Abstract
DNA barcoding potentially offers scientists who are not expert taxonomists a powerful tool to support the accuracy of field studies involving taxa that are diverse and difficult to identify. The taxonomy of rays has received reasonable attention in Australia, although the fauna in remote locations such as Ningaloo Reef, Western Australia is poorly studied and the identification of some species in the field is problematic. Here, we report an application of DNA-barcoding to the identification of 16 species (from 10 genera) of tropical rays as part of an ecological study. Analysis of the dataset combined across all samples grouped sequences into clearly defined operational taxonomic units, with two conspicuous exceptions: the Neotrygon kuhlii species complex and the Aetobatus species complex. In the field, the group that presented the most difficulties for identification was the spotted whiptail rays, referred to as the ‘uarnak’ complex. Two sets of problems limited the successful application of DNA barcoding: (1) the presence of cryptic species, species complexes with unresolved taxonomic status and intra-specific geographical variation, and (2) insufficient numbers of entries in online databases that have been verified taxonomically, and the presence of lodged sequences in databases with inconsistent names. Nevertheless, we demonstrate the potential of the DNA barcoding approach to confirm field identifications and to highlight species complexes where taxonomic uncertainty might confound ecological data.
Introduction
Taxonomic misidentification and the presence of cryptic species can seriously compromise the veracity of ecological, fisheries and conservation-related research and management [1]–[4]. These problems are further compounded by the ‘greying’ of the taxonomic workforce and the decline in the teaching of taxonomy and training of field biologists at universities, both issues identified as major impediments to the conduct of biodiversity science and conservation biology [5]. Within this context, a key question is: how much confidence can be placed in the application of correct scientific names of taxa reported in ecological studies? In a review of high-ranking ecological journals, Bortolus [4] reported that 62.5% of papers did not provide any supporting information justifying or guaranteeing the correct identification of the organisms under investigation.
The challenges for ecologists seeking verification of their field-based identifications are not trivial. Even when adequate taxonomic keys and field guides are available, it is often difficult to identify organisms in the field with confidence, as ecologists can be dealing with juveniles, undocumented geographic variants, or sexual dimorphism, such that accurate identification might require examination of microanatomy or measurements of a complex combination of morphometric attributes. Handling, examining and measuring individuals is often impractical, inappropriate for ethical reasons, or simply dangerous, thus exacerbating the problem of securing accurate identification. Furthermore, even if experienced taxonomists have studied the target organisms, it is unlikely that they can be encouraged to assist in the field, especially in remote locations. Voucher specimens can be taken for subsequent lodgement in museums; however, this is often impractical for large species, samples obtained in remote locations and studies involving multiple species. Even where voucher specimens can be obtained, it will not necessarily guarantee reliable and timely identification.
DNA barcoding potentially offers scientists who are not expert taxonomists a powerful tool to support the efficiency and accuracy of field studies involving the challenging identification of diverse taxa [6]. The proponents of this approach mostly advocate the use of a single gene for global identification of animals based on the availability of a library of sequences linked to voucher specimens, thus making these sequences, in effect, a DNA barcode [7], [8]. A 650-base fragment of the cytochrome c oxidase I (COI, cox1) is proposed as a ‘global’ standard because the variation in COI within species is lower relative to that among species. While the DNA barcoding approach has its critics when touted as a solution to impediments presented by traditional taxonomy [9], [10], it does potentially provide a quick and reliable means to confirm the identification of individuals in the field and to identify groups where there is discordance in the delineation of species boundaries that require further research. In their paper on DNA Barcoding Australian chondrichthyans, Ward et al., [6] recommend this approach for marine ecologists working on chondrichthyans in the absence of expert taxonomists.
While the taxonomy of rays has received reasonable attention in some parts of the world, including Australia [11] where DNA information is accumulating, the fauna in remote locations such as Ningaloo Reef, Western Australia remains relatively poorly studied. It is now becoming apparent that the field identification of some species without access to taxonomic expertise or the ability to evaluate diagnostic traits (e.g. morphometrics or microanatomy) is problematic. Recent studies indicate that morphologically cryptic elasmobranchs might be common, as some groups show ontogenetic colour variation and colour pattern similarities among different species [12]–[15]. For example, a recent revision of the ‘whiptail ray complex’ found that coloration patterns changed with life stage and different habitats [16], thus complicating field identification.
Here, we report an application of DNA-barcoding to confirm the identification of rays as part of ecological studies at Ningaloo Reef. The establishment of the Ningaloo Reef Ecosystem Tracking Array (NRETA), which is part of the Australian Animal Tagging and Monitoring System (AATAMS, www.imos.org.au/aatams.html), a national network of acoustic stations, provided the opportunity to address the lack of knowledge of the spatial ecology of these animals by enabling a study of the fine-scale movement of a diverse community of rays inhabiting this reef system (Cerutti-Pereyra et al. unpublished data) In these studies, 70 individual rays including both juveniles and adults representing 17 presumed species were captured and fitted with acoustic tags and monitored for more than two years. Tissue samples were taken from each tagged individual for DNA barcoding. We therefore present 67 new COI sequences from these 17 putative species of rays to confirm field identification based on sequences deposited in the GenBank database. Our over-arching aim was to assess the potential of DNA barcoding as an aid to batoid species identification for the tagging study.
Methods
Study group
Rays, or batoids, include a variety of fishes closely related to sharks. Recent immunological and molecular studies show an ancient split between the two groups, where batoids are a sister group to the clade consisting of all shark orders [17]–[19]. Even though the monophyly of batoids is widely accepted, interrelationships within batoids remain controversial. Although early research established six orders, recent work now recognizes five: electric rays (Torpediniformes), skates (Rajiformes), guitar fishes (Rhinobatiformes), sawfishes (Pristioformes), and stingrays (Myliobatiformes) [20], [21]. Worldwide, there are between 507 and 630 species, many of them poorly known and requiring further taxonomic studies. Recent molecular evidence focuses on relationships among elasmobranch orders, but few studies have addressed interrelationships within the rays, e.g. [21], [22], [23].
The central Indo-Pacific is a major centre of origin and radiation of stingrays [19] and within this region, the Indo-Australian archipelago contains 30% of all species of sharks and rays worldwide [11], [24], including many species of tropical rays. Rays are exploited directly or indirectly in commercial fisheries; however, detailed data on landings and by-catch are often lacking. Global reviews of batoid fisheries indicate that in most cases there are large gaps in the basic biological information required to implement strategic management plans for stocks [25], [26] and over-fishing has been suggested to be one of the critical reasons for the decline and local extinction of populations of rays and sharks in both hemispheres [26]–[32].
DNA information for species of rays is accumulating, including COI sequences with 1255 lodged on GenBank to date. This suggests that there is now a sufficient DNA database available to at least partially support a DNA barcoding approach for taxonomic identification of batoids.
Study site
Ningaloo Reef is the largest fringing reef system in the Southern Hemisphere and extends along 270 km of coastline in the north of Western Australia. The reef is separated from the coast by a 0.2 to 7 km wide sandy lagoon, which is backed by a dry coastal plain [33], [34].
Sampling
We used gill and hand nets, hook and line, a Hawaiian sling with a modified tip [35], and indigenous spear fishing to obtain tissue samples of rays. These were stored in a salt-saturated dimethyl sulphoxide (DMSO) solution (20% DMSO, 0.25 M EDTA, saturated with NaCl) in the field, then at −80°C in the laboratory. We visually identified and took disc-width measurements of each animal during handling or prior to taking tissue samples in the case of free-swimming rays.
