Abstract
During renal development, the proper emergence of the ureteric bud (UB) from the Wolffian duct is essential for formation of the urinary system. Previously, we showed that expression of transcription factor GATA-2 in the urogenital primordium was demarcated anteroposteriorly into two domains that were regulated by separate enhancers. While GATA-2 expression in the caudal urogenital mesenchyme is controlled by the UG4 enhancer, its more-rostral expression is regulated by UG2. We found that anteriorly displaced budding led to obstructed megaureters in Gata2 hypomorphic mutant mice, possibly due to reduced expression of the downstream effector bone morphogenetic protein 4 (BMP4). Here, we report that UG4-driven, but not UG2-driven, GATA-2 expression in the urogenital mesenchyme significantly reverts the uropathy observed in the Gata2 hypomorphic mutant mice. Furthermore, the data show that transgenic rescue by GATA-2 reverses the rostral outgrowth of the UB. We also provide evidence for a GATA-2–BMP4 epistatic relationship by demonstrating that reporter gene expression from a Bmp4 bacterial artificial chromosome (BAC) transgene is altered in Gata2 hypomorphs; furthermore, UG4-directed BMP4 expression in the mutants leads to reduced incidence of megaureters. These results demonstrate that GATA-2 expression in the caudal urogenital mesenchyme as directed by the UG4 enhancer is crucial for proper development of the urinary tract and that its regulation of BMP4 expression is a critical aspect of this function.
INTRODUCTION
Urogenital development initiates with the bilateral formation of the mesonephric or Wolffian ducts (WDs) in the intermediate mesoderm of the mouse embryo on embryonic day 8.5 (E8.5) (27). Subsequently, the WDs extend caudally to contact the urogenital sinus, which later gives rise to the bladder and the urethra. On approximately E10.5, metanephric development begins when a ureteric bud (UB) sprouts from the posterior WD. Iterative branching of the UB in response to signals emanating from the adjacent metanephric mesenchyme (MM) forms the collecting ducts and the distal renal tubules of the metanephros, the definitive kidney. One of the essential regulators that is secreted by the MM is glial cell-derived neurotrophic factor (GDNF), which stimulates the outgrowth of the UB and the subsequent bifurcation and branching in the developing kidney by activating the c-Ret receptor tyrosine kinase signaling pathway (3). Bone morphogenetic protein 4 (BMP4) is secreted by the urogenital mesenchymal cells surrounding the UB and prevents the aberrant outgrowth of the UB from more-rostral sites on the WDs by suppressing potential hyperactivation of the GDNF/c-Ret pathway. Indeed, Bmp4 heterozygous mutant embryos exhibit rostrally shifted ectopic ureteric budding, leading to abnormal ureteral orifices close to the urethral outlet in the bladder and resulting in the blockage of urinary flow and hydroureters (20). Thus, deciphering the regulatory mechanisms that control Bmp4 expression in urogenital mesenchymal cells will fundamentally contribute to our understanding of urinary tract development and pathophysiology.
Gata2 encodes a transcription factor containing two GATA-type zinc fingers that serve as its DNA binding domain and are conserved among all six members of the GATA factor family (GATA-1 through GATA-6) (25). These zinc fingers bind most avidly to the consensus sequence 5′-(A/G)GATA(A/T)-3′ (14, 18). During embryogenesis, GATA-2 expression is first detected in the urogenital mesenchyme that envelops the WDs and the cloacae of E10.5 embryos (13). On E12.5, GATA-2 expression persists in the mesenchymal cells that surround the branching UB (13). The lethal hematopoietic defect encountered on E9.5 in Gata2-null mutant embryos hampered the further analysis of GATA-2 function in the adult urinary system (30). To circumvent the early embryonic lethality in the null mutants, we recently generated Gata2 hypomorphic mutant mice (Gata2fGN/fGN) in which GATA-2 expression was significantly reduced due to the germ line insertion of a neomycin resistance cassette into the Gata2 locus (10). Although Gata2fGN/fGN hypomorphs did not succumb to embryonic lethality like the Gata2-null mutants did, at approximately 3 weeks of age, most of the hypomorphs developed megaureters resembling the phenotypes that are typical of human congenital anomalies of the kidney and the urinary tract (CAKUT) (10). This anomaly has been associated with ectopic rostral ureteric budding, presumably due to attenuated BMP4 expression in the urogenital mesenchyme of E10.5 Gata2fGN/fGN embryos (10).
Previously, we found that compound mutant transgenic mice carrying a 271-kbp Gata2 yeast artificial chromosome (YAC) (bearing sequences from 5′ kbp −198 to 3′ kbp +73 of the Gata2 locus) fully rescued the primary lethal hematopoietic deficiency in Gata2-null embryos. However, these YAC-rescued Gata2-null mutant newborns died as a result of their megaureters, reflecting bilaterally defective ureter-bladder connections (32). We deduced that this YAC did not contain the Gata2 regulatory elements that direct urogenital mesenchyme-specific GATA-2 expression. This was the first indication that GATA-2 function is critical for proper urogenital development and that the regulatory elements controlling urogenital GATA-2 expression were located beyond the boundaries of the 271-kbp YAC (i.e., beyond kbp −198 or kbp +73 of the Gata2 locus). Subsequently, we identified two distal Gata2 enhancers using bacterial artificial chromosome (BAC)-based transgenic reporter gene assays (13). One of these elements, designated UG4, is located 75 kbp 3′ to the Gata2 structural gene and directs GATA-2 expression in the caudal mesenchyme surrounding the UB and the urogenital sinus. The other enhancer element, designated UG2, is located 113 kbp 3′ to the Gata2 gene and directs Gata2 expression in the mesenchyme surrounding the WDs and the mesonephros. The two enhancers are separated by 30 kbp and share little sequence similarity, suggesting that the two distinct expression patterns might be regulated by different molecular pathways (13). However, the physiological significance of the separate GATA-2 expression domains in later urogenital development was unclear. Considering that Bmp4 is a critical downstream target of GATA-2, we attempted to determine which domain (if either) of GATA-2 urogenital expression, driven by either UG2 or UG4, is responsible for GATA-2-regulated BMP4-mediated urinary tract development. To this end, we conducted transgenic complementation rescue experiments by generating UG2- or UG4-driven GATA-2 or BMP4 transgenic lines and breeding them into the hypomorphic Gata2fGN/fGN background. The results clearly demonstrate that UG4-driven (caudal domain) expression of GATA-2 is critical for proper urinary tract development. Furthermore, UG4-driven transgenic BMP4 complementation partially corrects the megaureter phenotype in Gata2fGN/fGN mice, demonstrating that BMP4 is a functional downstream effector of GATA-2 in early urinary tract development.
MATERIALS AND METHODS
Transgenic mice.
Gata2 green fluorescent protein (GFP) knock-in (Gata2GFP/+) and Gata2fGN/fGN hypomorphic mutant mice were maintained in C57BL/6J genetic background (10, 28). Bmp4-BAC-lacZ transgenic mice were described previously (2). Gata3 mutant mice and their pharmacological rescue were described previously (15).
Generation of expression plasmids.
To generate pTKUG2-LacZ and pTKUG4-LacZ plasmids, UG2 or UG4 DNA fragments (1.2 or 2.8 kbp, respectively) (13) were cloned 3′ to the herpes simplex virus (HSV) thymidine kinase (TK) promoter in the pTKβ vector (Clontech) to mimic their native 3′ position in the Gata2 locus (13). To construct pTKUG2-GATA-2 and pTKUG4-GATA-2 expression plasmids, full-length mouse GATA-2 cDNA was used to replace the LacZ cDNA in the pTKUG2-LacZ and pTKUG4-LacZ vectors, respectively. To construct a pTKUG4-BMP4 plasmid, mouse BMP4 cDNA (a kind gift from Kate Barald of the University of Michigan) was used to replace LacZ cDNA in the pTKUG4-LacZ plasmid. To construct a pTKUG4-GATAm3-LacZ plasmid, the substituted mutations were generated using a PCR-based strategy.
The UG2 transgenic construct was linearized with XmnI/SalI, and the UG4 construct was linearized with an XhoI/BglII double digestion. The constructs were purified for microinjection into fertilized ova from BDF1 (C57BL/6 × DBA/2) mice using standard procedures (23). The mice were maintained in a pathogen-free mouse facility. All of the animal experiments were executed after approval by the Tohoku University Animal Care Committee.
RNA purification and quantitative RT-PCR.
