Abstract
There is growing interest in HIV-specific antibody-dependent cellular cytotoxicity (ADCC) as an effective immune response to prevent or control HIV infection. ADCC relies on innate immune effector cells, particularly NK cells, to mediate control of virus-infected cells. The activation of NK cells (i.e., expression of cytokines and/or degranulation) by ADCC antibodies in serum is likely subject to the influence of other factors that are also present. We observed that the HIV-specific ADCC antibodies, within serum samples from a panel of HIV-infected individuals induced divergent activation profiles of NK cells from the same donor. Some serum samples primarily induced NK cell cytokine expression (i.e., IFNγ), some primarily initiated NK cell expression of a degranulation marker (CD107a) and others initiated a similar magnitude of responses across both effector functions. We therefore evaluated a number of HIV-relevant soluble factors for their influence on the activation of NK cells by HIV-specific ADCC antibodies. Key findings were that the cytokines IL-15 and IL-10 consistently enhanced the ability of NK cells to respond to HIV-specific ADCC antibodies. Furthermore, IL-15 was demonstrated to potently activate “educated” KIR3DL1+ NK cells from individuals carrying its HLA-Bw4 ligand. The cytokine was also demonstrated to activate “uneducated” KIR3DL1+ NK cells from HLA-Bw6 homozygotes, but to a lesser extent. Our results show that cytokines influence the ability of NK cells to respond to ADCC antibodies in vitro. Manipulating the immunological environment to enhance the potency of NK cell-mediated HIV-specific ADCC effector functions could be a promising immunotherapy or vaccine strategy.
Introduction
The development of a safe and effective HIV vaccine is urgently needed. Traditional HIV vaccine constructs have focused on the induction of broadly neutralizing antibodies (BnAbs) and cytotoxic T-lymphocytes (CTL) [1]. However, several lines of evidence suggest that non-neutralizing HIV-specific antibodies could play an important role in preventing or controlling HIV infection. These antibodies can bind to infected cells and recruit innate immune effector cells, such as natural killer (NK) cells, to lyse infected cells through antibody-dependent cellular cytotoxicity (ADCC). A recent HIV vaccine efficacy trial based on a recombinant Canarypox virus prime and envelope protein boost showed partial protection from HIV infection, despite inducing only narrow neutralizing antibody responses and very modest CTL responses [2]. High levels of HIV-specific ADCC-competent antibodies were induced by this regimen, suggesting such responses may play a role in protective immunity [3], [4]. This idea is supported by elegant passive transfer experiments in Rhesus macaques, which demonstrate decreased effectiveness of BnAbs no longer capable of eliciting antibody constant region (Fc)-dependent ADCC responses [5]. Furthermore, ADCC responses have been associated with protective outcomes against immunodeficiency viruses [6], [7], in particular this has been observed in highly exposed seronegative intravenous drug users, HIV-infected elite controllers and vaccinated Rhesus macaques [8]–[10].
The potential effectiveness of infection-induced HIV-specific ADCC responses suggests that attempts to improve or modulate these responses could provide a therapeutic benefit or assist in protective immunity. Understanding the best mechanisms to present ADCC epitopes for immune recognition, or to improve the potency with which they activate NK cells, may be important for improving ADCC-based therapeutic or preventative strategies. Recent research has highlighted the importance of antibody specificity and the glycosylation of antibody Fc regions for driving efficient ADCC responses [11], [12]. It has also been demonstrated that NK cells are more likely to mediate ADCC if NK cell function was conferred through the interaction of inhibitory killer cell immunoglobulin-like receptors (KIR) and their major histocompatibility complex (MHC) class I (or HLA-I) ligands during the process of NK cell education [13]–[15]. Furthermore, soluble factors within plasma, such as cytokines, have been demonstrated to effect NK cell responsiveness [16] and have been associated with the rate of disease progression [17], potentially due to a synergistic effect on NK cell mediated anti-HIV ADCC.
Alterations in production and plasma/sera levels of cytokines during HIV infection are extensive. Investigators have reported increases in the ability of peripheral blood mononuclear cells (PBMC) to produce IL-4 [18] and IL-10 [19], while increased levels of soluble IL-10 [20], IL-7 [21], GM-CSF [22], and TNFα [23] have been observed in the plasma of infected individuals. Furthermore, reductions in the production of IL-12 and IL-15 have been reported in HIV-infected individuals [24], [25]. The presence of other soluble plasma factors has also been noted to be enhanced during HIV infection. Most notably, LPS levels are increased in HIV-infected individuals [26], and have been correlated with the level of immune activation and disease progression [27]. The influence of cytokines on NK cell-mediated HIV-specific ADCC has not been thoroughly studied. Such studies could ultimately lead to understanding how best to influence the immune environment to obtain optimal ADCC levels during therapeutic and/or prophylactic interventions.
NK cells are exquisitely sensitive to exogenous cytokines, which can increase or decrease multiple effector functions [16]. Most anti-HIV ADCC assays utilize whole serum or plasma that contains not only the antibody of interest but also variable levels of a suite of biologically activate cytokines. Indeed, chronic viral infections, such as HIV, substantially perturb plasma cytokine levels, which could plausibly influence the effectiveness of ADCC responses in vivo. Upon activation NK cells mediate a variety of effector functions, including the release of cytokines and chemokines, and degranulation to kill neighboring virus-infected cells. We hypothesized that exposure of NK cells to different exogenous cytokines prior to activation would differentially alter the effector functions mediated by activated NK cells. Furthermore, as educated NK cells are conferred with higher functional potential [28], we hypothesized that NK cells educated through KIR3DL1/HLA-Bw4 interactions would be more susceptible to the effects of exogenous cytokines than KIR3DL1+ NK cells from HLA-Bw6 homozygous individuals. We used a recently developed flow-based ADCC assay that measures NK cell activation (IFNγ synthesis and CD107a degranulation marker expression) to evaluate these hypotheses [29], [30]. Whole blood from HIV-uninfected healthy controls was incubated with HIV antigens and ADCC-competent plasma, serum or purified IgG from HIV-infected individuals. These incubations were done in the presence and absence of exogenous cytokines. NK cells responding to ADCC antibodies were evaluated for IFNγ production and expression of the CD107a degranulation marker.