We collected tissue samples from two individuals per species per site where possible. We also obtained samples from the Northern Territory, Lizard Island (Queensland), and Ha Long Bay (Vietnam) for comparison. The samples were collected by different researchers and fishermen; when possible, a provisional identification was made in the field. The individual samples, their geographic origin and initial taxonomic identification based on information provided by Last and Stevens [11], are shown in Table S1.
Laboratory procedures
We extracted genomic DNA from muscle tissue using DNeasy Blood & Tissue Kit, and amplified the COI gene by polymerase chain reaction (PCR) using the universal primers FishF2 (5′TCGACTAATCATAAAGATATCGGCAC3′) and FishR2 (5′ACTTCAGGGTGACCGAAGAATCAGAA3′) designed by Ward et al. [6]. Each 50 µl reaction contained 5 µl of DNA tissue (ca. 10 ng), 4 µl (0.2 mM) of total Bioline dNTPs, 3 µl (0.6 µM) of each primer, 0.1 µl of 5 U/µl Mango taq, 5 µl of 10x Mango buffer, and 2 µl (2 mM) of MgCl2. PCR cycle conditions were an initial 3 min denaturation at 94°C, followed by 35 cycles of 50 sec at 94°C, 2 min at 50°C, 1.5 min at 72°C and finished with 6 min at 72°C. We examined the PCR products on 1% agarose gels, purified with QIAGEN QIAquick PCR Purification kit and sequenced with the automated sequenced using the dye-termination method (BigDye Terminator v3.1, Applied Biosystems). We sequenced amplicons in both forward and reverse directions. Chromatograms were inspected for noisy and ambiguous base calling and translated to check for stop codons. Noisy tails were trimmed. Only those consisting of more than 519 bp were used for the analysis. Several sequences trimmed to less than 519 bp were excluded from the phylogenetic analysis but were submitted to online databases for identification. Sequences used for the phylogenetic analysis were submitted to GenBank database under the accession numbers given in Table S1.
Analysis
We assembled the sequence data using Mesquite 2.74 and revised our identification of samples after considering the results of two analyses. First, we submitted the sequences one at a time to the BOLD Identification Engine (www.boldsystems.org) and GenBank nucleotide database (www.ncbi.nlm.nih.gov/nucloetide). Both engines matched each uploaded sequence with every other sequence present in their databases and provided a percentage similarity with matching sequences (Table S2). In the second analysis, we constructed phylogenetic trees using ray sequences downloaded from both the GenBank nucleotide database and BOLD identification engine (Table S3). We chose sequences from GenBank/BOLD on the basis that they represented either the same species, a congeneric species, or they showed a high similarity to our sequences submitted to a blast search in GenBank or BOLD engines. If a species on GenBank displayed multiple divergent haplotypes, we chose sequences to represent this variation. We assembled these sequences with ours and aligned them using MEGA 4 [36].
The data set used for phylogenetic analysis was composed of only those sequences that consisted of a minimum of 519 bp after trimming. We used both neighbour-joining (NJ) and Bayesian methods of phylogenetic tree construction for analysis. Neighbour-joining has a strong track record of being able to rapidly analyze large datasets [37]. Modeltest 3.7 showed that the Hasegawa, Kishino and Yano [38] (HKY85) model of molecular evolution was the most appropriate for our dataset [39]. However, we also used the simple Kimura two-parameter model to estimate genetic distance [40] as it is the standard model of molecular evolution used in barcoding studies [41]. We used sequences from two species of sharks (Carcharhinus amblyrhyncos Bleeker, 1856 and C. plumbeus Nardo, 1827) and two species of rays (Pristis clavata Garman 1906 and Torpedo californica Ayres, 1855) from GenBank as outgroups in separate analyses. As the relationships at the level required for species discrimination did not change with the use of different outgroups, we only present the trees using shark taxa because we can be certain that these are an outgroup rather than an ingroup for batoids. We constructed trees using both nucleotide models with PAUP* 4.0b10 [42] and MrBayes [43]. As these provided similar outcomes, we only present results based on the neighbour-joining tree using the Kimura two-parameter model with bootstrap values and posterior probabilities.
We generated uncorrected pair-wise distances in PAUP* 4.0b10 [42], updating the name of the sequences used as detailed in Table S2. For initial species delineation, we grouped individuals that clustered with similarity <3.5% of divergence, which is the threshold recommended for COI of marine fish [6], [44]–[46] and equates to approximately 10x the intra-species variation proposed by Hebert et al., [8]. We also used multi-dimensional scaling (MDS) in SPSS to explore patterns of variation in groups displaying high intra-speciation or geographic variation. For ease of interpretation and readability, we present the neighbour-joining tree divided into 3 sections (Figures 1, 2, and 3).
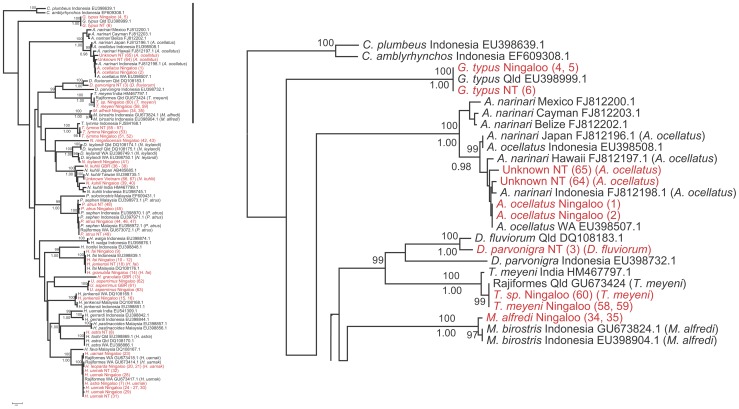
Figure 1. Phylogenetic relationship of rays Part I.
Reduced view of the neighbour-joining tree based on COI sequence data using Kimura-two-parameter substitution model (left); the first part of the tree (right). Names in red are the sequences obtained in this study, the corrected nomenclature is in () and given in Table S2.
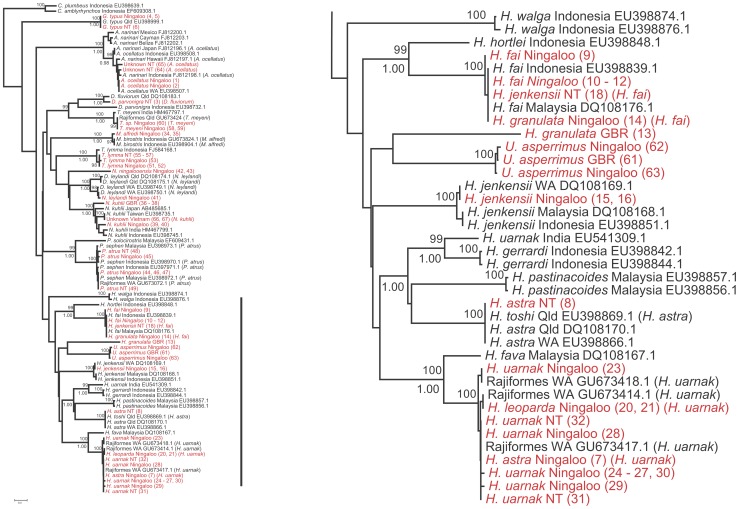
Figure 2. Phylogenetic relationship of rays Part II.
