Abstract
Over 1000 individuals were killed and 600,000 were displaced during post-election violence (PEV) in Kenya in 2008. Antiretroviral therapy (ART) depends on continuous access to medications which may have been interrupted due to PEV. In a mixed-methods retrospective review, treatment interruption of ART during PEV was measured among 2534 HIV-positive adults attending the Coptic Hope Center for Infectious Diseases in Nairobi, Kenya. Clients experiencing treatment interruption were compared between the PEV period (30 December 2007 to 28 February 2008) and the same time period one year earlier. Treatment interruption was defined as visiting the pharmacy ≥48 hours after antiretrovirals were calculated to have been completed. Despite clinical services remaining open throughout the PEV period, more clients (16.1%) experienced treatment interruption than during the comparison period (10.2%). Mean daily pharmacy visits were significantly lower (87 vs. 104; p < 0.006) and more variable (p = 0.03) during PEV. Among clients present at both periods (n = 1605), the odds of treatment interruption were 71% higher during PEV (95% confidence interval [CI], 34–118%). In multivariate analysis, men (odds ratio [OR], 1.37; 95% CI, 1.07–1.76) and clients traveling ≥3 hours to clinic (OR, 1.86; 95% CI, 1.28–2.71) were significantly more likely to experience treatment interruption. Clients affected by PEV were interviewed about factors associated with treatment interruption using semi-structured methods. Clients described fear, lack of transportation, and violence as contributing to treatment interruption. Widespread violence associated with the 2007 election in Kenya revealed the dependence of HIV patients on a stable civil society and infrastructure to access medications. Without the ability to maintain consistent HIV therapy, some patients face rapid treatment failure. HIV programs should have appropriate contingency plans wherever political instability may occur. Peace may be one of the most effective and most important public health interventions in Africa.
Keywords: violence, HAART, HIV therapy, interruption, conflict, Africa, Kenya, HIV
Introduction
Kenya held its fourth multi-party election on 27 December 2007. Soon afterwards, opposition politicians claimed that the election was rigged and foreign observers noted serious irregularities (European Union Election Observation Mission, 2008). Protests were called and became increasingly violent after the release of election results on 30 December. Nairobi, the capital of Kenya, became a focus of political protests. Much of the worst violence occurred in slums and informal settlements in Nairobi as well as the Rift Valley, a province located northwest of Nairobi. It is estimated that over 1000 individuals were killed and 600,000 displaced during the time period following the election (Department of State, 2008). At least 15,000 of the displaced individuals were HIV-positive (Allen, 2008). On 28 February 2008, a power-sharing agreement was signed, marking the end of the most violent period in recent Kenyan history.
HIV is a serious health problem in Kenya with an estimated adult HIV prevalence between 7.1 and 8.5% in 2007 (UNAIDS, 2008). Access to HIV treatment has accelerated in recent years and by the end of 2007 it was estimated that 172,000 Kenyans were on antiretroviral therapy (ART) (WHO, UNAIDS, & UNICEF, 2008). Despite this achievement, individuals on HIV therapy in Africa routinely face numerous obstacles to seeking treatment (Hardon et al., 2007; Tuller et al., 2009; Ware et al., 2009; Weiser et al., 2003) and are at risk of drug resistance if their access to ART is interrupted (Oyugi et al., 2007). Drug resistance may result in additional cost and treatment complexity, and death if second-line drugs are not available (Sendagire et al., 2009). The ramifications of unplanned treatment interruption at a population level could be significant.
This study evaluated the impact of Kenyan post-election violence (PEV) on interruption of ART among HIV-positive clients attending an HIV clinic in Nairobi, Kenya, and identified factors associated with treatment interruption.
Methods
This study was approved by the Ethical Review Committee of Kenyatta National Hospital (Nairobi, Kenya) and the Institutional Review Board of the University of Washington (Seattle, WA, USA). Research was conducted at the Coptic Hope Center for Infectious Diseases, a clinic providing free comprehensive HIV care services in Nairobi (Chung et al., 2009). The Hope Center provided care to nearly 7000 clients as of December 2007 with support from the President’s Emergency Plan for AIDS Relief (PEPFAR). Clients at the Hope Center were initiated on ART based on Kenyan national guidelines and attended adherence counseling prior to initiating treatment. Clients on ART were required to pick up antiretroviral medications from the pharmacist every 30 or 60 days. The number of pills dispensed was adjusted if clients missed doses, and additional adherence counseling was provided if needed. Clients were included in the study if they were 18 years of age or older at enrollment, were a Kenyan resident, had initiated ART, had data on the number of days of ART dispensed at last visit, and had an expected pharmacy visit during the period of PEV.
