Summary
Background
We have completed a randomised clinical trial of breastfeeding and formula feeding to identify the frequency of breastmilk transmission of HIV-1 to infants. However, we also analysed data from this trial to examine the effect of breastfeeding on maternal death rates during 2 years after delivery. We report our findings from this secondary analysis.
Methods
Pregnant women attending four Nairobi city council clinics were offered HIV tests. At about 32 weeks’ gestation, 425 HIV-1 seropositive women were randomly allocated to either breastfeed or formula feed their infants. After delivery, mother-infant pairs were followed up monthly during the first year and quarterly during the second year until death, or 2 years after delivery, or end of study.
Findings
Mortality among mothers was higher in the breastfeeding group than in the formula group (18 vs 6 deaths, log rank test, p=0·009). The cumulative probability of maternal death at 24 months after delivery was 10·5% in the breastfeeding group and 3·8% in the formula group (p=0·02). The relative risk of death for breastfeeding mothers versus formula feeding mothers was 3·2 (95% CI 1·3–8·1, p=0·01). The attributable risk of maternal death due to breastfeeding was 69%. There was an association between maternal death and subsequent infant death, even after infant HIV-1 infection status was controlled for (relative risk 7·9, 95% CI 3·3–18·6, p<0·001).
Interpretation
Our findings suggest that breastfeeding by HIV-1 infected women might result in adverse outcomes for both mother and infant.
Introduction
From 1992 to 1998, we did a randomised clinical trial of breastfeeding and formula feeding among infants of HIV-1 infected women in Nairobi, Kenya.1 We showed that the rate of breastmilk transmission of HIV-1 was 16·2%, and that breastfeeding accounted for 44% of all transmitted infection in the breastfeeding group. Formula feeding is usual for HIV-1 infected women in the industrialised world, and is also recommended for infected women in less-developed countries who can give formula safely (eg, women with access to clean water).2,3 Despite the risk of HIV-1 transmission, however, many seropositive women in less-developed countries continue to breastfeed because of financial constraints, little access to clean water and sanitation, fear of stigmatisation, cultural practices, or inadequate health care infrastructure.
Another major intervention to prevent mother-to-child transmission of HIV-1 is use of antiretroviral drugs. Large reductions in HIV-1 transmission have been shown in populations in which infants are formula fed4,5 and smaller but substantial reductions have been noted in breastfed infants.6–8 Short-course zidovudine or nevirapine regimens could potentially be provided to large numbers of HIV-1 infected women in less-developed countries, and could result in a substantial fall in the proportion of children who acquire HIV-1 infection.
But prevention of mother-to-child transmission of HIV-1 is only one of several interventions required to increase the likelihood that children survive. Findings from other studies in Africa have shown a three-fold to four-fold increased risk of death in children whose mothers have died.9,10 Thus, an additional challenge is to increase the likelihood of long-term survival of children born to HIV-1 infected women by lengthening the survival of their mothers.
The issue of breastfeeding by HIV-1 infected women has focussed exclusively on risk of HIV-1 transmission and of infant mortality and morbidity in relation to various infant feeding options. Also, breastfeeding might result in adverse outcomes for the HIV-1 infected mother. Lactation is a demanding metabolic process, and might be especially detrimental for women who are infected with HIV-1. Although not an aim of our original study, we postulated that breastfeeding might be associated with excess mortality among HIV-1 infected mothers, and did a secondary analysis to examine this hypothesis. Our original study1 offered the opportunity to examine the effect of lactation on maternal death among HIV-1 seropositive women.
Methods
Clinical procedures
Between Nov 6, 1992, and Oct 7, 1997, pregnant women who attended four Nairobi city council antenatal clinics were offered counselling and tests for HIV. We invited HIV-1 seropositive women to participate in the study. Women who were willing for their choice of infant feeding practice to be decided for them by a randomization process were enrolled at about 32 weeks’ gestation.
At enrolment, we interviewed participants with a standardised questionnaire and did a physical examination. We took blood to assess T-cell subsets, plasma viral load, and HIV-1 subtype. Women were examined for infections caused by sexually transmitted organisms, including syphilis, Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, bacterial vaginosis, and candida, as previously described.1 Infections were treated according to the standard recommended guidelines of the Kenyan Ministry of Health. We gave women iron and folate during pregnancy, and none received antiretroviral therapy.