Methods and Materials
Study Population
We studied ADCC induced NK cell activation responses elicited by sera samples from 32 HIV-infected subjects not on antiretroviral therapy recruited through the Kirby Institute (Sydney, Australia). Table 1 provides the demographics and clinical characteristics of these patients. Plasma and sera samples from an additional 19 HIV-infected individuals recruited from the Melbourne Sexual Health Centre and one HIV-infected individual from La Clinique l’Actuel, Montreal, Quebec, Canada were utilized to study envelope-specific ADCC responses. HIV-uninfected healthy laboratory volunteers provided whole blood, for assessment of NK cell function. All subjects provided informed consent for participating in this study and human research and ethics committees from all participating study sites approved this study.
Table 1. Clinical characteristics of HIV-infected donors.
| Mean (range) | |
| Number | 32 |
| Age | 41 (28–65) |
| Female/Male | 1/31 |
| CD4+ cell count (cells/µl) | 746.5 (504-1310) |
| Viral load (copies/ml) | 290 (<50-203100) |
HLA Typing
Kits from Atria Genetics was used to conduct sequence-based typing of HLA-B alleles. Otherwise, HLA-B typing was performed by the Victorian Transplant and Immunogenetics Service (Parkville, Australia), using sequence-based typing.
Anti-HIV ADCC NK Cell Activation Assay
As previously described [12], a whole blood assay was used to assess NK cell activation by ADCC Abs. Briefly, 150 µl of HIV-uninfected healthy control whole blood plus 50 µl of ADCC-competent HIV-infected plasma/serum (stored at −20°C), or purified IgG, was incubated at 37°C for 5 hours with 1 µg/ml of HIV Env peptide pool or whole gp140 protein, Brefeldin A (5 µg/ml, Sigma) and Monensin (6 µg/ml, Sigma). ADCC responses were assessed using either a peptide pool containing 15-mers that overlapped by 11 amino acids or whole gp140 protein. The peptide pool spanned the HIV-1 consensus subtype B Env protein (kindly supplied by the NIH AIDS Reagent Repository). As previous described, the gp140 protein was obtained from purification of the supernatant of HeLa or 293T cells transfected with the gp140 gene from the subtype B AD8 isolate [31]. After incubation, cells were surface stained with Per-CP-conjugated anti-CD3, FITC-conjugated anti-CD2, PE-conjugated anti-KIR3DL1, PE-Cy7-conjugated anti-CD56 and APC-conjugated anti-CD107a (All from BD Biosciences). Next, whole blood was treated with lysing solution (BD Biosciences) to remove red blood cells, and the remaining white blood cells were treated with permeabilization solution (BD Biosciences) and stained with Alexa700-conjugated anti-IFNγ antibody (BD Biosciences). Flow cytometry data was collected using a FACS Canto II Flow cytometer (BD Biosciences), and was analyzed using Flow Jo Version 9.2 software (Tree Star). We have previously shown this assay is not dependent on immune complexes activating NK cells [30]. The assay works efficiently using either overlapping peptides or whole Env protein as the HIV antigen.
IgG Purification and Depletion from Sera
To assess the impact of soluble serum/plasma factors on the skewing of NK cell activation profiles, we purified total IgG from serum over a protein G spin column (Thermo Fisher Scientific) to use in the ADCC ICS assay [12]. Sera were bound to protein G columns for 4 hours with end over end agitation before elution of IgG as per the manufacturer’s instruction. Purified IgG samples were then dialyzed in 2 ml mini dialysis tubes (GE Healthcare) before they were concentrated in 30 kDa Amicon ultra centrifugal filter devices (Millipore). IgG was also depleted from sera using protein G spin columns (Thermo Fisher Scientific). IgG-depleted sera was then combined with IgG from a single source and used in the ADCC ISC assay described above, to assess the effect of soluble sera factors on a known ADCC-mediated NK cell activation response.
Anti-Env IgG ELISA
100 ng of HIV-1AD8 Env gp140 purified from media conditioned by a stable gp140-expressing cell line (31) was absorbed onto the bottom of ELISA plate wells in coating buffer (20 mM Tris pH 8.8, 100 mM NaCl) overnight at 4°C. Wells were then blocked with blocking buffer (5% skim milk powder in PBS/0.1% Tween 20) for 1 hour. Patient samples were added as half log dilution series in block buffer and incubated for 4 hours at room temperature followed by washing with PBS/0.1% Tween 20. HRP conjugated antibody against human IgG in blocking buffer was then added and incubated for 1 hour. After washing ELISAs were developed using standard techniques. Background was measured by titration of HIV negative human sera. Wells were considered positive when OD was at least 5-fold higher than background. Endpoint titers (most dilute samples giving a positive reading) were averaged over two assays.
Cytokines
In vitro supplementation with the following cytokines and growth factors, for the five hour duration of the anti-HIV ADCC assay, was studied to assess their influence on NK cell activation profiles: IL-10 (50 ng/ml) (BD Biosciences), IL-15 (5 ng/ml) (R&D Systems), IL-4 (50 Units/ml) (BD Biosciences), GM-CSF (1 µg/ml) (BD Biosciences), IL-12 (100 ng/ml) (R&D Systems), IL-7 (50 ng/ml) (BD Biosciences), TNFα (200 ng/ml) (eBioscience) and LPS (1 µg/ml) (Sigma).
Statistical Analyses
Data analyses were performed using GraphPad Prism Version 4.0 software. Data sets were tested for normal distribution using the Kolmogorov-Smirnov test. Parametric data was analyzed using T-tests or paired T-tests. Non-parametric data was compared using Mann-Whitney tests, Wilcoxon Matched Pairs tests, or Spearman correlations.
