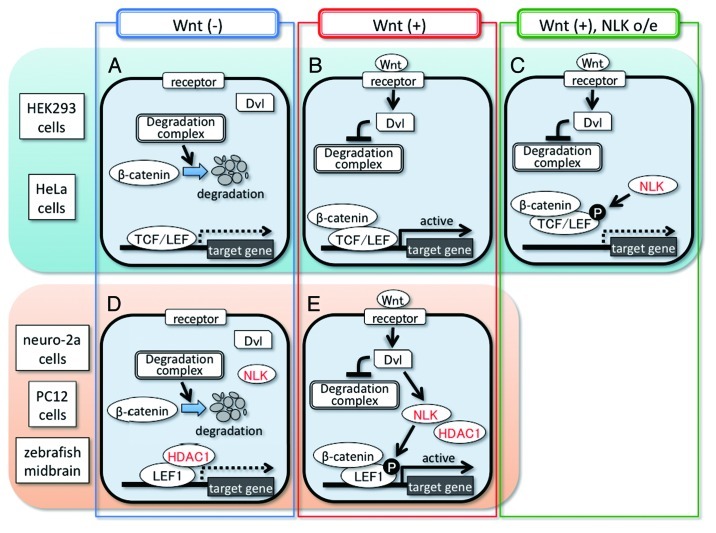
Wnt/β-catenin signaling controls cell proliferation and fate decision during embryogenesis and adult tissue homeostasis.1,2 In unstimulated cells, cytoplasmic β-catenin is destroyed by a degradation complex, and the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors represses the expression of Wnt/β-catenin signaling-target genes (Fig. 1A).1,2 The Wnt/β-catenin signaling is activated when the secreted glycoprotein Wnt binds to the cell surface receptor, thereby activating the cytoplasmic protein Dishevelled (Dvl). Activated Dvl inhibits the β-catenin degradation complex, resulting in cytoplasmic β-catenin stabilization. Stabilized β-catenin forms complexes with TCF/LEF in the nucleus, which then activate gene expression (Fig. 1B).1,2
Figure 1. Cell context-dependent positive and negative regulation of Wnt/β-catenin signaling by NLK. In the unstimulated condition, β-catenin is destabilized by a degradation complex and TCF/LEF represses the target gene expression in all types of cells (A and D). HDAC1 strongly inhibits LEF1 transcriptional activity in neuro-2a and PC12 cells and zebrafish midbrain NPCs but not in HEK293 and HeLa cells (A and D). The binding of the Wnt ligand to the receptor induces Dvl activation in all types of cells (B and E). In HEK293 and HeLa cells, Dvl induces the formation of a β-catenin–TCF/LEF complex through inhibition of a degradation complex, resulting in activation of target gene expression (B). NLK overexpression inhibits the Wnt ligand-induced target gene expression by blocking its DNA-binding activity (C). In neuro-2a and PC12 cells and zebrafish midbrain NPCs, Wnt ligand-activated Dvl promotes the expression of Wnt/β-catenin signaling target genes through induction of not only the formation of the β-catenin–LEF1 complex but also LEF1 phosphorylation by NLK and consequent dissociation of LEF1 from HDAC1 (E).
Nemo-like kinase (NLK/Nlk) is an evolutionarily conserved serine/threonine kinase. In Caenorhabditis elegans, the NLK homolog LIT-1 regulates the activity of POP-1, a C. elegans homolog of TCF/LEF, in a context-dependent manner. In endoderm induction, LIT-1 inhibits POP-1 nuclear localization by phosphorylating POP-1.3,4 LIT-1 also functions as a positive regulator of POP-1 in the fate specification of gonadal precursor cells.5-7 However, the mechanism underlying this positive regulation is unclear. Negative regulation of TCF/LEF by NLK has also been observed in the mammalian cell lines, HEK293 and HeLa. In these cell lines, NLK overexpression inhibits the DNA-binding activity of the β-catenin–TCF/LEF complex through TCF/LEF phosphorylation, resulting in a reduction of its transcriptional activity (Fig. 1C).8-10 In contrast, positive regulation of TCF/LEF by NLK has not yet been observed in vertebrates. In addition, the roles of NLK in Wnt/β-catenin signaling in vertebrate development are also unclear.
To investigate the roles of NLK in Wnt/β-catenin signaling in vivo, we used zebrafish. Inhibition of Nlk2, a zebrafish NLK homolog, or Lef1, a member of the TCF/LEF family, decreased the expression of a Wnt/β-catenin signaling target gene, zic2a and proliferation of neural progenitor cells (NPCs) in the presumptive midbrain, resulting in a reduction of the midbrain tectum size.10 Nlk2 phosphorylated Lef1 at the conserved threonine residue, and Nlk2 knockdown in zebrafish embryos decreased Lef1 phosphorylation.10 Furthermore, the phenotype caused by Nlk2 knockdown was suppressed by the expression of a Lef1 mutant that mimics a constitutively phosphorylated state.10 Thus, we demonstrated that Nlk2 is required for Wnt/β-catenin signaling through Lef1 phosphorylation in zebrafish midbrain NPCs. We also found that, inhibition of Wnt-1 in zebrafish reduced the Lef1 phosphorylation levels and caused the midbrain size reduction phenotype similar to that of Nlk2- or Lef1-knockdowned embryos,10 suggesting that Nlk2 phosphorylates Lef1 downstream of Wnt-1.
