Local community networks can mitigate pandemic influenza in the absence of vaccine and antiviral drugs.
Keywords: Influenza, social networks, social distance, computer simulation, nonlinear models, research
Abstract
Targeted social distancing to mitigate pandemic influenza can be designed through simulation of influenza's spread within local community social contact networks. We demonstrate this design for a stylized community representative of a small town in the United States. The critical importance of children and teenagers in transmission of influenza is first identified and targeted. For influenza as infectious as 1957–58 Asian flu (≈50% infected), closing schools and keeping children and teenagers at home reduced the attack rate by >90%. For more infectious strains, or transmission that is less focused on the young, adults and the work environment must also be targeted. Tailored to specific communities across the world, such design would yield local defenses against a highly virulent strain in the absence of vaccine and antiviral drugs.
At the start of an influenza pandemic, effective vaccine and antiviral drugs may not be available to the general population (1,2). If the accompanying illness and death rates of the virus strain are high, how might a community respond to protect itself? Closing roads, restricting travel, and community-level quarantine will enter discussions (3,4). However, within a community, influenza spreads from person to person through the social contact network. Therefore, understanding and strategically controlling this network during a period of pandemic is critical.
We describe how social contact network–focused mitigation can be designed. At the foundation of the design process is a network-based simulation model for the spread of influenza. We apply this model to a community of 10,000 persons connected within an overlapping, stylized, social network representative of a small US town. After study of the unmitigated transmission of influenza within the community, we change the frequency of contact within targeted groups and build combinations of strategies that can contain the epidemic. Finally, we show how infectivity of the strain and underlying structure of the infectious contact network influence the design of social distancing strategies. In the absence of vaccine and antiviral drugs, design for specific communities would defend against highly virulent influenza.
Methods
The design process first creates an explicit social contact network in which persons are linked to others in a community. Spread of influenza within the network is then simulated by imposing behavioral rules for persons, their links, and the disease. These rules are modified to implement targeted mitigation strategies within the community, the effectiveness of which is evaluated (5).
Contact Network
A network is created by specifying groups of given sizes (or range of sizes) within which persons of specified ages interact (e.g., school classes, households, clubs). The average number of links per person within the group is also specified because cliques form or are imposed (e.g., seating in a classroom). This number is used to construct a within-group network that can take various forms. We used fully connected, random, or ring networks for each group. Random networks are formed by randomly choosing 2 persons within the group and linking them. This process is repeated until the number of links within the group yields the specified average (each person will have a different number of links). The ring is formed by first placing persons next to neighbors and linking them to form a complete circle. Additional links are then made to next nearest neighbors symmetrically around the ring. Finally, links within a group are given an average frequency of contact per day. With this approach, a network can be built from the experience of community members to exhibit the clustered yet small-world characteristics (6) and overlapping quality of a structured community (7,8).
Our network represented a stylized small US town and took advantage of the diverse backgrounds of the authors (1 of whom is a teenager). The population of 10,000 conformed to the 2000 Census (9) and consisted of children (<11 years of age, 17.7%), teenagers (12–18 years of age, 11.3%), adults (19–64 years of age, 58.5%), and older adults (>65 years of age, 12.5%). All persons belonged to multiple groups, each associated with a subnetwork of links that reflected their lives within the community (Figure 1, Table 1). Households were composed of families (adults with children or teenagers), adults, or older adults. The age-class makeup and size of households conformed to the 2000 Census (9). All persons within each household were linked to each other with mean link contact frequencies of 6/day. Every person also belonged to 1 multiage extended family (or neighborhood) group (mean membership 12.5, mean link contact frequency 1/day).
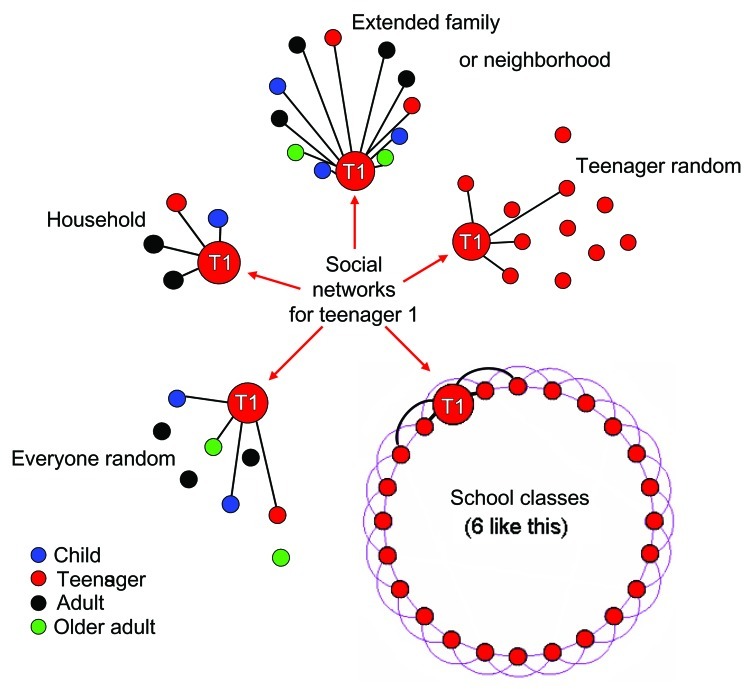
Figure 1.
Typical groups and person-to-person links for model teenager. The teenager (T1) belongs to a household (fully connected network, mean link contact frequency 6/day), an extended family or neighborhood (fully connected network, mean link contact frequency 1/day), and 6 school classes (ring network with connections to 2 other teenagers on each side as shown in black; purple links denote connections of other teenagers within the class; mean link contact frequency 1/day). Two random networks are also imposed, 1 within the age group (teenager random, average of 3 links/teenager, mean link contact frequency of 1/day), and 1 across all age groups (overall random average of 25 links/person [not all links shown], mean link contact frequency of 0.04/day).
Table 1. Groups, membership, networks, and mean frequencies of contact per link.
| Group (no. groups in community) | Membership | Average no. links per member | Network type and parameters | Mean frequency of contact per link per day |
|---|---|---|---|---|
| Households without older adults (2,730) | 1–2 adults, 0–4 children, 0–4 teenagers, mean size 3.13 | 2.13 | Fully connected | 6 |
| Households with older adults (742) | 1–2 older adults, mean size 1.75 | 0.75 | Fully connected | 6 |
| Extended families or neighborhoods (800) | 0–2 older adults, 0–8 adults, 0–8 teenagers, 0–8 children, mean size 12.5 | 11.5 | Fully connected | 1 |
| Child classes (69) | 1 class per child, 20–35 children in each | 4 | Ring network, 2 neighbors on either side | 6 |
| Child random (1) | All children | 3 | Random network link density 3/1,769 | 1 |
| Teenager classes (264) | 6 classes per teenager, 20–35 teenagers in each | 4 | Ring network, 2 neighbors on either side | 1 |
| Teenager random (1) | All teenagers | 3 | Random network link density of 3/1,129 | 1 |
| Adult work (351) | 1 work group per adult, 10–50 adults in each | 6 | Ring network, 3 neighbors on either side | 1 |
| Adult random (1) | All adults | 3 | Random network link density of 3/5,849 | 1 |
| Older adult gathering (156) | 1 gathering per person, 5–20 persons in each | 4 | Ring network, 2 neighbors on either side | 1 |
| Older adult random (1) | All older adults | 3 | Random network link density of 3/1,249 | 1 |
| Overall random (1) | All age classes | 25 | Random network link density of 25/9,999 | 1/25 a day |
All children and teenagers attended preschool or school; children attended 1 class/day, while teenagers attended 6 (classes of 20 to 35 children or teenagers). All adults went to work daily, where they interacted with other adults (work group size 10–50), and all older adults attended gatherings with other older adults (gathering size 5–20). For links within school classes, work, and gatherings of older adults, we assumed the simplest subnetwork that imposes local clustering: a ring lattice in which a person is linked to 2 (for children or teenager classes and gatherings of older adults) or 3 (adult work) neighboring persons on each side along the ring. Mean link contact frequencies for children in a class are 6/day. Teenager classes, adult work, and gatherings of older adults have mean link contact frequencies of 1/day.
