Abstract
We conducted a retrospective study of 155 children who received unrelated donor hematopoietic cell transplantation (HCT) between 1990 and 2005 for acute lymphoblastic leukemia (ALL) in third remission. Median age of patients was 11 years, median time from diagnosis to first relapse was 36 months and median time from first to second relapse was 26 months. Stem cell sources were bone marrow (n=115), peripheral blood (n=11) or cord blood (n=29). All patients received a myeloablative transplant-conditioning regimen. The 5-year estimates of leukemia-free survival (LFS), relapse and non-relapse mortality were 30%, 25% and 45%, respectively. In multivariate analysis, the only risk factor associated with relapse was interval between first and second relapse. Second relapses that occurred late, >26 months from first relapse, were associated with lower risk for post-HCT relapse compared to second relapses ≤26 months (RR 0.4; p=0.01). Relapse risks were lowest when late second relapse was preceded by late first relapse (> 36 months from diagnosis) as shown by a 3-year relapse rate of 9%, p=0.0009. Long-term LFS can be achieved for children with ALL in third remission using unrelated donor HCT, especially when the second relapse occurred late.
Keywords: Bone marrow transplant, hematopoietic cell transplant, acute lymphoblastic leukemia, unrelated donor
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) has been used for decades to treat childhood acute lymphoblastic leukemia (ALL). The outcome for children treated with chemotherapy alone for newly-diagnosed ALL is excellent, with 10-year leukemia-free survival (LFS) rates of over 70%.(1-3) Most patients with ALL who fail initial therapy or suffer a first relapse have relatively good chances of achieving a second remission with rescue therapy including chemotherapy alone or chemotherapy followed by allogeneic HCT.(4-7) The current consensus is to recommend allogeneic HCT for patients with ALL in second remission for whom a matched sibling donor is available or consider alternative donor transplantation for those with early first relapse (≤36 months) or otherwise at high risk for relapse based on disease characteristics or response to re-induction therapy.
Outcomes for children and adolescents with ALL who experience a second relapse after chemotherapy are inferior to those with earlier stages of disease.(8) Fewer patients achieve a third remission and the use of allogeneic HCT for patients who are unable to achieve remission show very poor outcomes.(9, 10) When third remissions are attained they are not expected to be sustainable by chemotherapy alone, and the recommendation has generally been to proceed to HCT with any available donor, usually an unrelated volunteer adult donor or cord blood. Scarce reports on a limited number of patients (11-13) suggest there is a role for allogeneic HCT in children with ALL in third remission, but this has not been studied in a larger cohort.
The goals of this study were to determine the outcome of children undergoing unrelated donor HCT for ALL in third remission and to identify prognostic factors that impact post-HCT relapse and LFS using data reported to a large, international cooperative registry.
PATIENTS, MATERIALS AND METHODS
Patient and Disease Characteristics
This report is based on data contributed by 57 transplant centers to the Center for International Blood and Marrow Transplant Research. The 155 patients who were analyzed were aged 1 - 18 years and received an unrelated donor HCT between January 1990 and December 2005 for ALL in third complete remission. Twenty-one recipients of prior HCT who relapsed and then received a second HCT in third remission were excluded because there are too few of these patients to be analyzed in a separate category. Eighteen of the 21 patients are dead; recurrent leukemia was the most frequent cause of death and others died of organ failure or infection. Almost all deaths occurred within the first year after the second transplantation. The characteristics of patients included in the current analysis are summarized in Table 1. The majority of patients (74%) had B-cell lineage ALL. Half of the patients experienced a first relapse within 36 months of the initial diagnosis, and half of the patients experienced a second relapse within 26 months of the first relapse. The median time from diagnosis to transplant was 61 months (11-167 months). Table 2 summarizes the sites of first and second relapse for all patients. For 83% of patients, the site of second relapse was the bone marrow with or without associated extramedullary involvement.
Table 1.