Total RNA was extracted from the ureter, bladder, or oviduct using ISOGEN (Nippon Gene). First-strand cDNA was synthesized using random hexamers and a Superscript III polymerase kit (Invitrogen). Real-time reverse transcription-PCR (RT-PCR) was performed using an ABI PRISM 7300 sequence detector system (Applied Biosystems) and 2× SYBR green PCR master mix (Invitrogen). The primer sequences for GATA-2, BMP4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are listed in Table S1 in the supplemental material. The abundance of each cDNA was determined based on the threshold cycle (CT) values and was experimentally determined by the amplification efficiency of each primer set, normalized to the abundance of GAPDH.
X-Gal staining.
Embryos collected on E11.5 were fixed in 3% formalin and 0.2% glutaraldehyde prior to overnight staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). For whole-mount embryo photography, the embryos were cleared in 80% glycerol or benzyl alcohol-benzyl benzoate (2:1). The embryos were fixed and equilibrated in 20% sucrose overnight before they were embedded in optimum-cutting-temperature compound (OCT). The embryo cryosections were rinsed with phosphate-buffered saline (PBS) and incubated in X-Gal solution overnight at 37°C. The next day, the sections were counterstained with nuclear fast red.
In situ hybridization.
In situ hybridization was performed as previously described (31). Briefly, digoxigenin-dUTP-labeled antisense riboprobes were prepared from a PCR-amplified mouse c-Ret cDNA template (sense primer, 5′-ACCTGAGTGCACCAAGCTTC-3′; antisense primer, 5′-CAGCTAAGTCCCGATGTA-3′) using total RNA from E10.5 embryos. Wnt11 cDNA from a cDNA clone collection was inserted into the pCR4-TOPO vector (Open Biosystems). The fragment was subcloned into the pGEM-T Easy vector (Promega), and antisense and sense riboprobes for each gene were synthesized using Sp6 and T7 polymerases, respectively. Embryos were collected on E11 and were fixed overnight in 4% paraformaldehyde in PBS at 4°C and then treated with proteinase K for a minimum of 10 min depending on the embryo stage. Afterwards, the embryos were prehybridized and then sequentially incubated with riboprobes and horseradish peroxidase (HRP)-conjugated antidigoxigenin antibody (Roche Molecular Biochemicals). The hybridization signals were visualized using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate as chromogens.
Immunoblotting analyses.
Nuclear extracts were prepared from mononuclear bone marrow cells by the Dignam method (4). Immunoblotting analysis was performed using anti-GATA-2 (C-20; Santa Cruz) and anti-lamin B (M-20; Santa Cruz) antibodies as reported previously (19). The band intensities were quantified using ImageJ software (NIH Image).
Luciferase reporter analyses.
The pUG4-LUC (LUC stands for luciferase) and pUG4-GATAm3-LUC reporters were constructed using the pGL3 promoter plasmid (Promega) as the base vector. The expression vector for GATA-2, pEF-GATA-2, was described previously (22). The pCMV-mSOX9 (CMV stands for cytomegalovirus) expression vector was purchased from Open Biosystems. HEK293T and QT6 cells were maintained in Dulbecco's modified Eagle medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (HyClone), 50 U/ml penicillin, and 50 mg/ml streptomycin sulfate. Cells were cultured in 24-well plates prior to cotransfection with 20 ng of pUG4-LUC or pUG4-GATAm3-LUC in the presence or absence of pEF-GATA-2 (50 to 200 ng) or pCMV-SOX9 (10 to 100 ng) using FuGene 6 (Roche). A Renilla luciferase pRL-null plasmid (2 ng; Promega) was cotransfected as an internal control. The luciferase activity at 48 h after transfection was determined using the dual-luciferase reporter assay system (Promega).
Statistical analyses.
The data are expressed as means ± standard deviations (SDs). For statistical analyses, the pairwise comparisons were made using a Mann-Whitney U test. P values of <0.05 were considered statistically significant.
RESULTS
A Gata2 hypomorphic mutation disrupts normal vesicoureteral junction formation.
We previously generated Gata2fGN/fGN homozygous mutant mice (10) in which the GATA-2 protein level in multiple tissues, including the bone marrow, was reduced to approximately 20% of that in Gata2+/+ littermates (see Fig. S1 in the supplemental material; also data not shown). To determine the etiological basis of the megaureters observed in the Gata2fGN/fGN hypomorphs, we conducted a detailed examination of the urinary systems of 3-week-old wild-type and Gata2fGN/fGN mice. Unlike wild-type animals, which rarely exhibited ureteric dilation (4.2%, n = 48 ureters from 24 mice [Fig. 1A and Table 1]), 44.4% of Gata2fGN/fGN ureters (n = 126) were moderately (18.3%) or severely (26.1%) distended (Fig. 1B and C and Table 1). Further histological examination revealed that the renal parenchyma of mice displaying a milder megaureter phenotype looked normal compared to wild-type kidneys. However, the most severe examples showed an enlarged calyceal space with thinning of the renal parenchyma, which was associated with the gravely distended ureters (Fig. 1D to F).
Fig 1.
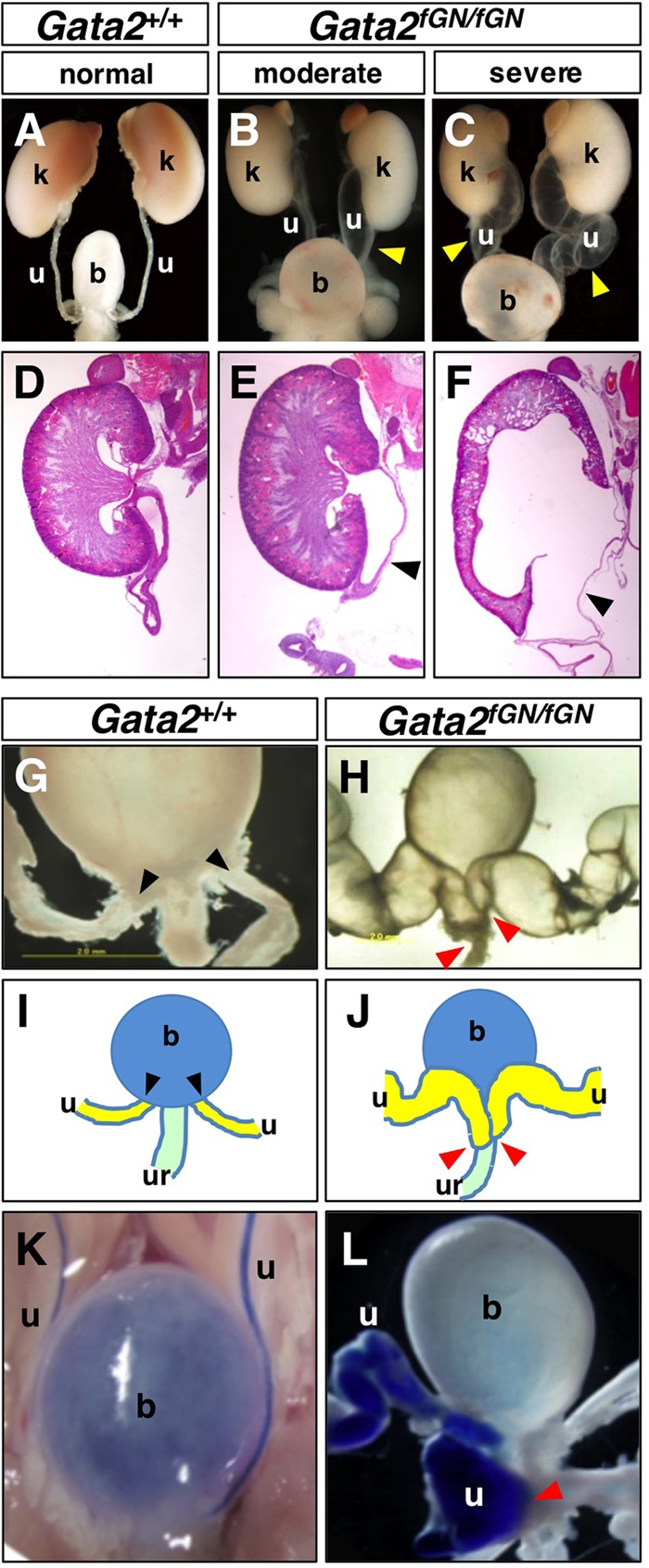
Gata2 hypomorphic mutant mice develop megaureters due to aberrant formation of the uretero-urethral junction. (A to F) Unlike Gata2+/+ mice, moderate to severe megaureters (arrowheads) were observed in Gata2fGN/fGN mice upon gross examination. Enlarged calyceal spaces with thinning of the renal parenchyma were observed in severe cases. (G) In Gata2+/+ mice, the ureteral orifices (arrowheads) were located at the posterolateral edge of the bladder. (H) In Gata2fGN/fGN mice, the aberrantly dilated ureters (arrowheads) were aberrantly connected to the urethra. (I and J) Schematic illustrations of the urinary tract systems shown in panels G and H, respectively. (K) Trypan blue dye injected into the renal pelvis flowed through the ureters to collect in Gata2+/+ bladders. (L) In Gata2fGN/fGN mice, trypan blue dye was visibly absent from the bladder but was present at the uretero-urethral junction (arrowhead). b, bladder; k, kidney; u, ureter; ur, urethra.
