Abstract
The presence of human immunodeficiency virus type 1 (HIV-1) in genital secretions may be a determinant of vertical HIV-1 transmission. Cervical and vaginal secretions from HIV-1–seropositive pregnant women were evaluated to determine prevalence and correlates of HIV-1–infected cells in the genital tract. HIV-1 DNA was detected by polymerase chain reaction in 32% of 212 cervical and 10% of 215 vaginal specimens. Presence of HIV-1 DNA in the cervix was associated with cervical mucopus and a significantly lower absolute CD4 cell count (354 vs. 469, P < .001). An absolute CD4 cell count <200 was associated with a 9.6-fold increased odds of cervical HIV-1 DNA detection compared with a count ≥500 (95% confidence interval, 2.8–34.2). Detection of vaginal HIV-1 DNA was associated with abnormal vaginal discharge, lower absolute CD4 cell count, and severe vitamin A deficiency. Presence of HIV-1–infected cells in genital secretions was associated with immunosuppression and abnormal cervical or vaginal discharge.
More than 2 million children worldwide are infected with human immunodeficiency virus type 1 (HIV-1), the majority of whom acquired the infection from their mothers [1]. Mother-to-child transmission of HIV-1 occurs in utero, intrapartum, and postnatally (through breast-feeding), with transmission rates ranging from 14% to 39% [2]. Several observations, including the differential infection rate of vaginally delivered twins, the protective effect of cesarean delivery on vertical transmission, and the association of prolonged ruptured membranes with vertical transmission, suggest that infant exposure to HIV-1 in the maternal genital tract during delivery is an important determinant of transmission [2]. The association between vertical transmission of HIV-1 and advanced maternal disease, immunosuppression, and plasma viremia or antigenemia, and the inverse association with antiretroviral therapy suggest that systemic maternal virus burden is an important factor in HIV-1 transmission [2–8]. Markers of systemic virus burden, however, may not necessarily reflect local virus burden, and the effect of genital HIV-1 shedding on vertical transmission may be distinct from the effect of plasma viremia on transmission.
HIV-1 has been cultured from cell-free and cellular fractions of vaginal and endocervical secretions of HIV-1–seropositive women [9, 10]. HIV-1–infected cells are detectable in female genital tract secretions by polymerase chain reaction (PCR) amplification. The prevalence of HIV-1 DNA in cervical secretions was 33% and 37% in two previously published studies from Nairobi and 17% in vaginal specimens in one of these studies [11, 12]. Correlates of HIV-1 DNA detection in the cervix have included oral contraceptives, cervical mucopus, ectopy, and cervical inflammation [11, 12]. In addition, two studies have shown an increased prevalence of HIV-1 DNA in the cervix of pregnant women, but these studies together included only 29 pregnant women at various stages of gestation [12, 13].
While detection of HIV-1 in semen has been inversely associated with CD4 cell count and zidovudine use, little is known about these factors in female genital tract shedding of HIV-1 [14, 15]. Moreover, correlates of genital shedding of HIV-1 in pregnancy have not been described. To characterize the prevalence and correlates of HIV-1 detection in cervical and vaginal specimens from pregnant women, we evaluated HIV-1–seropositive women participating in a breast-feeding transmission study in Nairobi.
Little is known about factors that predispose to virus localization within a host. We previously evaluated prevalence and correlates of HIV-1 pro virus in breast milk of women participating in this cohort [16]. To evaluate provirus shedding at different epithelial sites, we determined the relationship between HIV-1 shedding in the cervix and vagina and in breast milk.
Methods
Study population and procedures
Pregnant women attending three Nairobi City Council antenatal clinics underwent serologic testing for HIV-1 after receiving counselling. At the time of enrollment into the breast-feeding study, a standardized questionnaire was administered to assess demographic, sexual, obstetric, and medical histories.
