Introduction
Cystic fibrosis (CF) is a true multi-system disease and as life expectancy continues to increase, the spectra of symptoms and diagnoses seen in patients is ever expanding. Some of the conditions that are now seen in CF are specific to the underlying disease process, others are side-effects of long term medications and some are simply related to patients older age. Cancers of the digestive tract were previously extremely rare in patients with CF as they were unlikely to live to an age at which they would present. Over recent years they have been seen more frequently and research has shown that their incidence is higher in patients with CF when compared to the general population.1 In contrast, it has been known for some time that intussusception is more common in children with CF with the peak incidence at 3–12 months of age.2 More recently, in association with patients increased life expectancy, there have been a number of reports of intussusception in older children and adults with CF.3–7 Most of these reports have found the lead point to be inspisated faeces and none have identified a malignant lead point. We present the case of a 35-year-old woman with CF who presented with a sigmoid intussusception caused by 2 malignant polyps. At the time of diagnosis, metastases were identified in 2 local lymph nodes and the liver. As well as being the first reported case of intussusception in a patient with CF in which a malignant lead point has been identified, the case highlights a number of other important clinical, psychological and social issues.
Case History
A 35-year-old female with cystic fibrosis (CF) was admitted with a 3-week history of increasing abdominal pain, constipation and bloating that had not responded to simple laxatives. She had been diagnosed with CF aged 18 months due to failure to thrive, and her genotype was homozygous Phe508del. She normally received nocturnal non-invasive ventilation for type 2 respiratory failure but had been unable to tolerate this due to the abdominal bloating. She was under consideration for lung transplantation. On admission, forced expiratory volume in 1 second was 0.72 L (24% predicted) and her forced vital capacity was 1.45 L (40% predicted). She was known to have chronic Pseudomonas aeruginosa infection, CF related diabetes, osteopenia and iron deficiency but was not anaemic.
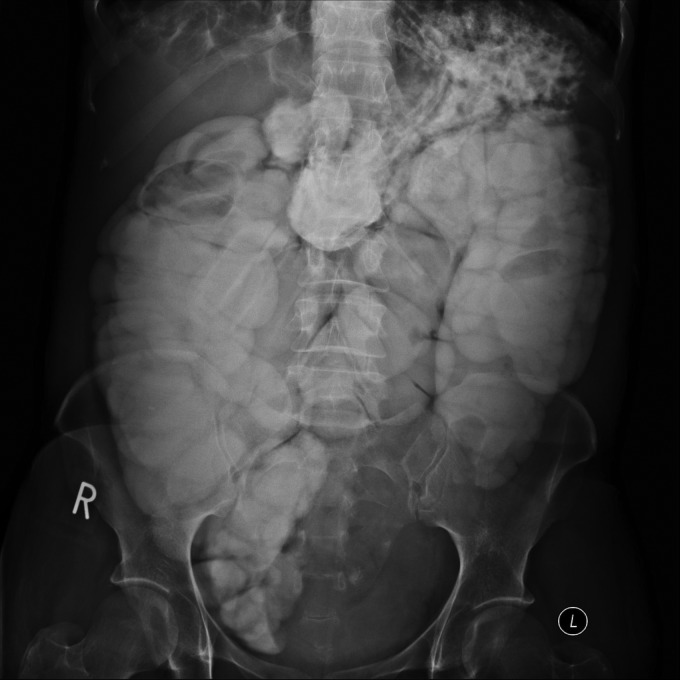
An initial abdominal radiograph (AXR) showed faecal loading but no evidence of distal intestinal obstruction syndrome (DIOS). She was commenced on intravenous (IV) fluids, compound macrogol and gastrografin for the constipation. IV Tobramycin and Meropenem were also started as her sputum was thicker and darker than normal. Despite this treatment she did not open her bowels in the next 36 hours and complained of increasing abdominal pain and bloating. A repeat AXR (essentially a contrast study due to treatment with gastrografin) showed dilated bowel with complete obstruction at the level of the sigmoid colon (Figure 1). On further discussion the patient admitted to intermittent rectal bleeding for 3 months prior to admission but had not reported this due to fears that her symptoms may be secondary to bowel cancer. Her father had died of colon cancer aged 40 years, but she had not discussed this with the CF team. Due to this family history she had previously been offered screening colonoscopies but declined this.