Characteristics of 155 patients undergoing unrelated hematopoietic cell transplant for acute lymphoblastic leukemia in third remission
| Characteristics | N(%) a |
|---|---|
| Age at transplant in years, median (range) | 11 (1- 18) |
| 1 – 10 years | 70 (45) |
| 11 – 18 years | 85 (55) |
| Male sex | 93 (60) |
| Pre-transplant performance score < 90%b | 31 (20) |
| Disease | |
| Pre-B or B-cell lineage ALL | 114 (74) |
| T-cell ALL | 8 (5) |
| Unknown | 33 (21) |
| Cytogenetic abnormalities prior to transplant c | |
| High risk | 8 ( 5) |
| Others | 43 (28) |
| No abnormalities | 42 (27) |
| Unknown | 62 (40) |
| Time from diagnosis to 1st relapse in months, median (range) | 35 (1-126) |
| ≤ 36 months | 78 (50) |
| > 36 months | 76 (49) |
| Unknown | 1 (1) |
| Time from 1st relapse to 2nd relapse in months, median (range) | 26 (4 – 116) |
| ≤ 26 months | 77 (50) |
| >26 months | 78 (50) |
| Time from diagnosis to transplant in months, median (range) | 61 (11 – 167) |
| ≤ 24 months | 20 (13) |
| 25 - 48 months | 34 (22) |
| 49 – 72 months | 38 (25) |
| 73 – 96 months | 29 (19) |
| > 96 months | 34 (22) |
Data is presented as N (%) unless otherwise specified.
Pre-transplant performance score was reported using Lansky-Play performance score for patients younger than 16 years and Karnofsky score for patients 16 and older.
Cytogenetic abnormalities were considered high-risk if any of the following abnormalities were present: t(4,11), t(9,22), hypodiploidy/near triploloidy or >5 abnormalities.
Table 2.
Sites of first and second leukemia relapse
| Site of 1st relapse | Site of 2nd relapse, N(%) | ||
|---|---|---|---|
| BM ± extramedullary | Isolated CNS | Other extramedullary | |
| BM ± extramedullary site | 47 (30) | 3 (2) | 0 |
| Isolated CNS | 6 (4) | 6 (4) | 0 |
| Other extramedullary sites | 6 (4) | 0 | 3 (2) |
| Unknown | 70 (45) | 11 (7) | 3 (2) |
Abbreviations: BM, bone marrow; CNS, central nervous system
Consent for reporting and inclusion in study reports was obtained by participating centers at time of transplantation. Data are reported to the Center for International Blood and Marrow Transplant Research at transplantation and at pre-defined intervals including annual follow-ups. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Transplant characteristics
Transplant characteristics are summarized in Table 3. The unrelated donor stem cell sources included bone marrow (74%), peripheral blood (PB, 7%) and cord blood (19%). Donor-recipient pairs considered well matched were defined as no known disparity between donor and recipient at HLA-A, -B, -C and DRB1, partially matched as one known or one likely disparity and mismatched as ≥2 disparities(14). Bone marrow and PB grafts were matched in 32% of cases, and mismatched at one or two loci in 68%. Cord blood grafts were all single units and 6/6 matched in 3% and mismatched at one or two loci in 97% of cases.
Table 3.
Transplant characteristics
| Characteristics | N(%) a |
|---|---|
| Conditioning regimen b | |
| - TBI + Cyclophosphamide ± other | 138 (89) |
| - TBI + other | 5 ( 3) |
| - Busulfan + Cyclophosphamide ± other | 10 ( 6) |
| - Busulfan + Melphalan | 2 ( 1) |
| Donor –Recipient sex match | |
| - Male donor/Male recipient | 50 (32) |
| - Male donor/Female recipient | 32 (21) |
| - Female donor/Male recipient | 43 (28) |
| - Female donor/Female recipient | 30 (19) |
| Donor-recipient CMV status | |
| - Donor (−) / recipient (−) | 64 (41) |
| - Donor (+)/ recipient (−) | 22 (14) |
| - Donor (−/+) / recipient (+) | 51 (33) |
| - Unknown | 18 (12) |
| Graft type/HLA matching c | |
| Bone marrow/PBSC | 115 (74) / 11 (7) |
| - Well matched | 40 (32) |
| - Partially matched | 36 (28) |
| - Mismatched | 50 (40) |
| Cord Blood | 29 (19) |
| - Well matched | 1 (3) |
| - Partially matched | 6 (21) |
| - Mismatched | 22 (76) |
| Year of transplant | |
| - 1990 – 1994 | 32 (21) |
| - 1995 – 1999 | 52 (34) |
| - 2000 – 2005 | 71 (46) |
| Therapy given as conditioning or GVHD prophylaxis | 80 (52) |
| Antithymocyte globulin | 80 (52) |
| Alemtuzumab | 9 (6) |
| GVHD prophylaxis d | |
| - T-cell depletion | 48 (31) |
| - Tacrolimus + other | 17 (11) |
| - Cyclosporine + Methotrexate ± other | 67 (43) |
| - Cyclosporine ± other | 23 (15) |
Abbreviations: TBI, total body irradiation; CMV, Cytomegalovirus; HLA, Human leukocyte antigen; ATG, anti-thymocyte globulin; GVHD, graft-versus-host disease; PB, peripheral blood
Data is presented as N (%) unless otherwise specified.
