Abstract
A challenge in ocular drug delivery is to maintain the therapeutic concentration of a drug at the site of action in the eye. The objective of the present study was to investigate the feasibility of micellar carrier systems for sustained drug delivery in transscleral iontophoresis in vitro. Simple and mixed micelles prepared using sodium taurocholate (TA) alone or with egg lecithin (LE) were the carrier systems studied. Dexamethasone (DEX), a poorly water soluble corticosteroid, was the model drug. The micellar carrier systems were first characterized for their solubilization and encapsulation of the drug. Passive and 2-mA iontophoretic (both cathodal and anodal) transport experiments were conducted using these micellar carrier systems in side-by-side diffusion cells with excised human sclera in vitro. Drug release studies were performed after the transport experiments. Saturated DEX solution without the micellar carriers was used as a control. It was found that the solubilization capacity of the micellar carrier systems increased as the total lipid concentration of the systems increased. Drug release from the sclera was significantly prolonged with the micellar carrier systems as compared to the control after passive and iontophoretic delivery. Less than ~ 20% of DEX was released from the sclera in approximately 2 hours after cathodal iontophoretic delivery of the micellar carrier systems, whereas more than ~ 50% of DEX was released from the control in the same time period under the same condition. Micellar carrier systems can be a suitable transscleral drug delivery system for poorly water soluble drugs by enhancing their aqueous solubilities and providing sustained drug delivery. These micellar carrier systems can be efficiently delivered into and across the sclera by iontophoresis for drug delivery.
Keywords: Human sclera, Dexamethasone, Mixed micelles, Simple micelles, Transscleral iontophoresis, Sustained release
1. Introduction
Posterior eye diseases account for the majority of blindness in the United States [1]. For example, it was reported that the number of patients suffering from posterior uveitis was more than 100,000 people in the United States (38 per 100,000 people yearly) [2]. The use of dexamethasone and other corticosteroids as anti-inflammatory agents to inhibit the inflammation in ocular tissues has been well documented [3-5]. Topical administration of corticosteroids is commonly used to treat anterior eye diseases [6-7], but is not effective in the treatment of posterior eye diseases due to the low levels of drugs delivered to the posterior eye from the administration site [8-9]. Intravitreal injection and the use of intraocular implants are effective methods to deliver drugs to the posterior parts of the eye, but these approaches are invasive [10-13]. Complications associated with intraocular injections, such as cataract, retinal detachment, vitreous hemorrhage, and endophthalmitis, have been reported [10-12]. The development of an effecitve and noninvasive method to deliver drugs to the posterior segment of the eye remains a challenge for ophthalmologists and pharmaceutical scientists.
Transscleral iontophoresis is a noninvasive technique and has been investigated to enhance drug delivery to the posterior segment of the eye [13-19]. A limitation of transscleral iontophoresis for the treatment of chronic eye diseass is the fast clearance of the drug from the eye to the systemic circulation such as through episcleral vessels and conjunctival lymphatic system, leading to the requirement of repeated iontophoresis administration. Also, short duration transscleral iontophoresis is not expected to directly deliver drugs to the posterior segment of eye due to the long transport path length from the application site to the back of the eye [20] and the ocular dynamic barrier [21]. Therefore, a sustained release carrier system for transscleral iontophoresis that can maintain therapeutic concentrations of the drug in the eye is desirable.
Nanoparticulate drug delivery systems such as micellar carriers have been studied to increase the solubility of poorly water soluble drugs [22-24]. The application of these carrier systems to improve therapeutic efficacy of various drugs has been established for different routes of drug administration, e.g., ocular, parenteral, oral, and dermal routes [25-31]. Micellar carrier systems have the properties such as being charged and nanosize for transscleral iontophoresis. In addition, mixed micelles composed of egg lecithin and taurocholate have been found to exhibit a phase transition from mixed micelles to liposomes upon aqueous dilution [32-33]. This phase transition property could be advantageous for sustained drug delivery.