To represent additional within-age class interactions, such as extracurricular activities, playgrounds, bowling leagues, or friends, persons are randomly linked to an average of 3 other persons of the same age class (mean link contact frequency 1/day). Finally, to emulate a somewhat patterned set of random contacts from commercial transactions and other ventures into public spaces, we impose a random overall network across all age classes with a mean of 25 links/person to yield 1 contact/person/day (mean link contact frequency 0.04/day).
Behavioral Rules
The spread of influenza within the contact network is modeled as a series of 2 classes of events: transition of a person between disease states and person-to-person transmission of influenza. Disease state transitions follow the natural history of influenza (Figure 2). After the latent state, an infected person transitions to an infectious presymptomatic state or an infectious asymptomatic state with probability pS or 1 – pS, respectively. Those with symptoms either stay home with probability pH, thus influencing their contacts, or continue to circulate with probability 1 – pH. Infected asymptomatic persons continue interacting without behavioral changes. Persons who are symptomatic die or become immune with probability pM or 1 – pM, respectively, and asymptomatic persons become immune. Because this final transition does not influence the spread of the disease, we use pM = 0.
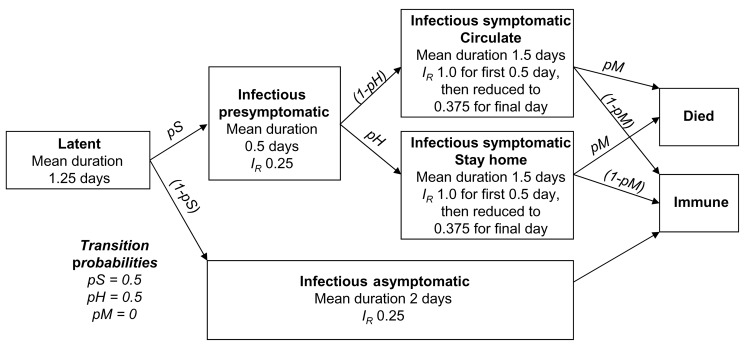
Figure 2.
Natural history of influenza in our model. Duration of each state for a given person is chosen from an exponential distribution. State relative infectivity (IR) and mean state duration were chosen to reflect the infectivity variation of Ferguson et al. (10,11) (see Figure 3). Transition probabilities between presymptomatic and postsymptomatic states are also noted. For symptomatic persons who stay at home, link frequencies outside the household are reduced by 90%.
Person-to-person transmission events are evaluated at the beginning of each period during which a person is infectious. Assuming contact events are statistically independent, a transmission time for each infectious person's links within the contact network is chosen from an exponential distribution with a mean of the link's contact frequency scaled by ID × IR × IA × SP × SA, where ID is the infectivity of the disease, IR is the relative infectivity of the disease state, SP is the susceptibility of people to the disease (here taken as 1.0), IA is the relative infectivity of the person who is transmitting, and SA is the relative susceptibility of the person receiving. If the transmission time is less than the period during which the person will be in an infectious state (also chosen from an exponential distribution with the prescribed means; Figure 2), transmission is scheduled at the chosen time. Otherwise, transmission along that link does not occur during that period. All transmission parameters and contact frequencies may be modified in each of the states, as well as varied among age classes by relative scaling factors such as IR. In this way, disease representations and mitigation strategies are implemented.
Most influenza-specific parameters used here reflect those of (10,11). We approximated normal influenza viral shedding data (15) with a time varying infectivity through choice of state periods and relative infectivity scaling factors (Figure 2 and Figure 3). The latent period is a constant (0.75 days) followed by a variable period (mean 0.5 days). The presymptomatic period (mean 0.5 days) has an IR of 0.25 after which it increased to 1.0 for the first part of the symptomatic period (mean 0.5 days), when viral shedding is maximum and coughing begins. IR is then reduced to 0.375 for the remainder of the infectious symptomatic period (mean 1 day). For infectious asymptomatic persons, IR was set at 0.25 for a mean period of 2.0 days, making these persons half as infective as those with symptoms. We chose pS as 0.5, pH as 0.5 for adults and older adults and pH as 0.9 for children and teenagers. When a person is in the symptomatic stay-home state, we reduce the frequency of all nonhousehold connections by 90%. Because children and teenagers have closer contact with others and are less likely to wash hands or control coughs (16), they are more infective and susceptible: IA and SA are both 1.5 for children, 1.25 for teenagers, and 1.0 for adults and older adults. Finally, ID is adjusted to yield specified attack rates within the community.
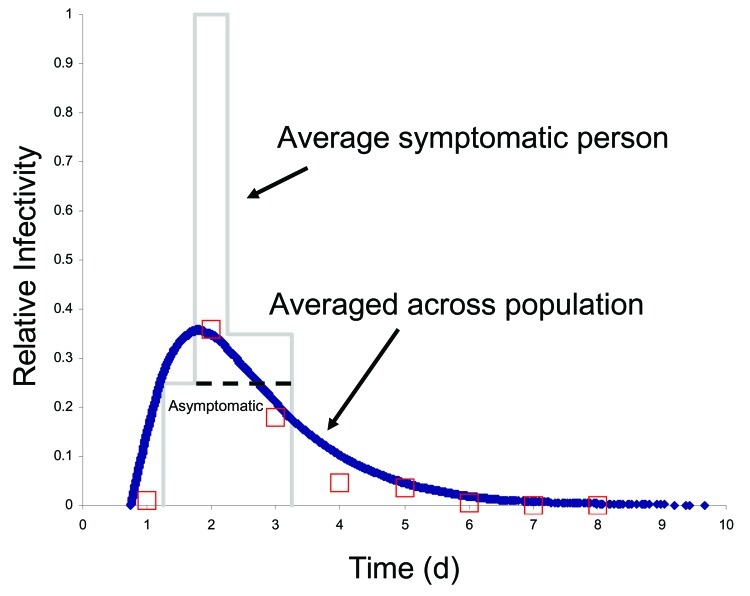
Figure 3.
