Progressive collapse and destruction of an entire lung is an uncommon but recognized manifestation of cystic fibrosis (CF).1 This devastating complication presents a considerable challenge to both the CF multi-disciplinary team and the transplant surgeon. The evidence base to guide management of this condition is sparse, and in our clinical experience it has proved impossible to reverse the underlying process. Case reports of surgical intervention for complete lung collapse have demonstrated a high incidence of adverse outcomes.1–3 We report, in detail, the case of one patient with progressive unilateral lung collapse followed by a summary of a seven comparable cases encountered at our unit.
Case report
Patient one was born in 1990. He was diagnosed with CF at the age of six months after presenting with an acute wheezing illness. Chest radiographs revealed collapse of the right upper lobe which resolved with antibiotic therapy. The sweat test was abnormal and DNA tests confirmed F508del homozygosity. His childhood was notable for the onset of chronic Pseudomonas aeruginosa infection at the age of seven years. He developed CF-related diabetes mellitus at fourteen. In 2006 he repeatedly isolated Mycobacterium abscessus in his sputum. Anti-mycobacterial chemotherapy was continued for approximately eighteen months before the patient stopped the treatment of his own volition. Since cessation of therapy there has been no further isolation of M. abscessus.
The patient transferred to our adult CF unit at the age of eighteen when his forced expiratory volume in one second (FEV1) was 37%-predicted. This had declined from approximately 90%-predicted at ten years and from 50%-predicted at fourteen. The chest radiograph revealed moderate bilateral changes consistent with cystic fibrosis but no evidence of lobar or segmental collapse. One year later he had developed increased consolidation in the right upper lobe with a mild degree of volume-loss. Blood gas analysis revealed type II respiratory failure and he was commenced on overnight non-invasive ventilation (NIV). Shortly after this, he began regular overnight nasogastric (NG) feeding with a baseline body mass index (BMI) of 16.4 kg/m2.
In November 2010 he was hospitalized with an exacerbation of CF due to rhinovirus infection. An average sputum load of 100 g/day was recorded at this time. One month later he was re-admitted with influenza A/H1N1 infection, a fall in FEV1 to 19%-predicted and persistently high sputum volumes. The chest radiograph demonstrated collapse and consolidation of the right lower lobe. He was treated with intravenous antibiotics, aminophylline, oseltamivir, NIV and intermittent positive pressure breathing (IPPB) to aid airway clearance. Inspiratory pressures of 20 cm H2O were used with IPPB. Nebulized therapy at this time included 7% hypertonic saline, dornase alfa, salbutamol and colistin, and a partial improvement in the lobar collapse was seen. Unfortunately, the lobar collapse recurred within two weeks of discharge.
In February 2011, his total IgE rose to 800 kIU/l with a specific IgE and IgG to Aspergillus fumigatus of 16.7 kIU/l and 93 mg/l respectively. Fibreoptic bronchoscopy revealed no evidence of mucus plugging or bronchial obstruction. He was commenced on oral itraconazole therapy and although his antibodies to Aspergillus declined substantially, there was no improvement in his lung function or radiology.
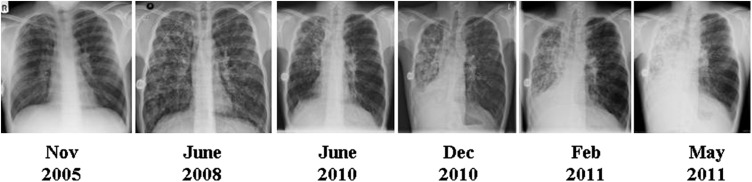
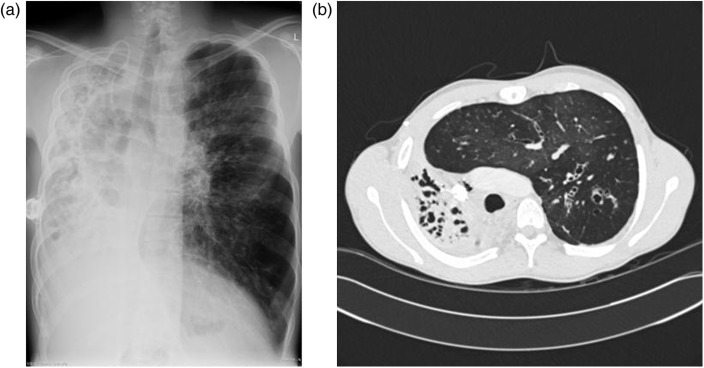
Over the following six months, chest radiographs documented an inexorable loss of volume of the right lung (see Figure 1). A high-resolution CT examination in July 2011 demonstrated complete destruction of the right lung with extensive mediastinal shift (see Figure 2). The patient currently has an FEV1 of 20%-predicted and a BMI of 18.7 kg/m2. He has been reviewed by the regional lung transplant service and after careful consideration of the risks posed by his thoracic asymmetry, he has been placed on the waiting list for double-lung transplantation.