Table 1.
Effects of TgUG2-G2 and TgUG4-G2 on megaureters in Gata2fGN/fGN mice
| Characteristic | Value for the following group of micea: |
||||||
|---|---|---|---|---|---|---|---|
| Gata2+/+ | Gata2fGN/fGN | Gata2fGN/fGN:TgUG2-G2 line 3 | Gata2fGN/fGN:TgUG2-G2 line 10 | Gata2fGN/fGN:TgUG4-G2 line 6 | Gata2fGN/fGN:TgUG4-G2 line 20 | Gata2fGN/fGN:TgUG2-G2 line 3 or 10, Gata2fGN/fGN:TgUG4-G2 line 6 or 20 | |
| No. of ureters examined | 48 | 126 | 50 | 36 | 26 | 44 | 42 |
| No. (%) of ureters moderately distended | 2 (4.2) | 23 (18.3) | 9 (18.0) | 6 (16.6) | 7 (26.9) | 4 (9.1) | 5 (11.9) |
| No. (%) of ureters severely distended | 0 (0) | 33 (26.1) | 13 (26.0) | 10 (27.8) | 1 (3.8**) | 1 (2.3**) | 3 (7.1**) |
| No. (%) of megaureters | 2 (4.2) | 56 (44.4) | 22 (44.0) | 16 (44.4) | 8 (30.7) | 5 (11.4**) | 8 (19.0**) |
Transgenic lines 3 and 6 express small amounts of GATA-2, and transgenic lines 10 and 20 express large amounts of GATA-2. Values that are significantly different (P < 0.01 by Mann-Whitney U test) compared to the values for Gata2fGN/fGN littermates are indicated by a pair of asterisks.
Ureters normally make a patent connection to the bladder wall posterolaterally such that the ureteral orifices (black arrowheads) and the medial urethral orifice define the trigonum vesicae (Fig. 1G and I). However, in 80% of the Gata2fGN/fGN mice with megaureters, the distal end of the ureters (red arrowheads) were instead fused to the urethra (Fig. 1H and J), while the remaining 20% of the hypomorphic mice exhibited abnormal ligation of the ureter to the vas deferens (data not shown). To visualize the point of obstruction in the former group of animals, we injected trypan blue dye into the renal pelvis (32). While the dye flowed through the ureters and collected in the bladders of wild-type mice (Fig. 1K), the dye flow was interrupted at the uretero-urethral junction in the Gata2fGN/fGN mice, indicating that the abnormal junction (red arrowhead) occluded the ureteral opening to the lower urinary tract (Fig. 1L). Thus, these data show that reduced GATA-2 activity leads to megaureters that result from the formation of an abnormal vesicoureteral junction in the majority of Gata2fGN/fGN mice.
Generation and characterization of urogenital mesenchyme-specific GATA-2 transgenic lines.
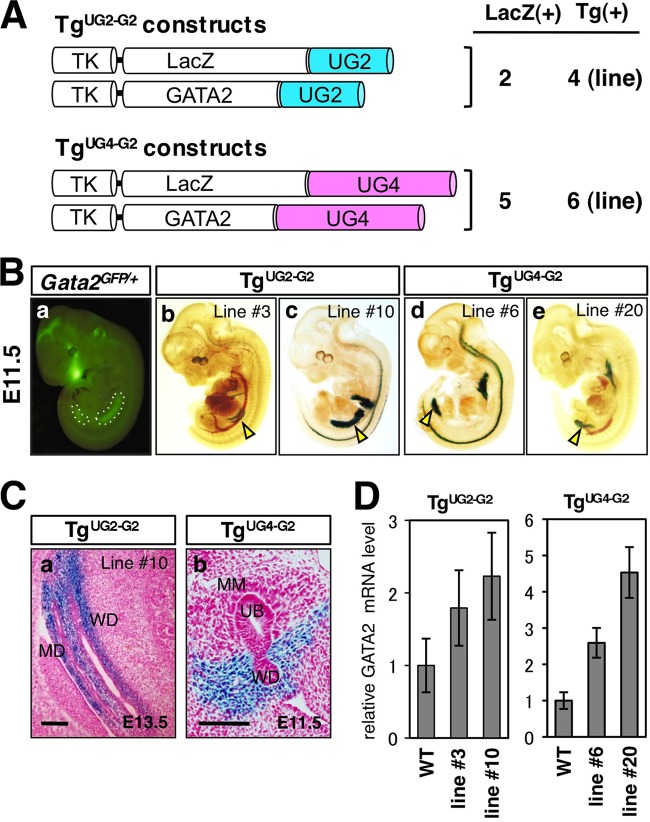
We previously identified two Gata2 urogenital enhancers, UG2 and UG4, that were able to direct lacZ expression in separate, discrete regions of the early urogenital mesenchyme in transgenic embryos (13). While UG2 was concluded to be responsible for GATA-2 expression in the mesonephric mesenchyme and in the mesenchyme surrounding the WDs, UG4 activates Gata2 in the mesenchyme surrounding the UB and the urogenital sinus.
To investigate whether reconstitution of GATA-2 activity in vivo using UG2 or UG4 to drive GATA-2 transgene expression could rescue the megaureter defects observed in Gata2fGN/fGN animals, the UG2 (1.2-kbp) and UG4 (2.8-kbp) enhancers were separately cloned 3′ to the HSV TK promoter and linked in cis to either LacZ or GATA-2 cDNA (Fig. 2A). UG2-LacZ or UG4-LacZ reporter construct was coinjected with UG2-GATA-2 or UG4-GATA-2 expression plasmid, respectively, to produce transgenic lines. Two lines of UG2-GATA-2/LacZ (TgUG2-G2 [Tg stands for transgenic]) and 5 lines of UG4-GATA-2/LacZ (TgUG4-G2) transgenic mice were generated (Fig. 2A). We first examined the expression of the cointegrated LacZ transgene in these lines. In comparison to endogenous urogenital GATA-2 expression as reflected by enhanced green fluorescent protein (eGFP) epifluorescence (outlined by white dots in Fig. 2B, panel a) in E11.5 Gata2 GFP knock-in (Gata2GFP/+) embryos (28), urogenital X-Gal staining patterns in E11.5 transgenic TgUG2-G2 (lines 3 and 10) and TgUG4-G2 (lines 6 and 20) embryos represented a subset of the overall eGFP fluorescence. More specifically, the urogenital β-galactosidase activity was detected more anteriorly or posteriorly in TgUG2-G2 or TgUG4-G2 embryos, respectively (arrowheads in Fig. 2B, panels b to e). Sagittal sections of an E13.5 TgUG2-G2 embryo exhibited LacZ expression in the mesonephric mesenchyme and the mesenchyme surrounding the WD and the Mullerian duct (MD), which gives rise to the male and female reproductive systems, respectively (Fig. 2C, panel a). In contrast, transverse sections of E11.5 TgUG4-G2 embryos showed that X-Gal staining was largely absent from the MM and the ureteric bud epithelium but was present in the urogenital mesenchyme surrounding the UB and the urogenital sinus (the caudal end of the WD [Fig. 2C, panel b]). In postnatal animals, we detected LacZ transgene expression in the male genital organs, including the vas deferens and epididymis, of TgUG2-G2 and TgUG4-G2 male neonates (see Fig. S2A, B, and G in the supplemental material) that partially recapitulates the endogenous GATA-2 expression pattern (13). In contrast, TgUG2-G2, but not TgUG4-G2, female transgenic mice showed LacZ expression in the oviduct (see Fig. S2C, E, and G), which is derived from the embryonic Mullerian duct and expresses abundant eGFP fluorescence in Gata2GFP/+ knock-in mice. UG4-driven, but not UG2-driven, LacZ expression was detected in the definitive bladder and ureter where endogenous GATA-2 is expressed regardless of gender (see Fig. S2D, F, and G). Overall, these observations indicate that UG2 recapitulates the expression of GATA-2 in the reproductive systems of both male and female mice, whereas urinary system (ureter/bladder)-specific GATA-2 expression is predominantly directed by UG4 (see Fig. S2G).
Fig 2.