Results
Skewed ADCC-induced NK Cell Activation Profiles Mediated by Sera from HIV-infected Individuals
NK cells exhibit a number of functions when activated by the Fc portion of ADCC antibodies, including the expression of cytokines and the degranulation and lysis of target cells. We hypothesized that ADCC-induced NK cell effector functions may be differentially regulated depending on the cytokine milieu of the plasma. To evaluate this hypothesis we simultaneously evaluated sera samples obtained from a cohort of 32 antiretroviral therapy naive HIV-infected subjects for their ability to activate fresh NK cells in blood obtained from a single healthy donor in the presence of Env peptide or protein antigens. We utilized a previously described flow cytometric assay of antibody-mediated NK cell activation [12], [15], [29], [30], [32]–[34]. This simple assay studies the ability of donor NK cells within whole blood to be activated by antibodies within HIV+ sera samples recognizing HIV peptides or Env proteins. The NK cell activation in this assay occurs only when both peptides/proteins and antibodies are present (Fig. 1a). Furthermore, this assay is not dependent on NK cell recognition of immune complexes.
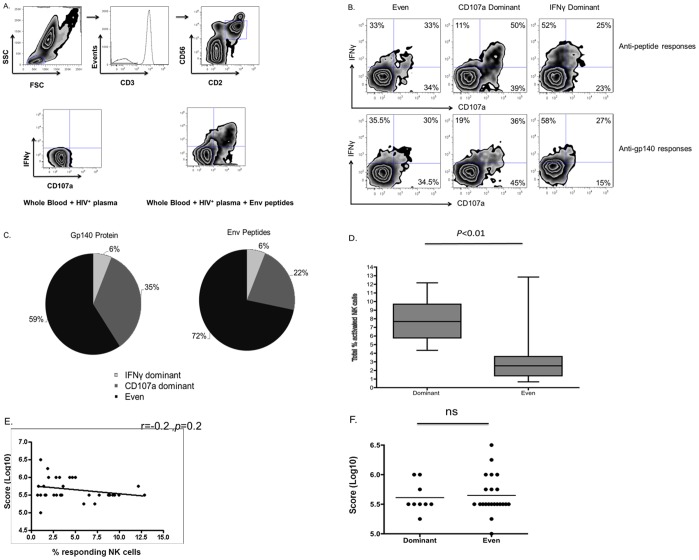
Figure 1. Differential NK cell activation patterns by HIV-specific ADCC.
The ability of NK cells to respond to anti-HIV ADCC antibodies was assessed using a flow-based assay. (A) Stimulated cells were stained with fluorochrome conjugated antibodies against CD3, CD2, CD56, CD107a and IFNγ. After collection on a FACS II Canto, lymphocytes were gated upon and NK cells were identified as CD3−CD2+CD56+. Cells within the NK cell population were assessed for IFNγ production and CD107a expression prior to and following activation. (B) Zebra plots depict examples of the diverse anti-HIV ADCC responses obtained when NK cells from a common donor are stimulated with sera from different HIV-infected individuals in the presence of Env peptides (top) or gp140 protein (bottom). The numbers in the quadrants represent the percentages of responding NK cells that are mediating IFNγ+CD107a−, IFNγ−CD107a+ and IFNγ+CD107a+ responses. (C) The pie chart on the left illustrates the frequency with which IFNγ dominant, CD107a dominant and even response profiles were observed, when sera samples from 32 HIV-infected individuals were used to stimulate NK cells from a common donor in the presence of gp140 protein. The pie chart on the right depicts the same analysis for stimulation with Env peptides. (D) The box and whiskers plot depicts the assessment of the relationship between the ability of different sera to induce diverse anti-HIV ADCC functional profiles against HIV gp140 and the magnitude of the total ADCC response. The total percent of NK cell activation was compared between sera that induced “even” or “skewed” ADCC responses, using a T-test. (E) The scatter plot illustrates the relationship between the levels of sera anti-HIV gp140 IgG and the percent of total NK cells, from a common donor, activated by the sera in the whole blood ADCC assay in the presence of gp140. This correlation was assessed with the Spearman correlation. (F) The scatter plot depicts the assessment of the impact of the level of sera-associated anti-HIV gp140 IgG on the functional profile induced by different sera, evaluated by a T-test.
We observed marked differences in the ability of NK cells from the same donor to express IFNγ, CD107a or both effector molecules in response to the same HIV Env antigens using different HIV+ sera samples - examples are shown in Fig. 1b. We quantified this difference by assessing whether there was markedly greater IFNγ expression, greater CD107a expression or similar expression of both molecules from NK cells. Quantification was performed by using a 1.5% differential to determine if responses were skewed towards a particular response profile. As such, responses that exhibited equal to or higher than 1.5% NK cell expression of one effector molecule than the other were considered skewed for that response, while responses with less than 1.5% differences were considered equal. For stimulations involving gp140 protein CD107a was the predominant effector molecule expressed in 22% of samples, whereas in a minority (6% of samples) IFNγ expression predominated (Fig. 1c). Similarly, for stimulations involving Env peptide stimulation CD107a was the predominant effector molecule expressed for 35% of samples, whereas in a minority (6% of samples) IFNγ expression predominated (Fig. 1c). It should be noted that plasma samples that induce skewed responses appear to induce similarly skewed responses regardless of the source of donor NK cells. We assessed anti-HIV ADCC using a single plasma source on 20 HIV-uninfected controls. We found that the same source of plasma consistently induced a CD107a-skewed NK cell functional profile; the mean ratio between percent of NK cells expressing CD107a and percent of NK cells expressing IFNγ was 3.0 (range 1.2–16.9) across the 20 subjects.
Previous research has suggested that individual NK cells within an organism obtain diverse functional potentials through ontological processes, such as NK cell education. As such, we questioned if the differential responses induced by different serum samples were reflective of the activation of different arrays of functionally competent NK cells. To answer this question we compared the total percentage of activated NK cells present in individuals that mediated “skewed” and “even” ADCC responses against HIV gp140. This analysis demonstrated that skewed responses were associated with higher percentages of activated NK cells (7.9+/−0.8 vs. 3.4+/−0.6, p<0.01, Mann-Whitney test) (Fig. 1d). It should be noted that a similar observation was made for ADCC responses against HIV Env peptides (Data not shown).