Second part of the Neighbour-joining tree based on COI sequence data using Kimura-two-parameter substitution model (left); the second part of the tree (right). Names in red are the sequences obtained in this study, the corrected nomenclature is in () and given in Table S2.
Figure 3. Phylogenetic relationship of rays Part III.
Third part of the Neighbour-joining tree based on COI sequence data using Kimura-two-parameter substitution model (left); the third part of the tree (right). Names in red are the sequences obtained in this study, the corrected nomenclature is in () and given in Table S2.
Results
General findings
We barcoded 67 individuals representing 17 putative ray species and five unidentified individuals for a fragment of the COI gene with an average length of ∼550 base pairs. When translated all sequences showed no stop codons, indication of heteroplasmy or NUMTs. All 67 sequences were compared with those in BOLD and GenBank databases (Table S2) to confirm the initial identification. Sixty one individuals with a minimum of 519 bp were included in the phylogenetic analysis verified by forward and reverse primers. Six sequences of less than 519 bp were excluded from this analysis. A neighbour-joining tree (Figures 1, 2, and 3) summarizes the relationships among samples from our study and matching sequences from the same or related species available on both nucleotide databases. After comparisons of our sequences with those on BOLD and GenBank databases, we present data for 16 species belonging to 10 genera, 3 families and 2 orders.
We barcoded 20 rays tagged as part of an ecological study at Ningaloo Reef (Cerutti-Pereyra etal. unpublished data) to confirm or correct field identifications (Table S2). Sequences of Himantura uarnak, H. fai, H. granulata, Aetobatus ocellatus, Pastinachus atrus, Taeniurops meyeni, Manta alfredi, Taeniura lymma, and Urogymnus asperrimus represent new sequences from Australia for the GenBank nucleotide database. Data for M. alfredi, and P. atrus represent new sequences from Australia for both BOLD and GenBank databases. Sequences for Neotrygon ningalooensis have no matching sequences in either the GenBank or BOLD databases and new sequences of N. kuhlii from Vietnam are also presented.
The average congeneric distance (D = 8.5%) was 14 times the average conspecific distance (D = 0.63%) (Table 1). These calculations excluded the aberrant samples D. parvonigra from Indonesia (D = 9%) (GenBank accession number EU398732) and H. uarnak from India (D = 12%) (GenBank accession number EU541309.1). Approximately 90% of within-species values had <2% divergence; ∼20% of these had <1% divergence and 10% had between 2 and 3% divergence.
Table 1. Means and ranges of K2P distance values (%) for the COI gene region at different taxonomic levels for the ray species analysed in this study.
| Comparisons | No. of comparisons | Mean | Minimum | Maximum |
| Between individuals within species | 60 | 0.63 | 0 | 3.00 |
| Between species within genera | 20 | 8.85 | 3.40 | 14.00 |
Taxonomic identification and barcoding
The COI sequences for the combined dataset (Figures 1, 2, and 3) grouped sequences into clearly defined operational taxonomic units, with two conspicuous exceptions. These consisted of what we refer to as N. kuhlii and Aetobatus species complexes. Spotted whip-tail rays presented the most difficulties for field identification and are referred to as the ‘uarnak’ complex. Complete consistency in field identification (often by different researchers) and the nomenclature of records held on GenBank and BOLD occurred for only one species, T. lymma, although the tree suggests a phylogeographic disjunction between the Indonesian and Australian samples of this species. Sequences of the recently described species Neotrygon ningalooensis [47] were placed in the same lineage with N. leylandi and N. kuhlii in the tree, but formed a clear and isolated cluster with an average genetic distance of 9% compared with other species within this genus.
Of the 67 sequences we tested, only 19 had consistent matches on both BOLD and GenBank (Table S2). As a consequence, there were a number of anomalies that meant that taxonomic identification was not straightforward or consistent. These anomalies were due to the presence of cryptic species, misidentification of species associated with sequences in the databases, or field misidentification of species in this study. We discuss these taxonomically complex groups and anomalies (Tables S1, S2) in more detail below:
Manta birostris (Walbaum, 1792)/Manta alfredi (Krefft, 1868) [48]
The submission of sequences identified as M. alfredi (# 34, 35) in both online databases produced matches of 99–100% with M. birostris. Sequences of this species showed a phylogeographic disjunction in the NJ tree between the Indonesian and Australian samples in the tree, but a genetic divergence of <1%.
Urogymnus asperrimus (Bloch & Schneider, 1801)
Sequences of this species from Ningaloo Reef (# 62, 63) and the Great Barrier Reef (# 61) clustered together in the tree and had an average genetic divergence of 0.32%. The submission of our sequences to GenBank produced either incorrect matches or matches only to the level of order (Table S2). Our sequences had matches of 98–100% in the BOLD database for U. asperrimus.
Glaucostegus typus (Bennet, 1830)
Sequences from Queensland (EU398732.1), Northern Territory (# 6), and Western Australia (#4, 5) were identical. Our sequences in GenBank had 99–100% similarity with G. typus and 100% similarity in BOLD with G. typus and Rhinobatos typus (senior synonym of Glaucostegus).
Dasyatis parvonigra (Last & White, 2008) [49]
A single specimen identified as D. parvonigra from Shoal Bay, Northern Territory (# 3) had a 98% similarity with a sequence on GenBank labelled as D. fluviorum (GenBank accession number DQ108183.1) from New South Wales, Australia and a 99% similarity with a sequence on BOLD labelled as Dasyatis sp. from Indonesia. Furthermore, a sequence from Indonesia recorded as D. parvonigra (EU398732.1), while placed in the same lineage, differed by 9%, whereas the average divergence with D. fluviorum from New South Wales was 1.6%.
Pastinachus sephen (Forsskal 1775)/Pastinachus atrus (Macleay, 1883)
Six sequences from rays identified by different researchers as P. atrus from Ningaloo Reef (# 44–47) and the Northern Territory (# 48, 49) clustered tightly with samples of P. sephen from Malaysia and Indonesia. The average genetic distance among samples was 0.29%. In GenBank, the most closely matched sequences were labelled P. sephen. In BOLD the highest matches (100%) included sequences identified as both P. atrus and P. sephen.
Taeniurops meyeni (Muller & Henle, 1841)
Of the three Taeniurops rays sampled from Ningaloo Reef, two were initially identified as Taeniurops meyeni [11] (# 58, 59) whereas the other was thought possibly to represent a new species because of an unusual colour pattern. The latter was provisionally referred to as Taeniurops sp (# 60). These sequences from Ningaloo Reef (n = 3) and one sequence under the name of Rajiformes (GenBank accession number GU673424.1) from Queensland were clustered tightly in the tree. There was a small difference between the Australian cluster and the sequence from India; however, the genetic distance among these sequences was low (0.36%). The matching entries in both GenBank and BOLD were labelled as Taeniura meyeni. Last and Stevens [11] revised the nomenclature of this species from Taeniura to Taeniurops.
Neotrygon leylandi (Last & White 2008) [50]
Sequences from Western Australia (n = 4) and Queensland (n = 3) for this species showed geographic variation with an average genetic distance among groups of 3% compared to 0.13% within groups. Our sequence from Ningaloo Reef, W.A. (# 41), matched 100% with sequences in BOLD labelled as N. leylandi and 99% with sequences in GenBank labelled Dasyatis leylandi (Last 1987) [51] (senior synonym of Neotrygon).