We also discovered that NLK functions as a positive regulator of Wnt/β-catenin signaling in the NPC-like mammalian cell lines, mouse neuro-2a and rat PC12. In HeLa and HEK293 cells, co-expression of an N-terminal region-deleted constitutively active β-catenin mutant (β-cateninΔN) with LEF1 (a mammalian Lef1 homolog)-induced strong activation of a Wnt/β-catenin signaling-responsive reporter, TOPFLASH.8-10 Interestingly, in neuro-2a and PC12 cells, co-expression of β-cateninΔN with LEF1 did not activate the TOPFLASH reporter; however co-expression of NLK with β-cateninΔN and LEF1 efficiently activated the reporter in a manner dependent on its kinase activity,10 suggesting that both NLK activation and β-catenin stabilization are required for activating LEF1-mediated transcription in the NPC-like cell lines. Furthermore, we showed that, in these cell lines, either Wnt-3a signaling or Dvl1 overexpression activated NLK kinase activity, which in turn induced LEF1 phosphorylation and transcriptional activation.10 NLK RNAi blocked Wnt-3a- and Dvl1-induced LEF1 phosphorylation and transcriptional activation.10 These observations suggest that Dvl has two functions that serve to activate LEF1-mediated transcription in the Wnt signaling pathway. One function is NLK activation, resulting in LEF1 phosphorylation, and the other is β-catenin stabilization (Fig. 1E).
How NLK activates β-catenin–LEF1 complex-mediated transcription remained unknown. We found that histone deacetylase 1 (HDAC1) strongly interacted with LEF1 in neuro-2a and PC12 cells by a co-IP assay. NLK-mediated LEF1 phosphorylation reduced the binding of LEF1 with HDAC1.10 In addition, treatment with trichostatin A (TSA), a specific inhibitor of HDAC1, strongly activated the TOPFLASH reporter in the presence of β-cateninΔN and LEF1 in neuro-2a cells.10 Thus, we showed that NLK promotes LEF1 dissociation from HDAC1 by phosphorylating LEF1, resulting in LEF1-mediated transcriptional activation (Fig. 1D and E). Furthermore, HDAC1 knockdown reversed the reduction in Lef1-mediated transcriptional activity observed in the Nlk2-knockdowned zebrafish embryo midbrain,10 suggesting that Nlk2 also activates Lef1 by cancelling HDAC1-mediated Lef1 inhibition in the zebrafish midbrain.
Why NLK is required for activating LEF1-mediated transcription in zebrafish midbrain NPCs and NPC-like mammalian cell lines but not in HEK293 and HeLa cells was unclear. We found that the inhibitory effect of HDAC1 on LEF1-mediated transcription is relatively weak in HEK293 and HeLa cells.10 The difference in the effect of HDAC1 may answer the abovementioned question. However, the molecular mechanisms underlying these dual and opposite roles of NLK in Wnt/β-catenin signaling remain unclear. Comparison of LEF1-binding proteins and LEF1 phosphorylation sites between these cell lines will help elucidate this mechanism.
Our findings suggest that vertebrates have at least two Wnt/β-catenin signaling pathways: one is the NLK-independent pathway (Fig. 1A and B), and the other is the NLK-dependent pathway (Fig. 1D and E). NLK is not only expressed in the brain but also in the other tissues such as the liver and lung,10,11 suggesting the possibility that NLK-dependent Wnt/β-catenin signaling may function in these tissues.
Glossary
Abbreviations:
- Nemo-like kinase
NLK
- Lymphoid enhancer factor
LEF
- T cell factor
TCF
- Dishevelled
Dvl
- histone deacetylase 1
HDAC1
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20183
References
- 1.Clevers H. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Logan CY, et al. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.Meneghini MD, et al. Nature. 1999;399:793–7. doi: 10.1038/21666. [DOI] [PubMed] [Google Scholar]
- 4.Rocheleau CE, et al. Cell. 1999;97:717–26. doi: 10.1016/S0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- 5.Herman M. Development. 2001;128:581–90. doi: 10.1242/dev.128.4.581. [DOI] [PubMed] [Google Scholar]
- 6.Siegfried KR, et al. Development. 2002;129:443–53. doi: 10.1242/dev.129.2.443. [DOI] [PubMed] [Google Scholar]
- 7.Siegfried KR, et al. Genetics. 2004;166:171–86. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishitani T, et al. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 9.Ishitani T, et al. Mol Cell Biol. 2003;23:131–9. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ota S, et al. EMBO J. 2012 In Press. [Google Scholar]
- 11.Brott BK, et al. Proc Natl Acad Sci U S A. 1998;95:963–8. doi: 10.1073/pnas.95.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]