Next, we evaluated if the differences in magnitude and functional profiles of NK cell-mediated ADCC responses to different plasmas could be explained by different levels of anti-HIV IgG present in the sera, or if it was related to other soluble sera factors. As such, an anti-Env IgG ELISA was utilized to assess the levels of anti-Env IgG in 31/32 sera samples (Due to limited sample availability). Figure 1E illustrates that no correlation was observed between the level of sera anti-Env IgG and the magnitude the anti-HIV ADCC response (r = 0.2, p = 0.2, Spearman correlation). Figure 1F illustrates that no difference in sera anti-Env IgG was observed between sera mediating “skewed” responses and those mediating “even” responses (p>0.05, Mann-Whitney test). These observations suggest that plasma factors in additional to Env-specific IgG levels are influencing the magnitude and functional profile of NK cell-mediated anti-HIV ADCC responses.
Previous research has demonstrated that soluble plasma factors, such as cytokines, can alter the immune responsiveness of NK cells [16]. To assess if plasma-derived factors were responsible for these skewed ADCC responses, we next purified IgG from the sera of 10 HIV-infected subjects with predominantly CD107a expression profiles and assessed the Env-specific ADCC-induced activation profiles of NK cells activated with purified IgG in comparison to whole sera. Four representative samples from these experiments are shown in figure 2A. Despite having no effect on the total percentage of responding NK cells (p>0.05, Wilcoxon Matched Pairs Test) (Fig. 2B), IgG purification was consistently observed to alter the response towards a more IFNγ+ response profile (Fig. 2C). This is illustrated by the shift from plasma induced responses that consisted of 94% of activated NK cells expressing CD107a and 36% of activated NK cells producing IFNγ to pure IgG induced responses that consisted of 80% of activated NK cells expressing CD107a and 63% of activated NK cells producing IFNγ. There was significantly increased synthesis of IFNγ in ADCC mediated by purified IgG compared to that mediated by sera (63+/−3.3 vs. 36+/−5.1, p = 0.0001, paired T-test) and significantly decreased expression of CD107a in ADCC mediated by purified IgG compared to that mediated by sera (80+/−2.9 vs. 94+/−1.0, p = 0.001, paired T-test). These results suggest that soluble factors, other than IgG, within sera, are affecting the cytokine expression/degranulation profile of NK cells following ADCC activation.
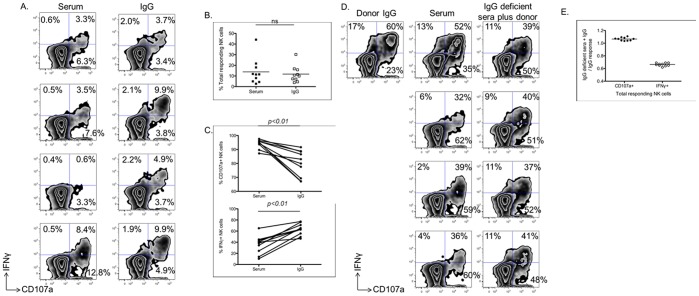
Figure 2. Effect of IgG purification on NK cell-mediated ADCC.
The impact of non-IgG soluble sera factors was assessed by activating NK cells for ADCC functionality in the presence of whole sera, purified IgG, or common purified IgG in the presence of IgG-depleted sera from a series of donors. (A) Zebra plots depict the anti-HIV ADCC mediated by NK cells when incubated with whole sera or IgG purified from the same sera. The values depicted represent the percentages of total NK cells mediating CD107a+IFNγ−, CD107a−IFNγ+, or CD107a+IFNγ+ functional profiles. (B) The scatter plot depicts a comparison of the percentage of total NK cells mediating ADCC-induced effector functions after stimulation of NK cells from a common donor with sera or IgG purified from sera. This difference was assessed with a paired T-test. (C) The graph on the top illustrates the percent of responding NK cells expressing CD107a after stimulation with sera or IgG purified from the same sera. The graph on the bottom illustrates the percent of responding NK cells producing IFNγ after stimulation with sera or IgG purified from the same sera. Differences were assessed with paired T-tests. (D) Zebra plots depict the response of NK cells from a common donor to anti-HIV ADCC when incubated with purified IgG from a common donor, sera from a series of donors, or purified IgG from a common donor combined with IgG-depleted sera from the series of donors. The values depicted represent the percentages of responding NK cells mediating CD107a+IFNγ−, CD107a−IFNγ+, or CD107a+IFNγ+ functional profiles. (E) The graph illustrates the alteration of the NK cell-mediated ADCC functional profile induce by the common purified IgG after the addition of IgG-depleted sera from a series of donors.
To confirm that the alteration of the NK cell responses towards higher IFNγ and lower CD107a expression following IgG purification was due to soluble factors present within the plasma, we next performed a set of experiments that involved separately adding IgG-depleted sera from 10 different HIV-infected donors that exhibited CD107a dominant responses to the purified IgG from a single donor. Four representative examples of these experiments are depicted in figure 2D. Similar to the results suggesting that ADCC responses induced by whole plasma are characterized by higher CD107a expression and lower IFNγ synthesis than ADCC responses mediated by purified IgG, the addition of IgG-depleted plasma from different donors to a common source of purified IgG increased the responding NK cells that expressed CD107a and decreased the responding NK cells that produced IFNγ (Fig. 2E). Cumulatively, these results demonstrate that soluble factors other than IgG, which are present within the plasma/sera of HIV-infected individuals, influence the functional profile of NK cells mediating anti-HIV ADCC.
Influence of Cytokines on HIV-specific ADCC Activation of NK Cells
As our initial results suggested that soluble plasma factors have an impact on NK cell-mediated effector functions, we selected a series of soluble factors to assess their influence on the anti-HIV ADCC induced activation profiles of NK cells. As discussed in the introduction, these factors were chosen due to their previously observed relevance to HIV infection [18]–[27]. These factors were added into a standard culture of healthy donor whole blood, Env antigens and plasma from an HIV-infected donor with known anti-Env ADCC antibodies. This experiment was repeated with whole blood from four HIV-uninfected donors. We observed consistent alterations in the profile of NK cell activation in the presence of several cytokines (Figure 3). GM-CSF and IL-4 both exhibited an inhibitory effect on overall Env-specific ADCC-induced NK cell activation, decreasing responses in all donors studied. IL-12, TNF-α and IL-7 had little overall effect on ADCC-mediated NK cell activation in the donors. However, both IL-10 and IL-15 had a markedly positive effect on ADCC-induced NK cell activation, increasing ADCC responses in all 4 donors. These results suggest that cytokines can alter the immunological microenvironment within which anti-HIV ADCC responses occur, increasing or decreasing the potency of these anti-viral responses.