A physical examination, including pelvic examination, was done during the middle of the third trimester, close to the 32nd week of gestation. During the speculum examination, the area of cervical ectopy was visually estimated, and cervical and vaginal specimens were collected. Cervical specimens were obtained for Gram’s staining, Neisseria gonorrhoeae culture, Chlamydia trachomatis antigen detection (Clearview Chlamydia; Unipath, Nepean, Canada, or Microtrak; Syva, San Jose, CA), and HIV-1 DNA amplification by PCR. Vaginal specimens were collected for potassium hydroxide microscopy for candidiasis, Gram’s staining for bacterial vaginosis, and HIV-1 DNA amplification by PCR. Syphilis serologic testing was done using the rapid plasma reagin assay for screening and Treponema pallidum hemagglutination assay for confirmation. Blood was obtained for complete blood cell count and white cell differential count, including enumeration of CD4 and CDS lymphocytes using monoclonal antibodies (Becton Dickinson, Erembodegem-Aalst, Belgium) and flow cytometry (FACScan, Becton Dickinson, San Jose). Vitamin A levels were measured using high-performance liquid chromatography on serum or plasma samples. Vitamin A deficiency was defined by levels <30 µg/dL and severe vitamin A deficiency as levels <20 µg/dL [17].
To determine the relationship between Trichomonas vaginalis infection and vaginal HIV-1 shedding, a nested case-control study was done in which 21 vaginal specimens with detectable HIV-1 DNA and 42 randomly selected control vaginal specimens without detectable HIV-1 DNA were analyzed for T. vaginalis by PCR [18].
Detection of HIV-1 DNA in cervical and vaginal specimens
Cervical specimens for PCR assays were obtained by gently rotating a Dacron swab within the cervical os, after the initial swab for Gram’s staining was collected. Vaginal specimens were obtained by rolling a swab against the vaginal wall in an area free of exudate. Swabs were delivered to the laboratory within 2 h of collection, stored in liquid nitrogen, and subsequently transported to the University of Washington.
After removal from liquid nitrogen, the swabs were vortexed briefly in lysis buffer (10 mM TRIS HCl, pH 8.3, 50 mM KCl, 0.01% [wt/vol] gelatin, 0.45% NP-40, 0.45% Tween 20, and 0.6 mg/mL proteinase K), incubated at 56°C for 90 min, and then boiled for 15 min to inactivate proteinase K. After removal of the swabs, PCR assays were done using a nested primer method with primers specific for HIV-1 gag DNA, as previously described [19]. The sensitivity of this assay was 99.2%, and the rate of false-positive reactions due to contamination was 0.30% [19]. Breast milk samples from a subset of these women were previously evaluated for HIV-1 DNA; the methods of collection and processing are described elsewhere [16].
Data analysis
Data were analyzed using SPSS-PC (SPSS, Chicago) and Epi-Info (USD, Stone Mountain, GA) statistical programs. To assess correlates of cervical and vaginal HIV-1 DNA, the Student’s t test and the Wilcoxon rank sum test were used for continuous variables, and Pearson’s χ2 test and Fisher’s exact test were used for categorical variables. Multivariate analyses were performed using logistic regression to control for potential confounding.
Results
Characteristics of the study population
Two hundred twenty-three HIV-seropositive pregnant women were evaluated. The median age was 23 years, and 73% were married. The median life-time number of sex partners was 3; the median number during pregnancy was 1, and the median monthly frequency of sexual intercourse during the last trimester of pregnancy was 2. Thirty-seven percent of women were primigravidas. The median gestational age at evaluation was 32 weeks (range, 17–40).
On speculum examination, 32% of 212 women had mucopurulent cervical discharge, and 51% had abnormal vaginal discharge. Most women (82%) had cervical ectopy, and cervical friability was noted in 43%. The prevalence of gonorrhea was 7%; chlamydial infection, 8%; genital warts, 11% candidiasis, 32%; and bacterial vaginosis, 53%.