Functional behavior of IR with time. Although infectivity of an asymptomatic person is constant with time (IR 0.25), infectivity of a symptomatic person changes from infectious presymptomatic (IR 0.25) to early infectious symptomatic (IR 1.0) to late symptomatic (IR 0.375). A symptomatic person with mean state periods as denoted in Figure 2 is shown in gray (asymptomatic with dashed line). Because state periods are different for each person (given by exponential distributions) and half of the infected persons are asymptomatic, the average population scale IR in time is smoothed as shown in blue. Both disease state periods and IR values were chosen to honor the clinically derived natural history of influenza (12–14), selected viral shedding data shown as open red squares (15), and the model of Ferguson et al. (10,11).
Results
We first show the spread of influenza within our unmitigated base case defined with parameters specified above and with ID chosen to yield an infected attack rate ≈50% to reflect the 1957–58 Asian influenza pandemic (10). Unless otherwise noted, we report infected attack rates and refer to them as simply attack rates rather than reporting the illness attack rate which is half of this value (pS = 0.5). We then demonstrate the design of effective local mitigation strategies for the base case that focus on targeted social distancing. Finally, we extend these results to design strategies for more infectious strains and for changes to the underlying infectious contact network that deemphasize the role of children and teenagers.
All simulations are initialized by infecting 10 randomly chosen adults with the assumption that adults are first to be infected through business travel or interaction with visitors from outside the community (5). Some of these initial infections instigate others and grow into an epidemic. Results vary across multiple realizations of the community network and random choice of initially infected adults (controlled by random number seed) not all of which yield an epidemic, defined when the number infected is >1% of the population. For every set of parameters, we conducted >100 simulations with different random number seeds and collected statistics for all simulations and for only those that result in epidemics (Table 2).
Table 2. Results for base case and miigation strategies*.
| Strategy | Averages for all simulations |
Averages for simulations with epidemics |
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. simulations | Total infected | Total time (d) | Peak infected | Time to peak (d) | No. epidemics | Total infected | Total time (d) | Peak infected | Time to peak (d) | ||||||||||||||||||||||||||||
| Case 1: Base case pandemic influenza | |||||||||||||||||||||||||||||||||||||
| Average | 1,000 | 4,908 | 81 | 688 | 35 | 978 | 5,018 | 82 | 703 | 36 | |||||||||||||||||||||||||||
| SD | 748 | 14 | 121 | 8 | 153 | 11 | 66 | 6 | |||||||||||||||||||||||||||||
| Case 2: Schools closed after 10 symptomatic cases, compliance 90% | |||||||||||||||||||||||||||||||||||||
| Average | 100 | 3,877 | 113 | 326 | 48 | 99 | 3,916 | 114 | 329 | 48 | |||||||||||||||||||||||||||
| SD | 468 | 22 | 64 | 13 | 259 | 19 | 56 | 12 | |||||||||||||||||||||||||||||
| % reduction from base case | 21 | -40 | 53 | -36 | 22 | -39 | 53 | -34 | |||||||||||||||||||||||||||||
| Case 3: Schools closed after 10 symptomatic cases, nonschool contacts doubled, compliance 90% | |||||||||||||||||||||||||||||||||||||
| Average | 100 | 5,604 | 76 | 850 | 34 | 95 | 5,898 | 79 | 894 | 35 | |||||||||||||||||||||||||||
| SD | 1,293 | 18 | 206 | 9 | 122 | 10 | 72 | 6 | |||||||||||||||||||||||||||||
| % reduction from base case | -14 | 6 | -24 | 4 | -18 | 4 | -27 | 2 | |||||||||||||||||||||||||||||
| Case 4: Schools closed after 10 symptomatic cases, children and teenagers kept home, household contacts doubled, compliance 90% | |||||||||||||||||||||||||||||||||||||
| Average | 100 | 341 | 60 | 43 | 16 | 93 | 361 | 62 | 45 | 17 | |||||||||||||||||||||||||||
| SD | 209 | 25 | 20 | 12 | 203 | 24 | 19 | 12 | |||||||||||||||||||||||||||||
| % reduction from base case | 93 | 26 | 94 | 53 | 93 | 25 | 94 | 52 | |||||||||||||||||||||||||||||
| Case 5: Schools closed after 10 symptomatic cases, children and teenagers kept home, household contacts doubled, compliance 50% | |||||||||||||||||||||||||||||||||||||
| Average | 100 | 1,551 | 135 | 90 | 47 | 95 | 1,630 | 141 | 94 | 49 | |||||||||||||||||||||||||||
| SD | 692 | 49 | 40 | 31 | 614 | 42 | 37 | 30 | |||||||||||||||||||||||||||||
| % reduction from base case | 68 | -67 | 87 | -33 | 68 | -72 | 87 | -36 | |||||||||||||||||||||||||||||
| Case 6: Schools closed after 10 symptomatic cases, children kept home, household contacts doubled, compliance 90% | |||||||||||||||||||||||||||||||||||||
| Average | 100 | 2,539 | 116 | 199 | 49 | 96 | 2,642 | 120 | 206 | 51 | |||||||||||||||||||||||||||
| SD | 661 | 30 | 66 | 17 | 433 | 23 | 56 | 14 | |||||||||||||||||||||||||||||
| % reduction from base case | 48 | -44 | 71 | -38 | 47 | -46 | 71 | -40 | |||||||||||||||||||||||||||||
| Case 7: All with symptomatic cases stay at home, compliance 90% | |||||||||||||||||||||||||||||||||||||
| Average | 100 | 3,692 | 91 | 408 | 41 | 94 | 3,926 | 95 | 433 | 43 | |||||||||||||||||||||||||||
| SD | 1,031 | 25 | 130 | 14 | 458 | 17 | 85 | 10 | |||||||||||||||||||||||||||||
| % reduction from base case | 25 | -12 | 41 | -16 | 22 | -16 | 38 | -20 | |||||||||||||||||||||||||||||
*Cases 2–7 are targeted social distancing strategies. Negative percent reductions reflect percent increases. Epidemics are defined as >100 infected. SD, standard deviation.
Unmitigated Base Case
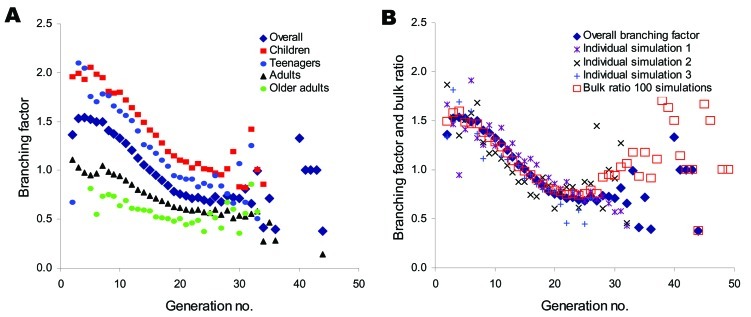
The sequence of infected persons can be represented as an expanding network of infectious transmissions (Figure 4). The number of secondary infections produced by an infected person, or branching factor, is easily visualized within the infectious contact network. The average branching factor depends on the person's age class and generation during the epidemic (Figure 5A). The maximum value within the first 10 generations is 2.05 (standard deviation [SD] 0.57) for children, 2.09 (SD 0.72) for teenagers, 1.11 (SD 0.43) for adults, 0.81 (SD 0.47) for older adults, and 1.54 (SD 0.36) for the entire population. Variability (large SD, especially for specific age classes) reflects the heterogeneity inherent within community contact networks of this size (Figure 5B).