Figure 3. Effect of exogenous cytokines on ADCC-induced NK cell activation.
A suite of 7 cytokines and LPS was added separately to a mix of HIV+ plasma co-incubated with healthy donor blood and HIV-1 Env antigens. Gated CD3−CD2+CD56+ NK cells were studied for CD107a and IFNγ expression. These experiments were repeated using whole blood from four separate donors (A) Zebra plots depict the anti-HIV ADCC mediated by NK cells when incubated with cytokines in the absence of anti-HIV antibodies and Env antigens, incubated with anti-HIV antibodies and Env antigens with no cytokines added, or with the addition of cytokines. The values shown represent the percentages of total NK cells expressing both IFNγ and CD107a. (B) The effect of all cytokines and LPS on ADCC-driven NK cell activation is shown, expressed as a ratio of the response with cytokine added to the response without cytokine. The effect of cytokine addition on both total CD107a+ and total IFNγ+ responses is shown. Error bars represent variation between different whole blood donors.
Effects of IL-10 and IL-15 on Anti-HIV ADCC Induced NK Cell Activation
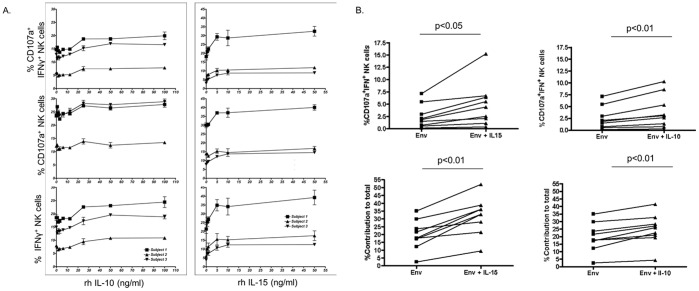
As IL-10 and IL-15 both enhanced NK cell-mediated anti-HIV ADCC effector functions, we performed a detailed assessment of the influences of these cytokines on NK cell-mediated anti-HIV ADCC. Since we observed an impact of these cytokines on the ability of NK cells to both produce IFNγ and express CD107a, we first performed titrations to elucidate the concentrations at which these cytokines are active (Figure 4A). These titrations also served to confirm the consistency of the effect of IL-10 and IL-15 across NK cells from several donors. While IL-10 required relatively high levels (i.e., 25–50 ng/ml) to enhance anti-HIV ADCC induced NK cell IFNγ production and degranulation, IL-15 maximally increased both NK cell effector functions at low concentrations (i.e., 5 ng/ml). These results demonstrate that cytokines can influence the effector functions of NK cells at concentrations that could be achievable through therapeutic mechanisms.
Figure 4. Consistent effects of IL-10 and IL-15 across numerous donors.
(A) Titration curves depict the effects of adding IL-10 or IL-15 to donor NK cells from three donors, which were all stimulated with the same HIV+ plasma in the presence of HIV-1 Env antigen. (B) NK cells from a common donor were stimulated with plasma from 9 HIV-infected individuals in the presence of HIV-1 Env antigen, with or without exogenous cytokines. Graphs on the top demonstrate the consistency of the effect of exogenous IL-10 and IL-15 on bifunctional (IFNγ+CD107a+) response profiles, regardless of the source of the plasma. Graphs on the bottom depict the consistency of the effect of exogenous IL-10 (50 ng/ml) and IL-15 (5 ng/ml) on the percentage contribution bifunctional responses make to the total NK cell response.
Control of viral infections has been associated with simultaneous mediation of several cell-based effector functions [35]–[37]. Indeed, natural control of HIV-infection has been associated with polyfunctional CTL and NK cell responses. As polyfunctional, rather than monofunctional, NK cell-mediated responses would be preferable to obtain by prophylactic and/or therapeutic interventions, we next assessed the impact of exogenous cytokines on the ability of NK cells to mediate more than one effector function simultaneously. Since different plasmas contain different arrays of biologically active cytokines that could influence the effects of exogenous cytokines, we assessed if the effects of adding IL-10 and IL-15 were consistent across a series of HIV-infected plasmas. Regardless of the plasma used in the whole blood ICS assay, we observed a consistent increase in the frequency of cells capable of mediating bifunctional (IFNγ+CD107a+) responses when IL-10 (2.5+/−0.8 vs. 3.9+/−1.2, p<0.01, paired T-test) and IL-15 (2.5+/−0.8 vs. 4.9+/−1.5, p<0.05, paired T-test) were added (Figure 4B). Indeed, exogenous IL-10 (19.7+/−3.2 vs. 24.8+/−3.4, p<0.01, paired T-test) and IL-15 (19.7+/−3.2 vs. 32.0+/−3.9, p<0.01, paired T-test) increased the contribution that bifunctional responses made to the total NK cell response (Figure 4B). The observation that exogenous cytokines have consistent effects across a series of HIV-infected plasmas suggests that therapeutic use of exogenous cytokines would have a similar effect across many HIV-infected individuals. Overall, these results suggest that polyfunctional cellular responses, which have been associated with natural protection from HIV disease progression [1], [35]–[37], are achievable through anti-HIV ADCC antibodies, especially in the presence of exogenous cytokines.