We randomly assigned women to breastfeeding or formula feeding groups with a computer generated list of random numbers and sealed envelopes. All women were counselled with respect to optimum feeding practices. Women assigned to the formula group were provided with powdered formula and asked to use boiled water for reconstitution, and a cup to feed. We followed up women with an interim questionnaire and a brief physical examination every 2 weeks until 36 weeks’ gestation, then every week until delivery. We saw mother-infant pairs monthly in the first year after delivery and quarterly in the second year until death, 2 years after delivery, or end of study. We obtained information about maternal morbidity using a questionnaire and physical examination at each visit. For women who failed to attend scheduled visits, we attempted to trace them to their home in Nairobi or to their rural home. For those who died we used hospital records or asked relatives to ascertain the cause of death.
Our original study was monitored by a data and safety monitoring board. In July, 1997, the board recommended that the study should continue until only 6 months after the last delivery in both groups. In April, 1998, because of differences in HIV-1 infection risk in the two groups, the board recommended that all women who breastfed be advised to formula feed and that the study continue until July 31, 1998 (6 months after the last delivery in both groups) to complete the data set. Our study was reviewed and approved by the institutional review boards of the University of Washington and University of Nairobi, and all women gave oral consent.
Laboratory methods
Laboratory methods used in this study have been previously described.1 Briefly, we screened blood for HIV-1 with an HIV ELISA assay (Behring, Ausgabe, Germany). Positive samples were confirmed with a second ELISA assay (Cambridge, Biotech, Rockville, MD, USA). We quantified plasma viral load of the mothers with Gen-Probe HIV RNA assays (Gen-Probe, La Jolla, CA, USA), and HIV-1 subtype was investigated with heteroduplex mobility assays, sequence analysis, or both.11 We measured CD4 and CD8 counts with monoclonal antibodies (Becton Dickinson, Erembodegen-Aalst, Belgium) and flow cytometry. Serum vitamin A concentrations were measured by high performance liquid chromatography.
Data analysis
We did intention-to-treat analyses using SPSS version 8.0. Differences in changes in bodyweight during follow-up in the two groups, and differences between women who were lost and those who were not lost to follow-up were tested with Pearson’s χ2 test or Fisher’s exact test for binary variables, and the Mann-Whitney U test or t test for continuous variables. We defined women as lost to follow-up if their vital status was unknown within 3 months of the completion of the study or 24 months after delivery, whichever came first.
Differences in mortality rates between the two groups at different times postpartum were tested with z-tests calculated from the cumulative mortality and standard error estimates obtained from an intent-to-treat Kaplan-Meier survival analysis. We obtained risk ratios for differences in maternal death rates between groups, other potential correlates of maternal death, and differences in infant mortality with Cox proportional hazards regression. The attributable risk of maternal death due to breastfeeding was calculated with the formula (RR-1)/RR, where RR is the relative risk of death for mothers in the breastfeeding versus formula feeding group, from a Cox proportional hazards model.
Results
425 women were randomly assigned—212 to the breastfeeding group and 213 to the formula feeding group. The characteristics of the study population have been previously described in detail.1 Briefly, median age was 23 years, and 325 (76%) were married. The women had few previous births, with a median parity of one (IQR zero to two). The median age at first coitus was 17 years, and the women had a lifetime median of three sexual partners. The median haemoglobin was 109 g/L and 207 (51%) were anaemic (haemoglobin <110 g/L). The median CD4 count was 407 cells/mL (IQR 268–528). CD4 cell counts were less than 200 cells/mL in 47 (12%) women, 200–499 in 218 (57%), and greater than 500 in 116 (31%). The median HIV-1 viral load was 42 700 virions/mL (IQR 10 245–161 884). HIV-1 subtyping was done for 320 women, of whom 225 (70%) had subtype A, 65 (20%) had D, 22 (7%) had C, 1 (0·3%) had G, and 7 (2%) had recombinant subtypes. The median vitamin A concentration was 0·86 µmol/L and 216 (70%) women had concentrations less than 1·05 µmol/L. Women who were randomly assigned to breastfeed or formula feed had closely similar baseline and delivery characteristics, as previously described (table).1
Characteristics of women in breastfeed and formula feed groups at baseline.
