. The human Brm protein is cleaved during apoptosis: the role of cathepsin G. Proc. Natl. Acad. Sci. U. S. A. 98: 3814– 3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bordoli L, et al. 2001. Functional analysis of the p300 acetyltransferase domain: the PHD finger of p300 but not of CBP is dispensable for enzymatic activity. Nucleic Acids Res. 29: 4462– 4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a. Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401– 415 [DOI] [PubMed] [Google Scholar]
- 8. Brizuela BJ, Elfring L, Ballard J, Tamkun JW, Kennison JA. 1994. Genetic analysis of the brahma gene of Drosophila melanogaster and polytene chromosome subdivisions 72AB. Genetics 137: 803– 813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan HM, La Thangue NB. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114: 2363– 2373 [DOI] [PubMed] [Google Scholar]
- 10. Cho YW, et al. 2007. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J. Biol. Chem. 282: 20395– 20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Creyghton MP, et al. 2010. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. U. S. A. 107: 21931– 21936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crump N, et al. 2011. Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. Proc. Natl. Acad. Sci. U. S. A. 108: 7814– 7819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daubresse G, et al. 1999. The Drosophila kismet gene is related to chromatin-remodeling factors and is required for both segmentation and segment identity. Development 126: 1175– 1187 [DOI] [PubMed] [Google Scholar]
- 14. Elfring L, et al. 1998. Genetic analysis of brahma: the Drosophila homolog of the yeast chromatin remodeling factor SWI2/SNF2. Genetics 148: 251– 265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farrona S, Hurtado L, Reyes JC. 2007. A nucleosome interaction module is required for normal function of Arabidopsis thaliana BRAHMA. J. Mol. Biol. 373: 240– 250 [DOI] [PubMed] [Google Scholar]
- 16. Florence B, McGinnis W. 1998. A genetic screen of the Drosophila X chromosome for mutations that modify deformed function. Genetics 150: 1497– 1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodman RH, Smolik S. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14: 1553– 1577 [PubMed] [Google Scholar]
- 18. Herz HM, et al. 2010. The H3K27me3 demethylase dUTX is a suppressor of Notch- and Rb-dependent tumors in Drosophila. Mol. Cell. Biol. 30: 2485– 2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong S, et al. 2007. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc. Natl. Acad. Sci. U. S. A. 104: 18439– 18444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Issaeva I, et al. 2007. Knockdown of ALR (MLL2) reveals ALR target genes and leads to alterations in cell adhesion and growth. Mol. Cell. Biol. 27: 1889– 1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalkhoven E, et al. 2003. Loss of CBP acetyltransferase activity by PHD finger mutations in Rubinstein-Taybi syndrome. Hum. Mol. Genet. 12: 441– 450 [DOI] [PubMed] [Google Scholar]
- 22. Kalkhoven E, Teunissen H, Houweling A, Verrijzer CP, Zantema A. 2002. The PHD type zinc finger is an integral part of the CBP acetyltransferase domain. Mol. Cell. Biol. 22: 1961– 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karlic R, Chung HR, Lasserre J, Vlahovicek K, Vingron M. 2010. Histone modification levels are predictive for gene expression. Proc. Natl. Acad. Sci. U. S. A. 107: 2926– 2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai JS, Herr W. 1992. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc. Natl. Acad. Sci. U. S. A. 89: 6958– 6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lan F, et al. 2007. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature 449: 689– 694 [DOI] [PubMed] [Google Scholar]
- 26. Lau PN, Cheung P. 2011. Histone code pathway involving H3 S28 phosphorylation and K27 acetylation activates transcription and antagonizes Polycomb silencing. Proc. Natl. Acad. Sci. U. S. A. 108: 2801– 2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lavigne M, et al. 2009. Interaction of HP1 and Brg1/Brm with the globular domain of histone H3 is required for HP1-mediated repression. PLoS Genet. 5: e1000769 doi:10.1371/journal.pgen.1000769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee MG, et al. 2007. Demethylation of H3K27 regulates Polycomb recruitment and H2A ubiquitination. Science 318: 447– 450 [DOI] [PubMed] [Google Scholar]