The objective of the present study was to investigate the properties of sustained release drug delivery systems of micelles for transscleral iontophoresis. Particularly, dexamethasone (DEX) was chosen as the model drug and DEX-loaded simple and mixed micellar carrier systems prepared using taurocholate and egg lecithin were the model micellar systems investigated. The physical properties of these micellar carrier systems, such as drug encapsulation, size, charge, conductivity, viscosity, and osmolarity, were characterized. The feasibility of transscleral delivery of these carrier systems by passive and iontophoretic transport methods was evaluated using cadaveric human sclera in vitro. Drug release profiles were determined after the transport experiments in vitro to examine the potential of these micelles as novel sustained release systems for poorly water soluble drugs.
2. Materials and Methods
2.1 Materials
3H-dexamethasone was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA) with purity of at least 97%. Sodium taurocholate (TA) was purchased from Sigma–Aldrich (St. Louis, MO). Lecithin (LE, from eggs, purity > 90%) was purchased from Indofine Chemical (Hillsborough, NJ). Dexamethasone was purchased from Letco Medical (Decatur, AL). Filter membrane (0.22 μm) was purchased from Fisher Scientific (Pittsburgh, PA). Dialysis membrane of molecular weight cutoff 1000 Da (MWCO 1000) was purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA). Phosphate-buffered saline (PBS, pH 7.4, consisting of 0.01 M phosphate buffer, 0.0027 M potassium chloride, 0.137 M sodium chloride) was prepared by dissolving PBS tablets (Sigma-Aldrich, St. Louis, MO) in distilled, deionized water. High performance liquid chromatography (HPLC) grade acetonitrile was purchased from Pharmaco-AAPER (Shelbyville, KY).
2.2 Preparation of micellar carrier systems
Mixed micellar solution at a ratio of LE to TA of 1:4 (mole ratio) was prepared by first dissolving an appropriate amount of TA in PBS to obtain a clear solution and then adding an appropriate amount of LE to make the mixed micelle solution of desired total lipid concentration. The concentration of the mixed micelle solution was 95 mg/mL in all experiments unless otherwise stated. DEX was formulated in the mixed micelles at two different concentrations: mixed micelles containing saturated (SMM) and unsaturated (UMM) DEX. In the preparation of SMM, excess amounts of DEX were added to the mixed micelle solution followed by equilibration at 36 ± 1°C for 24 to 48 hours. After equilibration, the mixture was filtered through 0.22 μm filter membrane to obtain clear SMM solution. In the preparation of UMM, an appropriate amount of DEX was added to the mixed micelle solution to a final DEX concentration of 0.1 mg/mL.
Since both simple and mixed micelles coexisted in the mixed micellar carrier system, saturated simple micellar carrier system (SSM) was also prepared and tested in the present study. SSM was prepared by dissolving an appropriate amount of TA in PBS followed by saturating the system with excess DEX. The solution was equilibrated in a circulating waterbath at 36 ± 1°C for 24 to 48 hours. After equilibration, the undissolved drug was separated from the solution by filtration using 0.22 μm filter membranes. The concentration of TA in the SSM solution was 28 mg/mL in all experiments unless otherwise stated. This concentration was selected because it was the same TA concentration as of the simple micelles co-existing in the 95 mg/mL mixed micelle system according to calculations based on a previous study [34].
Control solution of saturated DEX was prepared by adding an excess amount of DEX in PBS followed by equilibration in a circulating waterbath at 36 ± 1°C for 24 to 48 hours. After equilibration, the undissolved drug was separated from the solution by filtration using 0.22 μm filter membranes. The concentration of DEX in the solution (control) was found to be 0.1 mg/mL.
2.3 Preparation of the sclera
Cadaver eyes were obtained from National Disease Research Interchange (NDRI, Philadelphia, PA). The tissues were stored in moisture chambers at 4°C. Before the experiments, the tissue was cleaned at room temperature and adhering tissues on the sclera including the retina and choroid were removed with a pair of forceps. The sclera was then rinsed with PBS, cut into approximate size, and equilibrated in PBS at room temperature for 30 min before its use. The use of human tissues was approved by the Institutional Review Board at the University of Cincinnati, Cincinnati, OH.