Himantura fai, Jordan & Seale, 1906/H. jenkinsii (Annandale, 1909)
Three samples (# 15–17) from Ningaloo Reef identified in the field as H. jenkinsii matched sequences (99–100%) in both GenBank and BOLD. However, a different sample from the Northern Territory (# 18) also initially identified as H. jenkinsii matched a different species in GenBank (H. fai) and both Himantura fai and H. jenkinsii in BOLD. Four other individuals identified in the field as H. fai (# 9–12) and H. granulata (# 14) also clustered with this sample and were identified as H. fai in GenBank and H. fai and H. jenkinsii in BOLD.
The average conspecific genetic distances for H. fai (including a sample initially identified as H. granulata, #14) and H. jenkinsii were 0.03 and 0.4% respectively, while the average genetic distance between H. fai and H. jenkinsii was ∼ 13%. H. jenkinsii showed phylogeographic disjunction between samples from Indonesia/Southeast Asia and Australia, but a small genetic distance of <1%. Another sample, also identified as H. granulata (# 13) was clearly divergent in the tree, and matched H. hortlei [52] on GenBank (86%) and H. granulata on BOLD (99%).
Neotrygon kuhlii complex [50]
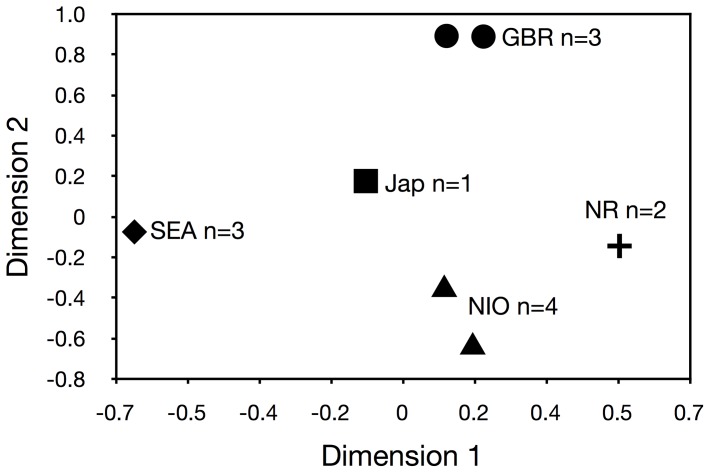
(formerly Dasyatis kuhlii). Our sequences of N. kuhlii had overall levels of similarity of 99–100% with sequences in both GenBank and BOLD databases. Sequences of unidentified rays from Vietnam (# 66–67) matched closely with N. kuhlii (99–100% similarity) on BOLD. The sequences (n = 11) provisionally assigned to this species formed five distinct subgroups in the tree and multi-dimensional scaling analysis (Fig. 4) and had an average genetic distance of ∼ 3%. These subgroups were: Great Barrier Reef (# 36–38), Ningaloo Reef (# 39-0), Japan (AB485685.1), northern Indian Ocean (HM467799.1), Indonesia (EU398745.1), and Southeast Asia (Vietnam: # 66–67; Taiwan: EU398735.1). Average distances among and within these groups were 3 and 0.15%, respectively. The most divergent lineage was from the Great Barrier Reef, which had an average genetic difference of 3.5% from the other sequences from this species. While there was generally a correspondence between the genetic distance and geographical proximity, the two Australian lineages from the western and eastern coasts had the greatest genetic distance (3.8%).
Figure 4. Multidimensional Scaling (MDS) of Neotrygon kuhlii.
Ningaloo Reef (NR), northern Indian Ocean (NIO), Great Barrier Reef (GBR), Japan (Jap), Southeast Asia (SEA);.
Aetobatus complex: A. narinari Euphrasen 1790/A. ocellatus Kuhli 1823
Sequences from Ningaloo Reef identified by different researchers in the field as A. ocellatus (# 1–2) and from unidentified samples from the Northern Territory (# 64–65), were identified as A. narinari using GenBank and as A. narinari and A. ocellatus using BOLD. Sequences of the species commonly referred to as the white-spotted eagle ray from the Caribbean region (Cayman Islands, Belize, and South-East Mexico) (Table S3) and sequences from the Indo-Pacific (Hawaii, Japan, Indonesia, and Australia) showed genetic differences ( = 3.4%). The genetic distance of sequences from A. narinari within the Indo-Pacific, including sequences of A. ocellatus from Australia was low ( = 0.86%). While the name A. narinari is consistently applied to what might be a distinct biological entity in the Caribbean, the names A. narinari and A. ocellatus seemed to be applied interchangeably to a different biological entity that is widespread in the Indo-Pacific.
The ‘uarnak’ complex: Himantura uarnak (Forsskal 1775), H. leoparda [16], H. astra [53], H. toshi (Whitley, 1939)
Samples of this group were identified by several researchers in the field. They were identified as H. leoparda (# 19–21), H. astra (# 7, 8) and H. uarnak (# 22–33) and all (except #19) were grouped together within the tree with an average genetic distance of 0.15% (ignoring the aberrant sequence of H. uarnak from India). Comparisons with the BOLD database identified all these sequences as H. uarnak. In contrast, identifications from GenBank were either uninformative or misleading and applied only the name of the order (Rajiformes) (Table S2). The aberrant sequence of H. uarnak from India had a genetic distance of 12% from this lineage. The BOLD database identified this sequence as H. uarnak (similarity of 100%), H. gerrardi [54] (similarity of 99%) and Dasyatis microps (similarity of 99%). A sequence from an individual collected from Shoal Bay identified as H. astra (# 8) matched sequences labelled as H. toshi in GenBank (100%) and as H. toshi and H. astra (98%) in BOLD. This sequence had an average genetic distance of 0.1% with both H. astra and H. toshi, suggesting these two species may refer to the same biological species.
Discussion
Our aim to investigate the applicability of DNA barcoding for confirming field-based identifications of rays, was at best, a partial success. Two kinds of problems limited the successful application of DNA barcoding to rays. First, biological and taxonomic issues included: a) the presence of cryptic species, b) species complexes with a number of named species of uncertain or unresolved taxonomic status and c) widespread species with substantial intra-specific geographical variation. The second set of problems involved the limitations associated with the online databases including: a) insufficient numbers of taxonomically verified entries on GenBank and BOLD databases; and b) the presence of lodged sequences with incorrect, duplicated, outdated, inconsistent or unhelpful names (e.g. insufficient taxonomic resolution). Nevertheless, our study has demonstrated the potential power of the DNA Barcoding approach to confirm field identifications, detect misidentifications, and discover cryptic species and species complexes with taxonomic issues.
As with other barcoding studies of rays [6], the COI gene region was effective for their taxonomic identification and delineation. This was particularly the case for species in which the complexity of their colour patterns made identification difficult without the input from an expert taxonomist. The average intra-specific genetic distance within species (0.63%) we obtained was larger than that reported for Australian chondrichthyans (0.37%) by Ward et al. [6]. This could have arisen because we increased the geographic extent of sampling for a number of species. In contrast, the average congeneric distance we recorded (7.5%) was similar (7.4%) to that found by Ward et al. [6]. Twenty species of rays representing 9 species tagged as part of an ecological project were correctly and consistently identified using BOLD, albeit with some inconsistent nomenclature (Table S2). In a study of marine fish, Zemlak et al. [46] suggested that similarity below 96.5% could be used as a rule of thumb for discriminating species. All of these samples had BOLD matches ≥98%, with these levels well within the tolerance range for intra-specific genetic divergence.