TBI+ Cyclophosphamide ± other (n=106), TBI + Cyclophosphamide + Etoposide (n=32), TBI+other (n=4), TBI+ Etoposide (n=1); TBI dose ≤ 1300 Gy (n=65), TBI dose ≤1300 Gy (n=78).
Donor-recipient well matched was defined as no known disparity between donor and recipient at HLA A,B,C and DRB1, partially matched as one known or one likely disparity and mismatched as ≥2 disparities. Seventy patients had high-resolution HLA typing information.
Cyclosporine + corticosteroids (n=10), Cyclosporine + corticosteroids + ATG (n=5), Cyclosporine alone (n=4), Cyclosporine + Mycophenolate (n=3), Cyclosporine + ATG (n=1).
All patients received myeloablative preparative regimens. Preparative regimens varied, but 92% of the regimens contained total body irradiation (TBI); of the 143 patients who received TBI, 65 (45%) received TBI dose <1300 cGy and 78 (55%), ≥1300 cGy. Most patients (69%) received a calcineurin inhibitor for graft-versus-host disease prophylaxis. Fifty seven percent (89 of 155) transplant recipients received in vivo T-cell depletion; achieved in 80 cases using antithymocyte globulin (ATG). Forty two percent (48 of 115) of bone marrow grafts were T-cell depleted using ex vivo methods. Both forms of T-cell depletion were used for 21% of the transplants because 33 recipients of ex vivo T-cell depleted marrow received ATG.
Statistical Analysis
Neutrophil recovery was defined as the first of three consecutive days with an absolute neutrophil count greater than 0.5 × 109/L. Platelet recovery was defined as the first of three consecutive days with a platelet count greater than 20 × 109/L and transfusion-independent for at least seven days. Acute and chronic graft-versus-host disease (GVHD) were graded using methods previously described. (15, 16) Non-relapse mortality (NRM) was defined as death due to causes other than recurrent leukemia. Probabilities of overall survival (OS) and LFS were calculated using Kaplan-Meier product limit estimates. (17) Relapse or death was considered failure for the end point of LFS. Cumulative incidence estimates were used to calculate the rates of hematopoietic recovery, GVHD, relapse and NRM. (18) Cox proportional hazard regression models were fit for the end points LFS, relapse, NRM and GVHD.(19) Exploratory variables that were chosen for regression models included the following: patient age, gender and pre-transplant performance score; disease characteristics such as time from diagnosis to first relapse, time from first relapse to second relapse and site(s) of second relapse; transplant characteristics such as use of TBI, donor-recipient sex match, donor-recipient CMV status, degree of HLA-match, graft type, year of transplant, and use of T-cell depletion or ATG. A backward stepwise model selection approach was used to identify potential significant risk factors. Variables that reached a 5% level of significance were kept in the final model. The effect of acute and chronic GVHD on relapse and LFS was evaluated by treating GVHD as a time-dependent covariate in the final regression models. All p-values are two-sided. Analyses were done using SAS 9.1 (Cary, North Carolina).
RESULTS
Hematopoietic recovery
One hundred and forty seven of 155 patients achieved neutrophil recovery and the median time to recovery was 18 days. The day-28 and day-100 incidence of neutrophil recovery was 84% (95% CI 77 -89) and 95% (95% CI 90-98), respectively. All 8 patients who failed to achieve neutrophil recovery died from a transplant related cause before day-100 post-transplant. Seven of these patients received mismatched bone marrow (n=3) and cord blood (n=4) grafts; anti-thymocyte globulin was included in the transplant-conditioning regimen for four patients. Ninety-four of 155 patients achieved platelet recovery and the median time to recovery was 32 days. The day-100 incidence of platelet recovery was 64% (95% CI 56-72).