Of 213 women, 56 (26%) ad HIV-1–related signs or symptoms (a history of diarrhea, cough, or fever for >1 month, pruritic rash, tuberculosis, shingles, or oral thrush). Of 182 women with T cell subset determinations, absolute CD4 cell counts were <200 in 14%, between 200 and 499 in 56%, and ≥500 in 30%. The mean CD4 cell count was 428/mm3. Fifty-two (24%) of 213 women had anemia (hemoglobin <10 g); the mean hemoglobin was 11.1 g. Of 212 women who had vitamin A assays, 136 (64%) had levels <30 µg/dL, and 50 (24%) had levels <20 µg/dL; the mean vitamin A level was 26 µg/dh. There was no significant correlation between vitamin A levels and absolute CD4 cell count (R = .07).
Prevalence of HIV-1 DNA in cervical and vaginal secretions
Cervical and vaginal specimens were obtained from 212 and 215 women, respectively. HIV-1 DNA was detected in 67 (32%) of 212 cervical samples and 21 (10%) of 215 vaginal samples. The prevalence of cervical shedding was significantly higher than vaginal shedding (P < .001). There was a significant association between HIV-1 detection at the two sites. Among the 204 women who had both sites sampled, vaginal HIV-1 DNA was present in 11 (17%) of 64 women with cervical shedding and 9 (6%) of 140 women without cervical shedding (odds ratio [OR], 3.0; 95% confidence interval [CI], 1.2–7.7). In addition, there was a significant association between cervical HIV-1 DNA shedding and the presence of HIV-1 DNA in breast milk samples obtained during the first week postpartum (OR, 3.3; 95% CI, 1.2–9.2). In multivariate analysis, a trend for an association was still seen after adjusting for absolute CD4 cell count (OR, 2.7; 95% CI, 0.9–8.1).
Correlates of cervical HIV-1 DNA detection
Detection of HIV-1 DNA in the cervix was not associated with age, marital status, or frequency of sex during pregnancy. The presence of cervical mucopus was significantly associated with HIV-1 shedding (OR, 2.1; 95% CI, 1.1–3.9) (table 1). Cervical mucopus was associated with gonorrhea (OR, 6.6; 95% CI, 2.0–21.6) and tended to be associated with chlamydia (OR, 2.0; 95% CI, 0.7–5.5). There was a higher prevalence of gonorrhea and chlamydia among women with HIV-1 DNA in the cervix, but this was not statistically significant. There was no correlation between HIV-1 DNA in the cervix and cervical ectopy.
Table 1.
Correlates of cervical HIV-1 DNA shedding.
| Correlate | PCR positive | PCR negative | Odds ratio (95% CI) |
P |
|---|---|---|---|---|
| Demographic, sexual, and obstetric histories | ||||
| Age, median (range) | 23 (17–38) | 23 (16–36) | .41 | |
| No. of sex partners this pregnancy, median | 1 | 1 | .13 | |