The PEV period was defined as 30 December 2007 through 28 February 2008. The comparison period was defined as 30 December 2006 through 28 February 2007 to address seasonal confounding. Treatment interruption was defined as returning to the pharmacy 48 hours or more after the last dispensed pill was finished. The date when the last pill was finished was calculated by retrieving the date of the most recent pharmacy visit for each client prior to 30 December 2007, and adding the number of days of medicine dispensed at that visit. A client receiving a 30-day supply of medicine on 21 December would be expected to consume his or her last pill on 20 January, and the individual would experience treatment interruption starting on 22 January.
The number of daily pharmacy visits during the PEV period was evaluated against the comparison period using a two-tailed t-test to identify statistically significant differences. The percentage of clients experiencing a treatment interruption was measured during the PEV period and the comparison period. In addition, the odds ratio (OR) for treatment interruption was calculated using matched methods for all clients present during both time periods; McNemar’s test was used to assess statistical significance.
Logistic regression was used to identify factors associated with treatment interruption. Data for this analysis originated from existing patient records. Variables analyzed included gender, income, travel time to clinic, having a treatment supporter, marital status, level of education, age, time since ART initiation, and most recent CD4 count. Variables with p-values below 0.10 were added to a multivariate logistic regression model to address confounding. The significance level was set at p ≤ 0.05 in this analysis. Analysis was conducted in SPSS version 16 (SPSS Inc, Chicago, IL, USA) and STATA version 10 (STATA, College Station, TX, USA).
Qualitative data collection took place in March 2009, approximately one year after the political settlement. In-depth interviews were conducted with staff and clients from the Hope Center who experienced a treatment interruption during the PEV period. These interviews collected information on disruption of services, human resources, stock-outs, coping mechanisms, and barriers or facilitators to treatment for clients. Interviews were recorded, transcribed, and coded for analysis. ATLAS.ti (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) was used for analysis of qualitative data.
Results
As of 29 December 2007, 4563 clients had initiated ART at the Hope Center: of whom 693 (15%) were 17 or younger; 886 (19%) were lost to follow up; 37 (1%) were missing data on ART doses at most recent pharmacy visit; 3 (<1%) were not resident in Kenya; and 410 (9%) were not expected at the pharmacy during the PEV period. The remaining 2534 clients were included in the quantitative analysis. Clients had a median age of 37 years (IQR, 32–43), 63.8% were female, and 79.1% reported having a treatment supporter. The median time from home to clinic was 1–2 hours and the median duration of treatment was 19.5 months (IQR, 9.8–28.5 months). This cohort bore similar characteristics to 2167 clients examined in the comparison period.
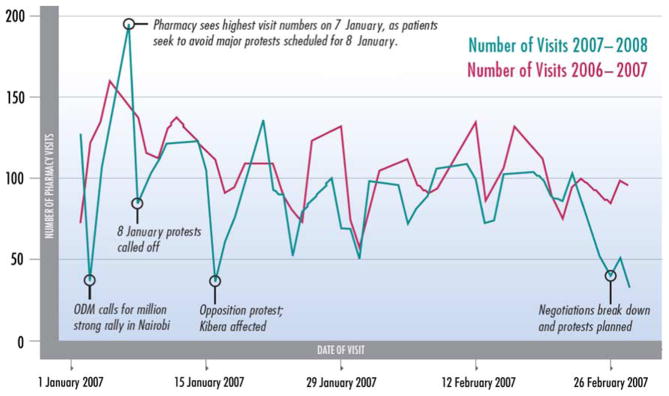
The Hope Center remained open for the entire period of PEV in Kenya. There was an observed temporal association between specific political events and the number of pharmacy visits (Figure 1). Pharmacy visits dropped from 127 on 2 January, to 37 on 4 January, a day when the opposition called for a mass protest. The busiest day during the period was 7 January, when 195 people visited the pharmacy. A protest that was planned for 8 January had been announced several days earlier and this may have motivated individuals to visit the pharmacy before the protest. Additional days with depressed visit numbers were temporally associated with the breakdown of political negotiations in late February. During the PEV period, the mean number of pharmacy visits was significantly lower (87 vs. 104; p = 0.006) and the day-to-day variability in pharmacy attendance (standard deviation [SD], 30.9 vs. 22.0) was significantly higher (p = 0.03) than the comparison period.