| Breastfeed group |
Formula feed group |
|
|---|---|---|
|
Age (years) |
23 (20–27) |
23 (21–27) |
|
Weight (kg) |
63 (58–69) |
63 (58–69) |
|
Gravidity |
2 (1–3) |
2 (1–3) |
|
Weeks’ gestation |
29 (25–32) |
29 (26–33) |
|
Vitamin A (µmol/L) |
0·86 (0·64–1·01) |
0·88 (0·64–1·14) |
|
Anaemia (Hb <110 g/L) |
93/200 (47%) |
114/207 (55%) |
|
Absolute CD4 count (cells/mL) |
399 (262–535) |
415 (282–528) |
| Absolute CD4 count (cells/mL) | ||
| <200 | 22/187 (12%) | 25/194 (13%) |
| 200–499 | 109/187 (58%) | 109/194 (56%) |
| >500 |
56/187 (30%) |
60/194 (31%) |
|
Plasma HIV-1 RNA viral load (virions/mL ×103) |
48 (13–172) |
37 (8–151) |
|
Cervical HIV-1 DNA positive |
66/182 (36%) |
67/190 (35%) |
|
Vaginal HIV-1 DNA positive |
40/186 (22%) |
29/187 (16%) |
|
Hours of labour |
9 (5–14) |
10 (6–15) |
|
From rupture of membranes to delivery >4 h |
59/189 (31%) |
51/187 (27%) |
|
Caesarean section |
17/193 (9%) |
15/193 (8%) |
| Twins | 5/201 (2%) | 7/207 (3%) |
Values are median (IQR) or proportion (%). Hb=haemoglobin.
Of the 212 women in the breastfeeding group, one died during pregnancy, eight were lost to follow-up before delivery, and six had no vital status information after delivery (figure 1). Of the 213 women in the formula feed group, five were lost to follow-up before delivery and eight had no vital status information after delivery. Of the remaining 397 women, 24 died; 15 during the first year and nine during the second year of follow-up. Our analysis dataset includes only those women who were alive at the time of delivery and for whom information about vital status after delivery was available. Thus, 197 women in the breastfeeding group and 200 in the formula group were included in the analysis of the relation between lactation and maternal death.
Figure 1. Trial profile.
Of these 397 women, 39 (20%) of 197 women in the breastfeeding group and 33 (17%) of 200 in the formula feeding group were lost to follow-up. The most usual reason for loss to follow-up was infant death (21 [54%] women in the breastfeeding and 19 [58%] in the formula feeding group, p=0·8), because those who lost an infant were no longer requested to come to the research clinic, although they were given the option of doing so. Most other women who were lost to follow-up changed residence or left Nairobi for their rural homes. Median time of lost to follow-up was 10·2 months in the breastfeeding group and 8·6 months in the formula group (p=0·3). Women classified as lost to follow-up were not substantially different from those who completed the study with respect to age, parity, or any baseline physical examination or laboratory test characteristics. However, those lost to follow-up were of lower socioeconomic status because they were less likely to live in a home with more than one room (22% [16/92] vs 42% [136/324], p=0·002), less likely to have flush sanitation (65% [47/72] vs 77% [251/325], p=0·03), more likely to have a shared toilet (88% [63/72] vs 73% [236/325], p=0·008), and less likely to have a refrigerator (zero vs 5% [17/325], p=0·05) than those who completed the study. A stratified analysis of correlates of loss to follow-up in each group had similar results, except that not all differences in socioeconomic status indicators were significant.
Compliance with breastfeeding was defined by any use of breastmilk, and that with formula feeding by total absence of breastfeeding. Compliance with the randomly assigned feeding practice was 189/197 (96%) in the breastfeeding group and 141/200 (71%) in the formula group (p=0·001). Of compliant women in the breastfeeding group, 95% were breastfeeding at 3 months, 90% at 6 months, and 80% at 12 months. On the basis of maternal report, the proportion of infants in the breastfeeding group who received 50% or more of their feeds as breastmilk was 79% at 6 months, 32% at 12 months, and 2% at 18 months. Median duration of breastfeeding was 17 months (range <1 week to >24 months), and median age of the infants at introduction of weaning foods was 3·8 months.