| Frequency of sexual intercourse/month in last trimester, median | 2 | 2 | .28 | |
| Primigravida | 20/66 (30) | 57/141 (40) | 0.6 (0.3–1.2) | .21 |
| Gestational age, median | 33 | 32 | .13 | |
| Physical examination | ||||
| HIV-related signs and symptoms | 21/63 (33) | 27/138 (20) | 2.1 (1.1–4.0) | .05 |
| Cervical discharge | 28/63 (44) | 38/138 (28) | 2.1 (1.1–3.9) | .03 |
| Vaginal discharge | 35/63 (56) | 70/138 (51) | 1.2 (0.7–2.2) | .63 |
| Genital warts | 6/63 (10) | 15/137 (11) | 0.9 (0.3–2.3) | .95 |
| Ectopy | 51/62 (82) | 110/136 (81) | 1.1 (0.5–2.4) | .97 |
| Cervical friability | 28/63 (44) | 57/138 (41) | 1.1 (0.6–2.1) | .79 |
| Oral thrush | 2/63 (3) | 1/138 (1) | 4.5 (0.4–50.5) | .23 |
| Laboratory | ||||
| Cervical WBC count, median | 15 | 16 | .69 | |
| Chlamydia trachomatis | 6/62 (10) | 11/134 (8) | 1.2 (0.4–3.4) | .95 |
| Neisseria gonorrhoeae | 7/65 (11) | 7/132 (5) | 2.2 (0.7–6.4) | .24 |
| Bacterial vaginosis | 29/58 (50) | 63/133 (47) | 1.1 (0.1–2.2) | .74 |
| Candidiasis | 21/57 (37) | 36/123 (29) | 1.4 (0.7–2.7) | .40 |
| Rapid plasma reagin assay | 5/65 (8) | 6/138 (4) | 1.8 (0.5–6.2) | .33 |
| Hemoglobin <10 g | 23/65 (35) | 25/137 (18) | 2.5 (1.2–5.1) | .007 |
| WBC count | 6.8 | 7.2 | .15 | |
| CD4 % cell | 22 | 27 | <.001 | |
| Absolute CD4 cell count | 354 | 469 | <.001 | |
| CD4:CD8 ratio | 0.4 | 0.6 | .002 | |
| CD8 % cell | 52 | 48 | .01 | |
| Absolute CD8 cell count | 817 | 874 | .32 | |
| Vitamin A level (µg/dL) | 27.0 | 25.5 | .47 | |
| Vitamin A <20 µg/dL | 14/62 (23) | 34/140 (24) | 0.9 (0.4–2.0) | .93 |
| Vaginal HIV-1 DNA | 11/64 (17) | 9/140 (6) | 3.0 (1.2–7.7) | .03 |
| Breast milk HIV-1 DNA | 17/25 (68) | 18/46 (39) | 3.3 (1.2–9.2) | .04 |
NOTE. Data are no. of subjects/no. total (%) unless otherwise indicated. CI, confidence interval; WBC, white blood cell.
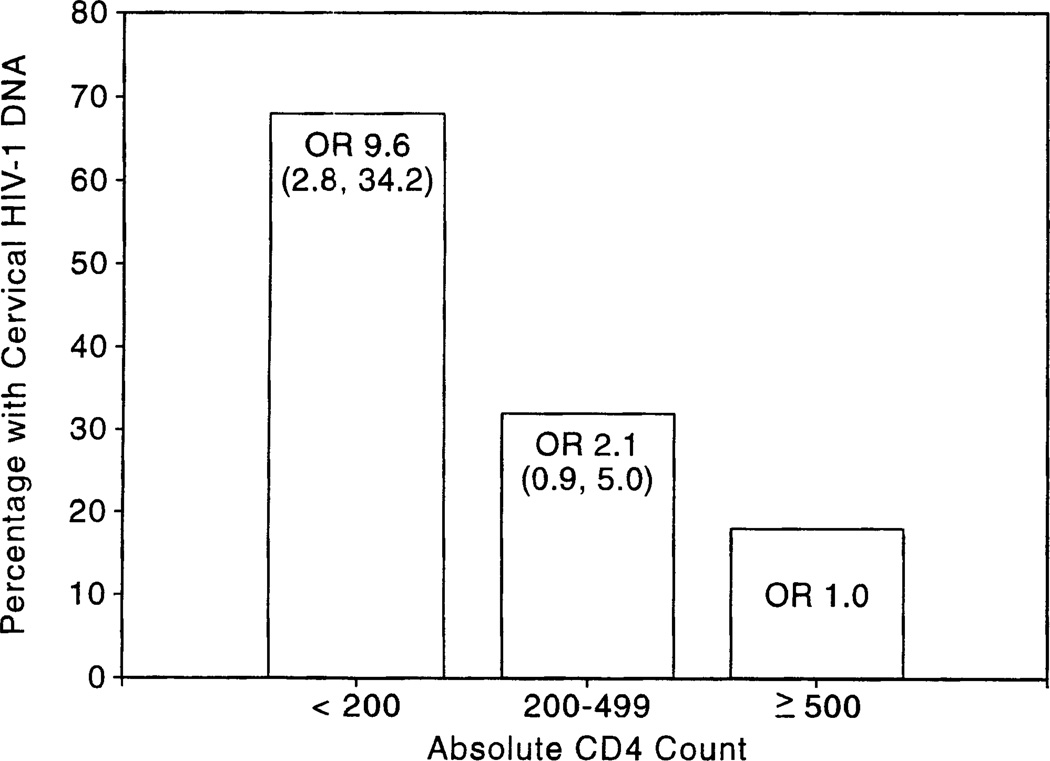
Women with HIV-1 DNA in their cervix had a significantly lower mean CD4 cell count (354 vs. 469, P < .001), CD4 cell percent (22% vs. 27%, P < .001), and CD4:CD8 ratio (0.5 vs. 0.6, P = .002) and a higher CD8 cell percent (52% vs. 48%, P = .01) than did women without HIV-1 DNA in their cervix. Women with absolute CD4 cell counts <200 had a 9.6-fold higher prevalence of HIV-1 DNA in their cervix than did women with CD4 cell counts ≥500 (OR, 9.6; 95% CI, 2.8–34.2) (figure 1). Women with cervical HIV-1 provirus had a significantly higher prevalence of HIV-related signs and symptoms (OR, 2.1; 95% CI, 1.1–4.0) and anemia (OR, 2.5; 95% CI, 1.3–4.8). The association with anemia did not appear to be mediated by immunosuppression, since anemia was not associated with absolute CD4 cell count or CD4 cell percent in this cohort. Neither vitamin A deficiency nor severe vitamin A deficiency was associated with cervical shedding in the group as a whole or after stratifying by CD4 cell counts.