Generation and characterization of UG2- and UG4-driven transgenic lines. (A) Schematic diagrams of the LacZ and GATA-2 expression constructs under the transcriptional control of the herpes simplex virus thymidine kinase (TK) promoter and 3′ UG2 (1.2-kbp) or UG4 (2.8-kbp) enhancers. The number of transgenic lines that are positive for LacZ staining in each domain of the urogenital mesenchyme [LacZ(+)] and the total number of established transgenic lines [Tg(+)] (where LacZ and GATA-2 cDNA positivity is demonstrated by PCR genotyping) are shown to the right of the schematic diagrams. (Ba) Discrete endogenous GATA-2 expression domains as reflected by eGFP epifluorescence (outlined by white dots) in an E11.5 Gata2GFP/+ embryo. (Bb to Be) Whole-mount TgUG2-G2 (lines 3 and 10) and TgUG4-G2 (lines 6 and 20) transgenic embryos showed X-Gal staining (arrowheads) in the rostral and caudal domains of the urogenital mesenchyme, respectively. (Ca) A transverse section of an E13.5 TgUG2-G2 (line 10) embryo revealed LacZ expression in the mesenchyme surrounding the Wolffian and Mullerian ducts (WD and MD, respectively). (Cb) A transverse section revealing LacZ expression in the mesenchymal cells surrounding the WD of an E11.5 TgUG4-G2 (line 6) embryo. Note that LacZ expression is absent from the metanephric mesenchyme (MM) and the ureteric bud (UB). Bars, 50 μm. (D) GATA-2 mRNA levels normalized to GAPDH mRNA levels in the oviduct or ureter/bladder of TgUG2-G2 or TgUG4-G2 transgenic mice, respectively, are shown. The cDNA samples were prepared using total RNA from three postnatal day 3 to 5 pups from each line. The data are presented as the means ± SDs (error bars). WT, wild type.
UG4-driven GATA-2 expression restores normal urinary tract development in Gata2 hypomorphs.
Prior to attempting transgenic rescue of Gata2fGN/fGN mice, we first quantified GATA-2 mRNA expression in the oviducts of TgUG2-G2 female mice and in the ureters and bladders of TgUG4-G2 mice by real-time quantitative RT-PCR (qRT-PCR). Of the four transgenic lines that were examined, GATA-2 mRNA was expressed from 1.8- to 4.7-fold more abundantly than in the wild-type littermates (Fig. 2D). Accordingly, TgUG2-G2 line 3 and TgUG4-G2 line 6 were categorized as low-GATA-2-expressing lines, while TgUG2-G2 line 10 and TgUG4-G2 line 20 were categorized as high-GATA-2-expressing lines. Each transgene was then bred into the Gata2fGN genetic background to generate Gata2fGN/fGN compound mutants carrying either TgUG2-G2 or TgUG4-G2.
To evaluate the ability of each transgene to correct the urogenital tract disorders in the transgenic Gata2fGN/fGN compound mutants, we scored the incidence and severity of megaureters in 3-week-old littermates. While neither the low- nor high-GATA-2-expressing UG2-driven transgenic lines (line 3 or 10, respectively) modified the incidence or severity of megaureters in the Gata2fGN/fGN:TgUG2-G2 compound mutant mice (Fig. 3A and Ba and Bb and Table 1), Gata2fGN/fGN:TgUG4-G2 mice displayed a spectrum of partially rescued phenotypes. In the latter animals, the frequency and severity of megaureters correlated with the transgene-derived GATA-2 levels (Fig. 3A, Bc, and Bd and Table 1). The overall incidence of megaureters diminished from 44.4% (56/126 ureters) in Gata2fGN/fGN mice to 30.7% (8/26 ureters) in Gata2fGN/fGN:TgUG4-G2 line 6 mice and to 11.4% (5/44 ureters) in Gata2fGN/fGN:TgUG4-G2 line 20 mice with higher GATA-2 expression (Fig. 3A and Table 1). We also noted that the occurrence of severe megaureters diminished drastically from 26.1% (33/126 ureters) in Gata2fGN/fGN mice to 3.8% (1/26 ureters) or 2.3% (1/44 ureters) in Gata2fGN/fGN:TgUG4-G2 mice that express lower (line 6) or higher (line 20) levels of transgenic GATA-2, respectively, (Fig. 3A and Table 1). Coinheritance of both TgUG2-G2 and TgUG4-G2, regardless of transgene expression level, yielded no additional improvement compared to rescue with TgUG4-G2 alone (Fig. 3A and Table 1). The Kaplan-Meier survival curves showed that only 24% of the Gata2fGN/fGN mice survived beyond the first 10 days after birth (Fig. 3C). Of note, we rarely observed anemia in the surviving Gata2fGN/fGN mice, indicating that a low level of GATA-2 expression (20% compared to the wild-type level) is sufficient to maintain their peripheral blood cell number (see Table S2 in the supplemental material). Interestingly, the viability of Gata2fGN/fGN:TgUG4-G2 mice was significantly improved (37% lethality within the first 10 days after birth [P = 0.023, n = 20]; Fig. 3C) compared to Gata2fGN/fGN:TgUG2-G2 mice (63% lethality within the first 10 days after birth [P = 0.50, n = 20]) (Fig. 3C). Taken together, these studies show that complementation of GATA-2 expression when driven by Gata2 UG4, but not by UG2, in the caudal urogenital mesenchyme greatly improved the survival and urogenital deficiency of Gata2fGN/fGN mice.
Fig 3.
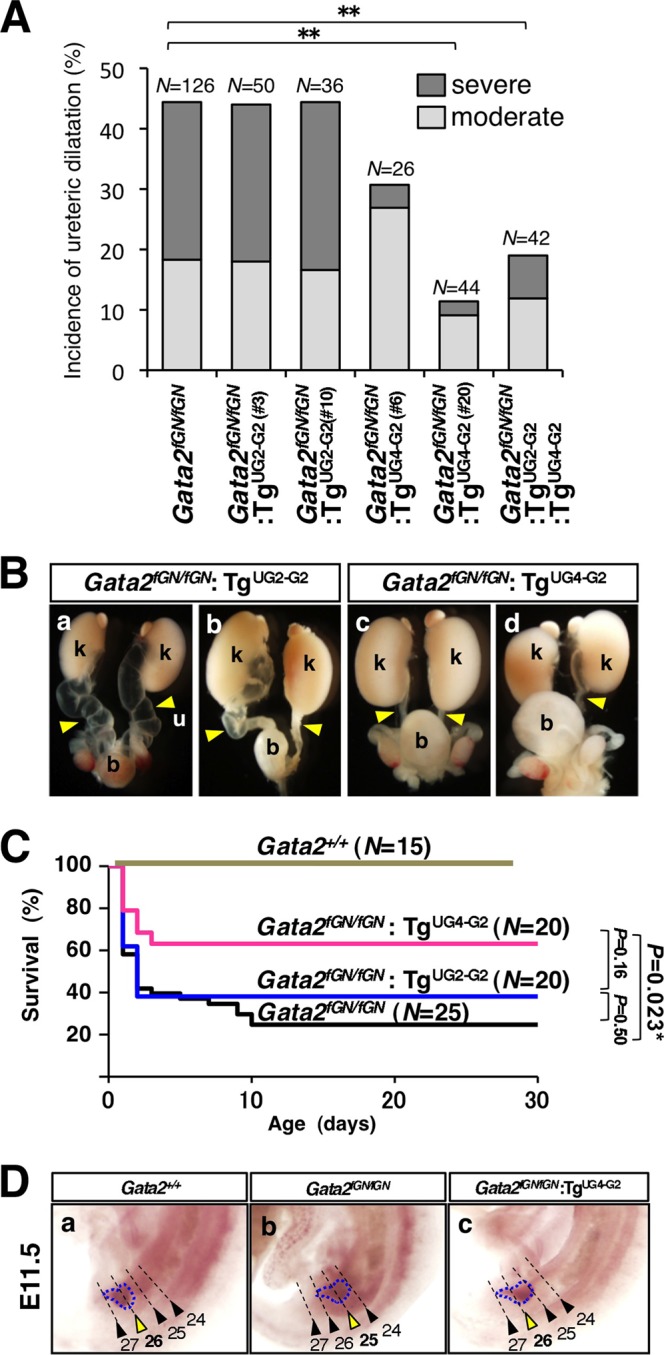
UG4-driven GATA-2 complementation reduces megaureters in Gata2fGN/fGN mice. (A) Frequencies of moderate or severe megaureters in 3-week-old animals of various Gata2 genotypes. Note that the reduction of megaureters in Gata2fGN/fGN:TgUG4-GATA2 mice correlates directly with the transgene-derived GATA-2 levels, as the reduction is greater in the high-GATA-2-expressing line 20 than in the low-GATA-2-expressing line 6. In contrast, neither TgUG2-GATA2 line has any effect on megaureter formation in Gata2fGN/fGN mice. Coexpression of TgUG2-GATA2 (line 3 or 10) and TgUG4-GATA2 (line 6 or 20) does not yield any additive improvements. The values that are statistically significantly different (P < 0.01 by a Mann-Whitney U test) for mice of various genotypes are indicated by bars and a pair of asterisks. (B) Representative urinary tract systems dissected from two Gata2fGN/fGN:TgUG2-GATA2 mice (a and b) or two Gata2fGN/fGN:TgUG4-GATA2 mice (c and d) are shown. Note that Gata2fGN/fGN:TgUG2-GATA2 mice display a more severe genitourinary anomaly. b, bladder; k, kidney; u, ureter. (C) The Kaplan-Meier survival curves for mice of various Gata2 genotypes during the first 30 days after birth are displayed. The statistical significance of the difference between two groups of animals as evaluated by the log rank test is shown. (D) The anteroposterior position of UB outgrowth (outlined by a blue broken line) relative to the somites (numbered and indicated by arrowheads) along the Wolffian duct in E11.5 embryos is indicated by a yellow arrowhead. The somites and the UB are labeled by Wnt-11 and c-Ret riboprobes, respectively. Wild-type control embryos displayed normal ureteric bud positioning at the 26th somite (a), whereas Gata2fGN/fGN embryos showed more-rostral ectopic ureteric budding at the 25th somite (b). In Gata2fGN/fGN:TgUG4-GATA2 embryos, the ureteric bud position was restored to the 26th somite (c).