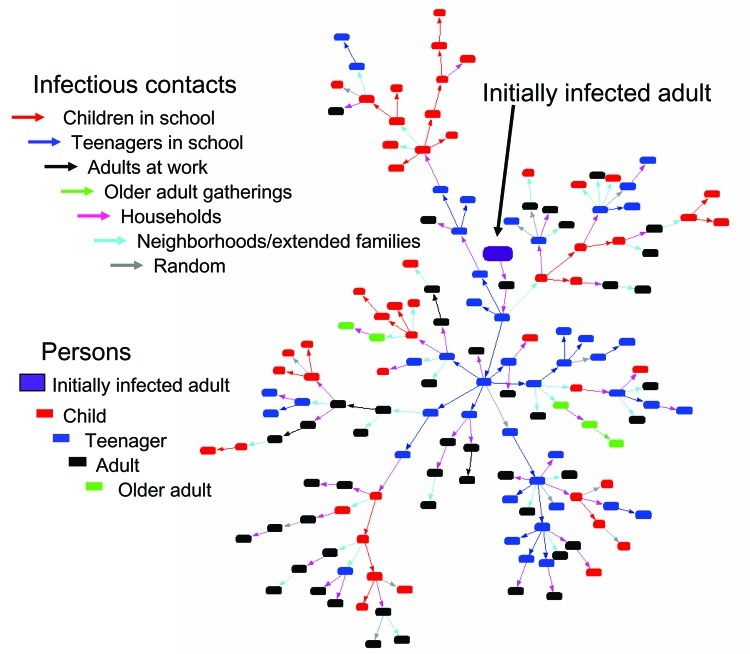
Figure 4.
Initial growth of an infectious contact network. Colored rectangles denote persons of designated age class, and colored arrows denote groups within which the infectious transmission takes place. In this example, from the adult initial seed (large purple rectangle), 2 household contacts (light purple arrows) bring influenza to the middle or high school (blue arrows) where it spreads to other teenagers. Teenagers then spread influenza to children in households who spread it to other children in the elementary schools. Children and teenagers form the backbone of the infectious contact network and are critical to its spread; infectious transmissions occur mostly in the household, neighborhood, and schools.
Figure 5.
Branching factor and the approximation of the reproductive number Ro. A) Overall and age class–specific branching factors as a function of generation averaged over 100 simulations. The standard deviations of these averages can be large (<0.72 at the peak value for teenagers) and reflect the heterogeneity within the person contact networks and from community to community. B) Branching factors for overall average and 3 example simulations compared with the bulk ratio of infections in a generation to those in the previous generation pooled across 100 simulations. We chose the maximum value of the bulk ratio (1.6) as an approximation of the reproductive number Ro.
The backbone of infectious contact networks is formed primarily of children and teenagers with infectious transmissions mostly in the household, neighborhood, and schools. Infectious transmissions are highest in households without older adults (39%, SD 3%), followed by extended families or neighborhoods (25%, SD 1%), schools (19%, SD 1%), work (7%, SD 2%), combined random groups (9%, SD 2%), and households with older adults (1%, SD 0.1%). On average, 78% (SD 2%) of children and 71% (SD 3%) of teenagers become infected. Adults (attack rate 44% of adults, SD 2%) get influenza mainly from children, teenagers, and other adults within the family. Older adults, who contact children and teenagers only through the extended family or neighborhoods and the random overall network, are relatively isolated (attack rate 23% of older adults, SD 2%).
Children and teenagers compose only 29% of the population yet they are responsible for 59% (SD 4.5%) of infectious contacts, adults for 38% (SD 7.9%), and older adults for 3% (SD 0.6%) (Table 3). Approximately half of infectious contacts of either children or teenagers are within the same age class (19%, SD 0.8% and 9%, SD 0.7%, respectively). Adults get influenza from children or teenagers at approximately the same frequency (24%, SD 1.6%) as from other adults (26%, SD 5.9%). Older adults are equally likely to get influenza from children or teenagers as from adults or older adults (2%, SD 0.3%). Transmission to children or teenagers from adults is 10% (SD 1.8%) and nearly none by older adults. These transmission results are supported by recent field studies that show children who go to preschool or school are more likely to contact influenza and their family members are also more likely to become ill (17,18) as well as that a person is also more likely to be infected when exposed to children or teenagers than to adults (14).
Table 3. Unmitigated base case infectious contact fractions (% of the total no. of infectious contacts) between age classes*.
| Class | To children | SD | To teenagers | SD | To adults | SD | To older adults | SD | Total | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| From children | 18.6 | 0.8 | 2.9 | 0.3 | 16.1 | 1.1 | 1.2 | 0.2 | 38.8 | 2.4 |
| From teenagers | 2.4 | 0.8 | 9.1 | 0.7 | 8.0 | 0.5 | 0.6 | 0.1 | 20.1 | 2.1 |
| From adults | 6.0 | 0.6 | 3.8 | 1.2 | 26.0 | 5.9 | 2.1 | 0.4 | 38.0 | 7.9 |
| From older adults | 0.2 | 0.1 | 0.2 | 0.1 | 0.9 | 0.9 | 1.8 | 0.3 | 3.1 | 0.6 |
| Total | 27.3 | 2.2 | 16.0 | 2.2 | 50.9 | 7.7 | 5.8 | 0.9 |
*SD, standard deviation.
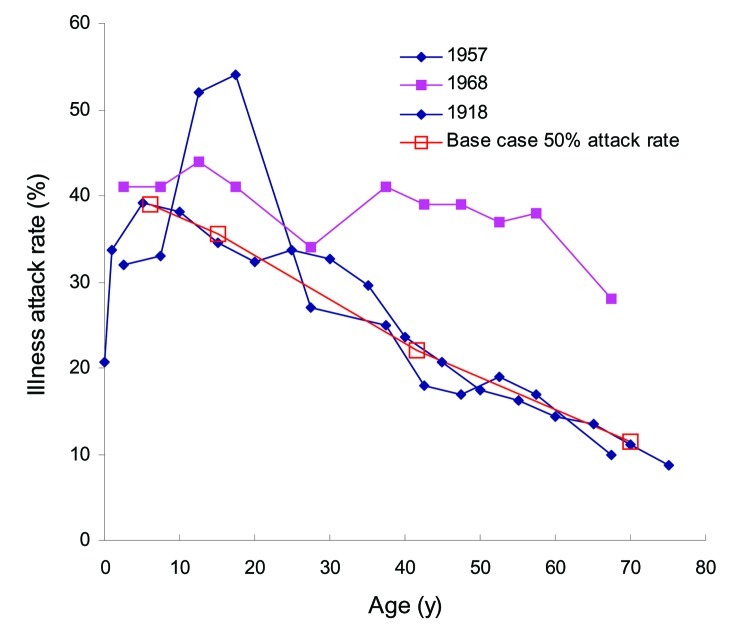
Reasonable correspondence is observed (Figure 6) between age class–specific attack rates and those of past pandemics (19–21). Infections transmitted within each environment are also consistent with other simulation studies (10–14). The maximum value of the overall branching factor (Figure 5) reflects the often-cited reproductive number Ro. However, how Ro should be calculated from small-community data such as ours is ambiguous (10,11,14). To estimate Ro, we pooled results across 100 communities (simulations) to reflect a population of 1 million (Figure 5B). The maximum value of the bulk ratio (new infections to old) within the first 10 generations is 1.6, and we choose it as our estimate of Ro. An Ro of 1.6 with an attack rate of 50% matches recent pandemic simulation results (10,14) and lies within the range (1.5–1.7) for the 1957–58 influenza pandemic (Figure 5B) (10).