Figure 1.
Prevalence of cervical HIV-1 DNA by absolute CD4 cell count. OR = odds ratio.
Correlates of vaginal HIV-1 DNA detection
Detection of vaginal HIV-1 DNA was not associated with age, marital status, frequency of sex during pregnancy, or HIV-related signs or symptoms. Women with HIV-1 DNA in the vagina had a higher frequency of abnormal vaginal discharge (OR, 3.3; 95% CI, 1.1–9.4) (table 2). There was no association between detection of vaginal HIV-1 DNA shedding and presence of cervical mucopus, genital warts, bacterial vaginosis, trichomonas, gonorrhea, or chlamydia. Women with vaginal HIV-1 DNA had a higher prevalence of candidiasis, but this association did not reach statistical significance. Women with vaginal shedding had a significantly lower mean CD4 cell percent (2% vs. 25%, P = .01), absolute CD4 cell count (334 vs. 438, P = .03), and CD4:CD8 ratio (0.4 vs. 0.6, P = .02). Severe vitamin A deficiency was associated with detection of HIV-1 DNA in the vagina (OR, 2.6; 95% CI, 1.1–6.1). In a multivariate model, adjusting for absolute CD4 cell count, there was a trend for an association between severe vitamin A deficiency and vaginal HIV-1 shedding (OR, 2.5; 95% CI, 0.8–7.5).
Table 2.
Correlates of vaginal HIV-1 DNA shedding.
| Correlate | PCR positive | PCR negative | Odds ratio (95% CI) |
P |
|---|---|---|---|---|
| Physical examination | ||||
| Cervical discharge | 8/20 (40) | 57/186 (31) | 1.5 (0.6–3.9) | .55 |
| Vaginal discharge | 15/20 (75) | 89/186 (48) | 3.3 (1.1–9.4) | .04 |
| Genital warts | 2/20 (10) | 20/185 (11) | 0.9 (0.2–4.2) | 1.0 |
| Laboratory | ||||
| Trichomonas vaginalis | 8/21 (38) | 17/42 (41) | 0.9 (0.3–2.7) | 1.0 |
| Bacterial vaginosis | 9/19 (47) | 86/176 (49) | 0.9 (0.3–2.7) | .90 |
| Candidiasis | 9/19 (47) | 52/167 (31) | 2.0 (0.8–5.2) | .24 |
| WBC count | 6.1 | 7.1 | .02 | |
| CD4 % cell | 21 | 25 | .03 | |
| Absolute CD4 cell count | 334 | 438 | .01 | |
| CD4:CD8 ratio | 0.4 | 0.6 | .02 | |
| CD8 % cell | 53 | 49 | .07 | |
| Absolute CD8 cell count | 842 | 859 | .84 | |
| Vitamin A <20 µg/dL | 8/18 (44) | 41/187 (26) | 2.6 (1.1–6.1) | .04 |
| Cervical HIV-1 DNA | 11/20 (55) | 53/184 (29) | 3.0 (1.2–7.7) | .03 |
| Breast milk HIV-1 DNA | 3/5 (68) | 34/68 (50) | 1.5 (0.2–9.6) | 1.0 |
NOTE. Data are no. of subjects/no. total (%) unless otherwise indicated. CI, confidence interval; WBC, white blood cell.