Graft-versus-Host Disease
Ninety patients developed grade B-D acute GVHD grade. The day-100 incidence of grade B-D acute GVHD was 58% (95% CI 50-66). In multivariate analysis, the risk of acute GVHD was higher among male recipients of female donors, recipients of non-TBI containing regimens, patients with low pre-transplant performance scores, and those who did not receive ATG (Table4a). Forty-one patients developed chronic GVHD; the 5-year incidence of chronic GVHD was 28% (95% CI 21-35). In multivariate analysis, graft type was the only risk factor identified; transplantation of PB grafts was associated with the highest risk (Table 4b).
Table 4.
Multivariate analysis of acute (a) and chronic (b) GVHD
| Independent Variables | N | Relative Risk (95% CI) | p-value |
|---|---|---|---|
| a. Acute GVHD | |||
| Donor recipient sex match | |||
| - Female donor-male recipient | 43 | 1.00 | |
| - Other | 110 | 0.53 (0.33-0.85) | 0.009 |
| Conditioning regimen | |||
| - TBI-containing | 142 | 1.00 | |
| - Non-TBI | 11 | 3.25 (1.57 – 6.73) | 0.001 |
| Pre-transplant performance score | |||
| - ≤90% | 31 | 1.00 | |
| - >90% | 117 | 0.43 (0.25 – 0.73) | 0.002 |
| ATG given | |||
| - No | 73 | 1.00 | |
| - Yes | 80 | 0.65 (0.42 – 0.99) | 0.044 |
| b. Chronic GVHD | |||
| Graft type | P-overall= 0.011 | ||
| - Bone Marrow | 109 | 1.00a | |
| - PBSC | 11 | 2.85 (1.18 – 6.92) | 0.02 |
| - Cord Blood | 29 | 0.43 (0.15 -1.21) | 0.11 |
Abbreviations: GVHD, graft-versus-host disease; TBI, total body irradiation; ATG, anti-thymocyte globulin; PBSC, peripheral blood stem cells
Survival, Relapse and Non-relapse Mortality
At last follow-up 47 of 155 patients were alive and the median follow-up was 75 (range 44-170) months. The 5-year probabilities of OS and LFS were 31% (95% CI 24-39) and 30% (95% CI 22-37), respectively. Thirty of 155 patients had died from relapse and the 5-year incidence of post-transplant relapse was 25% (95% CI 19-33). Seventy-eight of 155 (50%) patients had died from causes other than leukemia recurrence. The day-100, 1-year and 5-year incidences of NRM were 19% (95% CI, 14%-26%), 41 (95% CI, 33%-49%) and 45% (95% CI, 37%-53%), respectively. Causes of death are summarized in Table 5. Pulmonary and other organ toxicity accounted for 33 (42%) of the NRM and these deaths were distributed evenly before and after day 100. Infection and GVHD accounted for 18% and 17% of deaths attributed to NRM, respectively.
Table 5.
Causes of death
| Cause of death | Number | ≤ Day 100 | > Day 100 |
|---|---|---|---|
| Total Deaths, number | 108 | 33 | 75 |
| Leukemia | 30 | 1 | 29 |
| Graft Failure | 3 | 2 | 1 |
| Infection | 14 | 5 | 9 |
| Pulmonary Toxicity (IPS, ARDS) | 16 | 8 | 8 |
| Other (non-lung) Organ Failure/Toxicity | 17 | 7 | 10 |
| Secondary Malignancy | 5 | 0 | 5 |
| Hemorrhage/Vascular | 8 | 4 | 4 |
| GVHD ± infection | 13 | 6 | 7 |
| Unknown | 2 | 0 | 2 |
Abbreviations: IPS, idiopathic pneumonitis syndrome; ARDS, acute respiratory distress syndrome; GVHD, graft-versus-host disease
Legend: Five patients died from secondary cancers including 3 brain tumors, one EBV-induced lymphoproliferative disease and one of unknown type.