Figure 1.
Serial chest radiographs of patient one illustrating progressive consolidation and loss of volume of the right lung with associated mediastinal shift
Figure 2.
(a) Chest radiograph and (b) high-resolution CT scan image from July 2011 demonstrating complete destruction of the right lung with contralateral lung hyperinflation and mediastinal shift
Case series
In addition to the case described above, we have identified a further seven patients with progressive unilateral lung collapse who attended our centre between 1995 and 2011. Table 1 summarizes the key characteristics of these patients. Six out of eight (75%) cases affected the right lung and a similar number were male. The patients isolated a variety of organisms in their sputum with Pseudomonas aeruginosa the most common pathogen. Only patient one met established diagnostic criteria for allergic bronchopulmonary aspergillosis (ABPA).
Table 1.
Characteristics of eight patients with CF treated for unilateral complete lung collapse/consolidation at Manchester Adult CF Centre between 1995 and 2011
| Patient No. | Age (years) | Sex | Affected Lung | Infecting Organism | Long-term NIV | Enteral Feed | Peak Total IgE (kIU/l) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | M | Right | Pseudomonas aeruginosa | Yes | Yes – NG | 800 | On Transplant List |
| 2 | 27 | M | Left | Pseudomonas aeruginosa | No | Yes – NG | 310 | Alive |
| 3 | 18 | M | Right | Burkholderia multivorans | Yes | Yes – PEG | 36 | Alive |
| 4 | 29 | F | Right | Ralstonia pickettii Staphylococcus aureus | Yes | No | 7 | Alive |
| 5 | 40 | M | Right | Pseudomonas aeruginosa | No | No | 39 | Alive |
| 6 | 33 | F | Right | Pseudomonas aeruginosa | Yes | Yes – PEG | 130 |
Dead (Not suitable for lung transplant due to comorbidities) |
| 7 | 32 | M | Right | Pseudomonas aeruginosa | Yes | No | 22 |
Dead (Attempted bilateral lung transplant – died on table) |
| 8 | 20 | M | Left | Pseudomonas aeruginosa | Yes | No | n/a |
Dead (Died immediately post-pneumonectomy) |
NG: nasogastric tube; PEG: percutaneous endoscopic gastrostomy
Comprehensive details of the therapies received by individual patients are not available given the retrospective nature of this series. However, in line with standard practice on our unit, each patient was offered extensive specialist physiotherapy input, nebulized mucolytics and antibiotics alongside management of complications of CF such as diabetes mellitus. Despite these efforts, we have been entirely unsuccessful in our attempts to reverse the lung collapse in each case.
Surgical intervention has been attempted in two of these patients with an adverse outcome in both cases. Patient seven underwent attempted bilateral lung transplantation, but considerable technical difficulties were experienced and he died on the operating table. Patient eight was referred for a pneumonectomy in advance of a subsequent contralateral single lung transplant. He deteriorated substantially following the pneumonectomy and died before transplantation could be performed.
Discussion
The cases described above reflect a challenging scenario in the care of adult patients with CF. Unilateral complete lung collapse has been described in the CF literature,1–5 but there is no consensus as to the aetiology or optimal management of this condition. Below we discuss the potential causes of unilateral lung collapse and review the options for management.
Aetiology and pathophysiology
Lobar atelectasis is a common manifestation of CF6 and complete lung collapse may simply reflect a natural progression of this phenomenon. Impaction of thick mucus in the small airways is thought to play a major role in the development of localized disease. This process may be further altered by factors increasing the viscosity of CF sputum such as hyperglycaemia and haemoptysis. Sub-optimal adherence with medication and airway clearance is relevant in some cases. A congenital predisposition towards localized CF lung disease may be important in others, as suggested by patient one's infantile presentation with right upper lobe collapse.
The role of respiratory pathogens in localized CF lung disease is not clear but they are likely to be an important factor. The majority of patients in our series were chronically infected with Pseudomonas aeruginosa which has been clearly associated with progression of CF lung disease. Our first case also suffered acute infection with rhinovirus and influenza A in the three months before complete loss of the right lung. In vitro studies have demonstrated that viral infection enhances adherence of bacterial pathogens to airway epithelial cells7 which could conceivably lead to focal lung damage.