Figure 6.
Comparison of simulated age class–specific illness attack rates with past pandemics. Simulated illness attack rates (half the infectious attack rate) for the unmitigated base case are close to those found in studies of historic pandemics in 1957 (19), 1968 (20), and 1918 (21). Notable differences are the 1968 Hong Kong flu, which had less effect on youth and 1957–58 Asian flu, which had greater effect; however, historic data are inherently uncertain. Closer correspondence to either of these 2 cases could be achieved through changes in IA or SA or modification of the underlying social contact network (see Results) because the network was likely different from that of a small town of today.
Base Case–Targeted Social Distancing
High infectiousness and a high number of contacts, many like-to-like, create a zone of high infectious contact centered on children and teenagers within the community's social network. Targeting this zone can protect the community at large.
First, we examined closing schools. Although contacts in classes are removed, those in all other groups may increase because children and teenagers spend more time at home, in neighborhoods, with friends, and in public spaces. We assume that school closure at a minimum doubles household contacts. Closing schools with 90% compliance the day after 10 symptomatic cases reduces the attack rate to 22% (Table 2). However, if we assume that school closure doubles all link contact frequencies for children or teenagers within their nonschool groups, attack rates are increased by 18% (Table 2).
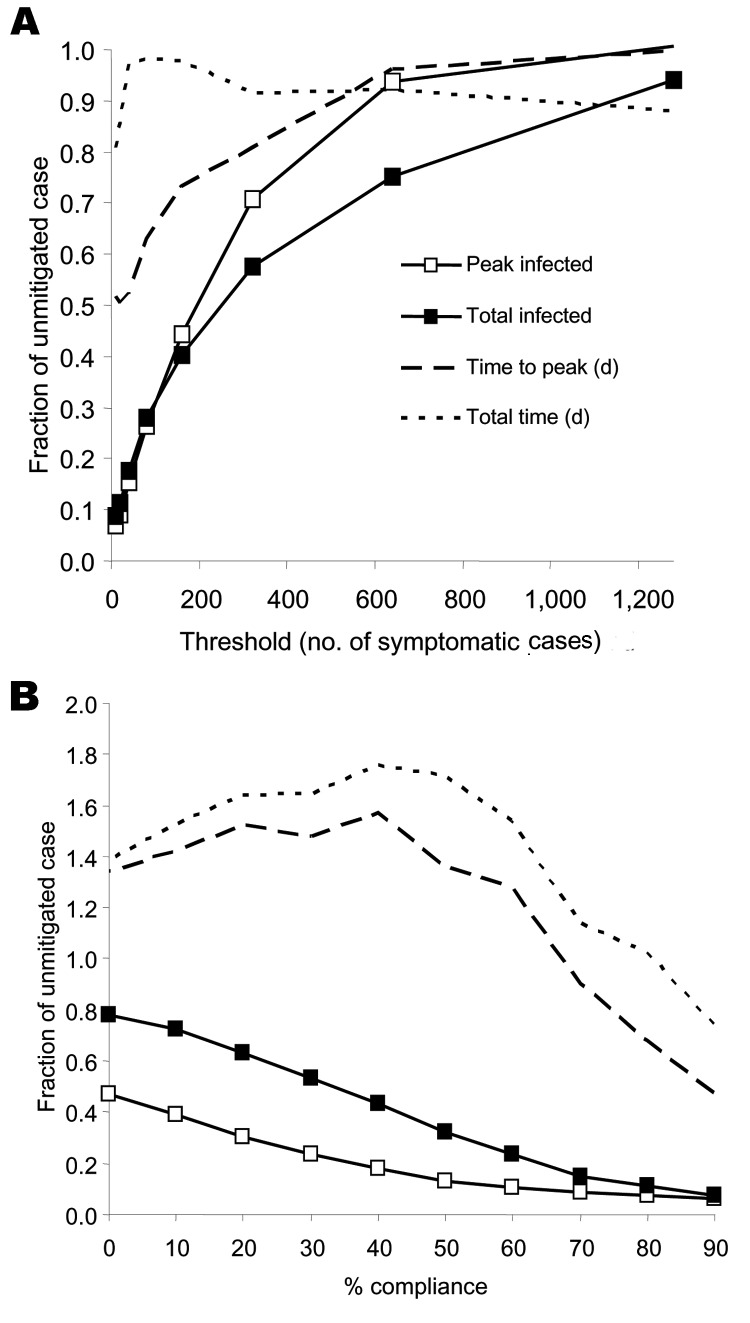
Alternatively, we send all children and teenagers home after school closure to remain for the duration of the pandemic. Now contact frequencies are reduced by 90% for all groups that contain only children or teenagers (classes and their random networks) and doubled, as before, for children or teenagers in households. In the extended family or neighborhood and the random overall networks, child or teenager contact frequencies are also reduced by 90%. Thus, although children and teenagers are restricted to the home, adults and older adults go about their day-to-day routines, except that they avoid children or teenagers who are not household members. Imposing this strategy the day after 10 symptomatic cases reduces attack rates by 93% (Table 2). Waiting until 80 symptomatic cases reduces attack rates by 73% (Figure 7A).
Figure 7.
Fraction of unmitigated base case attack rate for targeted social distancing of children and teenagers as a function of A) implementation policy threshold given by the number of symptomatic cases (compliance at 90%) and B) compliance with staying at home (implementation policy threshold at 10 symptomatic cases, 0% compliance closes schools alone). Each point represents the average of simulations of 100 that yielded epidemics (>100 infected). Standard deviations for variation of threshold are <3% of the total population. However, for compliance variation, standard deviations increase to a maximum of 7% of the total population at a compliance of 30%.
To evaluate the tradeoff between effectiveness and public compliance, we reduced the percentage of nonschool and nonhousehold contacts that have their frequencies reduced with the child and teenager stay-at-home policy (Figure 7B). At 50% compliance, attack rates can be reduced by 68% (Table 2). Reduction in compliance also increases the time scales for the epidemic. Epidemics lengthen above the base case and reach a factor of ≈1.8 at 40% compliance (Figure 7B).
Other social distancing strategies can be considered. Because children outnumber teenagers and children are more infective and susceptible, what happens if only children are distanced, while teenagers attend school and follow their usual routines? At 90% compliance, this strategy reduces the attack rates by 47% (Table 2). What if all sick persons remain at home when symptomatic? At 90% compliance this strategy reduces attack rates by 20% (<25% of infectious persons are influenced as pS × pH = 0.25 for adults only) (Table 2).
More Infective Strains and Contact Networks with Less Emphasis on the Young
We have modeled an influenza strain with an infectivity representative of the 1957–58 Asian influenza pandemic and a social contact network reflective of a stylized US town. Although results for the unmitigated base case yield age class–specific attack rates similar to those for past epidemics (Figure 6), will the targeted social distancing strategies found above remain effective if 1) the strain is more infective or 2) the importance of the young is deemphasized?