UG4-driven GATA-2 complementation remedies aberrant ureteric budding in Gata2fGN/fGN mice.
Multiple studies have shown that the precise positioning of UB sprouting along the anteroposterior axis of the WD can have profound consequences on later urinary tract development (12, 29). We therefore performed in situ hybridization for c-Ret (a ureteric bud marker) and Wnt11 (a somite marker) in E11.5 Gata2fGN/fGN embryos expressing TgUG2-G2 or TgUG4-G2 to visualize and assess UB outgrowth. While ureteric budding was observed at the 26th somite level in E11.5 wild-type embryos, most Gata2fGN/fGN embryos exhibited more-rostral ureteric budding at the axial level (corresponding to the 25th somite [Fig. 3D, panels a and b]) (10). Notably, in nearly all Gata2fGN/fGN:TgUG4-G2, but not in Gata2fGN/fGN:TgUG2-G2, compound mutant embryos, localization of the UB was correctly restored to the 26th somitic segment (Fig. 3D, panel c; also data not shown). Therefore, these observations unequivocally demonstrated that caudal GATA-2 urogenital expression, which is under the transcriptional influence of the UG4 enhancer, is essential for proper ureteric bud sprouting, while UG2-driven rostral mesonephric GATA-2 expression plays no apparent role in UB positioning.
TgBMP4–BAC-LacZ expression is suppressed in the caudal urogenital mesenchyme of Gata2fGN/fGN mice.
The soluble growth and developmental regulator BMP4 is known to promote proper UB positional information along the WD by suppressing rostral (ectopic) ureteric budding (20). We previously demonstrated that the level of BMP4 mRNA was lower in the intermediate mesoderm of E10.5 Gata2fGN/fGN embryos than in comparable wild-type embryos (10). Furthermore, in cotransfection assays, GATA-2 potentiates luciferase reporter gene expression via a conserved GATA binding site in the second intron of Bmp4 where in vivo GATA-2 binding has been demonstrated in cardiomyocytes and embryoid bodies by chromatin immunoprecipitation analysis (10, 16, 21).
To determine whether reduced GATA-2 activity could impact BMP4 expression in the urogenital mesenchyme surrounding the UB in Gata2fGN/fGN mice, we employed a BAC LacZ reporter (harboring 199 kbp of 5′ flanking sequence and 28 kbp of 3′ flanking sequence at the Bmp4 locus) transgenic murine line (referred to as TgBMP4-BAC-LacZ). TgBMP4-BAC-LacZ mice, which have been shown to faithfully recapitulate endogenous BMP4 expression in the urogenital region (2), were bred into the Gata2fGN background to generate Gata2fGN/fGN:TgBMP4-BAC-LacZ mice. In E11.5 Gata2+/+:TgBMP4-BAC-LacZ embryos, LacZ expression was observed in the urogenital mesenchyme surrounding the UB and the WD, but not in the MM (Fig. 4A and B), mirroring the pattern observed with UG4-driven LacZ expression (Fig. 2C, panel b). Interestingly, X-Gal staining in the corresponding region of Gata2fGN/fGN:TgBMP4-BAC-LacZ was less intense in the stained area when viewed at either the whole-mount or cellular level (compare Fig. 4C and D with Fig. 4A and B). However, LacZ expression in other BMP4-expressing tissues, such as the limb bud and the forebrain, was not affected (Fig. 4C and D; also data not shown). These observations, coupled with our previous in vitro studies (10), strongly imply that BMP4 expression in the urogenital mesenchyme is under the direct regulatory influence of GATA-2.
Fig 4.
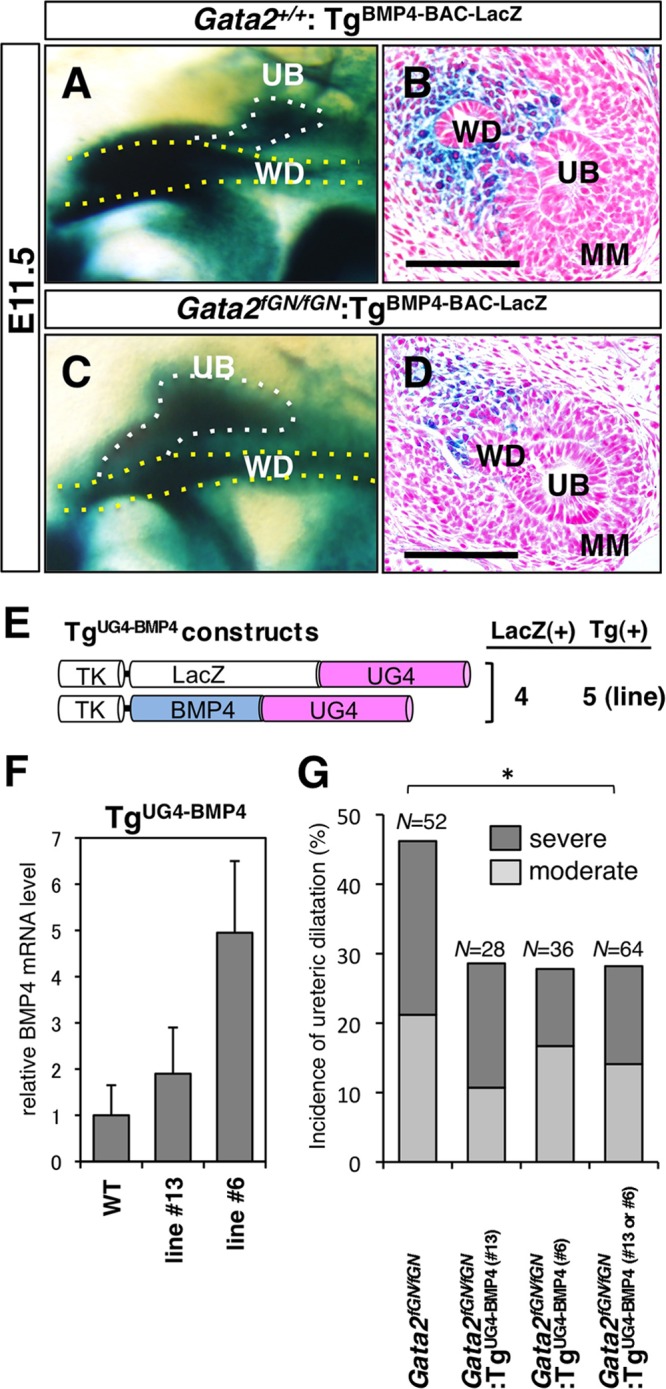
BMP4 is a downstream effector of GATA-2 in the urogenital mesenchyme. (A and B) LacZ reporter expression was abundant in the mesenchymal cells around the Wolffian duct (WD) and the ureteric bud (UB) of E11.5 Gata2+/+:TgBMP4-BAC-LacZ embryos. Bar, 50 μm. (C and D) In E11.5 Gata2fGN/fGN:TgBMP4-BAC-LacZ mutant embryos, LacZ reporter expression was reduced in the corresponding mesenchyme. Bars, 50 μm. (E) Schematic diagram of the TgUG4-LacZ and TgUG4-BMP4 constructs. The UG4 enhancer (2.8 kbp) was cloned 3′ of BMP4 or LacZ cDNA under the control of the herpes simplex virus thymidine kinase (TK) promoter. The number of transgenic lines that were positive for LacZ staining in the caudal domain of the urogenital mesenchyme [LacZ(+)] and the total number of established transgenic lines [Tg(+)] (where LacZ and BMP4 cDNA positivity is demonstrated by PCR genotyping) are shown to the right of the schematic diagram. (F) The BMP4 mRNA levels (normalized to GAPDH mRNA levels) in the ureter/bladder of postnatal day 3 to 5 pups (n = 3) with or without TgUG4-BMP4 are shown. The data are presented as means plus SDs (error bars). (G) The total incidence and severity of megaureters were evaluated in 3-week-old Gata2fGN/fGN mice with or without TgUG4-BMP4. The degree of severity of the megaureters (severe or moderate) is indicated. Both TgUG4-BMP4 transgenic lines (line 6 or 13) rescued the urinary tract abnormalities. The statistically significant difference (P < 0.05 by a Mann-Whitney U test) between the values for Gata2fGN/fGN and Gata2fGN/fGN:TgUG4-BMP4 (line 13 or 6) mice is indicated by the bar and asterisk (see Table 2).