Figure 1.
Plain abdominal radiograph post gastrograffin. Note the dilated colon and the absence of contrast past the level of the sigmoid colon
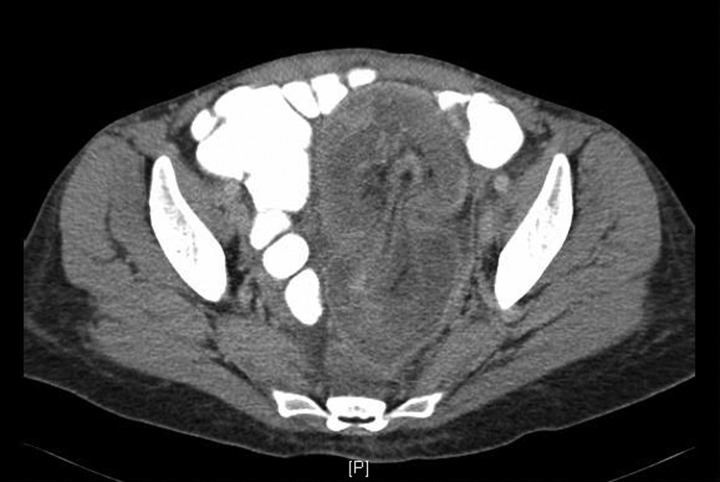
An urgent ultrasound scan was undertaken which suggested a colonic intussusception. A subsequent computerized tomography (CT) scan confirmed a long segment intussusception at the level of the sigmoid colon (Figure 2). The affected bowel was noted to be thick walled and oedematous. An attempt to reduce the intussusception by air insufflation was unsuccessful. The patient therefore proceeded to a Hartmann's procedure under general anaesthesia. During the operation, 45 cm of non-viable, intussuscepted sigmoid colon was removed and an end colostomy formed. The resected bowel contained 2 large polyps. Histology later confirmed that both polyps contained moderately differentiated adenocarcinoma; one of which had invaded across the muscularis propria. Adenocarcinoma was identified in 2 of 22 local lymph nodes.
Figure 2.
Abdominal CT scan. Intussusception seen at the level of the sigmoid colon with thick, oedematous bowel wall
Whilst awaiting a staging CT scan, the patient was reviewed by the Oncology team. They informed the patient that if there were no distant metastases then chemotherapy could be used to prevent recurrence and would normally be of benefit to 5–10 patients in 100. Despite this, due to the patients CF it was thought that the risk of overwhelming infection secondary to chemotherapy induced neutropenia may outweigh any possible benefits. Unfortunately the staging CT scan identified 4 hypodense liver lesions which were later confirmed by magnetic resonance imaging and thought to be pathognomonic of metastases. The patient was re-reviewed by the Oncology team and treatment options re-discussed. Chemotherapy could have slowed disease progression and extended life, but again these benefits were thought to be outweighed by the risk of overwhelming infection. Local treatment of the liver lesions was also considered but not thought likely to be of benefit.
After discussions with her partner, the patient subsequently decided that although she wanted to continue to receive full treatment for her CF, she did not want any further treatment, investigations or discussion regarding her cancer. As recent malignancy is an absolute contraindication to lung transplantation, discussions regarding this were discontinued. Although the patient was initially keen to have the stoma reversed, this was not pursued. Thirteen months after the initial presentation the patient is stable and has had no further abdominal symptoms. Although her other liver function tests have remained normal, her alkaline phosphatase has increased from 50 IU/L to 401 IU/L. She has required a number of prolonged admissions for recurrent haemoptysis, but her lung function remains stable and her weight has increased. She has also married her partner.
Discussion
Increasing life expectancy in adults with CF has resulted in an array of diseases becoming apparent that were not previously associated with CF. Intussusception was previously thought to only affect children with CF, and adults with CF rarely lived to an age at which digestive tract tumours would present. It is now accepted that adults with cystic fibrosis can not only present with these two illnesses but that they are at increased risk compared to the general population.1,2 Despite this, we believe this is the first reported case of colonic intussusception in a patient with cystic fibrosis in which a malignant lead point has been identified.