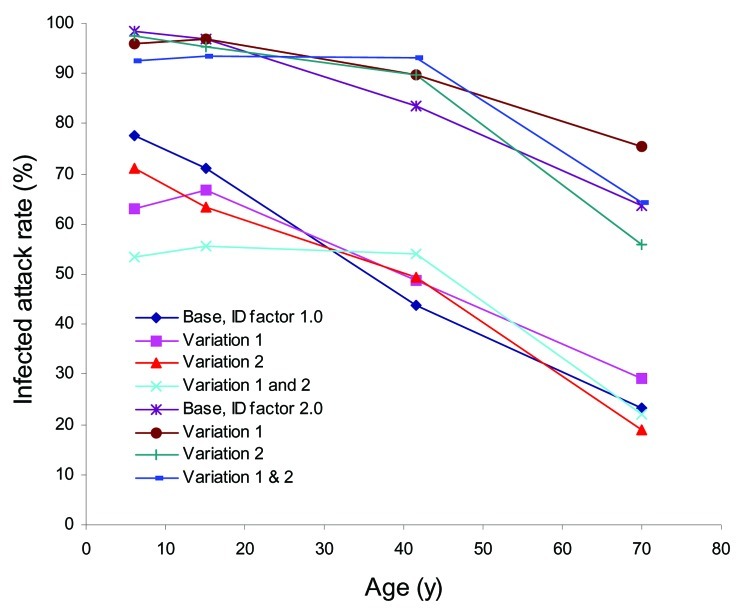
To explore these questions, we considered 3 increases in disease infectivity ID by factors of 1.25 (attack rate ≈66%, Ro ≈ 1.8), 1.5 (attack rate ≈75%, Ro ≈ 2.0), and 2.0 (attack rate ≈86%, Ro ≈2.4). These increases encompass and exceed the 1918–19 Spanish influenza pandemic (Ro 1.8–2.0) (10). We also sequentially removed enhanced transmission by children and teenagers and thus the zone of high infectious contact that we have designed social distancing strategies to target. We created 3 variations: the first removed relative infectivity and susceptibility enhancement of children and teenagers (IA and SA 1.0) (variation 1); the second increased frequency of contact within the work environment by a factor of 4 to give adults the same number of contacts as younger persons (variation 2); and the third combined variations 1 and 2. For each of the resulting set of 4 cases (base, variation 1, variation 2, and variation 1 and 2), ID was altered slightly to maintain the reference of ≈50% infected attack rate for Ro ≈1.6.
As ID increases, age specific–attack rates increase (Table 4). As we remove differences in the number of contacts and/or relative infectivity and susceptibility (IA, SA) between young and adults, the infected attack rates systematically shift from young persons to adults (Figure 8). These results suggest that for such situations, social distancing strategies must be devised that focus on more than children and teenagers alone.
Table 4. Unmitigated case results for Ro and average attack rates (%) for increasing ID and base case, variation 1, variation 2, and variations 1 and 2 combined*.
| Type | ID factor | Ro | Attack rates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | SD | Children | SD | Teenagers | SD | Adults | SD | Older adults | SD | |||
| Base case | 1.0 | 1.6 | 51 | 1.7 | 79 | 2.3 | 72 | 2.7 | 45 | 1.8 | 23 | 2.0 |
| 1.25 | 1.8 | 66 | 1.2 | 90 | 1.1 | 85 | 1.7 | 61 | 1.5 | 36 | 2.1 | |
| 1.5 | 2.0 | 75 | 0.7 | 95 | 0.6 | 92 | 1.1 | 71 | 0.9 | 47 | 1.9 | |
| 2.0 | 2.4 | 86 | 0.6 | 99 | 0.3 | 97 | 0.5 | 84 | 0.7 | 64 | 2.0 | |
| Variation 1 | 1.0 | 1.5 | 52 | 2.2 | 65 | 2.8 | 68 | 3.2 | 50 | 2.2 | 30 | 2.5 |
| 1.25 | 1.7 | 70 | 1.3 | 82 | 1.4 | 84 | 1.8 | 68 | 1.5 | 47 | 2.3 | |
| 1.5 | 1.9 | 80 | 0.8 | 90 | 1.0 | 91 | 0.9 | 79 | 0.9 | 60 | 2.0 | |
| 2.0 | 2.4 | 90 | 0.5 | 96 | 0.6 | 97 | 0.7 | 90 | 0.6 | 76 | 1.6 | |
| Variation 2 | 1.0 | 1.5 | 52 | 1.9 | 72 | 2.6 | 64 | 2.9 | 50 | 2.1 | 19 | 1.7 |
| 1.25 | 1.8 | 68 | 1.0 | 87 | 1.3 | 81 | 1.6 | 68 | 1.3 | 31 | 2.1 | |
| 1.5 | 1.9 | 78 | 0.8 | 93 | 0.9 | 89 | 1.2 | 79 | 1.0 | 41 | 2.2 | |
| 2.0 | 2.3 | 88 | 0.5 | 98 | 0.4 | 96 | 0.7 | 90 | 0.7 | 57 | 1.9 | |
| Variations 1 and 2 combined | 1.0 | 1.5 | 52 | 2.0 | 55 | 2.3 | 57 | 2.7 | 56 | 2.3 | 23 | 1.7 |
| 1.25 | 1.8 | 70 | 1.1 | 74 | 1.8 | 76 | 1.9 | 75 | 1.2 | 37 | 2.0 | |
| 1.5 | 2.0 | 80 | 0.8 | 84 | 1.2 | 85 | 1.2 | 85 | 0.8 | 48 | 2.0 | |
| 2.0 | 2.4 | 90 | 0.4 | 93 | 0.6 | 94 | 0.9 | 94 | 0.5 | 65 | 1.8 | |
*Variation 1 is removal of relative infectivity and susceptibility; variation 2 is an increase in work group frequency of contact to give all children, teenagers, and adults the same overall contact frequencies. Average attack rates accumulate over only those simulations that resulted in epidemics (>100 infected). RO, reproductive number; ID, disease infectivity; SD, standard deviation.
Figure 8.
Unmitigated age-specific attack rate results for disease infectivity (ID) factors of 1.0 and 2.0 and base case, variation 1 (removal of relative infectivity and susceptibility), variation 2 (increase in work group frequency of contact to give all children, teenagers, and adults the same overall contact frequencies), and variations 1 and 2 combined. Illness attack rates shown in Figure 6 are half these values.
To find effective targeted social distancing strategy combinations across the range of disease infectivity and infectious contact networks, we formulated 5 strategies and applied them individually and in combination: 1) school closure (S) where the contact frequency within schools was reduced 90% and children and teenager's household contacts were doubled; 2) children and teenagers social distancing (CTsd) where their contact frequencies in all nonhousehold and nonschool groups were reduced 90% and their household contacts doubled; 3) adult and older adult social distancing (AOAsd) where their contact frequencies in all nonhousehold and nonwork groups were reduced 90% and household contacts doubled; 4) liberal leave (LL), where all children and teenagers and 90% of adults withdraw to the home when symptomatic; and 5) work social distancing (Wsd) where the contact frequency within work groups was reduced 50%. For each combination, we implemented the strategy(ies) the day after 10 symptomatic cases and conducted 100 simulations.