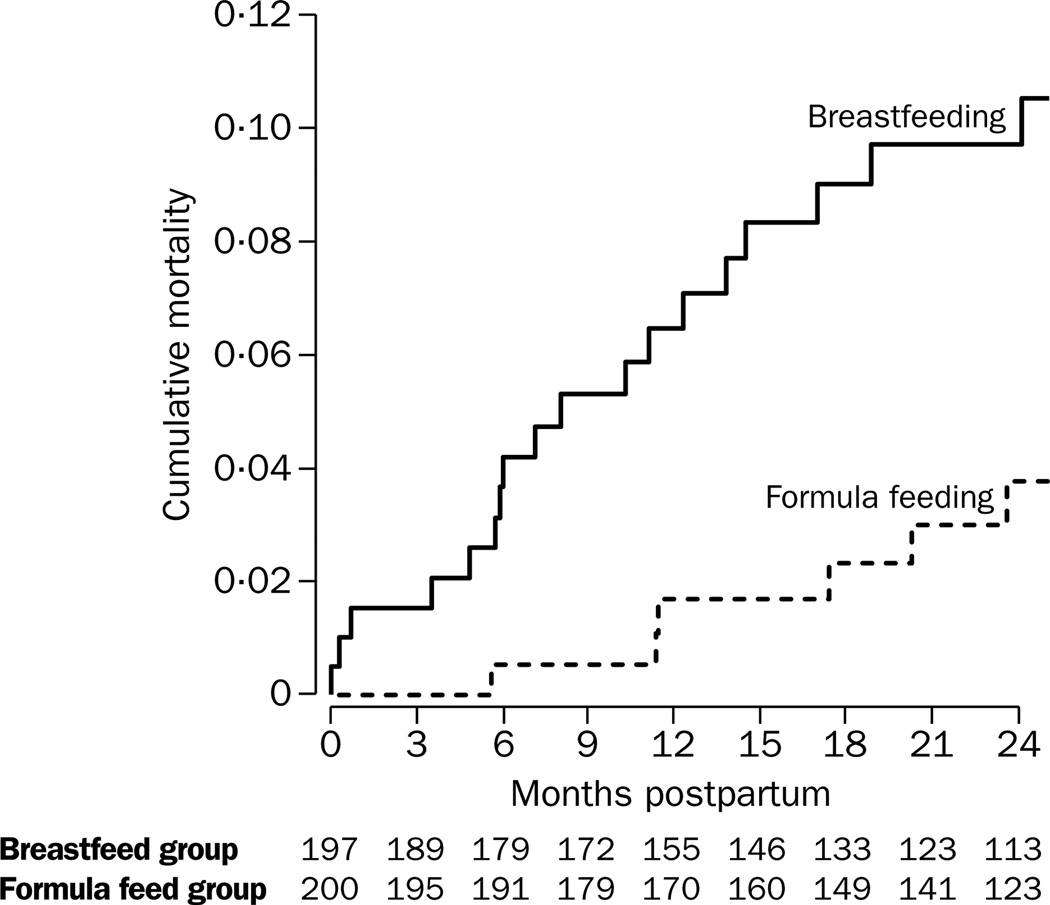
18 of the 197 women in the breastfeeding group and six of the 200 women in the formula group died during follow-up. The cumulative probability of death was higher in the breastfeeding group than in the formula group (figure 2, log rank test, p=0·009). At 24 months, the cumulative mortality was higher in women in the breastfeeding group than in those in the formula group, with Kaplan Meier survival analysis (11% vs 4%, p=0·02). The difference in the cumulative mortality rates in the two groups was significant by as early as 6 months after delivery (p=0·02). 22% of the overall excess mortality had taken place by 6 weeks, 55% by 6 months, 72% by 1 year, and 100% by 18 months. Overall, there was a three-fold increased risk of dying for women in the breastfeeding group (RR 3·2, 95% CI 1·3–8·1, p=0·01), with Cox proportional hazard regression models. 69% of maternal deaths in the breastfeeding group were attributable to breastfeeding. In Cox regression models, the association between random assignment to the breastfeeding group and maternal death persisted after log plasma viral load and log CD4 count at enrolment were controlled for (4·7, 1·8–12·0, p=0·001).