Intussusception is 10 times more common in those with CF than in the general population with an incidence of around 1%.2 As in the general population the peak incidence is 3 months to one year, but those with CF also have a second peak around ten years of age and there are increasing numbers of reported cases in adult patients.3–7 The lead points commonly identified in patients with CF are the faecal bolus associated with DIOS and the appendix which can become distended with inspissated muco-faeculant contents.5 The older the patient, the more likely a lead point will be identified.6
Although the overall risk of cancer is not increased in patients with CF, there is an increase in the incidence of the digestive tract tumours1,8 and cases have been reported in patients as young as 13 years.9 The largest study to assess the risk of cancer in patients with CF included 28,511 patients in the United States and Canada, and approximately 24,500 patients across 17 European countries between 1985 and 1992.1 In both geographical areas the overall risk of cancer was similar to that of the general population, but the risk of cancer of the digestive tract was significantly increased (Odds ratio [OR] ≈6.5). The increased risk was most dramatic in those aged 20–29 years (OR > 20) and for cancer affecting the pancreas (OR 31.5), oesophagus (OR 14.3) and bowel (OR 9.3). This study did not support an earlier suggestion that the risk was highest for those patients homozygous for the Phe508 mutation or those presenting with failure to thrive.10 Another large study performed by the same team of 28,858 patients with CF in North America between 1990 and 1999 produced very similar results.11
The reason for the increased risk of cancer of the intestinal tract in patients with CF is unknown but various hypotheses have been put forward.1,10,11 The organ-specific risks of cancer may be associated with the different localization and expression of CF transmembrane conductance regulator gene (CFTR), as high levels of expression are maintained into adult life in organs of the intestinal tract, in contrast to respiratory tissue. Other possible explanations relate to the effect that CF has on organs of the intestinal tract; Barrett's oesophagus has been reported in patients with CF and is known to be associated with cancer of the oesophagus, and hepatobilairy tract cancers often occur in patients with gallstones which are a frequent finding in patients with CF. Steatorrhoea, decreased expression of mucin genes (MUC3, MUC11 and MUC12) and malabsorption leading to nutritional/antioxidant deficiencies have also been implicated.
An additional increase in the incidence of digestive tract tumours is seen in those patients who have undergone transplant procedures, presumably related to the sustained, intense immunosuppressive therapy. In the large study mentioned earlier of patients with CF in North America, (28,858 patients, 1068 post-transplantation) the standardized incidence ratio (SIR) for all cancers in patients post transplantation was 6.3 and for digestive tract tumours was 21.2.11 At one North American transplant centre 4/65 (6.2%) of CF patients surviving beyond 6 months post lung transplant developed fatal colonic adenocarcinoma.12 This high incidence prompted the initiation of screening colonoscopies for all adults with CF post lung transplantation. Furthermore, 7/20 (35%) of patients who underwent screening colonoscopies had polyps ranging from 2 to 30 mm in diameter.
The increased incidence of digestive tract malignancies1 and also the increased incidence of coeliac disease13 in patients with CF, mean that their physicians must be vigilant in monitoring for the signs and symptoms of gastrointestinal pathology, and instigating appropriate investigations. It should be remembered, however, that there is a high incidence of iron deficiency in patients with CF,14 and so iron deficiency in the absence of anaemia and other signs or symptoms should not trigger further investigations.
Positive avoidance is a recognized cognitive model of adjustment to illness. It involves the active, conscious behavioural and cognitive avoidance which allows the patient to concentrate on everyday life without constantly thinking about their illness.15 The capacity to put the illness out of their thoughts for at least some of the time, gives the patient control and seems to be a characteristic of those who cope well. In this case, the initial acceptance and understanding of the diagnosis and its implications defines her coping strategy as positive avoidance rather than denial. Despite the patients rising alkaline phosphatase, her stable lung function and increasing weight would normally have lead to the Oncology team repeating the imaging of her liver to monitor progression of the metastases. In line with her wishes this has not been performed but highlights the potential difficulties in monitoring patients who use positive avoidance as a cognitive model of adjustment to illness. In patients with CF the difficulties associated with the diagnosis of malignancy are accompanied by an additional ‘loss of hope’, as this is an absolute contraindication to transplantation. This can undoubtedly have an additional psychological effect.