As ID increases, more strategies must be combined to keep the attack rate <10% (Table 5, shaded values). As children and teenagers become less prominent, targeting adults becomes important, even at an ID factor of 1. For an ID factor of 1.5 (as infective as the 1918-19 Spanish influenza pandemic) and across all variations, both the young and adults must be targeted and all strategies must be implemented to effectively mitigate the epidemic. However, for an ID factor of 2.0, we can at best reduce the attack rate to 20 –40% through full strategy combination, not ideal but still a significant benefit.
Table 5. Mitigated case average attack rates (%) for increasing ID and base case, variation 1, variation 2, and variations 1 and 2 combined*.
| No. | Strategy combination |
Base case ID factor |
Variation 2 ID factor |
Variation 1 ID factor |
Variations 1 and 2 ID factor |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | CTsd | AOAsd | LL | Wsd | 1 | 1.25 | 1.5 | 2 | 1 | 1.25 | 1.5 | 2 | 1 | 1.25 | 1.5 | 2 | 1 | 1.25 | 1.5 | 2 | |
| 1 | 51 | 66 | 75 | 86 | 52 | 68 | 78 | 88 | 52 | 70 | 80 | 90 | 52 | 70 | 80 | 90 | |||||
| 2 | Wsd | 48 | 63 | 72 | 84 | 41 | 60 | 71 | 83 | 47 | 66 | 77 | 88 | 35 | 58 | 72 | 86 | ||||
| 3 | LL | 41 | 57 | 67 | 79 | 37 | 57 | 68 | 82 | 36 | 57 | 70 | 84 | 28 | 55 | 69 | 84 | ||||
| 4 | LL | Wsd | 39 | 55 | 65 | 78 | 30 | 49 | 62 | 77 | 30 | 53 | 67 | 82 | 12 | 42 | 60 | 78 | |||
| 5 | AOAsd | 38 | 51 | 59 | 70 | 40 | 58 | 68 | 79 | 25 | 46 | 58 | 72 | 33 | 56 | 69 | 80 | ||||
| 6 | AOAsd | Wsd | 35 | 48 | 56 | 66 | 30 | 47 | 58 | 71 | 18 | 39 | 51 | 66 | 9 | 37 | 53 | 71 | |||
| 7 | AOAsd | LL | 32 | 46 | 55 | 66 | 28 | 48 | 60 | 73 | 13 | 36 | 50 | 67 | 11 | 40 | 57 | 74 | |||
| 8 | AOAsd | LL | Wsd | 30 | 43 | 52 | 63 | 21 | 40 | 51 | 66 | 10 | 32 | 46 | 62 | 4 | 23 | 42 | 64 | ||
| 9 | CTsd | 41 | 58 | 69 | 82 | 45 | 64 | 75 | 86 | 41 | 63 | 76 | 88 | 46 | 67 | 78 | 88 | ||||
| 10 | CTsd | Wsd | 37 | 55 | 66 | 79 | 31 | 53 | 66 | 80 | 32 | 57 | 71 | 85 | 21 | 52 | 67 | 83 | |||
| 11 | CTsd | LL | 29 | 48 | 60 | 75 | 26 | 50 | 64 | 78 | 20 | 47 | 63 | 80 | 19 | 49 | 65 | 81 | |||
| 12 | CTsd | LL | Wsd | 27 | 45 | 57 | 72 | 16 | 40 | 55 | 72 | 14 | 41 | 58 | 77 | 6 | 32 | 53 | 74 | ||
| 13 | CTsd | AOAsd | 29 | 46 | 56 | 68 | 34 | 55 | 66 | 78 | 15 | 40 | 54 | 70 | 27 | 54 | 67 | 79 | |||
| 14 | CTsd | AOAsd | Wsd | 26 | 42 | 52 | 64 | 20 | 41 | 54 | 69 | 9 | 31 | 45 | 63 | 5 | 30 | 50 | 69 | ||
| 15 | CTsd | AOAsd | LL | 22 | 39 | 51 | 64 | 18 | 42 | 56 | 72 | 7 | 29 | 45 | 64 | 7 | 35 | 55 | 73 | ||
| 16 | CTsd | AOAsd | LL | Wsd | 20 | 37 | 48 | 61 | 10 | 32 | 47 | 63 | 5 | 22 | 39 | 58 | 3 | 16 | 37 | 61 | |
| 17 | S | 41 | 61 | 73 | 85 | 45 | 66 | 77 | 87 | 47 | 68 | 79 | 90 | 51 | 69 | 80 | 90 | ||||
| 18 | S | Wsd | 36 | 57 | 70 | 83 | 30 | 54 | 68 | 83 | 38 | 62 | 75 | 88 | 29 | 56 | 71 | 85 | |||
| 19 | S | LL | 23 | 47 | 62 | 78 | 23 | 49 | 65 | 80 | 20 | 50 | 66 | 83 | 22 | 51 | 67 | 83 | |||
| 20 | S | LL | Wsd | 19 | 44 | 59 | 76 | 9 | 38 | 55 | 74 | 13 | 44 | 62 | 80 | 6 | 35 | 55 | 76 | ||
| 21 | S | AOAsd | 26 | 47 | 59 | 74 | 34 | 56 | 69 | 81 | 16 | 44 | 60 | 76 | 34 | 58 | 70 | 82 | |||
| 22 | S | AOAsd | Wsd | 20 | 41 | 55 | 70 | 14 | 41 | 57 | 73 | 8 | 35 | 52 | 71 | 7 | 36 | 55 | 74 | ||
| 23 | S | AOAsd | LL | 11 | 35 | 51 | 68 | 12 | 40 | 57 | 74 | 5 | 28 | 48 | 69 | 8 | 38 | 57 | 75 | ||
| 24 | S | AOAsd | LL | Wsd | 9 | 32 | 47 | 65 | 5 | 27 | 45 | 66 | 4 | 20 | 41 | 64 | 3 | 14 | 39 | 65 | |
| 25 | S | CTsd | 4 | 26 | 50 | 73 | 15 | 47 | 64 | 80 | 12 | 46 | 64 | 82 | 34 | 58 | 71 | 84 | |||
| 26 | S | CTsd | Wsd | 3 | 15 | 40 | 68 | 3 | 21 | 46 | 71 | 5 | 32 | 55 | 78 | 6 | 36 | 56 | 77 | ||
| 27 | S | CTsd | LL | 2 | 7 | 29 | 60 | 3 | 21 | 45 | 70 | 3 | 17 | 43 | 70 | 5 | 33 | 54 | 75 | ||
| 28 | S | CTsd | LL | Wsd | 2 | 6 | 20 | 54 | 2 | 6 | 24 | 57 | 2 | 9 | 31 | 64 | 2 | 9 | 33 | 64 | |
| 29 | S | CTsd | AOAsd | 2 | 4 | 13 | 44 | 4 | 24 | 48 | 70 | 2 | 4 | 15 | 49 | 8 | 37 | 56 | 73 | ||
| 30 | S | CTsd | AOAsd | Wsd | 2 | 3 | 7 | 30 | 2 | 5 | 16 | 49 | 2 | 3 | 6 | 28 | 2 | 5 | 20 | 54 | |
| 31 | S | CTsd | AOAsd | LL | 2 | 3 | 9 | 34 | 2 | 7 | 27 | 58 | 2 | 3 | 7 | 36 | 3 | 11 | 35 | 63 | |
| 32 | S | CTsd | AOAsd | LL | Wsd | 2 | 3 | 6 | 25 | 2 | 3 | 8 | 37 | 2 | 2 | 5 | 20 | 2 | 3 | 9 | 39 |
*Variation 2 is an increase in work group frequency of contact to give all children, teenagers, and adults the same overall contact frequencies; variation 1 is removal of relative infectivity and susceptibility. ID, disease infectivity; S, school closure; CTsd, children and teenagers social distancing; AOAsd, adults and older adults social distancing; LL, liberal leave; Wsd, work social distancing. Shaded numbers denote strategy combinations that reduce the attack rate to <10% of the population (illness attack rate <5%). Average attack rates accumulate over only those simulations that resulted in epidemics (>100 infected). Average standard deviation across the entire set of simulations was 2.2% with a maximum of 7.6%.