Figure 1.
Number of pharmacy visits by date during the post-election violence time period and one year earlier.
Of the 2534 clients examined during the PEV period, 16.1% (n = 408) experienced treatment interruption. This was higher than 10.2% (n = 222) who experienced treatment interruption during the comparison period. One thousand six hundred and five individuals were enrolled and receiving care at the Hope Center during both the PEV period and the comparison period. In a matched analysis of these individuals, the odds of treatment interruption were 1.71 times higher during the PEV period (95% confidence interval [CI], 1.34–2.18; p < 0.0001).
Several factors were associated with treatment interruption in the context of PEV in univariate analysis (Table 1). Individuals traveling three or more hours to clinic had 1.91 times greater odds of treatment interruption than individuals traveling 1–2 hours (95% CI, 1.31–2.77). Each additional year on ART was associated with 21% lower odds of treatment interruption (OR, 0.79; 95% CI, 0.70–0.89). Gender, travel time to clinic, and duration of time on ART were included in a multivariate logistic regression model. In this model, male gender was associated with 1.37 times greater odds of treatment interruption (95% CI, 1.07–1.76), and travel times of three or more hours were associated with 1.86 times greater odds of treatment interruption compared to the referent group (95% CI, 1.28–2.71). Duration of time on ART was not associated with treatment interruption in the multivariate regression model. During the comparison period, none of these variables was significantly associated with treatment interruption.
Table 1.
Characteristics associated with treatment interruption during the post-election time period.
| Characteristic | Experienced treatment interruption
|
Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Yes (n = 408) | No (n = 2126) | |||
| Gender | ||||
| Male | 39.7% (162) | 35.2% (749) | 1.21 (0.97–1.51) | 1.37 (1.07–1.76)* |
| Female | 60% (245) | 64.5% (1372) | 1.0 | 1.0 |
| Missing | 0.2% (1) | 0.2% (5) | ||
| Income (Ksh/month) | ||||
| < 5000 | 49.5% (202) | 46.3% (985) | 1.0 | |
| ≥ 5001 | 43.4% (177) | 46.8% (995) | 0.87 (0.70–1.08) | |
| Missing | 7.1% (29) | 6.9% (146) | ||
| Time from home to clinic | ||||
| < 1 hour | 27.9% (114) | 22.5% (479) | 1.26 (0.96–1.67) | 1.19 (0.89–1.58) |
| 1–2 hours | 31.4% (128) | 31.9% (679) | 1.0 | 1.0 |
| 2–3 hours | 7.1% (29) | 9.3% (198) | 0.78 (0.50–1.20) | 0.79 (0.51–1.22) |
| ≥3 hours | 12.3% (50) | 6.5% (139) | 1.91 (1.31–2.77)** | 1.86 (1.28–2.71)** |
| Missing | 21.3% (87) | 29.7% (631) | ||
| Has a treatment supporter | ||||
| Yes | 80.2% (327) | 78.9% (1678) | 1.09 (0.80–1.49) | |
| No | 13.7% (56) | 14.8% (314) | 1.0 | |
| Missing | 6.1% (25) | 6.3% (134) | ||
| Marital status | ||||
| Married | 49.5% (202) | 48.4% (1028) | 1.0 | |
| Single | 12.0% (49) | 15.4% (328) | 0.76 (0.54–1.06) | |
| Other | 34.1% (139) | 32.5% (691) | 1.02 (0.81–1.30) | |
| Missing | 4.4% (18) | 3.7% (79) | ||
| Education | ||||
| < 5 years | 4.9% (20) | 5.4% (114) | 1.10 (0.68–1.80) | |
| ≥ 5 years | 89.0% (363) | 88.3% (1877) | ||
| Missing | 6.1% (25) | 6.4% (135) | ||
| Age | ||||
| 18–24 | 3.7% (15) | 2.9% (61) | 1.0 | |
| 25–34 | 37.0% (151) | 32.8% (697) | 0.88 (0.49–1.59) | |
| 35–44 | 37.3% (152) | 43.3% (921) | 0.67 (0.37–1.21) | |
| ≥ 45 | 22.1% (90) | 21.0% (447) | 0.82 (0.45–1.51) | |
| Median years on treatment | ||||
| CD4 count | 1.40 | 1.66 | 0.79 (0.70–0.89)*** | 0.84 (0.70–1.02) |
| < 350 | 64.7% (264) | 62.5% (1328) | 1.07 (0.86–1.35) | |
| ≥ 350 | 35% (143) | 36.5% (776) | 1.0 | |
| Missing | 0.3% (1) | 1.0% (22) | ||
p = 0.011;
p = 0.001;
p < 0.001.