Figure 2. Mortality of mothers in breastfeeding and formula feeding groups.
Breastfeeding was associated with a higher risk of death in mothers than formula feeding, p=0·009.
Of the women who died, ten (48%) had CD4 counts of less than 200 cells/mL at enrolment and nine (43%) had counts of 200–499 cells/mL. Viral loads were high, with 12 (71%) of 17 having enrolment viral loads greater than 100 000 virions per mL, including four with loads more than 1 000 000. There was a significant association between maternal death, and CD4 counts and viral load at enrolment. Compared with women who had CD4 counts of greater than 500 cells/mL the relative risk of death for those with counts of 200–499 cells/mL was 2·4 (0·5–11·2, p=0·3) and 14·7 (3·2–67·4, p=0·001) for those with counts less than 200 cells/mL. Women with a viral load at enrolment of more than the median of 42 700 virions per mL had an 8-fold increased risk of dying (8·0, 1·8–34·8, p=0·006).
Precise information about causes of maternal death was missing because of restricted availability of diagnostic tests and reliance on verbal autopsies for deaths occurring outside Nairobi. However, 15 (63%) of 24 women who died were classified as having AIDS on the basis of clinical diagnoses of Kaposi’s sarcoma, wasting syndrome, tuberculosis, and cryptococcal meningitis. An additional five women (21%) had illness possibly related to HIV-1 infection (eg, pneumonia, chronic diarrhoea, or chronic cough) at the time of death. For the remaining four women, no information about cause of death was available.
The difference between weight shortly after delivery and weight at 6 months postpartum has been used as a measure of the metabolic demands of breastfeeding.12 We compared every women’s weight at her earliest postnatal visit, between 0·5 and 3 months, with weight at the latest visit, between 5 and 9 months after delivery. Women in the breastfeeding group lost more weight during this postpartum period than did those in the formula feeding group (mean 0·17 vs 0·00 kg per month, 95% CI for difference 0·02–0·33, p=0·03). There was a significant relation between weight loss during follow-up and mortality, with a RR of 3·4 (2·0–5·8) for each kg lost per month. The median weight loss per month was 0·7 kg for women who died and 0·05 kg for women who remained alive (p=0·03). After adjustment for weight change during follow-up, there was still an association between breastfeeding and maternal death (2·9, 1·1–7·6).
Of the 24 mothers who died during follow-up, one had an infant whose mortality status was unknown, 12 had infants still alive at the latest follow-up, and 11 had infants who died during follow-up. Of these 11 infants, six died after the mother’s death, one died at the same time as the mother, and four died before the mother. Maternal death was associated with infant death overall (3·1 for infant death, 1·6–5·9, p<0·001). There was an even stronger association between maternal death and subsequent infant death (5·6, 2·4–13·1, p<0·001), even after controlling for infant HIV-1 infection status (7·9, 3·3–18·6, p<0·001).
Discussion
In our randomised clinical trial of breastfeeding and formula feeding practices, we noted that random assignment to the breastfeeding group was associated with a greater than three-fold increased mortality rate among HIV-1 infected mothers during 2 years of follow-up. Our study might underestimate the true risk of maternal death associated with breastfeeding because almost a third of the women in the formula group did not comply with their assigned feeding practice.
We postulate two potential mechanisms for our finding. First, the combined metabolic burdens of HIV-1 infection and breastfeeding in a population that has inadequate nutritional intake could result in substantial nutritional impairment. During exclusive breastfeeding, a woman makes 600–700 mL of breastmilk per day, which requires about 2637 kJ daily.13 Without adequate nutritional intake, lactation is maintained by the energy derived from breakdown of body fat and muscle, which might lead to maternal weight loss and maternal depletion syndrome.14 Lactation-associated maternal weight loss might be more pronounced in HIV-1 infected than in healthy women. HIV-1 infection increases the basal metabolic rate. The energy expenditure of HIV-1 infected individuals while resting is about 10% more than in uninfected individuals (about 628kJ per day), and even higher if the person has superimposed opportunistic infection or high viral loads.15,16
We noted that women in the breastfeeding group had greater weight loss postpartum than women in the formula feeding group. Additionally, weight loss was associated with maternal death. Thus, the excess weight loss in the breastfeeding group might have contributed to mortality. However, the association between breastfeeding and maternal death persisted after controlling for weight loss during follow-up, suggesting that weight loss does not fully account for this association.