Importance of NK Cell Education for Effects of Exogenous Cytokines on NK Cell-mediated Anti-HIV ADCC
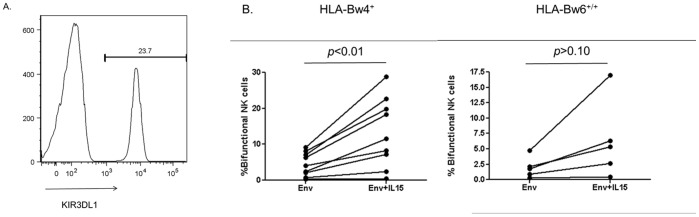
The ability of NK cells to mediate effector functions is controlled by a developmental process known as education [28]. In short, this process ensures self-tolerance of NK cells by only conferring functional potential upon NK cells that express inhibitory receptors for self-ligands. Recent research suggests that the stronger the inhibitory signal delivered to NK cells during this process, the stronger and wider array of effector functions mediated by the NK cell [38]. Indeed, polyfunctional NK cell responses occur more frequently in NK cells that are educated by KIR3DL1/HLA-Bw4 combinations that are protective against HIV infection and/or disease progression [36], [37]. Interestingly, KIR3DL1+ NK cells from HLA-Bw4 carriers have also been demonstrated to mediate higher bifunctional anti-HIV ADCC responses than the same NK cell subset from HLA-Bw6 homozygotes [15]. As we observed a greater effect of exogenous cytokines on bifunctional NK cells, we next assessed if NK cells educated through KIR3DL1/HLA-Bw4 interactions are more sensitive than KIR3DL1+ NK cells from HLA-Bw6 homozygotes to exogenous cytokine treatment. Figure 5a illustrates the gating strategy we employed to distinguish KIR3DL1+/− NK cells. Since exogenous IL-15 induced the largest increase in bifunctional ADCC responses, we assessed the role of educated NK cells in exogenous cytokine enhanced functionality using IL-15. We assessed groups of 9 HLA-Bw4+ individuals and 5 HLA-Bw6 homozygous donors and found that exogenous IL-15 treatment significantly increased the bifunctional activity of KIR3DL1+ NK cells from HLA-Bw4 carriers (4.5+/−1.1 vs. 13.3+/−3.2, p<0.01, paired T-test), but not from HLA-Bw6 homozygotes (1.98+/−0.8 vs. 6.4+/−2.8, p>0.05, paired T-test) (Figure 5B). Although increases in bifunctional activity were consistently observed HLA-Bw6 homozygotes, the reason these changes did not reach significance could reflect the lower responsiveness of non-educated NK cells to cytokine stimulation [14], [39].
Figure 5. Role of NK cell education in exogenous cytokine stimulation.
Whole blood from nine HLA-Bw4 carriers and five HLA-Bw6 homozygous healthy controls was incubated with ADCC-competent plasma in the presence of HIV-1 Env antigen, with or without exogenous IL-15. (A) CD3− CD56+ NK cells were gated upon and assessed for KIR3DL1 expression. (B) KIR3DL1+ NK cells from HLA-Bw4 carriers and HLA-Bw6 homozygotes were assessed for their ability to mediate bifunctional (i.e., CD107a+IFNγ+) anti-HIV ADCC responses in the absence and presence of IL-15. The graph represents the influence of IL-15 on the bifunctionality of NK cells from the KIR3DL1+ subset in both groups. The impact of IL-15 on the bifunctionality of these NK cells was assessed using paired T-tests.
Discussion
Several studies have linked NK cell-mediated anti-HIV ADCC to protection against HIV infection and/or disease progression [6]–[10]. Although several assays exist to evaluate anti-HIV ADCC, the readouts of these experimental systems are influenced by several factors that could confound associations of ADCC with HIV disease progression. Most assays measuring anti-HIV ADCC analyze plasma or sera from HIV-infected individuals. As HIV infection is associated with immune activation and perturbations of plasma cytokine levels [16], these samples often contain a suite of biologically active factors that can skew the ADCC measurement. Indeed, using a NK cell activation anti-HIV ADCC assay we observed that, depending on the source of serum used, NK cells from a common donor mediated a series of responses either skewed towards cytokine production, degranulation or equally spread across both effector functions. These skewed responses were dependent upon soluble plasma/sera factors and not solely due to intrinsic qualities of anti-HIV antibodies, as purification of IgG was associated with alteration of NK cell effector functions. Exogenous addition of IL-10 or IL-15 markedly increased NK cell-mediated anti-HIV ADCC effector functions. The effects of IL-15 were observed most potently on NK cells educated through co-expression of KIR3DL1 and HLA-Bw4, which have previously been associated with protection against HIV infection and/or disease progression [40], [41]. Of the molecules tested, IL-4 and GM-CSF had a negative influence on NK cell activation, and IL-4 has been implicated in HIV pathogenesis [18]. Surprisingly, no effect was observed for IL-12 on anti-HIV ADCC. This could reflect differences in the methodology utilized in the current manuscript compared to other investigations. For example, the current investigation added IL-12 to the NK cells for a brief five hour period. Other investigators generally add IL-12 to the NK cells for an overnight period prior to NK cell functional assays [42].
The effects of cytokines on NK cell-mediated anti-HIV ADCC effector functions are potently observed within the KIR3DL1 educated NK cell subset mediating both IFNγ production and degranulation as assessed by CD107a expression. As educated NK cells mediating polyfunctionality have been associated with protection from HIV infection and/or disease progression [36], [37], the ability of cytokines to alter the functionality of these NK cells may partially explain the association of certain cytokine perturbations with protection from HIV infection or advancement of HIV disease. Indeed, the functional observations of this study suggest this possibility. We observed that IL-10 and IL-15 enhanced NK cell functionality. Previous research has demonstrated that both IL-10 and IL-15 can enhance NK cell functions, including ADCC [14], [43]–[48]. The current investigation, however, has built upon these previous studies by investigating the role for IL-15 in a novel assay, as well as the ability of this cytokine to improve upon the functions of “educated” and “uneducated” NK cells. Interestingly, IL-15 has been demonstrated to be increased in the breast milk that is fed to exposed uninfected infants [49]. Meanwhile, exposed-uninfected individuals and slow progressing individuals have been demonstrated to carry IL-10 promoter polymorphisms that result in high levels of plasma IL-10 [50], [51]. Although IL-10 and IL-15 could act through multiple mechanisms to assist in control of HIV, we speculate that an important mechanism could be their ability to enhance NK cell responsiveness to ADCC antibodies. Future studies should evaluate the association of overlap between NK cell education and plasma cytokine levels with protection from HIV infection.