Thirteen in-depth interviews were conducted with clients at the Hope Center to provide insight into factors associated with treatment interruption during the PEV period. Eight (62%) of the clients interviewed were women, all resided in the greater Nairobi area, and the age of clients ranged from 30 to 63 with a median age of 39. All clients had initiated ART before the 2007 election. The most common occupation for clients was small business or trading. Treatment interruptions lasted from two days to two months.
PEV had a number of health impacts on informants, and many clients felt their HIV treatment had been compromised. A 45-year-old widow with three children described how the experience affected her health, “After the post-election violence, my CD4 count went down to 179. I was really affected during this period because I was unable to come to the hospital the whole of January and February.”
There were a number of barriers to treatment that were unique to the PEV period. Six of the 13 clients interviewed had personal experience of being evicted, attacked by mobs, or having homes or businesses burned. One informant, a 39-year-old widow with two children, was evicted from her home in Nairobi, and sought refuge with family in the town of Naivasha (76 km west of Nairobi) only to be evicted a second time. “Yes, we were evicted! You see, Kikuyus were evicting Luos, Luos were evicting Luhyas, Luhyas were also evicting Luos, etc., etc. – each ethnic group wanted to evict all the other ones; they burnt our houses and all our belongings, so I fled with my children…”
Lack of transportation was another major barrier to treatment during this period. A 37-year-old farmer from the outskirts of Nairobi had a hard time finding transport, “I left the house on at least three different occasions but I’d get to the bus stop and find that matatus [minibus taxis] were not commuting; I’d then go back home and try again the following day.” When service was available, fares were at a premium depending on the safety of the route. A 43-year-old widow had difficulty affording taxi fares, “The bus fares were really high…the fares back to Kibera were even more expensive because no one was going back into the ‘battle zone’ so they would charge even more, citing greater risk.”
A variety of factors facilitated access to treatment for clients. A powerful commitment to treatment was demonstrated through words and actions. A 63-year-old widow explained, “It’s actually upon each one of us to make every sacrifice to get to hospital.” Family members were an important treatment facilitator. During the PEV period, family provided refuge, food, moral support, and money for transportation and drugs. A 35-year-old married woman from Nairobi was able to travel to hospital because her husband sent her money through a mobile phone banking system, “I called my husband and explained to him what had happened. I asked him to send me money which he did through M-pesa and in this way, I was able to get the bus fare that I needed to get to the hospital to collect my medication.” Several informants received calls or visits from the Hope Center social work team and credited them as a reason for returning to the clinic. One client who was profoundly impacted by PEV explained, “Had they [social workers] not come to my house, I don’t know what I would eventually have done – at the time I was feeling totally lost and confused.”
Discussion
The period of PEV was associated with a 71% increase in the odds of treatment interruption among Hope Center clients who had been on ART for at least one year. Treatment interruption has significant implications for HIV patients. Treatment interruption is associated with the development of drug resistance, treatment failure, opportunistic infections, and death (DART Trial Team, 2008; Oyugi et al., 2007; Strategies for Management of Antiretroviral Therapy (SMART) Study Group, 2008). By disrupting a patient’s access to ART, political violence in Kenya may therefore have increased long-term morbidity and mortality among those living with HIV.
We found that men had a 37% higher odds of treatment interruption than women. Political demonstrations in the country appeared to involve more men than women, with men more likely to be targets of violence. The ability of men to access treatment may therefore have been more severely affected by PEV. Specific attention to retaining male clients may be warranted in situations of political instability and violence.