Discussion
In this study of 223 HIV-1–seropositive pregnant women recruited from antenatal clinics in Nairobi, HIV-1 DNA was detected in 32% of cervical and 10% of vaginal samples. In previous studies of nonpregnant cohorts in Kenya, HIV-1 DNA was detected in 33%–37% of cervical samples and 17% of vaginal samples [11, 12]. These data corroborate and extend our earlier findings that virus-infected cells are more commonly present in cervical than vaginal secretions. Although two small studies found an association between pregnancy and cervical HIV-1 DNA, our current results do not demonstrate that pregnant women have an increased prevalence of cervical HIV-1 DNA compared with nonpregnant cohorts. Our samples were obtained at a single time point during the last trimester of pregnancy. Serial evaluations over the course of pregnancy will be useful for determining potential effects of hormonal and other physiologic changes in pregnancy on genital shedding of HIV-1.
We found that cervical mucopus was associated with cervical shedding of HIV-1 DNA, and abnormal vaginal discharge was associated with vaginal shedding of HIV-1. Cervical mucopus was associated with gonorrhea and chlamydia, and there was a higher, albeit not statistically significant, prevalence of gonorrhea and chlamydia among women with cervical shedding of HIV-1. In a previous study, we found that gonorrhea was associated with urethral HIV-1 DNA shedding in men, and that the prevalence of HIV-1 DNA declined by 50% following treatment [19]. Sexually transmitted diseases (STDs) have been consistently identified as risk factors for heterosexual transmission of HIV-1 [20, 21], but the effect of STDs on vertical transmission has not been conclusively documented [4, 8, 22]. Defining the effect of STDs on vertical transmission of HIV-1 will be important for developing potential interventions. While routine antenatal STD screening and treatment may be impractical in resource-poor settings, examination for cervical mucopus is inexpensive and may identify women at higher risk for STDs and HIV-1 shedding.
The presence of HIV-1 DNA or HIV-1 RNA in the semen of HIV-1–seropositive men is associated with immunosuppression [14, 15]. An association between cervical or vaginal shedding with immunosuppression has not been previously reported. Our study demonstrated a significantly lower absolute CD4 cell count, CD4 cell percent, and CD4:CD8 ratio among women with cervical or vaginal shedding of HIV-1. Our data suggest that the association between maternal immunosuppression and vertical transmission of HIV-1 may be mediated in part by increased prevalence of genital provirus. If the effects of maternal immunosuppression or antiretroviral therapy on vertical transmission are due to the effect on genital virus load, it is possible that local interventions to decrease genital load (e.g., topical antivirals or antiseptics administered antenatally and peripartum) may reduce transmission as effectively as systemic interventions but at lower cost, with fewer side effects, and with less induction of resistant strains.
Cervical shedding of HIV-1 DNA was associated with anemia in this cohort, a potentially important observation given that anemia was associated with perinatal transmission in one large study from Zaire [8]. Anemia may simply be a marker of advanced HIV-1 clinical disease or may reflect underlying nutritional deficiencies that compromise mucosal integrity or immune responsiveness. Folate deficiency, a common cause of anemia in pregnancy, is associated with megaloblastic changes in epithelial surfaces, including cervical epithelium. Deficiencies of iron, vitamin B6, and zinc are associated with both immune dysfunction and anemia.