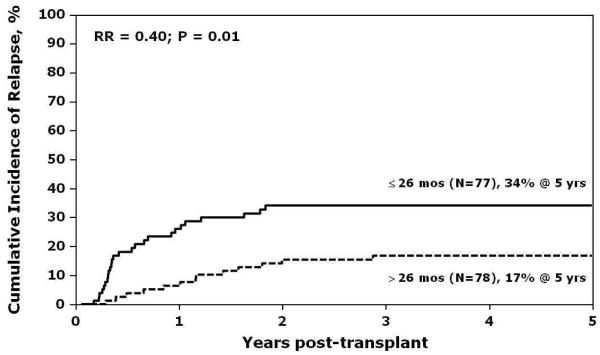
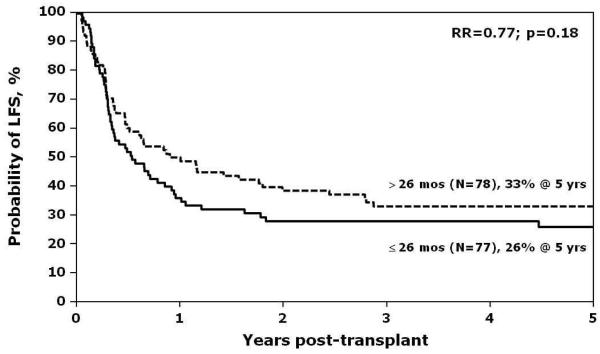
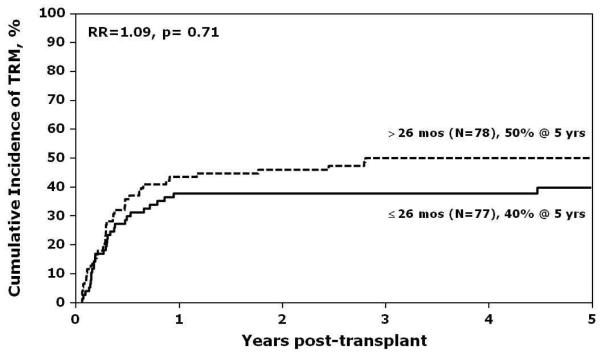
In multivariate analysis, the only significant risk factor associated with relapse was the interval between first and second relapse (Figure 1). Patients were considered as having an early second relapse if the interval between first and second relapse was 26 months or earlier, and as late relapse, if the interval between first and second relapse was greater than 26 months. Patients with a late second relapse had a lower risk for relapse compared to those with early second relapse (RR 0.4, 95% CI 0.21 - 0.78, p=0.01). The effect of time from diagnosis to first relapse was also examined (Table 6). Patients were considered as having an early first relapse if the time from diagnosis to first relapse was 36 months or less, and as late relapse if time was greater than 36 months. The lowest incidence of relapse post-transplant was observed in patients who experienced late first relapse and late second relapse (5-year relapse of 9%; 95% CI, 3-19%; p=0.0009). There were no significant differences in sites of relapse across groups experiencing late or early first relapse, or late or early second relapse. Estimates of LFS and NRM are shown in Figures 2 and 3. Other variables that were tested in the multivariate models for LFS and NRM and found not to be statistically significant were: time from diagnosis to first relapse, site of second relapse, recipient age and sex, performance score, degree of HLA-match, graft source, use of ATG or ex vivo T-cell depletion, and presence of acute or chronic GVHD.
Figure 1.
Cumulative incidence of post-transplant relapse
Table 6.
Probability of outcomes across groups defined by time from diagnosis to first relapse and time from first to second relapse.
| Months diagnosis to 1strelapse | ≤ 36 | ≤ 36 | > 36 | > 36 | |
|---|---|---|---|---|---|
| Months 1st to 2nd relapse | ≤ 26 | > 26 | ≤ 26 | > 26 | |
| N | 55 | 23 | 22 | 54 | |
| Probability (95% CI) | p-value | ||||
| 3-year LFS | 32 (20-44) | 30 (13-49) | 18 (6-36) | 35 (23-48) | 0.43 |
| 3-year OS | 34 (21-46) | 43 (23-62) | 20 (6-40) | 39 (26-51) | 0.32 |
| 3-year Relapse | 33 (21-46) | 35 (17-55) | 36 (17-56) | 9 (3-19) | 0.0009 |
| 3-year NRM | 35 (23-47) | 35 (17-54) | 45 (24-64) | 56 (41-68) | 0.12 |
Abbreviations: CI, confidence interval; LFS, leukemia-free survival; OS, overall survival; NRM, nonrelapse mortality
Figure 2.
Estimates of leukemia-free survival
Figure 3.