The observation that IL-10 and IL-15 can enhance anti-HIV NK cell activity suggests that these cytokines may be potential resources for prophylactic and/or therapeutic interventions. Although the concentrations of exogenous cytokines that are required to observe increases in NK cell functionality are above what is observed in vivo [52], it is worth noting that long-term changes in in vivo concentrations of cytokines could still be mediating the skewed responses mediated by several plasma samples. Previous research has demonstrated that the effects of cytokines are synergistic [53]. Furthermore, neutralization of plasma cytokines has been demonstrated to rescue NK cells from the effects of inhibitory endogenous cytokines, such as TGF-β [54]. Lastly, although the high concentrations of exogenous cytokines required to observe NK cell activation may not reflect in vivo concentrations, the concentrations may still be safely utilized for preventative or therapeutic interventions. The levels of IL-15 required to activate NK cells to mediate anti-HIV ADCC are particularly intriguing, as it may be feasible to therapeutically administer similar levels of IL-15 to HIV-infected individuals [55]. Although, the levels of exogenous IL-10 required to stimulate increased anti-HIV ADCC are much higher, they too could likely be supplied safely from an exogenous source [56]. The fact that IL-10 enhances NK cell-mediated anti-HIV ADCC, however, makes it a particularly attractive target. Although some previous research has associated IL-10 with poor viral control, this is mostly due the detrimental effects of IL-10 on CTL [19]. Future research should investigate IL-10 as a component of an antibody containing anti-HIV microbicide that utilizes the ADCC effector function of NK cells. Our results, in combination with previous demonstrations of high-producing IL-10 promoter polymorphisms in exposed uninfected individuals [50], suggest that IL-10 may be particularly well-suited to protect against HIV infection.
Demonstrating that exogenous cytokines can enhance NK cell-mediated anti-HIV ADCC responses that have been associated with protection from HIV infection and/or disease progression is an important step towards understanding how to design better ADCC-based therapeutics or vaccines. Our data suggests vaccines co-expressing IL-15 [57] could result in strongly enhanced ADCC potency. While this study investigated the effect of exogenous cytokines on a small number of NK cell effector functions, future research should elucidate the effector functions involved in viral suppression/control and evaluate the effects of exogenous cytokine stimulation on these functions. Additionally, analysis of monoclonal ADCC antibodies could also evaluate the influence of Fc region glycosylation patterns on NK cell activation. With a broader understanding of the factors determining the potency of anti-HIV ADCC responses, such as NK cell education, antibody specificity and soluble plasma factors, enhanced ADCC-based therapies and vaccines can be more rationally designed.
Acknowledgments
We are grateful to A. Chung, M. Navis, J. Silvers and H. Kent for helpful assistance, the physicians caring for the HIV+ subjects recruited (T Read, M Chen, C Fairley, T Schmidt, C Bradshaw, R Moore, R McFarlane, D Baker, M McMurchie, D Cooper, S Pett, A Carr, R Finlayson, D Smith, TM Soo, M Kelly, J Patten, B Anderson, S Marlton, D Smith, M Bloch, N Doong, N Roth), and to all the subjects recruited to donate blood samples for this study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by Australian National Health and Medical Research Council awards 510448 and 455350, Australian Research Council award LP0991498, and National Institutes of Health award R21AI081541. MSP is supported by a Canadian Institutes of Health Research Vanier Scholarship and a Michael Smith Foreign Study Supplement. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Wren L, Kent SJ. HIV Vaccine efficacy trial: glimmers of hope and the potential role of antibody-dependent cellular cytotoxicity. Hum Vaccin. 2011;7:473. doi: 10.4161/hv.7.4.14123. [DOI] [PubMed] [Google Scholar]
- 4.Karnasuta C, Paris RM, Cox JH, Nitayaphan S, Pitisuttithum P. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine. 2005;23:2529. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol. 2011;85:6912. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2173. [PubMed] [Google Scholar]
- 8.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 9.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brocca-Cofano E, McKinnon K, Demberg T, Venzon D, Hidajat R. Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine. 2011;29:3319. doi: 10.1016/j.vaccine.2011.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel D, Guo X, Ng S, Melchior M, Balderes P. IgG isotype, glycosylation, and EGFR expression determine the induction of antibody-dependent cellular cytotoxicity in vitro by cetuximab. Hum Antibodies. 2010;19:99. doi: 10.3233/HAB-2010-0232. [DOI] [PubMed] [Google Scholar]
- 12.Chung AW, Isitman G, Navis M, Kramski M Center RJ, et al. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A. 2011;108:7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons MS, Zipperlen K, Gallant M, Grant M. Killer cell immunoglobulin-like receptor 3DL1 licenses CD16-mediated effector functions of natural killer cells. J Leukoc Biol. 2010;88:912. doi: 10.1189/jlb.1009687. [DOI] [PubMed] [Google Scholar]
- 14.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Parsons MS, Wren L, Isitman G, Navis M, Stratov I. HIV infection abrogates the functional advantage of natural killer cells educated through KIR3DL1/HLA-Bw4 interactions to mediate anti-HIV antibody-dependent cellular cytotoxicity. J Virol. 2012. [DOI] [PMC free article] [PubMed]
- 16.Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: II. viral strategies for evasion and lessons for immunotherapy and vaccination. J Leukoc Biol. 2008;84:49. doi: 10.1189/jlb.0907649. [DOI] [PubMed] [Google Scholar]
- 17.Roberts L, Passmore JA, Williamson C, Little F, Bebell LM. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS. 2010;24:831. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srikanth P, Castillo RC, Sridharan G, John TJ, Zachariah A. Increase in plasma IL-10 levels and rapid loss of CD4+ T cells among HIV-infected individuals in south India. Int J STD AIDS. 2000;11:51. doi: 10.1258/0956462001914904. [DOI] [PubMed] [Google Scholar]