We found that clients traveling three or more hours had 86% greater odds of treatment interruption than patients who traveled between one and two hours to clinic. During the PEV period, roads were blocked and vehicles were stopped by some groups to search for and kill members of opposing tribes. Interviews described how patients who relied on public transportation found that services had stopped running and how they also feared violence on the way. Travel time and cost is an important factor in clinic retention even during times of relative calm (Hardon et al., 2007; Tuller et al., 2009). During violence, the impact of travel time appears to be exacerbated. Decentralization of ART services may help clients access care during periods of political unrest.
Treatment programs can also minimize the impact of political violence on care by preparing for variations in patient demand. This study demonstrated temporal patterns of clinic utilization that closely mirrored the timing of political events. We found that clinic attendance increased substantially ahead of dangerous events such as political rallies. Our data suggest that clinic managers can make use of news and political announcements to bring additional staff to work on days when more patients are expected.
Despite enormous challenges, patients demonstrated a strong dedication to their health and were creative in accessing treatment. This is similar to results from studies of HIV treatment in other conflict-affected regions in East and Central Africa (Culbert et al., 2007; Garang, Odoi, & Kalyango, 2009; Kiboneka et al., 2008, 2009; Mills, Ford, Singh, & Eyawo, 2009; Olupot-Olupot et al., 2008; Reid, van Engelgem, Telfer, & Manzi, 2008; Vreeman et al., 2009), where HIV treatment programs continue despite great odds. The ability of patients at the Coptic Hope Center to continue their care during PEV appears to reflect a strong personal commitment, possibly based on thorough adherence counseling. Clients in the program also described a heavy reliance on social and family networks to meet their livelihood needs and to access treatment during PEV. The strength of familial and social networks has been cited as a factor in improving antiretroviral adherence during peaceful times (Ware et al., 2009), and may be even more important in the context of conflict.
Our study had several strengths including a large cohort, detailed information on characteristics of the cohort, an objective measure of treatment interruption, and a mixed-methods approach which combined quantitative and qualitative data. There were also limitations to the study. We may have overestimated treatment interruption because we were not able to account for clients seeking care at other facilities. We were not able to interview clients who never returned to clinic following PEV; these individuals may have revealed even more important barriers to treatment access. Finally, although an association between the PEV period and treatment interruption was demonstrated, the events may not have been causal.
As demonstrated in this study, the health of people living with HIV is vulnerable to violent conflict and HIV programs should have appropriate contingency plans wherever political instability may occur. Without the ability to maintain consistent HIV therapy, patients face rapid treatment failure. Continued political conflict may erase gains made by the Kenyan Ministry of Health, PEPFAR, and the Global Fund to increase antiretroviral coverage and reduce HIV morbidity and mortality. Peace may be one of the most effective and most important public health interventions in Africa.
Acknowledgments
L. Pyne-Mercier implemented and designed the study, and participated in all data analysis. M. Chung and G. John-Stewart helped to design the study, interpret the data, and write the paper. B. Richardson assisted with statistical analyses and helped to design the study and write the paper. J. Thiga, N. Kist, and H. Noshy helped to conduct the study in the field. N. Kagondu was responsible for qualitative data collection. We would like to acknowledge the contributions of the research personnel and data management teams in Nairobi, Kenya, and Seattle, WA, USA; and the staff and clinicians at the Coptic Hope Center in Nairobi, Kenya, for providing a conducive environment for HIV care and research. Most of all, we thank the participants who were willing to share their experiences following the 2007 election.
Footnotes
Conflict of interest statement
This study was conducted at a treatment program funded through the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). L. Pyne-Mercier, M. Chung, G. John-Stewart, B. Richardson are employed by the University of Washington, which receives PEPFAR funding. N. Kist, J. Thiga, and H. Noshy are employed by the Coptic Hope Center for Infectious Diseases, which receives PEPFAR funding through the University of Washington and was the site of the research.
References
- Allen K. AIDS patients hit by Kenya crisis. BBC News. 2008 Feb 15; Retrieved from http://newsvote.bbc.co.uk/mpapps/pagetools/print/news.bbc.co.uk/2/hi/africa/7245190.stm.