DNA Barcoding has also been useful when only parts of an animal are available for identification e.g. [45], [55], [56]. The value of barcoding in this context was confirmed by the identification of ‘unknown’ species from tissues samples obtained from rays in markets near Ha Long Bay, Vietnam (# 66, 67) and from fishers in the Northern Territory (# 64, 65) as belonging to the N. kuhlii and A. narinari species complexes, respectively. In both cases, the match between our sequences and the BOLD database was ≥99%. Furthermore, as both species groups displayed significant geographic variation, the confidence of identifications was enhanced due to lodgements on data bases of sequences from individuals from a range of geographic localities.
Barcoding has been used successfully to aid in the identification of species with morphological complexity e.g. [12], [57], [58]. In our study we found that not all field identifications were correct or reliable, with a total of nine specimens representing four species identified incorrectly. Field identification was particularly challenging in the ‘uarnak’ complex group due to similarities in colour patterns among species. While the DNA sequences as summarized in the tree indicated clear taxonomic groupings, the fact that identical reference sequences on the BOLD database were labelled with two different names further complicated taxonomic identification. Lastly, one specimen thought to be a possible new species of Taeniurops (# 60) based on colour patterns was unambiguously identified from the BOLD database as Taeniura meyeni and was genetically identical to other samples of this species from Ningaloo Reef. Another example of ambiguous taxonomy, which limited the value of barcoding for rays, involved the species P. atrus and P. sephen. The low sequence divergence and the absence of any geographic structure in the relationships among the sequences of P. atrus and P. sephen indicated that the sequences available online under these different names are most likely the same species. Furthermore, the close relative found in the Red Sea that was originally named as Pastinachus sephen was morphologically different from the Indo-Pacific form [11], [59].
The databases were uninformative for two species. D. parvonigra (# 3) was identified simply as Dasyatis sp in the BOLD database while GenBank matched an entirely different species, D. fluviorum to our sequence. Neotrygon ningalooensis (# 42–43) represents a new species [47] for which sequences are not yet available in the databases with no matching sequences greater than a similarity of 89%. Overall, these results show that a great deal of care must be taken when using DNA barcoding to confirm field identifications, particularly with groups that have a recent history of nomenclatural changes. When the online search engines gave ambiguous responses to our sequence submissions, the phylogenetic tree and genetic distances analyses proved useful aids to identification.
The misidentification of several species belonging to the genus Himantura on the basis of morphology confirms the taxonomic complexity of the genus, which has been continuously reviewed for the last 10 years [11], [16], [53], [60]. The ‘uarnak’ complex is a group of whip-rays with spotted, ocellated and reticulated dorsal patterns that up until 2008, had 7 valid nominal species [53]. Identification of members of this complex was further complicated due ontogenetic changes in colour patterns that can lead to misidentification of different life-history stages of the same species [16]. We found field identification of species within this group challenging because of the similarities in colour patterns among H. uarnak, H. leoparda, and H. astra. The clustering of H. leoparda as H. uarnak in the tree suggests that these two named species represent the same biological species in this study. While the Australian samples are clearly a distinct species, a sequence from India (GenBank accession number EU541309.1) lodged under the same name is genetically quite different (12%) when compared with the rest of H. uarnak sequences and may represent a new species more closely related to H. gerrardi.
Himantura fai and H. jenkinsii also proved difficult to distinguish in the field. As discussed above, the sequences we obtained matched both H. fai and H. jenkinsii in the BOLD database; however, the tree clearly showed that these are distinct species, suggesting that a revision of the names attached to sequences in the BOLD database is required. Sequences assigned to H. astra and H. toshi also need to be reviewed [53]. The tree suggests there is only one species, but the BOLD database again produced ambiguous results with our sequences being identified as both H. toshi and H. astra with similarities of 100%.
Confusion in taxonomy was also a problem for the genus Aetobatus. Aetobauts narinari represents a widespread species complex and the pattern of geographic variation in COI indicates that there are two closely related forms. One distinct species, A. narinari, occurs in the north Atlantic and the other that occurs in the Indo-Pacific should be referred to A. ocellatus [61], [62]. To add to the uncertainty involving these species, the BOLD database identified our sequences as both A. narinari and A. ocellatus. Our results were consistent with those of Richards et al. [62] and Schluessel et al. [63] who analysed sequences of cytochrome b and COI and found that individuals of A. narinari from the west Atlantic formed a distinct lineage compared with those from the Indo-Pacific. Based on a morphological review, White et al. [61] proposed that A. ocellatus is a separate species restricted to the Indo-West Pacific and distinct from the A. narinari complex. The average genetic distance between sequences of A. narinari from the Caribbean Sea and sequences from the Indo-Pacific region labelled as A. narinari in our study was 3.4%, consistent with the idea that the Atlantic and Indo-Pacific lineages are separate species. This pattern and geographical divergence between Atlantic and Pacific stocks has been observed in other elasmobranchs such as Squalus acanthias [64].
The Neotrygon kuhlii species complex is also widespread, with the maximum divergence close to the rule of thumb for discriminating species. Geographic differences in genetic divergences indicate the possibility of three differentiated clades consisting of a) east Asia (Vietnam, Taiwan, and Japan), b) the eastern Indian Ocean (India, Indonesia, and Ningaloo Reef, Australia) and c) the Great Barrier Reef (Australia). This is consistent with the suggestion by Ward et al. [6] of the possibility of cryptic species within N. kuhlii. Further research is required to determine geographic boundaries and to examine variation in other genes (e.g. microsatellite loci) to establish if this group is undergoing incipient speciation.
We increased the geographic spread of genetic sampling for several rays in the tropical Indo-Pacific and a number of contrasting patterns have emerged that might be of taxonomic or biological importance. Several species were noteworthy for having little genetic divergence over large (1000s of km) distances. For example, Glaucostegus typus (# 4–6) shared haplotypes between Ningaloo Reef, Western Australia and Northern Territory; U. asperrimus (# 61 63) shared haplotypes between Ningaloo Reef, Western Australia and Queensland; H. fai (# 9–12, 18) and P. atrus (# 44–49) both shared haplotypes between Ningaloo Reef, the Northern Territory and Malaysia. These results suggest that these species all have high vagility, at least at generational time scales.
In contrast, T. lymma (# 50–53, 56, 57), H. jenkinsii (# 15, 16), and M. alfredi (# 34, 35) showed little (<1%) but potentially biologically relevant variation in sequences between Australia and Indonesia. While our sample sizes were small, this result implies that the biogeographic factors responsible for population differentiation could potentially act on the three species in a similar way. The possibility of population differentiation in M. alfredi is supported by the observations of strong residency patterns in Indonesia [65] and in Ningaloo Reef (F. McGregor, pers. comm.) based on acoustic tagging and photo-identification studies. An individual misidentified in the field as D. parvonigra (# 3) from the Northern Territory was in fact a new record of D. fluviorum, a species that was previously thought to occur only along the eastern coast of Australia [11].
The extent of genetic divergence within several species (N. kuhlii, N. leylandi) from the north-west and east of Australia might reflect historical isolation when the land bridge between New Guinea and northern Australia formed during the Holocene and late Pleistocene [66]. A number of marine and coastal species (including elasmobranchs) show this pattern of differentiation caused by vicariant events [14], [15], [67]–[69]. Further investigation of this idea would require intensive sampling of these rays for both nuclear and mitochondrial markers between Torres Strait and the Arafura-Timor Seas to understand the geographic basis for genetic differentiation. It was, however, surprising to find such discordance between genetic differentiation and body size in some rays (e.g. T. lymma vs M. alfredi) because it is generally assumed that body size and dispersal capacity are correlated in elasmobranchs [70]. Several genetic studies have found surprisingly strong population structure in sharks and rays considered vagile that might be related to site fidelity in both adults and juveniles or deep water acting as barriers to dispersal [14], [15], [71], [72].