Second, lactation might affect HIV-1 replication. Lactation is associated with raised prolactin, an immunomodulating hormone that might be immunosuppressive at high concentrations.17 The effect of prolactin on HIV-1 replication in vitro warrants assessment. Mastitis is associated with raised viral load in breastmilk, but whether there is a concomitant rise in plasma viral load is unknown.18 Nor do we know whether other local factors associated with breastfeeding (eg, nipple cracking or candida infection), or breastmilk production itself are associated with enhanced HIV-1 replication. Our study was not designed to assess the effect of lactation on maternal death or nutritional status and therefore we obtained only a few data that allowed us to investigate the mechanism for the association.
There are several possible drawbacks to our study. First, although there was an association between random assignment to the breastfeeding group and mortality of mothers, the number of maternal deaths was small and CIs of the relative risk estimates were, therefore, large. Second, 18% of women were lost to follow-up. Although loss patterns were similar in the two groups (we have no reason to believe that mortality in women lost differed from those retained) bias might have been introduced. Third, participants in the trial were a highly select subgroup. Thus these results might not be generalisable to all populations of HIV-1 infected women. The mortality rate recorded in our study was similar to rates previously reported for HIV-1 infected women in Africa, but results might be different for women with different mortality risk (eg, with various stages of HIV-1).19
We need to further assess the association between breastfeeding and maternal death, although it could be difficult to interpret data from observational studies. Mothers’ decisions about infant feeding are affected by many factors, including the mother’s health status. We noted, for example, that women randomly assigned to the formula feeding group who decided to breastfeed had less advanced HIV-1 disease (lower enrolment plasma HIV-1 RNA) than those who complied with formula feeding. Plasma viral load was also a predictor of maternal death. Thus, an analysis of our data based on actual feeding modality rather than assigned group would underestimate mortality risk. We did an intent-to-treat analysis plan, as recommended for randomised clinical trial data, to avoid the potential confounding that might otherwise bias results.20
An important finding was that maternal death was associated with an increased risk of subsequent infant deaths. Factors contributing to maternal death (eg, advanced HIV-1 infection and high viral loads) might also have been associated with risk of infant HIV-1 infection and subsequent mortality.21,22 However, when we controlled for HIV-1 infection status in the infant, we showed that infants of mothers who died had an eight-fold increase in the likelihood of subsequent death. When considering HIV-1 infection in women of reproductive age in less developed countries, promotion of the survival of the mother is important, in addition to the reduction of risk of HIV-1 infection to the infant.
Our finding that breastfeeding is associated with maternal death has important implications for public health policy. Our results lend support to the aim of offering HIV-1 tests to all pregnant women to ensure that infected women are identified and counselled. Counselling, with respect to infant feeding choices, should include discussion of potential risks to the mother’s health from breastfeeding as well as the risk of transmitting HIV-1 to the infant. For HIV-1 infected women who choose to breastfeed, further research is needed into the mechanism of the association between lactation and maternal death, including assessment of the efficacy of nutritional supplements to women who breastfeed to reduce excess mortality. Our aim should be to ensure that all HIV-1 infected mothers are able to feed their infants in a way that keeps risk to a minimum for both mother and child.
Acknowledgments
We thank mothers and children who participated in the trial and research nurses, laboratory technicians, and data management teams. We thank Marie Reilly for assistance with data management and analysis; Julie Overbaugh for virological assays; Nairobi City Council; the Division of Obstetrics and Gynaecology at Kenyatta National Hospital; Makadara Maternity Centre; and the Departments of Medical Microbiology and Paediatrics at the University of Nairobi. We thank the members of the data and safety monitoring board: Thomas Fleming, Connie Celum, James Hughes, Claudes Kamenga, Helen McGough, and Heather Watts for their encouragement and advice. This study was supported by a grant from the National Institutes of Health (NICHD-23412). R Nduati, G John, D Mbori-Ngacha, and T Mwatha were investigators in the International AIDS Research and Training Programme, supported by the Fogarty International Center, National Institutes of Health (D43-TW00007, T22-TW00001).