Estimates of nonrelapse mortality
DISCUSSION
Risk-stratification of newly diagnosed childhood ALL has changed the role of allogeneic HCT for ALL in first remission.(20, 21) Thus allogeneic HCT for first remission ALL is generally confined to those children who are considered to be at very high risk for relapse.(22) Children whose ALL relapses within 36 months from diagnosis and achieve a second remission usually undergo allogeneic HCT if a matched sibling donor is available. However, practice variation exists across the country and 50% of the children in our cohort with early (mostly marrow) relapse did not proceed to HCT in second remission, which would have been considered best practice. These patients lack an HLA-matched sibling and the treating physician deferred unrelated donor transplantation. For a child who does not undergo HCT in second remission but relapses and achieves a third remission, alternative donor HCT has been considered the option offering the best chance for cure, despite the limited data available. As ALL therapy continues to be risk-adapted it is reasonable to expect that pediatric referrals for alternative donor HCT to treat ALL in third remission will increase in coming years.
A randomized or prospective therapeutic study of this patient group has never been conducted, and retrospective reviews to date have been limited to small studies.(11-13) Borgmann and colleagues reported on a subset of 33 children undergoing BMT for ALL in third remission in Europe from 1983 to 1995.(11) They found that LFS was approximately 48% and NRM was 30%, with worse outcomes when second relapse occurred within six months from completion of previous therapy. In contrast, patients whose second relapse occurred later had LFS of 61%, comparable to that seen in the same study for patients who underwent HCT for ALL in second remission. Afify and colleagues reported on their single institution experience with a similar group of patients in the UK. (12) They found that shorter duration of second remission (< 30 months) was associated with lower LFS and higher NRM. Shorter duration of remission and the presence of extramedullary disease were also associated with a higher risk of post-transplant relapse. Most recently, Gassas and colleagues reported on the Canadian experience.(13) They found that LFS in a group of 22 children was 32% and that most leukemia-free survivors had chronic GVHD, suggesting a role for graft-versus-leukemia effect in survivors.
Our study represents the largest most comprehensive analysis to date of the outcome of children receiving unrelated donor HCT for ALL in third remission. We observed LFS rates of 30% and it was not influenced by disease characteristics, donor or stem cell source, conditioning or graft manipulation. It was of particular interest that only 26% of the cohort had well matched grafts yet HLA matching and graft source did not influence LFS. Assessment of donor-recipient HLA-matching typically requires thousands of patients to efficiently address its impact on transplantation outcomes. Consequently, donor selection practices should follow the recommendations of those reports. One caveat for the current analysis is that information on site of relapse (first and second) was not reported for a substantial number of patients. While it is possible that the relative small numbers did not allow for adequate comparison between subgroups, these results support the recommendation that patients with ALL in third remission, especially those with long intervals between first and second relapse, should proceed to HCT as soon as they achieve third complete remission with the best alternative donor available.
Relapse rates were lowest in the group of children who experienced late first relapse and late second relapse, likely indicative of more sensitive disease compared to other cohorts. We did not observe a survival advantage for patients with chronic GVHD, as suggested by other reports. (13) However, others have demonstrated graft-versus-leukemia effects after allogeneic HCT for ALL and our inability to demonstrate this phenomenon may be explained by the relatively small sample size of patients experiencing chronic GVHD in this report.
The most sobering observation was perhaps that NRM was high in all patients regardless of time to relapse and in the group of patients with the lowest relapse risk the higher NRM negated any survival advantage. We observed NRM rates higher than those reported for patients with ALL in second remission following matched sibling or unrelated donor HCT.(5-7) Forty-two percent of non-relapse deaths were attributable to organ toxicity, suggesting that the cumulative effects of pre-transplant re-induction therapies might mitigate some of the survival benefits provided by allogeneic HCT for third remission ALL. Perhaps another important message from this observation of high NRM rates after HCT in third remission ALL is to preferably transplant as many children as possible in second rather than third complete remission. Our cohort was notable for the 50% in whom first relapse occurred <36 months from diagnosis and assuming that most of these children had marrow relapses they would have met the conventional criteria for transplant in second remission. It is possible that absence of a well-matched donor might have been the reason that some children did not undergo HCT in second remission but this rationale would now be questionable given current outcomes for alternative donor HCT.