The general limits and pitfalls of DNA barcoding as a stand-alone tool for identifying species and delimiting taxonomic boundaries have been dealt with elsewhere [10], [73], [74]–[76]. However, it is worth reiterating that taxonomic decision-making solely on the basis of a single maternally inherited marker will not identify all biological species. Other studies of rays have found that mtDNA sequences have not been useful for delimiting species boundaries since haplotypes can be shared, particularly between newly evolved species [12]. Conversely, it is possible that some species with higher genetic distances that approach the (arbitrarily defined) species-level thresholds might be able to interbreed. Such rules-of-thumb for genetic distance will vary in their usefulness among gene regions and across taxonomic groups and will inevitably be a “one-way” test for species discrimination [6], [77], [78].
While we found that barcoding for rays was largely successful as an identification tool, there were several limitations. To succeed, barcoding must be able to reference a stable and well-defined taxonomy and have access to a sufficient number of barcodes lodged on databases that have been verified taxonomically [77]. We discovered that several species groups require taxonomic review both to define confidently species boundaries and revise nomenclatures. Furthermore, the continued updating of sequences lodged on GenBank and BOLD is a vital, but a rarely considered issue in the practical application of barcoding. The specimens from which sequences are derived must first be identified by a competent taxonomist. The names assigned to sequences need to be updated on the online genetic data bases when taxonomies are revised and names changed. Fifty-eight percent of our sequences did not matched entries on GenBank and 30% showed ambiguous results on BOLD due to confusing nomenclature (Table S2). For example, in the cases of H. fai versus H. jenkinsii and H. astra versus H. toshi, the BOLD search engine showed a 99–100% similarity with both names in each case, thereby invalidating the simple use of BOLD as an identification tool. Furthermore, a number of ray sequences on GenBank were identified only to genus or family level making them uninformative for DNA barcoding-based identification.
Conclusions
DNA barcoding was successful in validating field identifications and correcting misidentifications of tagged rays at Ningaloo Reef, WA, although application of the technique was somewhat problematic due to the inconsistency and ambiguity of taxonomic information available on the online data bases. Our genetic analyses have resulted in a better understanding of intra-species diversity and biogeographic patterns along the coast of northern Australia and at localities across the Indo-Pacific that will ultimately be useful for delimiting species boundaries, fisheries management and conservation of tropical rays.
In the future, the usefulness of ray barcoding will be directly related to the quantity and geographic representation of sequences, the number of sequences from taxonomically verified specimens, taxonomic revisions of key species complexes and a revision of the taxonomic nomenclature assigned to existing sequences on genetic data bases. With these advances, together with the recent production of COI sequences and taxonomic studies in Australia [6], [11] and Indonesia [79], [80], barcoding for species identification of rays will become far less problematic, at least for this region. Such an approach needs to be extended to areas with high diversity of rays around the world.
Supporting Information
Specimen collection details for all sequences obtained in this study.
(DOCX)
Identification of sampled rays using GenBank and BOLD databases.
(DOCX)
Online sequences used in this study with their GenBank accession numbers.
(DOCX)
Acknowledgments
We thank P. Last and B. Ward for help with species identification and W. White for constructive comments on the manuscript. We thank A. Tan and D. McGaffin for tagging and sampling equipment. We are very appreciative of the fieldwork support given to the study by F. McGregor, M. Gray, G. Enver and the Anindilyakwa Rangers from Groote Eylandt.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: We thank the Australian Institute of Marine Science and Charles Darwin University for financial support, and CONACYT-México for an international grant to FCP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Austin CM, Ryan SG. Allozyme evidence for a new species of freshwater crayfish of the genus Cherax Erichson (Decapoda: Parastacidae) from the south-west of Western Australia. Invertebr Syst. 2002;16:357–367. [Google Scholar]
- 2.Moura T, Silva MC, Figueiredo I, Neves A, Munoz PD, et al. Molecular barcoding of north-east Atlantic deep-water sharks: species identification and application to fisheries managment and conservation. Mar Freshwat Res. 2008;59:214–223. [Google Scholar]
- 3.Vecchione M, Mickevich MF, Fauchald K, Collette BB, Williams AB, et al. Importance of assessing taxonomic adequacy in determining fishing effects on marine biodiversity. ICES Journal of Marine Science: Journal du Conseil. 2000;57:677–681. [Google Scholar]
- 4.Bortolus A. Error cascades in the biological sciences: The unwanted consequences of using bad taxonomy in ecology. AMBIO: A Journal of the Human Environment. 2008;37:114–118. doi: 10.1579/0044-7447(2008)37[114:ecitbs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Khuroo AA, Dar GH, Khan ZS, Malik AH. Exploring an inherent interface between taxonomy and biodiversity: current problems and future challenges. J Nat Conserv. 2007;15:256–261. [Google Scholar]
- 6.Ward RD, Holmes BH, White WT, Last P. DNA barcoding Australasian chondrichthyans: results and potential uses in conservation. Mar Freshwat Res. 2008;59:57–71. [Google Scholar]
- 7.Ward RD, R Hanner, Hebert PDN. The campaign to DNA barcode all fishes, FISH-BOL. J Fish Biol. 2009;74:329–356. doi: 10.1111/j.1095-8649.2008.02080.x. [DOI] [PubMed] [Google Scholar]
- 8.Hebert PDN, Stoeckle MY, Zemlak TS, Francis C. Identification of birds through DNA Barcodes. PLoS Biology. 2004;2:e312–e312. doi: 10.1371/journal.pbio.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moritz C, Cicero C. DNA Barcoding: Promise and Pitfalls. PLoS Biol. 2004;2:e354. doi: 10.1371/journal.pbio.0020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tautz D, Arctander P, Minelli A, Thomas RH, Vogler AP. A plea for DNA taxonomy. Trends Ecol Evol. 2003;18:70–74. [Google Scholar]
- 11.Last PR, Stevens JD. Sharks and Rays of Australia 2nd edition. Melbourne: CSIRO Publishing. 644 p. 2009.
- 12.Toffoli D, Hrbek T, Goes-de-Araujo M, Pinto-de-Almeida M, Charvet-Almeida P, et al. A test of the utility of DNA in the radiation of the freshwater stingray genus Potamotrygon (Potamotrygonidae, Myliobatiformes). Genet Mol Biol. 2008;31:324–336. [Google Scholar]
- 13.Wynen L, Larson H, Thorburn D, Peverell S, Morgan D, et al. Mitochondrial DNA supports the identification of two endangered river sharks (Glyphis glyphis and G. garricki) across northern Australia. Mar Freshwat Res. 2009;60:554–562. [Google Scholar]
- 14.Sandoval-Castillo J, Rocha-Olivares A. Deep mitochondrial divergence in Baja California populations of an aquilopelagic elasmobranch: the golden cownose ray. J Hered. 2011;102:269–274. doi: 10.1093/jhered/esr004. [DOI] [PubMed] [Google Scholar]
- 15.Sandoval-Castillo J, A Rocha-Olivares, C Villacivencio-Garayzar, Balart E. Cryptic isolation of Gulf of California shovelnose guitarfish evidenced by mitochondrial DNA. Mar Bio. 2004;145:983–988. [Google Scholar]
- 16.Manjaji-Matsumoto BM, Last PR. Last PR, White WT, Pogonoski JJ, editors. Himantura leoparda sp. nov., a new whipray (Myliobatoidei: Dasyatidae) from the Indo–Pacific. 2008. Descriptions of New Australian Chondrichthyans: CSIRO Marine and Atmospheric Research Paper 293-302.