UG4-driven transgenic BMP4 expression also rescues megaureters in Gata2fGN/fGN mice.
If the hypothesis that BMP4 is an epistatically downstream functional effector of GATA-2 during early urinary tract development is correct, then BMP4 complementation is predicted to rectify the urinary tract development in Gata2fGN/fGN mice. To test this hypothesis, we generated BMP4 transgenic mice by coinjecting UG4-driven BMP4 and UG4-driven LacZ constructs into fertilized ova (TgUG4-BMP4 [Fig. 4E]). Of four TgUG4-BMP4 transgenic lines that were generated, we chose two transgenic lines (lines 6 and 13) that most faithfully recapitulated UG4-driven gene expression (data not shown) for use in attempted transgenic rescue experiments. Using qRT-PCR to analyze total RNA from neonatal ureters/bladders, we determined the transgenic BMP4 expression level of the two TgUG4-BMP4 lines. Line 6 expressed more BMP4 (4.9-fold) than line 13 did (1.9-fold), compared to wild-type tissue (Fig. 4F). These two BMP4-expressing lines were separately bred into the Gata2fGN background. Again, we scored the frequency and severity of megaureters in 3-week-old Gata2fGN/fGN:TgUG4-BMP4 compound mutant mice. Importantly, both TgUG4-BMP4 transgenic mouse lines partially rescued both aspects of the urinary tract abnormalities in Gata2fGN/fGN mice. Improvement of the total incidence of megaureters in Gata2fGN/fGN:TgUG4-BMP4 (lines 6 and 13) mice was statistically significant in comparison to Gata2fGN/fGN mice (Fig. 4G and Table 2). These observations confirm that BMP4 is responsible for downstream signaling events under the regulation of GATA-2 that promote early urinary tract morphogenesis.
Table 2.
Effects of TgUG4-BMP4 on megaureters in Gata2fGN/fGN mice
| Characteristic | Value for the following group of micea: |
||||
|---|---|---|---|---|---|
| Gata2+/+ | Gata2fGN/fGN | Gata2fGN/fGN:TgUG4-BMP4 line 13 | Gata2fGN/fGN:TgUG4-BMP4 line 6 | Gata2fGN/fGN:TgUG4-BMP4 line 6 or 13 | |
| No. of ureters examined | 28 | 52 | 28 | 36 | 64 |
| No. (%) of ureters moderately distended | 1(3.6) | 11 (21.2) | 3 (10.7) | 6 (16.7) | 9 (14.1) |
| No. (%) of ureters severely distended | 0 (0) | 13 (25.0) | 5 (17.9) | 4 (11.1) | 9 (14.1) |
| No. (%) of megaureters | 1 (3.6) | 24 (46.2) | 8 (28.6) | 10 (27.8) | 18 (28.1*) |
Transgenic line 13 expresses a small amount of GATA-2, and transgenic line 6 expresses a large amount of GATA-2. The value that is significantly different (P < 0.05 by Mann-Whitney U test) compared to the value for Gata2fGN/fGN littermates is indicated by an asterisk.
The tissue specificity of UG4-driven transgene expression does not require a GATA factor.
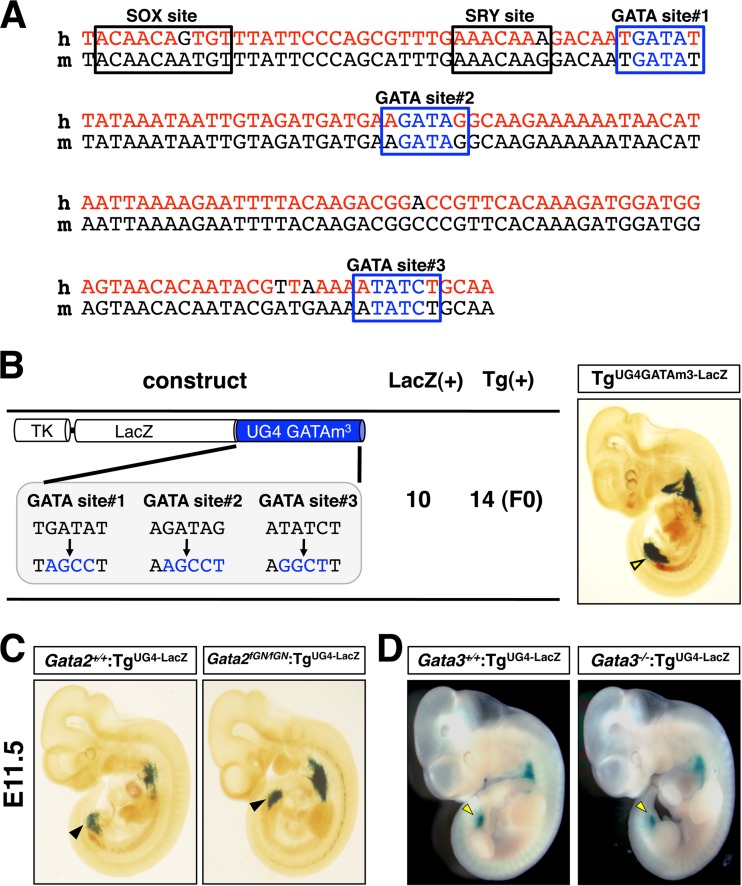
We next sought to identify potential upstream regulators of the UG4 enhancer. We searched for putative transcription factor binding sites using the TFSEARCH database (www.cbrc.jp/research/db/TFSEARCH) and identified three evolutionarily conserved GATA binding sites within the UG4 element (Fig. 5A). We hypothesized that GATA-2 might autoregulate itself via the three conserved GATA sites in UG4. To determine the functional importance, if any, of these GATA motifs, we generated UG4-driven luciferase constructs in which either all three or none of the GATA sites were mutated with nucleotide substitutions (see Fig. S3A in the supplemental material). The constructs were cotransfected with a GATA-2 expression vector into HEK293T cells, a human embryonic kidney-derived cell line. The data revealed that while the control wild-type UG4-LUC reporter expression was induced with increasing concentrations of GATA-2 expression plasmid, this phenomenon was abolished when all three GATA sites were mutated in the UG4-GATAm3-LUC reporter (see Fig. S3B). UG4 also contains putative binding sites for the sex-determining region Y (SRY)-related high-mobility-group box (SOX) family transcription factors (Fig. 5A). Hence, we examined the responsiveness of the UG4-LUC reporter to SOX9, which is also expressed in the caudal urogenital mesenchyme of mouse embryos (26). Of note, both the UG4-LUC and the UG4-GATAm3-LUC reporters were similarly activated by SOX9 cotransfection in a dose-dependent manner (see Fig. S3B). These data suggest that either GATA-2 or another GATA factor is a key regulator of UG4 transcription and highlight the possible involvement of SOX family factors in regulating urogenital GATA-2 expression (see Discussion).
Fig 5.