- Chung MH, Drake AL, Richardson BA, Reddy A, Thiga J, Sakr SR, John-Stewart GC. Impact of prior HAART use on clinical outcomes in a large Kenyan HIV treatment program. Current HIV Research. 2009;7(4):441–446. doi: 10.2174/157016209788680552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert H, Tu D, O’Brien DP, Ellman T, Mills C, Ford N, Venis S. HIV treatment in a conflict setting: Outcomes and experiences from Bukavu, Democratic Republic of the Congo. PLoS Medicine. 2007;4(5):e129. doi: 10.1371/journal.pmed.0040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DART Trial Team. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts <200 cells/ml. AIDS. 2008;22:237–247. doi: 10.1097/QAD.0b013e3282f2d760. [DOI] [PubMed] [Google Scholar]
- Department of State. Background notes: Kenya. 2008 Retrieved from http://www.state.gov/r/pa/ei/bgn/2962.htm.
- European Union Election Observation Mission. Kenya final report: General elections 27 December 2007. 2008 Retrieved from http://www.eueomkenya.org/Main/English/PDF/Final_Report_Kenya_2007.pdf.
- Garang P, Odoi A, Kalyango N. Adherence to antiretroviral therapy in conflict areas: A study among patients receiving treatment from Lacor Hospital, Uganda. AIDS Patient Care and STDs. 2009;23(9):743–747. doi: 10.1089/apc.2009.0073. [DOI] [PubMed] [Google Scholar]
- Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, Laing R. Hunger, waiting time and transport costs: Time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- Kiboneka A, Nyatia RJ, Nabiryo C, Anema A, Cooper CL, Fernandes KA, Mills EJ. Combination antiretroviral therapy in population affected by conflict: Outcomes from large cohort in northern Uganda. BMJ. 2009;338:b201. doi: 10.1136/bmj.b201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiboneka A, Nyatia RJ, Nabiryo C, Olupot-Olupot P, Anema A, Cooper C, Mills E. Pediatric HIV therapy in armed conflict. AIDS. 2008;22(9):1097–1098. doi: 10.1097/QAD.0b013e32830163c0. [DOI] [PubMed] [Google Scholar]
- Mills E, Ford N, Singh S, Eyawo O. Providing antiretroviral care in conflict settings. Current HIV/AIDS Reports. 2009;6:201–209. doi: 10.1007/s11904-009-0027-7. [DOI] [PubMed] [Google Scholar]
- Olupot-Olupot P, Katawera A, Cooper C, Small W, Anema A, Mills E. Adherence to antiretroviral therapy among a conflict-affected population in northeastern Uganda: A qualitative study. AIDS. 2008;22(14):1882–1884. doi: 10.1097/QAD.0b013e3283112ba6. [DOI] [PubMed] [Google Scholar]
- Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, Kityo C, Bangsberg DR. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21(8):965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- Reid T, van Engelgem I, Telfer B, Manzi M. Providing HIV care in the aftermath of Kenya’s post-election violence Medecins Sans Frontieres’ lessons learned January–March 2008. Conflict and Health. 2008;2:5. doi: 10.1186/1752-1505-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendagire H, Easterbrook PJ, Nankya I, Arts E, Thomas D, Reynolds SJ. The challenge of HIV-1 antiretroviral resistance in Africa in the era of HAART. AIDS Reviews. 2009;11:59–70. [PMC free article] [PubMed] [Google Scholar]
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: Role of CD4+ cell counts and HIV RNA levels during follow-up. Journal of Infectious Diseases. 2008;197(8):1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in Southwestern Uganda: A qualitative study. AIDS and Behavior. 2009;14:778–784. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Epidemiological factsheets on HIV/AIDS and sexually transmitted infections: Kenya (Report No: EFS 2008 Kenya) 2008 Retrieved from http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_KE.pdf.
- Vreeman RC, Nyandiko WM, Sang E, Musick BS, Braitstein P, Wiehe SE. Impact of the Kenya post-election crisis on clinic attendance and medication adherence for HIV-infected children in western Kenya. Conflict and Health. 2009;3(1):5–10. doi: 10.1186/1752-1505-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware NC, Idoko J, Kaaya S, Biraro IA, Wyatt MA, Agbaji O, Bangsberg DR. Explaining adherence success in sub-Saharan Africa: An ethnographic study. PLoS Medicine. 2009;6(1):e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser S, Wolfe W, Bangsberg DR, Thior I, Gilbert P, Makhema J, Marlink R. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. Journal of the Acquired Immune Deficiency Syndrome. 2003;34(3):281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- WHO, UNAIDS, & UNICEF. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. 2008 Retrieved from http://www.who.int/hiv/pub/2008progressreport/en/