- 17.Douady CJ, Dosay M, Shivji MS, Stanhope MJ. Molecular phylogenetic evidence refuting the hypothesis of Batoidea (rays and skates) as derived sharks. Mol Phylogen Evol. 2003;26:215–221. doi: 10.1016/s1055-7903(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 18.Lawson R, SJ Burch, SM Oughterson, Heath S, Davies DH. Evolutionary relationships of cartilaginous fishes: an immunological study. J Zoo. 1995;237:101–106. [Google Scholar]
- 19.Winchell CJ, Martin AP, Mallatt J. Phylogeny of elasmobranchs based on LSU and SSU ribosomal RNA genes. Mol Phylogen Evol. 2004;31:214–224. doi: 10.1016/j.ympev.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 20.McEachran JD, Aschliman N. Carrier JC, Musick JA, Heithaus MR, editors. Phylogeny of batoidea. 2004. pp. 79–114. Biology of sharks and their relatives. Boca Raton: CRC Press.
- 21.Rocco L, Liguori I, Costagliola D, Morescalchi MA, Tinti F, et al. Molecular and karyological aspects of Batoidea (Chondrichthyes, Elasmobranchi) phylogeny. Gene. 2007;389:80–86. doi: 10.1016/j.gene.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Lovejoy NR. Systematics of myliobatoid elasmobranchs: with emphasis on the phylogeny and historical biogeography of neotropical freshwater stingrays (Potamotrygonidae: Rajiformes). Zool J Linn Soc. 1995;117:207–257. [Google Scholar]
- 23.Sezaki K, Begum RA, Wongrat P, Srivastava MP, SirKantha S, et al. Molecular phylogeny of Asian freshwater and marine stingrays based on the DNA nucleotide and deduced amino acid sequences of the cytochrome b gene. Fish Sci. 1999;65:563–570. [Google Scholar]
- 24.Last PR, White WT. Biogeographic patterns in the Australian chondrichthyan fauna. J Fish Biol. 2011;79:1193–1213. doi: 10.1111/j.1095-8649.2011.03095.x. [DOI] [PubMed] [Google Scholar]
- 25.Bonfil R. Fowler SL, Reed TM, Dipper FA, editors. Trends and patterns in world and Asian elasmobranch fisheries. 1997. pp. 15–24. Elasmobranch biodiversity, conservation and management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 IUCN SSC Shark Specialist Group. Gland, Switzerland and Cambridge, UK: IUCN.
- 26.Frisk MG. Carrier JC, Musick JA, Heithaus MR, editors. Life history strategies of batoids. 2010. pp. 283–316. The biology of sharks and their relatives. Boca Raton: CRC Press.
- 27.Stevens JD, Bonfil R, Dulvy NK, Walker PA. The effects of fishing on sharks, rays and chimaeras (Chondrichthyans) and the implications for marine ecosystems. ICES J Mar Sci. 2000;57:476–494. [Google Scholar]
- 28.White WT Dharmadi. Species and size compositions and reproductive biology of rays (Chondricthyes, Batoidea) caught in target and non-target fisheries in eastern Indonesia. J Fish Biol. 2007;70:1809–1837. [Google Scholar]
- 29.Dulvy NK, Metcalfe JD, Glanville J, Pawson MG, Reynolds JD. Fishery stability, local extinctions, and shifts in community structure in skates. Conserv Biol. 2000;14:283–293. [Google Scholar]
- 30.Field IC, Meekan MG, Buckworth RC, Bradshaw CJA. David WS, editor. Susceptibility of sharks, rays and chimaeras to global extinction. 2009. pp. 275–363. editor. Advances in Marine Biology Academic Press. [DOI] [PubMed]
- 31.Brander K. Disappearance of common skate Raja batis from the Irish Sea. Nature. 1981;290:48–49. [Google Scholar]
- 32.Graham KJ, Andrew NL, Hodgson KE. Changes in relative abundance of sharks and rays on Australian South East Fishery trawl grounds after twenty years of fishing. Mar Freshwat Res. 2001;52:549–561. [Google Scholar]
- 33.Taylor JG, Pearce AF. Ningaloo Reef currents: implications for coral spawn dispersal, zooplankton and whale shark abundance. J R Soc West Aust. 1999;82:57–65. [Google Scholar]
- 34.Leprovost Dames Moore. Ningaloo Marine Park (CommonwealthWaters) Literature review. Accessed 15 May 2012. 2000. Available: http://www.environment.gov.au/coasts/mpa/publications/pubs/ningaloo-marine-resources.pdf.
- 35.Bilgmann K, Griffiths OJ, Allen SJ, Möller LM, et al. A biopsy pole system for bow-riding dolphins: sampling success, behavioral responses, and test for sampling bias Mar Mamm Sci. 2007;23:218–225. [Google Scholar]
- 36.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Gadagkar SR. Efficiency of the Neighbor-Joining method in reconstructing deep and shallow evolutionary relationships in large phylogenies. J Mol Evol. 2000;51:544–553. doi: 10.1007/s002390010118. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa M. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 39.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 40.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 41.Hebert PD, S Rarnasingham, deWard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society B (Supplement) 2003;270:s96–s99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (* and Other Methods), Beta version 4b10. 2002. Beta version 4b10 ed. Sunderland: Sinauer Associates.
- 43.Ronquist F, Huelsenbeck JP, Mark Pvd. MrBayes 3.1 Manual: Florida State University & University of San Diego. 2005.
- 44.Ward RD, Zemlak TS, Innes BH, Last PR, Hebert DN. DNA barcoding Australia's fish species. Philos Trans R Soc Lond, Ser B: Biol Sci. 2005;360:1847–1857. doi: 10.1098/rstb.2005.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes BH, Steinke D, Ward RD. Identification of shark and ray fins using DNA barcoding. Fish Res. 2009;95:280–288. [Google Scholar]
- 46.Zemlak TS, Ward RD, Connell AD, Holmes BH, Hebert PDN. DNA barcoding reveals overlooked marine fishes. Mol Ecol Resour. 2009;9:237–242. doi: 10.1111/j.1755-0998.2009.02649.x. [DOI] [PubMed] [Google Scholar]
- 47.Last PR, White WT, Puckridge M. Neotrygon ningalooensis n. sp. (Myliobatoidei: Dasyatidae), a new maskray from Australia. Aqua. 2010;16:37–50. [Google Scholar]
- 48.Marshall AD, LJV Compagno, Bennett MB. Redescription of the genus Manta with resurrection of Manta alfredi (Krefft, 1868) (Chondrichthyes; Myliobatoidei; Mobulidae). Zootaxa. 2009;2301:1–28. [Google Scholar]
- 49.Last PR, White WT. Last PR, White WT, Pogonoski JJ, editors. Dasyatis parvonigra sp. nov., a new species of stingray (Myliobatoidei: Dayatidae) from the tropical eastern Indian Ocean. 2008. Descriptions of New Australian Chondrichthyans: CSIRO Marine and Atmospheric Research Paper 275-282.