Footnotes
Contributors
Ruth Nduati and Joan Kreiss were co-principal investigators and were jointly responsible for the study. Ruth Nduati, Grace John, and Dorothy Mbori-Ngacha were responsible for clinical field work, Barbra Richardson and Anthony Mwatha for data management and biostatistical analysis, and Jeckoniah Ndinya-Achola and Job Bwayo for laboratory assays.
References
- 1.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1985;34:721–731. [PubMed] [Google Scholar]
- 3.Joint United Nations Program on HIV/AIDS. HIV and infant feeding. Wkly Epidemiol Rec. 1996;71:289–291. [PubMed] [Google Scholar]
- 4.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 6.Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d’Ivoire: a randomised trial. Lancet. 1999;353:781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 7.Dabis F, Msellati P, Meda N, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d’Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. Lancet. 1999;353:786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 8.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 9.Taha TET, Miotti P, Liomba G, Dallabetta G, Chiphangwi J. HIV, maternal death and child survival in Africa. AIDS. 1996;10:111–112. doi: 10.1097/00002030-199601000-00021. [DOI] [PubMed] [Google Scholar]
- 10.McDermott JM, Slutsker L, Steketee RW, Wirima JJ, Breman JG, Heymann DL. Prospective assessment of mortality among a cohort of pregnant women in rural Malawi. Am J Trop Med Hyg. 1996;55:66–70. doi: 10.4269/ajtmh.1996.55.66. [DOI] [PubMed] [Google Scholar]
- 11.Neilson JR, John GC, Carr JK, et al. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J Virol. 1999;73:4393–4403. doi: 10.1128/jvi.73.5.4393-4403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Raaij JMA, Schonk CM, Vermaat-Miedema SH, Peek MEM, Hautvast JGAJ. Energy cost of physical activity throughout pregnancy and the first year postpartum in Dutch women wih sedentary lifestyles. Am J Clin Nutr. 1990;52:234–239. doi: 10.1093/ajcn/52.2.234. [DOI] [PubMed] [Google Scholar]
- 13.Van Raaij JMA, Schonk CM, Vermaat-Miedema SH, Peek MEM, Hautvast JGAJ. Energy cost of lactation, and energy balances of well-nourished Dutch lactating women: reappraisal of the extra energy requirements of lactation. Am J Clin Nutr. 1991;53:612–619. doi: 10.1093/ajcn/53.3.612. [DOI] [PubMed] [Google Scholar]
- 14.Adair LS, Popkin BM. Prolonged lactation contributes to depletion of maternal energy reserves in Filipino women. J Nutr. 1992;122:1643–1655. doi: 10.1093/jn/122.8.1643. [DOI] [PubMed] [Google Scholar]
- 15.Melchior J-C, Raguin G, Boulier A, et al. Resting energy expenditure in human immunodeficiency virus-infected patients: comparison between patients with and without secondary infections. Am J Clin Nutr. 1993;57:614–619. doi: 10.1093/ajcn/57.5.614. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan K, Tai VW, Schambelan M, et al. Energy expenditure in human immunodeficiency virus infection. N Engl J Med. 1997;336:70–71. doi: 10.1056/NEJM199701023360115. [DOI] [PubMed] [Google Scholar]
- 17.Reber PM. Prolactin and immunomodulation. Am J Med. 1993;95:637–644. doi: 10.1016/0002-9343(93)90360-2. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 19.Lindan CP, Allen S, Serufilira A, et al. Predictors of mortality among HIV-infected women in Kigali, Rwanda. Ann Intern Med. 1992;116:320–328. doi: 10.7326/0003-4819-116-4-320. [DOI] [PubMed] [Google Scholar]
- 20.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. New York: Springer Verlag; 1998. pp. 284–322. [Google Scholar]
- 21.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 22.Lambert G, Thea DM, Pliner V, et al. Effect of maternal CD4+ cell count, acquired immunodeficiency syndrome, and viral load on disease progression in infants with perinatally acquired human immunodeficiency virus type 1 infection. J Pediatr. 1997;130:830–837. doi: 10.1016/s0022-3476(97)70274-9. [DOI] [PubMed] [Google Scholar]