Discussion
Results for our stylized small town suggest that targeted social distancing strategies can be designed to effectively mitigate the local progression of pandemic influenza without the use of vaccine or antiviral drugs. For an infectivity similar to that of the 1957–58 Asian influenza pandemic, targeting children and teenagers, by not only closing schools but also by keeping these age classes at home, was effective. However, given uncertainty in the infectivity of the influenza strain, underlying social contact network, or relative infectivity/susceptibility of the young versus adults, planning to implement strategies that also target adults and the work environment is prudent. To mitigate a strain with infectivity similar to that of the 1918–19 Spanish influenza pandemic, simulations suggest that all young and adults must be targeted regardless of the likely enhanced transmission by the young.
Implementation of social distancing strategies is challenging. They likely must be imposed for the duration of the local epidemic and possibly until a strain-specific vaccine is developed and distributed. If compliance with the strategy is high over this period, an epidemic within a community can be averted. However, if neighboring communities do not also use these interventions, infected neighbors will continue to introduce influenza and prolong the local epidemic, albeit at a depressed level more easily accommodated by healthcare systems.
Our design approach explicitly implements disease-host interaction within the social contact network where the disease spreads. Measuring contact networks within communities for the spread of infectious diseases requires focused research that combines sociology, public health, and epidemiology. Such networks will likely differ across cultures, between urban and rural communities, and with community size. With the aid of detailed demographic data, expert elicitation of social scientists and community members, behavioral surveys, and possibly experiments, a network could be constructed for any community of interest. Configurations that consider, for example, college campuses or military reservations may be of use given that the highest death rate of any group in the 1918–19 Spanish influenza pandemic was in young adults (22).
Acknowledgments
We thank Louise Maffitt, Paul Kaplan, Nancy Brodsky, Theresa Brown, George Barr, Richard Hatchett, Carter Mecher, and Neil Ferguson for discussions and suggestions.
This research was supported by the National Infrastructure Simulation and Analysis Center, a program of the Department of Homeland Security's Infrastructure Protection/Risk Management Division composed of a core partnership of Sandia National Laboratories and Los Alamos National Laboratory. Sandia is operated by Sandia Corporation, a Lockheed Martin Company of the US Department of Energy's National Nuclear Security Administration under contract DE-AC04-94AL85000.
Biography
Dr RJ Glass is a technical staff member at Sandia National Laboratories. His research focus is self-organized spatial/temporal pattern with current interest in complex adaptive infrastructures and behavioral systems.
Footnotes
Suggested citation for this article: Glass RJ, Glass LM, Beyeler WE, Min HJ. Targeted social distancing designs for pandemic influenza. Emerg Infect Dis [serial on the Internet]. 2006 Nov [date cited]. http://dx.doi.org/10.3201/eid1211.060255
References
- 1.World Health Organization. Avian influenza frequently asked questions. Geneva: The Organization; 2005. [PubMed] [Google Scholar]
- 2.Check E. Avian flu special: is this our best shot? Nature. 2005;435:404–6. 10.1038/435404a [DOI] [PubMed] [Google Scholar]
- 3.US Homeland Security Council. National strategy for pandemic influenza: implementation plan. Washington: US Department of Homeland Security; 2006. [Google Scholar]
- 4.US Department of Health and Human Services. Pandemic influenza plan HHS. Washington: The Department; 2005. [Google Scholar]
- 5.Glass RJ, Glass LM, Beyeler WE. Local mitigation strategies for pandemic influenza: prepared for the Department of Homeland Security under the National Infrastructure Simulation and Analysis Center. Report no. SAND2005–7955J. Washington: Department of Homeland Security; 2005.
- 6.Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature. 1998;393:440–2. 10.1038/30918 [DOI] [PubMed] [Google Scholar]
- 7.Palla G, Deranyi I, Farkas I, Vicsek T. Uncovering the overlapping community structure of complex networks in nature and society. Nature. 2005;435:814–8. 10.1038/nature03607 [DOI] [PubMed] [Google Scholar]
- 8.Newman ME, Park J. Why social networks are different from other types of networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:036122. 10.1103/PhysRevE.68.036122 [DOI] [PubMed] [Google Scholar]
- 9.US Census Bureau. United States Census 2000. Washington: The Bureau; 2000. [Google Scholar]
- 10.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. 10.1038/nature04795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–14. 10.1038/nature04017 [DOI] [PubMed] [Google Scholar]
- 12.Longini IM, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159:623–33. 10.1093/aje/kwh092 [DOI] [PubMed] [Google Scholar]
- 13.Longini IM, Nizam A, Xu SF, Ungchusak K, Hanshaoworakul W, Cummings DA, et al. Containing pandemic influenza at the source. Science. 2005;309:1083–7. 10.1126/science.1115717 [DOI] [PubMed] [Google Scholar]
- 14.Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103:5935–40. 10.1073/pnas.0601266103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection: relation to symptom formation and host defense. J Clin Invest. 1998;101:643–9. 10.1172/JCI1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boelle PY. Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;23:3469–87. 10.1002/sim.1912 [DOI] [PubMed] [Google Scholar]
- 17.Viboud C, Boelle PY, Cauchemez S, Lavenu A, Valleron AJ, Flahault A, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54:684–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Principi N, Esposito S, Gasparini R, Marchisio P, Crovari P; Flu-Flu Study Group. Burden of influenza in healthy children and their households. Arch Dis Child. 2004;89:1002–7. 10.1136/adc.2003.045401 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Chin TD, Foley JF, Doto IL, Gravelle CR, Weston J. Morbidity and mortality characteristics of Asian strain influenza. Public Health Rep. 1960;75:148–58. 10.2307/4590751 [DOI] [PubMed] [Google Scholar]
- 20.Davis LE, Caldwell GG, Lynch RE, Bailey RE, Chin TD. Hong-Kong Influenza: the epidemiologic features of a high school family study analyzed and compared with a similar study during the 1957 Asian influenza epidemic. Am J Epidemiol. 1970;92:240–7. [DOI] [PubMed] [Google Scholar]
- 21.Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18:64–76. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Death rates for selected causes by 10–year age groups, race, and sex: death registration states. Tables 1900–39. Atlanta: The Centers; 2005. [Google Scholar]