- 50.Last PR, White WT. Last P, White WT, Pogonoski JJ, editors. Resurrection of the genus Neotrygon Castelnau (Myliobatoidei: Dayatidae) with the description of Neotrygon picta sp. nov., a new species from northern Australia. 2008. Descriptions of New Australian Chondrichthyans: CSIRO Marine and Atmospheric Resarch Paper 315-325.
- 51.Last PR. New Australian fishes. Part 14. Two new species of Dasyatis (Dasyatididae). Memoirs of Museum of Victoria. 1987;48:57–61. [Google Scholar]
- 52.Last PR, Manjaji-Matsumoto M, Kailola PJ. Himantura hortlei n. sp., a new species of whipray (Myliobatiformes: Dasyatidae) from Irian Jaya, Indonesia. Zootaxa. 2006;1239:19–34. [Google Scholar]
- 53.Last PR, White WT, Pogonoski JJ. Last PR, White WT, Pogonoski JJ, editors. Himantura astra sp. nov., a new whipray (Myliobatoidei: Dasyatidae) from northen Australia. 2008. Descriptions of New Australian Chondrichthyans: CSIRO Marine and Atmospheric Research Paper 303-314.
- 54.Gray JE. List of the specimens of fish in the collection of the British Museum.Part I. Chondropterygii. London: British Museum of Natural History. 1851.
- 55.Valière N, Fumagalli L, Gielly L, Miquel C, Lequette B. Long-distance wolf recolonization of France and Switzerland inferred from non-invasive genetic sampling over a period of 10 years. Anim Conserv. 2003;6:83–92. [Google Scholar]
- 56.Domingo-Roura X, Marmi J, Ferrando A, López-Giráldez F, Macdonald DW, et al. Badger hair in shaving brushes comes from protected Eurasian badgers. Biol Conserv. 2006;128:425–430. [Google Scholar]
- 57.Ovenden JR, Morgan JAT, Kashiwagi T, Broderick D, Salini J. Towards better management of Australia's shark fishery: genetic analyses reveal unexpected ratios of cryptic blacktip species Carcharhinus tilstoni and C. limbatus. Mar Freshwat Res. 2010;61:253–262. [Google Scholar]
- 58.Amaral AR, Sequeira M, Coelho MM. A first approach to the usefulness of cytochrome c oxidase I barcodes in the identification of closely related delphinid cetacean species. Mar Freshwat Res. 2007;58:505–510. [Google Scholar]
- 59.Last PR, Manjaji-Matsumoto M. Last P, White WT, Pogonoski JJ, editors. Description of a new stingray, Pastinachus gracilicaudus sp. nov. (Elasmobranchii: Myliobatiformes), based on material from the Indo–Malay Archipelago. 2010. Descriptions of new sharks and rays from Borneo. Hobart: CSIRO Marine and Atmospheric Research 115-128.
- 60.Last PR, Stevens JD. Sharks and Rays of Australia. Melbourne: CSIRO Publishing. 513 p. 1994.
- 61.White WT, Last PR, Naylor GJP, Jensen K, Caira JN. Last PR, White WT, Pogonoski JJ, editors. Clarification of Aetobatus ocellatus (Kuhl, 1823) as a valid species, and a comparison with Aetobatus narinari (Euphrasen, 1790) (Rajiformes: Myliobatidae). 2010. Descriptions of new sharks and rays from Borneo. Hobart: CSIRO Marine and Atmospheric Research 141-164.
- 62.Richards VP, Henning M, Witzell W, Shivji MS. Species delineation and evolutionary history of the globally distributed spotted eagle ray (Aetobatus narinari). J Hered. 2009;100:273–283. doi: 10.1093/jhered/esp005. [DOI] [PubMed] [Google Scholar]
- 63.Schluessel V, Broderick D, Collin SP, Ovenden JR. Evidence for extensive population structure in the white-spotted eagle ray within the Indo-Pacific inferred from mitochondrial gene sequences. J Zool. 2010;281:46–55. [Google Scholar]
- 64.Ward RD, Holmes BH, Zemalak TS, Amith PJ. DNA barcoding discriminates spurdogs of the genus Squalus. . Last PR, White WT, Pogonoski JJ, editors. Hobart, Australia: CSIRO Marine and Atmospheric Research Paper. 2007;014:117–130. editors. Descriptions of new dogfishes of the genus Squalus (Squaloidea: Squalidae). [Google Scholar]
- 65.Dewar H, Mous P, Domeier M, Muljadi A, Pet J, et al. Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar Biol. 2008;155:121–133. [Google Scholar]
- 66.Voris HK. Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J Biogeogr. 2000;27:1153–1167. [Google Scholar]
- 67.Rawlings LH, Barker D, Donnellan SC. Phylogenetic relationships of the Australo-Papuan Liasis pythons (Reptilia: Macrostomata), based on mitochondrial DNA. Aust J Zool. 2004;52:215–227. [Google Scholar]
- 68.Avise JC. Molecular population structure and the biogeographic history of a regional fauna: a case history with lessons for conservation biology. Oikos. 1992;63:62–76. [Google Scholar]
- 69.Keenan CP. Recent evolution of population structure in Australian barramundi, Lates calcarifer (Bloch): An example of isolation by distance in one dimension. Mar Freshwat Res. 1994;45:1123–1148. [Google Scholar]
- 70.Bruce BD, Stevens JD, Malcolm H. Movements and swimming behaviour of white sharks (Carcharodon carcharias) in Australian waters. Mar Biol. 2006;150:161–172. [Google Scholar]
- 71.Keeney DB, Heupel M, Hueter RE, Heist EJ. Genetic heterogeneity among blacktip shark, Carcharhinus limbatus, continental nurseries along the U.S. Atlantic and Gulf of Mexico. Mar Biol. 2003;143:1039–1046. [Google Scholar]
- 72.Schrey AW, Heist EJ. Microsatellite analysis of population structure in the shortfin mako (Isurus oxyrinchus). Can J Fish Aquat Sci. 2003;60:670–675. [Google Scholar]
- 73.Ebach MC, Holdrege C. More taxonomy, not DNA barcoding. BioScience. 2005;55:823–824. [Google Scholar]
- 74.Valentini A, Pompanon F, Taberlet P. DNA barcoding for ecologists. Trends Ecol Evol. 2009;24:110–117. doi: 10.1016/j.tree.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Hajibabaei M, Singer GAC, Hebert PDN, Hickey DA. DNA barcoding: how itcomplements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 2007;23:167–172. doi: 10.1016/j.tig.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Rubinoff D. Utility of mitochondrial DNA barcodes in species conservation. Conserv Biol. 2006;20:1026–1033. doi: 10.1111/j.1523-1739.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 77.Meyer CP, Paulay G. DNA barcoding: error rates based on comprehensive sampling. PloS Biology. 2005;3:e422–e422. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munasinghe DHN, Murphy NP, Austin CM. Utility of mitochondrial DNA sequences from four gene regions for systematic studies of Australian freshwater crayfish of the genus Cherax (Decapoda: Parastacidae). J Crust Biol. 2003;23:402–417. [Google Scholar]
- 79.White WT, Last PR, Stevens JD, Yearsley G Fahmi. Economically important sharks and rays of Indonesia. Canberra: CSIRO. 330 p. 2006.
- 80.Last PR, White WT, Pogonoski JJ. Descriptions of new sharks and rays from Borneo. Hobart: CSIRO Marine and Atmospheric Research 173 p. 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specimen collection details for all sequences obtained in this study.
(DOCX)
Identification of sampled rays using GenBank and BOLD databases.
(DOCX)
Online sequences used in this study with their GenBank accession numbers.
(DOCX)