- 21.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 22.Hober D, Ajana F, Petit MC, Sartiaux C, Boniface M. Granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha in patients with human immunodeficiency virus (HIV) type 1 infection. Microbiol Immunol. 1993;37:792. doi: 10.1111/j.1348-0421.1993.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 23.Rautonen J, Rautonen N, Martin NL, Philip R, Wara DW. Serum interleukin-6 concentrations are elevated and associated with elevated tumor necrosis factor-alpha and immunoglobulin G and A concentrations in children with HIV infection. AIDS. 1991;5:1325. doi: 10.1097/00002030-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 24.D’Ettorre G, Forcina G, Lichtner M, Mengoni F, D’Agostino C. Interleukin-15 in HIV infection: immunological and virological interactions in antiretroviral-naive and -treated patients. AIDS. 2002;16:188. doi: 10.1097/00002030-200201250-00006. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JD, Chehimi J, Gri G, Kostman JR, Montaner LJ. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1011. [PubMed] [Google Scholar]
- 26.Gregson JN, Steel A, Bower M, Gazzard BG, Gotch FM. Elevated plasma lipopolysaccharide is not sufficient to drive natural killer cell activation in HIV-1-infected individuals. AIDS. 2009;23:34. doi: 10.1097/QAD.0b013e3283199780. [DOI] [PubMed] [Google Scholar]
- 27.Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:117. [PubMed] [Google Scholar]
- 28.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nat Rev Immunol. 2010;10:734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 29.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung AW, Rollman E Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 31.Center RJ, Wheatley AK, Campbell SM, Gaeguta AJ, Peut V, et al. Induction of HIV-1 subtype B and AE-specific neutralizing antibodies in mice and macaques with DNA prime and recombinant gp140 protein boost regimens. Vaccine. 2009;27:6612. doi: 10.1016/j.vaccine.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Isitman G, Chung AW, Navis M, Kent SJ, Stratov I. Pol as a target for antibody dependent cellular cytotoxicity responses in HIV-1 infection. Virology. 2011;412:116. doi: 10.1016/j.virol.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung AW, Navis M, Isitman G, Wren L, Silvers J. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr. 2011;58:131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson SE, Rollman E, Chung AW Center RJ, Hejdeman B, et al. NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol. 2011;24:368. doi: 10.1089/vim.2011.0025. [DOI] [PubMed] [Google Scholar]
- 35.Thobakgale CF, Streeck H, Mkhwanazi N, Mncube Z, Maphumulo L. Short Communication: CD8(+) T Cell Polyfunctionality Profiles in Progressive and Nonprogressive Pediatric HIV Type 1 Infection. AIDS Res Hum Retroviruses. 2011;27:1012. doi: 10.1089/aid.2010.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulet S, Song R, Kamya P, Bruneau J, Shoukry NH. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J Immunol. 2010;184:2064. doi: 10.4049/jimmunol.0902621. [DOI] [PubMed] [Google Scholar]
- 37.Parsons MS, Boulet S, Song R, Bruneau J, Shoukry NH. Mind the gap: lack of association between KIR3DL1*004/HLA-Bw4-induced natural killer cell function and protection from HIV infection. J Infect Dis. 2010;202:S360. doi: 10.1086/655966. [DOI] [PubMed] [Google Scholar]
- 38.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 39.Juelke K, Killig M, Thiel A, Dong J, Romagnani C. Education of hyporesponsive NK cells by cytokines. Eur J Immunol. 2009;39:2555. doi: 10.1002/eji.200939307. [DOI] [PubMed] [Google Scholar]
- 40.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008;22:1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- 41.Martin MP, Qi Y, Gao X, Yamada E, Martin JN. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roda JM, Joshi T, Butchar JP, McAlees JW, Lehman A. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13:6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 43.Lin SJ, Roberts RL, Ank BJ, Nguyen QH, Thomas EK. Effect of interleukin (IL)-12 and IL-15 on activated natural killer (ANK) and antibody-dependent cellular cytotoxicity (ADCC) in HIV infection. J Clin Immunol. 1998;18:345. doi: 10.1023/a:1023290932154. [DOI] [PubMed] [Google Scholar]
- 44.Moga E, Canto E, Vidal S, Juarez C, Sierra J. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Exp Hematol. 2011;39:1071. doi: 10.1016/j.exphem.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Loubeau M, Ahmad A, Toma E, Menezes J. Enhancement of natural killer and antibody-dependent cytolytic activities of the peripheral blood mononuclear cells of HIV-infected patients by recombinant IL-15. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:145. doi: 10.1097/00042560-199711010-00001. [DOI] [PubMed] [Google Scholar]
- 46.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115:1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 47.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur J Immunol. 1999;29:2665. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 48.Mocellin S, Panelli M, Wang E, Rossi CR, Pilati P. IL-10 stimulatory effects on human NK cells explored by gene profile analysis. Genes Immun. 2004;5:630. doi: 10.1038/sj.gene.6364135. [DOI] [PubMed] [Google Scholar]
- 49.Walter J, Ghosh MK, Kuhn L, Semrau K, Sinkala M. High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. J Infect Dis. 2009;200:1502. doi: 10.1086/644603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatterjee A, Rathore A, Sivarama P, Yamamoto N, Dhole TN. Genetic association of IL-10 gene promoter polymorphism and HIV-1 infection in North Indians. J Clin Immunol. 2009;29:77. doi: 10.1007/s10875-008-9220-5. [DOI] [PubMed] [Google Scholar]
- 51.Oleksyk TK, Shrestha S, Truelove AL, Goedert JJ, Donfield SM. Extended IL10 haplotypes and their association with HIV progression to AIDS. Genes Immun. 2009;10:322. doi: 10.1038/gene.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeBlaker-Hohe DF, Yamauchi A, Yu CR, Horvath-Arcidiacono JA, Bloom ET. IL-12 synergizes with IL-2 to induce lymphokine-activated cytotoxicity and perforin and granzyme gene expression in fresh human NK cells. Cell Immunol. 1995;165:43. doi: 10.1006/cimm.1995.1184. [DOI] [PubMed] [Google Scholar]
- 54.Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008;180:360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenblum IY, Johnson RC, Schmahai TJ. Preclinical safety evaluation of recombinant human interleukin-10. Regul Toxicol Pharmacol. 2002;35:71. doi: 10.1006/rtph.2001.1504. [DOI] [PubMed] [Google Scholar]
- 57.Boyer JD, Robinson TM, Kutzler MA, Vansant G, Hokey DA. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc Natl Acad Sci U S A. 2007;104:18653. doi: 10.1073/pnas.0709198104. [DOI] [PMC free article] [PubMed] [Google Scholar]