Semba et al. [17] in a study in Malawi, observed a significant association between vitamin A deficiency and vertical transmission of HIV-1, and this association was independent of CD4 cell count. Mothers with severe vitamin A deficiency (<20 µg/dL) had a mother-to-child transmission rate of 32.4%, while those with high vitamin A levels (>40 µg/dL) had a transmission rate of 7.2% (P < .0001). The mechanism for the increased transmission risk remains speculative, but vitamin A deficiency is known to be associated both with immunosuppression and with mucosal changes. In a previous study in our breast-feeding cohort, we observed an association between the presence of HIV-1 DNA in breast milk and vitamin A deficiency, but only among women with CD4 cell counts <400 [16]. We were particularly interested in determining whether vitamin A levels were correlated with genital HIV-1 shedding. In our current study, we found an association between vaginal HIV-1 shedding and severe vitamin A deficiency, suggesting that a possible protective effect of vitamin A on transmission may be mediated in part by the effect of vitamin A on vaginal shedding.
In this study, women with cervical shedding of HIV-1–infected cells had a significantly higher likelihood of HIV-1 shedding in the vagina and in breast milk. Simultaneous shedding of HIV-1 from multiple sites may be due to a high total virus burden. Alternatively, it is possible that certain HIV-1 strains, such as macrophage-tropic strains, are more likely to be shed from mucosal or epithelial surfaces or that there are host factors at the mucosal level that predispose some women to be “shedders.”
We view our results as an important first step in understanding the relationship between local virus and intrapartum transmission. Many questions follow, the most important of which is to determine whether the presence of HIV-1–infected cells in cervical or vaginal secretions is predictive of intrapartum transmission. It would also be of interest to quantify cell-associated and cell-free virus in genital secretions and to characterize the presence or levels of virus throughout the course of pregnancy. It is conceivable that in the future, antenatal PCR testing of genital secretions could identify women who would benefit from cesarean delivery. An improved understanding of genital tract shedding of HIV-1 during pregnancy is necessary to design rational interventions to reduce vertical transmission during the intrapartum period.
Acknowledgments
We thank the Nairobi City Council for permission to use clinics at Umoja and Jericho and the administration of Kenyatta National Hospital for permission to use clinical facilities. We thank the women in the study for their participation, the clinical and laboratory staff, the staff at the International AIDS Research and Training Program for their assistance with manuscript preparation, and Donald Riley for performing PCR analyses of vaginal specimens for T. vaginalis.
Grant support: NIH (HD-23412, TW-00001, TW-00007, and the Clinical Nutrition Research Unit, DK-35816).
Footnotes
Presented in part: IXth International Conference on AIDS and STDs in Africa, Kampala, Uganda, December 1995, (abstract B142); XIth International Conference on AIDS, Vancouver, Canada, July 1996 (abstract C331).
Informed consent was obtained from all study participants. Human experimentation guidelines from the US Department of Health and Human Services, University of Nairobi, and University of Washington were followed.