Given that reduced intensity conditioning (RIC) has now been explored for children and adults with ALL, such strategies might be considered for heavily pretreated children undergoing HCT for third remission ALL. (23-26) A question for the future will be whether LFS can be improved for these children if one attempts to balance the known higher relapse rates associated with reduced intensity conditioning and the expected abrogation of higher NRM rates that follow myeloablative conditioning. Unfortunately, the observation that NRM increased in our study cohort from 19% at day 100 to 45% at 5 years does not clearly support the hypothesis that RIC approaches will necessarily offset NRM and improve survival. The second major cause of NRM in our study was infection without GVHD (18% of NRM) with 9 of the 14 deaths occurring after day 100 and suggests that delayed reconstitution of immunity might be responsible. This is not surprising because at least half of the children had received in vivo or ex vivo T cell depletion and 21% had received both forms of T cell depletion. Taken together this suggests that it will be particularly important in this high risk group to try and minimize peritransplant approaches that impede immune reconstitution and to consider also approaches that might augment infection prophylaxis. Finding the right balance might be challenging because our observation that deaths from GVHD were considerably lower compared to other reports in children with ALL is also readily explained as a benefit of these T cell depletion strategies. (7, 9)
We conclude that long-term LFS is achievable in children after unrelated donor HCT for ALL in third remission. However, transplantation in third remission is not an optimal strategy because NRM rates are high which implies that when an HLA-matched sibling is lacking then a more desirable option is transplantation in second remission with the best available alternative donor. When considering transplantation in third remission it is important to consider carefully the impact of one or more T cell depletion approaches on delayed immune reconstitution and at the very least incorporate augmented infection prophylaxis strategies. In addition, post-transplant relapse prevention strategies should be considered, with possible options being use of donor lymphocyte infusions and/or small molecule inhibitors (e.g. tyrosine kinase inhibitors) or other new agents, either prophylactically or pre-emptively triggered by the results of sensitive and specific minimal disease monitoring.
ACKNOWLEDGMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Footnotes
Financial Disclosure Statement: The authors have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell C, Richards S, Harrison CJ, Eden T. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980-2001. Leukemia. 2010;24:406–418. doi: 10.1038/leu.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Raetz E, Zhang MJ, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgmann A, von Stackelberg A, Hartmann R, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101:3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 7.Bunin N, Carston M, Wall D, et al. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood. 2002;99:3151–3157. doi: 10.1182/blood.v99.9.3151. [DOI] [PubMed] [Google Scholar]
- 8.Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolfrey AE, Anasetti C, Storer B, et al. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood. 2002;99:2002–2008. doi: 10.1182/blood.v99.6.2002. [DOI] [PubMed] [Google Scholar]
- 10.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgmann A, Baumgarten E, Schmid H, et al. Allogeneic bone marrow transplantation for a subset of children with acute lymphoblastic leukemia in third remission: a conceivable alternative? Bone Marrow Transplant. 1997;20:939–944. doi: 10.1038/sj.bmt.1701013. [DOI] [PubMed] [Google Scholar]
- 12.Afify Z, Hunt L, Green A, Guttridge M, Cornish J, Oakhill A. Factors affecting the outcome of stem cell transplantation from unrelated donors for childhood acute lymphoblastic leukemia in third remission. Bone Marrow Transplant. 2005;35:1041–1047. doi: 10.1038/sj.bmt.1704958. [DOI] [PubMed] [Google Scholar]
- 13.Gassas A, Ishaqi MK, Afzal S, Dupuis A, Doyle J. Outcome of haematopoietic stem cell transplantation for paediatric acute lymphoblastic leukaemia in third complete remission: a vital role for graft-versus-host-disease/ graft-versus-leukaemia effect in survival. Br J Haematol. 2008;140:86–89. doi: 10.1111/j.1365-2141.2007.06840.x. [DOI] [PubMed] [Google Scholar]
- 14.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 16.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 17.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. Springer-Verlag; New York, N.Y.: 2003. [Google Scholar]
- 18.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 20.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nachman JB, La MK, Hunger SP, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children’s oncology group. J Clin Oncol. 2009;27:5189–5194. doi: 10.1200/JCO.2008.20.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balduzzi A, Valsecchi MG, Uderzo C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet. 2005;366:635–642. doi: 10.1016/S0140-6736(05)66998-X. [DOI] [PubMed] [Google Scholar]
- 23.Pulsipher MA, Boucher KM, Wall D, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114:1429–1436. doi: 10.1182/blood-2009-01-196303. [DOI] [PubMed] [Google Scholar]
- 24.Verneris MR, Eapen M, Duerst R, et al. Reduced-intensity conditioning regimens for allogeneic transplantation in children with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2010;16:1237–1244. doi: 10.1016/j.bbmt.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohty M, Labopin M, Volin L, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116:4439–4443. doi: 10.1182/blood-2010-02-266551. [DOI] [PubMed] [Google Scholar]
- 26.Marks DI, Wang T, Perez WS, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116:366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]