- 29. Lin N, et al. 2011. A barrier-only boundary element delimits the formation of facultative heterochromatin in Drosophila and vertebrates. Mol. Cell. Biol. 31: 2729– 2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martens JA, Winston F. 2002. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 16: 2231– 2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller SA, Mohn SE, Weinmann AS. 2010. Jmjd3 and UTX play a demethylase-independent role in chromatin remodeling to regulate T-box family member-dependent gene expression. Mol. Cell 40: 594– 605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohan M, et al. 2011. The COMPASS family of H3K4 methylases in Drosophila. Mol. Cell. Biol. 31: 4310– 4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohrmann L, et al. 2004. Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes. Mol. Cell. Biol. 24: 3077– 3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naidu S, Love I, Imbalzano A, Grossman S, Androphy E. 2009. The SWI/SNF chromatin remodeling subunit BRG1 is a critical regulator of p53 necessary for proliferation of malignant cells. Oncogene 28: 2492– 2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogryzko V, Schiltz R, Russanova V, Howard B, Nakatani Y. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87: 953– 959 [DOI] [PubMed] [Google Scholar]
- 36. Papoulas O, et al. 1998. The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development 125: 3955– 3966 [DOI] [PubMed] [Google Scholar]
- 37. Pasini D, et al. 2010. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res. 38: 4958– 4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petruk S, et al. 2001. Trithorax and dCBP acting in a complex to maintain expression of a homeotic gene. Science 294: 1331– 1334 [DOI] [PubMed] [Google Scholar]
- 39. Ragvin A, et al. 2004. Nucleosome binding by the bromodomain and PHD finger of the transcriptional cofactor p300. J. Mol. Biol. 337: 773– 788 [DOI] [PubMed] [Google Scholar]
- 40. Schnetz MP, et al. 2010. CHD7 targets active gene enhancer elements to modulate ES cell-specific gene expression. PLoS Genet. 6: e1001023 doi:10.1371/journal.pgen.1001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwartz YB, et al. 2006. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genetics 38: 700– 705 [DOI] [PubMed] [Google Scholar]
- 42. Schwartz YB, et al. 2010. Alternative epigenetic chromatin states of Polycomb target genes. PLoS Genet. 6: e1000805 doi:10.1371/journal.pgen.1000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwartz YB, Pirrotta V. 2007. Polycomb silencing mechanisms and the management of genomic programmes. Nat. Rev. Genet. 8: 9– 22 [DOI] [PubMed] [Google Scholar]
- 44. Shen W, et al. 2007. Solution structure of human Brg1 bromodomain and its specific binding to acetylated histone tails. Biochemistry 46: 2100– 2110 [DOI] [PubMed] [Google Scholar]
- 45. Smith ER, et al. 2008. Drosophila UTX is a histone H3 Lys27 demethylase that colocalizes with the elongating form of RNA polymerase II. Mol. Cell. Biol. 28: 1041– 1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srinivasan S, Dorighi KM, Tamkun JW. 2008. Drosophila Kismet regulates histone H3 lysine 27 methylation and early elongation by RNA polymerase II. PLoS Genet. 4: e1000217 doi:10.1371/journal.pgen.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tamkun JW, et al. 1992. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell 68: 561– 572 [DOI] [PubMed] [Google Scholar]
- 48. Thompson PR, Kurooka H, Nakatani Y, Cole PA. 2001. Transcriptional coactivator protein p300. Kinetic characterization of its histone acetyltransferase activity. J. Biol. Chem. 276: 33721– 33729 [DOI] [PubMed] [Google Scholar]
- 49. Tie F, et al. 2009. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development 136: 3131– 3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tie F, Furuyama T, Harte PJ. 1998. The Drosophila Polycomb-group proteins ESC and E(Z) bind directly to each other and co-localize at multiple chromosomal sites. Development 125: 3483– 3496 [DOI] [PubMed] [Google Scholar]
- 51. Tie F, Furuyama T, Prasad-Sinha J, Jane EP, Harte PJ. 2001. The Drosophila Polycomb group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development 128: 275– 286 [DOI] [PubMed] [Google Scholar]
- 52. Tie F, Prasad-Sinha J, Birve A, Rasmuson-Lestander A, Harte PJ. 2003. A 1 MDa ESC/E(Z) complex from Drosophila that contains Polycomblike and RPD3. Mol. Cell. Biol. 23: 3352– 3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tie F, Stratton CA, Kurzhals RL, Harte PJ. 2007. The N terminus of Drosophila ESC binds directly to histone H3 and is required for E(Z)-dependent trimethylation of H3 lysine 27. Mol. Cell. Biol. 27: 2014– 2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang W, et al. 1996. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 15: 5370– 5382 [PMC free article] [PubMed] [Google Scholar]
- 55. Wang W, et al. 1996. Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev. 10: 2117– 2130 [DOI] [PubMed] [Google Scholar]
- 56. Wang Z, et al. 2008. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 40: 897– 903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zeng L, Zhou MM. 2002. Bromodomain: an acetyl-lysine binding domain. FEBS Lett. 513: 124– 128 [DOI] [PubMed] [Google Scholar]
- 58. Zhang Q, et al. 2010. Biochemical profiling of histone binding selectivity of the yeast bromodomain family. PLoS One 5: e8903 doi:10.1371/journal.pone.0008903 [DOI] [PMC free article] [PubMed] [Google Scholar]