In summary this is the first reported case of intussusception in a patient with CF with a malignant lead point. It highlights the changing array of conditions seen in patients with CF due to their increasing life expectancy and specifically the increased incidence of digestive tract tumours and intussusception.
DECLARATIONS
Competing interests
None declared
Funding
Not applicable
Ethical approval
Not applicable
Guarantor
Not applicable
Contributorship
FG was the primary author of the article. AJ and RBT were involved in planning the case history and discussion in addition to editing the final document.
Acknowledgements
None
References
- 1.Neglia JP, FitzSimmons SC, Maisonneuve P, et al. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med 1995;332:494–9 [DOI] [PubMed] [Google Scholar]
- 2.Holsclaw DS, Rocmans C, Shwachman H Intussusception in patients with cystic fibrosis. Pediatrics 1971;48:51–8 [PubMed] [Google Scholar]
- 3.Low VH, Paulson EK Gastrointestinal case of the day. Cystic fibrosis with colonic intussusception. AJR Am J Roentgenol 1995;165:196 [DOI] [PubMed] [Google Scholar]
- 4.Khera G, Kumarage S, Strekozov B Adult intussusception in a cystic fibrosis patient–mimicking acute appendicitis. ANZ J Surg 2004;74:807–8 [DOI] [PubMed] [Google Scholar]
- 5.Nash EF, Stephenson A, Helm EJ, et al. Intussusception in Adults with Cystic Fibrosis: A Case Series with Review of the Literature. Digestive Diseases and Sciences [Internet] 2011. Jun 16; Available from: http://www.ncbi.nlm.nih.gov/pubmed/21678050 (last accessed November 9 2011)
- 6.Hafen GM, Taylor AC, Oliver MR, et al. Intussusceptions arising from two different sites in a child with cystic fibrosis. Pediatr. Pulmonol 2005;40:358–61 [DOI] [PubMed] [Google Scholar]
- 7.Webb AK, Khan A Chronic intussusception in a young adult with cystic fibrosis. J R Soc Med 1989;82:47–8 [PMC free article] [PubMed] [Google Scholar]
- 8.Maisonneuve P, Marshall BC, Lowenfels AB Risk of pancreatic cancer in patients with cystic fibrosis. Gut 2007;56:1327–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibele AR, Koplin SA, Slaughenhoupt BL, Kryger JV, Friedl A, Lund DP Colonic adenocarcinoma in a 13-year-old with cystic fibrosis. J Pediatr Surg 2007;42:E1–3 [DOI] [PubMed] [Google Scholar]
- 10.Neglia JP, Wielinski CL, Warwick WJ Cancer risk among patients with cystic fibrosis. J Pediatr 1991;119:764–6 [DOI] [PubMed] [Google Scholar]
- 11.Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW 3rd, Lowenfels AB Cancer risk in nontransplanted and transplanted cystic fibrosis patients: a 10-year study. J Natl Cancer Inst 2003;95:381–7 [DOI] [PubMed] [Google Scholar]
- 12.Meyer KC, Francois ML, Thomas HK, et al. Colon cancer in lung transplant recipients with CF: increased risk and results of screening. J Cyst Fibros 2011;10:366–9 [DOI] [PubMed] [Google Scholar]
- 13.Fluge G, Olesen HV, Gilljam M, et al. Co-morbidity of cystic fibrosis and celiac disease in Scandinavian cystic fibrosis patients. J Cyst Fibros 2009;8:198–202 [DOI] [PubMed] [Google Scholar]
- 14.Keevil B, Rowlands D, Burton I, Webb AK Assessment of iron status in cystic fibrosis patients. Ann Clin Biochem 2000;37:662–5 [DOI] [PubMed] [Google Scholar]
- 15.Moorley S, Greer S Cognitive behaviour therapy for people with cancer. Second edition Oxford: Oxford University Press; 2002 [Google Scholar]