2.4 Characterization of micellar carrier systems
2.4.1 Drug encapsulation: solubility study
Different concentrations of SMM and SSM were prepared using the method described in Section 2.2 to study the solubility of DEX in the micellar carrier systems. The total lipid concentrations (TA and LE) of SMM were 25, 40, 55 and 95 mg/mL in the SMM experiments, and the concentrations of TA were 29, 39, 47, 70 and 95 mg/mL in the SSM experiments. After equilibrating SMM and SSM solutions with excess amounts of DEX in a circulating waterbath at 36 ± 1°C for 24 to 48 hours, the undissolved drug was separated from the solution by filtration using 0.22 μm filter membranes. Aliquots of the filtered micelle solutions were then subjected to appropriate dilution with the mobile phase used in the HPLC assay. The diluted samples were assayed for the drug using an HPLC system (Prominence, Shimadzu, Columbia, MD) consisted of CBM-20A system controller, LC-20AT solvent delivery module, SIL-20A autosampler, and SPD-20A UV-Vis detector. The separation was performed with Microsorb C18 column (150 mm × 4.6 mm; Varian, Inc., Palo Alto, CA) at room temperature. The mobile phase consisted of acetonitrile and water (65:35, v/v) and was delivered at a flow rate of 1.0 mL/min. The detection wavelength for DEX was 284 nm. Each sample was analyzed at least twice, and the averages were used to calculate the concentrations of solubilized drug in the different systems.
2.4.2 Drug encapsulation: dialysis membrane study
Drug encapsulation in the micellar systems was also measured in a passive transport experiment with the MWCO 1000 dialysis membrane in a circulating waterbath at 36 ± 1°C. The dialysis membrane was mounted between the two half-cells of a side-by-side diffusion cell with an effective diffusion area of 0.64 cm2. The donor solutions were prepared by adding trace amounts of radiolabelled DEX (0.2 μCi/mL) into SMM, UMM, and the control solutions. The receptor solution was PBS. The volume of the donor and receptor solutions was 1.5 mL. The duration of the transport experiments was 90 minutes. Samples of 10 μL and 1 mL were withdrawn from the donor and receptor compartments at predetermined time intervals, respectively. The volume of the receptor was maintained constant by the addition of 1 mL fresh PBS after each sampling. The samples were then mixed with 10 mL of liquid scintillation cocktail (Ultima Gold™, PerkinElmer Life and Analytical Sciences, Shelton, CT) and assayed by a liquid scintillation counter (Beckman Coulter LS 6500, Fullerton, CA).
The apparent flux (J) was calculated from the slope (ΔQ/Δt) of the linear region of the plot of the cumulative amount (ΔQ) of the permeant transported across the membrane into the receptor chamber versus time (t) divided by the effective diffusion area (AD).
| (1) |
The apparent permeability coefficient (P) was calculated by normalizing the apparent flux by the donor concentration of the permeant (CD).
| (2) |
Encapsulation efficiency (EE) is defined as the fraction of the drug encapsulated into the mixed micelles with respect to the total amount of the drug in the micelle solution. As only the free drug can permeate across the dialysis membrane, EE was determined by:
| (3) |
where Pmm is the apparent permeability coefficient of the drug determined in the transport experiment of the micellar carrier system and Paq is the apparent permeability coefficient of the drug determined in the transport experiment of the control.
2.4.3 Measurements of conductivity, viscosity, osmolarity, size, and zeta potential
The conductivity, viscosity, osmolarity, effective size and zeta potential of the micellar carrier systems were measured at room temperature using a conductivity meter (510 series, Oakton Instruments, Vernon Hills, IL), Ostwald viscometer (Barnstead International/Thermo scientific, Dubuque, Iowa), osmometer (Model 3300, Advanced Instruments Inc, Norwood, MA), and Malvern Zetasizer® (Nano ZS, Malvern Instruments Ltd, Worcestershire, UK), respectively.