Allergic bronchopulmonary aspergillosis (ABPA) is well-recognized in CF and may present with mucus plugging of the airways.8 However, only one patient within our series met established criteria for the diagnosis of ABPA and five out of eight patients had a total IgE within the normal range. This suggests that ABPA is not the dominant driving force behind unilateral lung collapse in most cases.
Aspiration of gastric contents into the lungs must also be considered as a possible causative factor given the unilateral nature of pulmonary disease. Gastro-oesophageal reflux disease (GORD) is known to be common in patients with CF9 and four of the patients reported here required a regular proton-pump inhibitor. It is not known whether overnight enteral feeding poses an additional increased risk of aspiration.
Our case series of eight patients reflects a diverse mix of patients with no particular factor standing out as being a common cause. Larger studies examining explanted or post-mortem lung specimens are required to fully understand the pathophysiology of this process.
Management of impending whole lung collapse
The optimal management of progressive lung collapse and consolidation has not been determined. Our approach has been to look for, and correct where possible, the causative factors discussed above. A central part of the management revolves around effective clearance of sputum from the airways. Specialist physiotherapy input is crucial to this process in line with standard CF care.10 Aids to airway clearance include nebulized mucolytics such as dornase alfa and hypertonic saline. Case reports have documented instances of resolution of lobar atelectasis with both inhaled and bronchoscopically-administered dornase alfa,11,12 although there have been no instances of successful reversal of whole lung collapse with this medication to our knowledge. Attention to adequate hydration, control of hyperglycaemia and adherence with prescribed medication is also important to maximize the chances of effective airway clearance.
Bronchoscopy is an essential investigative and therapeutic procedure. Examination of the airways allows exclusion of a foreign body, obstructive lesion or mucus plug as a cause of lung collapse. In addition to obtaining microbiological samples, the bronchoscopist is also able to aspirate purulent secretions or mucus plugs from the affected lobes. Bronchoscopic instillation of dornase alfa or hypertonic saline may also be considered.
Intermittent positive pressure breathing (IPPB) has been used in a variety of respiratory conditions to aid sputum clearance, although there is no evidence to demonstrate its efficacy in CF.13 In the first case described above, IPPB led to a short-lived improvement in right lower lobe atelectasis but the benefit lasted less than two weeks. Non-invasive ventilation (NIV) is an alternative means of providing inspiratory positive pressure and is an established treatment for respiratory failure. NIV is also widely used as an adjunct to airway clearance in CF.14 All but one of our patients with complete lung collapse required nocturnal NIV to control hypercapnia. NIV does not appear to have prevented progression of the underlying lung collapse. A consideration with any form of positive airway pressure in this clinical scenario is the danger of contralateral hyperinflation, as seen in patient one, and the attendant risk of pneumothorax. The maximum ‘safe’ inspiratory pressure with only one functioning lung is not known.
Management of completed whole lung collapse
In our experience, progression to end-stage respiratory failure is inevitable following complete lung collapse. Difficult decisions regarding the feasibility of surgical intervention or lung transplantation are often encountered in these patients. It appears clear that all surgical options are associated with a high level of risk.
Pneumonectomy has been described in severe asymmetrical CF lung disease12–15 with the rationale that a destroyed lung is non-functional and does not contribute to the patient's pulmonary reserve. Outcomes in the few cases reported in the literature have varied from death in the early post-operative period1 through to survival at over four years in one patient.2
Lung transplantation in unilateral lung destruction raises further difficulties and the optimal approach is not clear. Adhesions around the consolidated, shrunken lung present a considerable technical challenge during lung explantation.16 Additionally, the marked asymmetry of the thorax leads to mismatch between the donor lungs and the affected hemithorax. Single lung transplantation with contralateral pneumonectomy has been reported as a means of dealing with this asymmetry. Pneumonectomy of the destroyed lung has been performed both simultaneously5,17 and up to eight months in advance of single-lung transplantation.3,16 Where single lung transplantation has been performed with a consolidated native lung left in situ the outcomes have been poor.16
A final option is to perform bilateral lung transplantation with intra-operative donor lung volume reduction on the side of the smaller hemithorax. Samano and colleagues described two cases of bilateral lung transplantation with lobectomy of one donor lung to allow accommodation within the asymmetrical thoracic cavity.18 Survival beyond one year was achieved in both cases. This approach may be the best option for patient one described in the case report above.