References
- 1.WHO/GPA/CNP/EVA/93.1. Geneva: WHO; 1993. Global program on AIDS. [Google Scholar]
- 2.Mofenson LM. Epidemiology and determinants of vertical HIV transmission. Semin Pediatr Infect Dis. 1994;5:253–265. [Google Scholar]
- 3.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type-1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 4.Boyer PJ, Dillon M, Navaie M, et al. Factors predictive of maternal-fetal transmission of HIV-1. Preliminary analysis of zidovudine given during pregnancy and/or delivery. JAMA. 1994;271:1925–1930. [PubMed] [Google Scholar]
- 5.European Collaborative Study. Risk factors for mother-to-child transmission of HIV-1. Lancet. 1992;339:1007–1012. doi: 10.1016/0140-6736(92)90534-a. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JB, Katasha P, Horn DI, et al. β2-microglobulin, HIV-1 p24 antibody and acid dissociated HIV-1 p24 antigen levels: predictive markers for vertical transmission of HIV-1 in pregnant women. AIDS. 1993;7:1475–1479. [PubMed] [Google Scholar]
- 7.Khouri YF, McIntosh K, Cavacini L, et al. Vertical transmission of HIV-1: correlation with maternal viral load and plasma levels of CD4 binding site anti–gpl20 antibodies. J Clin Invest. 1995;95:732–737. doi: 10.1172/JCI117720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Louis ME, Kamenga M, Brown C, et al. Risk for perinatal HIV-1 transmission according to maternal immunologic, virologic and placental factors. JAMA. 1993;269:2853–2859. [PubMed] [Google Scholar]
- 9.Vogt MW, Witt DJ, Craven DE, et al. Isolation patterns of human immunodeficiency virus from cervical secretions during the menstrual cycle of women at risk for the acquired immunodeficiency syndrome. Ann Intern Med. 1987;106:380–382. doi: 10.7326/0003-4819-106-3-380. [DOI] [PubMed] [Google Scholar]
- 10.Wofsy CB, Cohen JB, Hauer LB, et al. Isolation of AIDS-associated retrovirus from genital secretions of women with antibodies to the virus. Lancet. 1986;1:527–529. doi: 10.1016/s0140-6736(86)90885-8. [DOI] [PubMed] [Google Scholar]
- 11.Kreiss J, Willerford DM, Hensel M, et al. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J Infect Dis. 1994;170:1597–1601. doi: 10.1093/infdis/170.6.1597. [DOI] [PubMed] [Google Scholar]
- 12.Clemetson DBA, Moss GB, Willerford DM, et al. Detection of HIV DNA in cervical and vaginal secretions. JAMA. 1993;269:2860–2864. [PubMed] [Google Scholar]
- 13.Henin Y, Mandelbrot L, Henrion R, Pradinaud R, Coulaud JP, Montagnier L. Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J Acquir Immune Defic Syn. 1993;6:72–75. [PubMed] [Google Scholar]
- 14.Anderson DJ, O’Brien TR, Politch JA, et al. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA. 1992;267:2769–2774. [PubMed] [Google Scholar]
- 15.Mermin JH, Holodniy M, Katzenstein DA, et al. Detection of human immunodeficiency virus DNA and RNA in semen by the polymerase chain reaction. J Infect Dis. 1991;164:769–772. doi: 10.1093/infdis/164.4.769. [DOI] [PubMed] [Google Scholar]
- 16.Nduati RW, John GC, Richardson BA, et al. Human immunodeficiency virus type 1–infected cells in breast milk: association with immunosuppression and vitamin A deficiency. J Infect Dis. 1995;172:1461–1468. doi: 10.1093/infdis/172.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semba RD, Miotti PG, Chiphangwi JD, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet. 1994;343:1593–1597. doi: 10.1016/s0140-6736(94)93056-2. [DOI] [PubMed] [Google Scholar]
- 18.Riley DE, Roberts MC, Takayama T, Kreiger JN. Development of a polymerase chain reaction–based diagnosis of Trichomonas vaginalis. J Clin Microbiol. 1992;30:465–472. doi: 10.1128/jcm.30.2.465-472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss GB, Overbaugh J, Welch M, et al. Human immunodeficiency virus DNA in urethral secretions in men: association with gonococcal urethritis and CD4 depletion. J Infect Dis. 1995;172:1469–1475. doi: 10.1093/infdis/172.6.1469. [DOI] [PubMed] [Google Scholar]
- 20.Cameron BW, Simonsen JN, D’Costa LJ, et al. Female to male transmission of human immunodeficiency type 1: risk factors for seroconversion in men. Lancet. 1989;2:403–408. doi: 10.1016/s0140-6736(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 21.Laga M, Manoka AT, Kivuvu M, et al. Nonulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Nair P, Alger L, Hines S, et al. Maternal and neonatal characteristics associated with HIV infection in infants of seropositive women. J Acquir Immune Defic Syndr. 1993;6:298–302. [PubMed] [Google Scholar]