The UG4 enhancer activation is not dependent on a GATA factor. (A) Comparative alignment of the conserved 165-bp UG4 core sequence in humans (h) and mice (m). The three conserved GATA binding sites and the SRY/SOX sequences are indicated. (B) Schematic diagram of the TgUG4-GATAm3-LacZ construct showing the nucleotide substitutions introduced into the three GATA sites in UG4. The number of E11.5 founder (F0) transgenic embryos that were positive for LacZ staining in the caudal domain of the urogenital mesenchyme [LacZ(+)] and the total number of founder transgenic embryos [Tg(+)] are shown to the right of the schematic diagram. LacZ expression was maintained in the TgUG4-GATAm3-LacZ embryos (right). (C) LacZ expression pattern was unaltered in E11.5 Gata2fGN/fGN:TgUG4-LacZ embryos (right) compared to Gata2+/+:TgUG4-LacZ littermates (left). (D) LacZ expression was similarly unaffected in the Gata3−/−:TgUG4-LacZ embryos (right) in comparison with Gata3+/+:TgUG4-LacZ littermates (left).
To investigate a potential GATA factor autoregulatory mechanism in vivo, we generated a mutant UG4-LacZ reporter construct that harbored the same disruptive nucleotide substitutions in the three GATA sites (UG4-GATAm3-LacZ), injected fertilized ova, and then conducted founder (F0) transgenic reporter analysis. Concurrently, we bred a transgenic mouse line carrying UG4-LacZ (TgUG4-LacZ) alone into the Gata2fGN background to examine LacZ expression in the event of GATA factor depletion. Unexpectedly, most E11.5 TgUG4-GATAm3-LacZ transgenic embryos (10/14) still maintained caudal domain-specific LacZ expression in the urogenital mesenchyme (Fig. 5B). Similarly, tissue-specific TgUG4-LacZ expression was barely affected in E11.5 Gata2fGN/fGN:TgUG4-LacZ embryos in comparison to expression in Gata2+/+:TgUG4-LacZ littermates (Fig. 5C). Together, these results suggest that the tissue specificity of UG4-driven transgene expression is independent of the GATA factor binding sites, thus leaving the expression pattern unaltered even when GATA-2 expression is 80% depleted in vivo.
GATA-3, another urogenital GATA transcription factor, is expressed in E11.5 embryos in the UB and WD epithelium, forming a mutually exclusive pattern with that of the UG4-driven GATA-2 expression (see Fig. S4 in the supplemental material). Therefore, we surmised that GATA-3 could not exert a direct influence on UG4-driven Gata2 gene transcription in the early urogenital mesenchyme. To address this issue directly, we examined the same TgUG4-LacZ reporter transgenic mouse-derived LacZ expression in the E11.5 Gata3−/− mutant background. As expected, LacZ expression was essentially unaffected in the Gata3−/−:TgUG4-LacZ compound mutant embryos in comparison to Gata3+/+:TgUG4-LacZ littermates, indicating that UG4 directs tissue-specific Gata2 expression in a GATA-3-independent manner (Fig. 5D). Overall, these results indicate that UG4-driven GATA-2 expression in the caudal urogenital mesenchyme can be specified essentially independently from GATA-2 or GATA-3 expression there.
DISCUSSION
In the present study, we demonstrated that rostrally shifted ureteric budding during early embryogenesis led to a subsequent erroneous placement of the ureteral orifices in the urethra of postnatal Gata2fGN/fGN mice and that the molecular etiology underlying this developmental abnormality is disruption of the UG4-directed GATA-2–BMP4 axis (Fig. 6). Precision in ureteric bud outgrowth along the WD is paramount to the later development of a functional genitourinary system in adulthood (12, 17, 29). Generation of an ectopically rostral UB in Bmp4 heterozygous mutant mice led to the formation of an aberrant vesicoureteral junction (20). Other mouse models showing similar ectopic ureteral budding and accompanying urinary tract anomalies have been reported. For example, mice deficient for either SLIT2 or its receptor ROBO2, both of which are primarily known as axon guidance molecules, exhibit abnormal rostral extension of the GDNF expression domain in the urogenital mesenchyme. Consequently, this abnormal GDNF expression resulted in an increased number of UBs, which are associated with hydroureters at the perinatal stage (6). Sprouty1, a receptor tyrosine kinase (RTK) antagonist, inhibits GDNF signaling via the RTK (c-Ret)/coreceptor GFRα1 complex on the UB epithelium to ensure the formation of a proper, single ureteric bud. Sprouty1 deficiency leads to the outgrowth of numerous UBs and subsequently to multiple ureters in the definitive urinary system (1). These mutant mice exhibit a wide spectrum of urinary anomalies, including hydronephrosis, cystic kidneys, and duplex kidneys. Interestingly, Gata2fGN/fGN mice have highly characteristic abnormalities and develop a hydroureter without perturbed metanephrogenesis or ureteral multiplicity, presumably due to its specialized function for fine-tuning the precise localization of single ureteric budding. Given that similar anomalies are frequently observed in pediatric urology, we suspect that the Gata2fGN/fGN mouse may serve as a useful animal model to elucidate the molecular etiology underlying similar human urogenital pathologies.
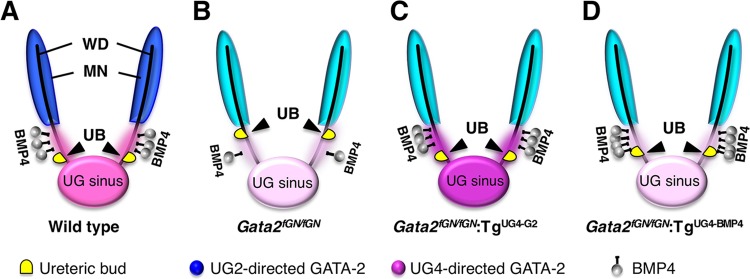
Fig 6.
A model depicting the role that the GATA-2–BMP4 axis plays in urinary tract development in the caudal domain of the urogenital mesenchyme. (A) In wild-type embryos, a normal level of GATA-2 expression induces sufficient secretion of BMP4 around the caudal urogenital mesenchyme to promote ureteric budding at the correct position along the Wolffian duct. (B) In Gata2 hypomorphic embryos, the reduced mesenchymal expression of BMP4 results in more rostral ectopic ureteric budding. (C) UG4-driven GATA-2 complementation restored the ureteric budding to the normal somite position by reconstituting mesenchymal BMP4 expression. (D) UG4-driven caudal mesenchymal BMP4 complementation partially rescued the urinary tract abnormalities in Gata2 hypomorphic embryos, presumably by correcting the ureteric budding position. The intensity of the blue (cranial) and pink (caudal) colors represents the GATA-2 expression level in each domain of the urogenital mesenchyme. MN, mesonephros; WD, Wolffian duct; UB, ureteric bud; UG sinus, urogenital sinus.
The initial discovery of two separate domains governing GATA-2 urogenital expression suggested the possibility that GATA-2 exerts different functions within each domain. Our initial expectation was that caudal GATA-2 expression around the urogenital sinus and the UB would be more critical for proper development of the urinary tract because it represents the original developmental site leading to the formation of the vesicoureteral junction. As anticipated, UG4-driven transgenic GATA-2 complementation significantly improved the hydronephrosis abnormality (Fig. 6), whereas TgUG2-GATA-2 transgenic mice failed to rescue this pathophysiology. UG2 directs transgene expression in the rostral domain of mesonephric mesenchymal cells, which subsequently gives rise to the reproductive system. In fact, UG2-LacZ reporter transgenic mice revealed postnatal LacZ expression in the definitive reproductive systems, including the male vas deferens and epididymis and the female oviduct, while LacZ was only faintly expressed in the urinary system. Presumably, GATA-2 expression in the rostral domain of the mesonephric mesenchyme contributes to the development and/or maintenance of the reproductive systems because we often observed infertility in the rare surviving male or female Gata2fGN/fGN mice (data not shown). Furthermore, a recent clinical report showing that GATA-2 mutations are frequently found in familial lymphedema (Emberger syndrome), which is often associated with genital dysplasia, highlights the potential importance of GATA-2 for the proper development of the reproductive organs (24). To further clarify the functional significance of the UG2 and UG4 elements, the consequences of the targeted disruption of each element would be of particular interest.
TgUG4-BMP4 transgenic mice partially rescued the urinary tract abnormalities in Gata2fGN/fGN mice, supporting an epistatic relationship between GATA-2 and BMP4, a functional downstream effector of GATA-2 in early urogenital development. However, even though transgenic UG4-driven BMP4 was expressed at levels almost 4-fold higher than those in wild-type controls, the urinary tract abnormalities in the Gata2fGN/fGN embryos were incompletely rescued. In contrast, another recent report showed that BMP4 induces GATA-2 expression, which mediates cellular differentiation signals in the hemangioblast (16). Therefore, BMP4 might require GATA-2 activity to fully exert its function during urogenital development. Given the subtle but significant differences between Gata2fGN/fGN and Bmp4 heterozygous mutant embryos with respect to morphological alterations in the urinary tract, we surmise that there are likely to be other GATA-2 target genes that also contribute to early urinary tract development (20). To comprehensively define the molecular network in early urinary tract development that is under the regulatory influence of GATA-2, a whole-transcriptome analysis using microdissected urogenital mesenchymal tissues from Gata2fGN/fGN embryos would be of potential value.