Conclusions
Unilateral complete lung collapse and consolidation is an uncommon but well-recognized complication of cystic fibrosis. Numerous questions and uncertainties remain as to the aetiology of this process and it provides a considerable therapeutic challenge to the CF team. We hope that by raising these issues within the forum of the Royal Society of Medicine's cystic fibrosis symposium, our shared experiences can help improve patient outcomes for the future.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
Written consent to publish was obtained from the patient or next of kin
Guarantor
WF
Contributorship
WF conceived the idea for the report, conducted data collection and wrote the first draft of the manuscript. JH conducted data collection, collated details of physiotherapy techniques employed in the cases and contributed to the writing of the manuscript. AKW identified the patients making up the case series, assisted with data collection and contributed to the writing of the manuscript.
Acknowledgements
None
References
- 1.Huisman C, de Graaff CS, Boersma WG Unilateral air bronchogram in a patient with cystic fibrosis. Chest 2002;121:1343–4 [DOI] [PubMed] [Google Scholar]
- 2.Häusler M, Franke E, Wendt G, Kentrup H, Döhmen H, Kusenbach G Pneumonectomy in cystic fibrosis. Pediatr Pulmonol 1999;28:376–9 [DOI] [PubMed] [Google Scholar]
- 3.Piotrowski JA, Splittgerber FH, Donovan TJ, Ratjen F, Zerkowski HR Single-lung transplantation in a patient with cystic fibrosis and an asymmetric thorax. Ann Thorac Surg 1997;64:1456–8; discussion 58–9 [DOI] [PubMed] [Google Scholar]
- 4.Thompson RD, Empey DW, Bailey CM Left recurrent nerve paralysis associated with complete lung collapse with consolidation in an adult with cystic fibrosis. Respir Med 1996;90:567–9 [DOI] [PubMed] [Google Scholar]
- 5.Forty J, Hasan A, Gould FK, Corris PA, Dark JH Single lung transplantation with simultaneous contralateral pneumonectomy for cystic fibrosis. J Heart Lung Transplant 1994;13:727–30 [PubMed] [Google Scholar]
- 6.Stern RC, Boat TF, Orenstein DM, Wood RE, Matthews LW, Doershuk CF Treatment and prognosis of lobar and segmental atelectasis in cystic fibrosis. Am Rev Respir Dis 1978;118:821–6 [DOI] [PubMed] [Google Scholar]
- 7.Ramphal R, Small PM, Shands JW Jr, Fischlschweiger W, Small PA Jr. Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infection and Immunity 1980;27:614–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis–state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003;37:S225–64 [DOI] [PubMed] [Google Scholar]
- 9.Ledson MJ, Tran J, Walshaw MJ Prevalence and mechanisms of gastro-oesophageal reflux in adult cystic fibrosis patients. J R Soc Med 1998;91:7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flume PA, Robinson KA, O'Sullivan BP, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care 2009;54:522–37 [PubMed] [Google Scholar]
- 11.Shah PL, Scott SF, Hodson ME Lobar atelectasis in cystic fibrosis and treatment with recombinant human DNase I. Respir Med 1994;88:313–5 [DOI] [PubMed] [Google Scholar]
- 12.Slattery DM, Waltz DA, Denham B, O'Mahony M, Greally P Bronchoscopically administered recombinant human DNase for lobar atelectasis in cystic fibrosis. Pediatr Pulmonol 2001;31:383–8 [DOI] [PubMed] [Google Scholar]
- 13.Bott J, Blumenthal S, Buxton M, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax 2009;64:i1–51 [DOI] [PubMed] [Google Scholar]
- 14.Moran F, Bradley JM, Piper AJ Non-invasive ventilation for cystic fibrosis. Cochrane Database Syst Rev 2009:CD002769 [DOI] [PubMed]
- 15.Marmon L, Schidlow D, Palmer J, Balsara RK, Dunn JM Pulmonary resection for complications of cystic fibrosis. J Pediatr Surg 1983;18:811–5 [DOI] [PubMed] [Google Scholar]
- 16.Souilamas R, Mostafa A, Guillemain R, Boussaud V, Amrein C, Chevalier P Single-lung transplantation for cystic fibrosis and metachronus pneumonectomy: case reports. Transplant Proc 2008;40:3594–5 [DOI] [PubMed] [Google Scholar]
- 17.Jougon J, Dromer C, Mac Bride T, Velly JF Synchronous left lung transplantation and right pneumonectomy for end-stage bronchiectasis through Clamshell approach. Specific problems. Eur J Cardiothorac Surg 2002;22:833–5 [DOI] [PubMed] [Google Scholar]
- 18.Samano MN, Waisberg DR, Villiger LE, Pêgo-Fernandes PM, Jatene FB Bilateral lung transplantation in asymmetric thorax: case reports. Transplant Proc 2008;40:872–4 [DOI] [PubMed] [Google Scholar]