To determine the molecular basis for the earliest aspects of urogenital specification during development, it is equally important to determine which upstream regulators of Gata2 are active in the caudal urogenital mesenchyme. Because there are three evolutionarily conserved GATA binding sites within the UG4 element, we initially hypothesized that Gata2 might autoregulate itself in the caudal domain of the urogenital mesenchyme through the conserved GATA binding sites. However, TgUG4-LacZ-derived LacZ expression was largely unaffected when those GATA sites were mutated, although the same substitution mutations diminished the GATA-2-dependent induction of UG4-directed luciferase (LUC) activity in cell-based transfection assays. One plausible explanation for this apparent discrepancy is that other transcription factors that bind to sequences within the UG4 element could have a greater responsibility for the specification of urogenital GATA-2 expression. We observed that SOX9 activated the UG4-LUC reporter in a manner independent of the GATA site mutations, and indeed, there have been several reports showing that the SOX family transcription factors contribute to urogenital development. For example, heterozygous mutation of human SOX9 causes campomelic dysplasia (CD), which is frequently characterized by hydroureters, hydronephrosis, and renal hypoplasia (11). Intriguingly, the expression pattern of SOX9 in the early embryonic urogenital mesenchyme is precisely coincident with that of GATA-2 (26). SOX17, which is also expressed in the urogenital primordium, has also been implicated in human urinary tract anomalies, and a recent report revealed the presence of dominant mutations in the SOX17 gene in a number of CAKUT cases (5). Given this clinical and experimental evidence, it is important to clarify the regulatory relationship between the SOX family transcription factors and GATA-2, if such a relationship exists, to reveal a possible etiological link with the CAKUT cases.
GATA-2 is predominantly, but not exclusively, expressed in the early urogenital mesenchyme surrounding the UB and the WD at E11.5, while GATA-3 expression arises exclusively in the epithelial cells of the UB and the WD at the same stage (9). In early kidney development, GATA-3 induces c-Ret expression in the epithelial cells of the WD, thereby facilitating the GDNF-mediated outgrowth of the UB (7, 8, 9). In contrast, we demonstrated that GATA-2 activates BMP4 expression in the urogenital mesenchyme, which modulates GDNF/c-Ret activity to potentially prevent ectopic budding. Consistent with the differential expression patterns of GATA-2 and GATA-3 and their respective target genes, a recent report showed that urogenital mesenchymal BMP4 expression was not significantly affected by WD-specific Gata3 deletion (8). The distinct expression patterns of these two different GATA factors in the early urogenital primordium may account for the different phenotypic anomalies observed in the respective mutant mice. GATA-3-deficient embryos that express a low level of transgenic GATA-3 (less than haploid abundance) using a Gata3 kidney enhancer to direct GATA-3 expression often exhibited hypoplastic metanephric kidneys (9). However, the Gata2fGN/fGN mice exhibited almost no disruption of metanephrogenesis (10) (this study). It will be necessary to further elucidate the distinct versus possibly overlapping functions of these two urogenital GATA factors to fully understand the molecular basis of urinary tract development.
Considering the crucial roles played by GATA-2 in proper urogenital development, we are currently searching for germ line regulatory single-nucleotide polymorphisms (SNPs) in CAKUT patients, particularly focusing on the UG4 region. Further investigation of the physiological function of GATA-2 in the urogenital primordium should provide additional insight into the developmental basis for the formation of the urogenital system and may lead to possible therapeutic approaches for the relatively common CAKUT anomalies in the field of pediatric urology.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michito Hamada, Mikiko Suzuki, and Yu Lei for helpful discussions.
This work was supported in part by Grants-in-Aids for Creative Scientific Research and Scientific Research from JSPS, the Tohoku University Global COE Program for the Conquest of Signal Transduction Diseases with “Network Medicine,” ERATO from JST, and the NAITO foundation.
Footnotes
Published ahead of print 9 April 2012
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Basson MA, et al. 2006. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev. Biol. 299:466–477 [DOI] [PubMed] [Google Scholar]
- 2. Chandler KJ, Chandler RL, Mortlock DP. 2009. Identification of an ancient Bmp4 mesoderm enhancer located 46 kb from the promoter. Dev. Biol. 327:590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costantini F, Shakya R. 2006. GDNF/Ret signaling and the development of the kidney. Bioessays 28:117–127 [DOI] [PubMed] [Google Scholar]
- 4. Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gimelli S, et al. 2010. Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract. Hum. Mutat. 11:1352–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grieshammer U, Ma L, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. 2004. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev. Cell 6:709–717 [DOI] [PubMed] [Google Scholar]
- 7. Grote D, Souabni A, Busslinger M, Bouchard M. 2006. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133:53–61 [DOI] [PubMed] [Google Scholar]
- 8. Grote D, et al. 2008. Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet. 4:e1000316 doi:10.1371/journal.pgen.1000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasegawa SL, et al. 2007. Dosage-dependent rescue of definitive nephrogenesis by distant Gata3 enhancer. Dev. Biol. 15:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoshino T, et al. 2008. Reduced BMP4 abundance in Gata2 hypomorphic mutant mice result in uropathies resembling human CAKUT. Genes Cells 13:159–170 [DOI] [PubMed] [Google Scholar]
- 11. Houston CS, et al. 1983. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al. in 1971. Am. J. Med. Genet. 15:3–28 [DOI] [PubMed] [Google Scholar]
- 12. Ichikawa I, Kuwayama F, Pope JC, Stephens FD, Miyazaki Y. 2002. Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int. 61:889–898 [DOI] [PubMed] [Google Scholar]
- 13. Khandekar M, Suzuki N, Lewton J, Yamamoto M, Engel JD. 2004. Multiple, distant Gata2 enhancers specify temporally and tissue-specific patterning in the developmental urogenital system. Mol. Cell. Biol. 24:10263–10276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ko LJ, Engel JD. 1993. DNA-binding specificities of the GATA transcription factor family. Mol. Cell. Biol. 13:4011–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim KC, et al. 2000. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat. Genet. 25:209–212 [DOI] [PubMed] [Google Scholar]
- 16. Lugus JJ, et al. 2007. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development 134:393–405 [DOI] [PubMed] [Google Scholar]
- 17. Mackie GG, Stephens FD. 1975. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J. Urol. 114:274–280 [DOI] [PubMed] [Google Scholar]
- 18. Merika M, Orkin SH. 1993. DNA-binding specificity of GATA family transcription factors. Mol. Cell. Biol. 13:3999–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minegishi N, Suzuki N, Kawatani Y, Shimizu R, Yamamoto M. 2005. Rapid turnover of GATA-2 via ubiquitin-proteasome protein degradation pathway. Genes Cells 10:693–704 [DOI] [PubMed] [Google Scholar]
- 20. Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. 2000. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Invest. 105:863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nemer G, Nemer M. 2003. Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev. Biol. 254:131–148 [DOI] [PubMed] [Google Scholar]
- 22. Nozawa D, et al. 2009. GATA2-dependent and region-specific regulation of Gata2 transcription in the mouse midbrain. Genes Cells 14:569–582 [DOI] [PubMed] [Google Scholar]
- 23. Onodera K, et al. 1997. GATA-1 transcription is controlled by distinct regulatory mechanisms during primitive and definitive erythropoiesis. Proc. Natl. Acad. Sci. U. S. A. 94:4487–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ostergaard P, et al. 2011. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 43:923–931 [DOI] [PubMed] [Google Scholar]
- 25. Patient RK, McGhee JD. 2002. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12:416–422 [DOI] [PubMed] [Google Scholar]
- 26. Reginensi A, et al. 2011. SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum. Mol. Genet. 20:1143–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saxen L. 1987. Organogenesis of the kidney. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 28. Suzuki N, et al. 2006. Combinatorial Gata2 and Sca1 expression defines hematopoietic stem cells in the bone marrow niche. Proc. Natl. Acad. Sci. U. S. A. 103:2202–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas JC, DeMarco RT, Pope JC. 2005. Molecular biology of ureteral bud and trigonal development. Curr. Urol. Rep. 6:146–151 [DOI] [PubMed] [Google Scholar]
- 30. Tsai FY, et al. 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371:221–226 [DOI] [PubMed] [Google Scholar]
- 31. Wilkinson DG, Nieto MA. 1993. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225:361–373 [DOI] [PubMed] [Google Scholar]
- 32. Zhou Y, et al. 1998. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J. 17:6689–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.