2.5 Transscleral transport study
Cadaveric human sclera was sandwiched between the two half-cells of a side-by-side diffusion cell with the choroid side facing the receptor. The diffusion cells had effective diffusion area of 0.2 cm2 and the temperature was maintained by a circulating waterbath at 36 ± 1°C. The volume of the donor and receptor solutions was 1.5 mL. Prior to the transport experiments, trace amounts of radiolabelled DEX (0.5-1 μCi/mL) were added into SMM, UMM, SSM, and the control solutions in the donor chamber. PBS was the receptor solution in all the transport experiments. Passive transport, anodal iontophoresis (anode in the donor), and cathodal iontophoresis (cathode in the donor) experiments were performed. In the iontophoresis experiments, 2 mA current was applied across the sclera with a constant current iontophoretic device (Phoresor II Auto, Model PM 850, Iomed, Inc., Salt Lake City, UT) using Ag/AgCl (cathode) and Ag (anode) as the driving electrodes. The duration of the transport experiment was 20 min. Samples of 10 μL donor solution and 1 mL receptor solution were taken at predetermined time intervals for assay. Fresh PBS of 1 mL was then added into the receptor to maintain a constant volume in the receptor. The samples were mixed with 10 mL of the liquid scintillation cocktail and assayed by the liquid scintillation counter.
In these transscleral transport experiments, the instantaneous fluxes were calculated from the changes in the cumulative amount of the permeant transported across the sclera into the receptor chamber and the effective diffusion area for all time points using Eq. 1. The instantaneous fluxes at the last two tme points in the transport experiments were averaged, and the apparent permeability coefficient (flux normalized by the concentration) was calculated by dividing the average flux by the donor concentration using Eq. 2. It was important to characterize the transscleral transport behavior of the micellar carrier systems under a controlled in vitro setting in these experiments although this setting might not predict transscleral drug delivery in vivo due to the absence of blood vasculature and lymphatic clearance.
In a separate study, transport experiments of DEX in SMM were conducted on the same sclera tissue using a three-stage protocol: first passive transport, cathodal iontophoresis, and second passive transport under the same experimental conditions of the transport experiments described above. The results of the first and second passive transport experiments were compared to examine possible irreversible electropermeabilization effects of iontophoresis upon human sclera.
2.6 Drug release study
Drug release study was carried out following the transscleral transport study with the same diffusion cells and sclera. Particularly, at the end of the transport experiments, both the donor and receptor solutions were removed and 1.5 mL of fresh PBS was added into the receptor chamber to start the release study. A stopper was used on the donor chamber to seal the donor chamber and maintain the pressure in the chamber. The donor and receptor chambers were not rinsed before the start of the release study to avoid potential washout of the drug from the sclera. The drug release study was performed for 6 days. At predetermined time intervals, 1 mL of receptor solution was taken from the receptor for assay followed by replenishing the receptor with 1 mL fresh PBS. The samples were mixed with 10 mL of the liquid scintillation cocktail and assayed by the liquid scintillation counter. While the results in the in vitro drug release study might be different from those in vivo due to the absence of clearance in vitro, the study was performed to assess the sustained release properties of the micellar carrier systems before future in vivo studies.
2.7 Drug extraction study
Drug extraction study of the sclera was performed following the drug release study to determine the amount of DEX remained in the sclera after the drug release study. At the end of the drug release experiment, the sclera was removed from the side-by-side diffusion cell assembly and was immersed in 2 mL ethanol solution in a vial for 24 hours. After 24 hours of extraction, 1 mL of sample was withdrawn from the vial, mixed with 10 mL of liquid scintillation cocktail, and assayed for DEX by the liquid scintillation counter. The total amounts of DEX loaded into the sclera with passive and iontophoretic delivery were calculated as the sum of the amounts of DEX released from the sclera in the drug release study and the amounts of DEX remained in the sclera.
2.8 Statistical analysis
All experiments were conducted with a minimum of three replicates using sclera tissues from different donors. The means ± standard deviations (SD) of the data are presented. Data were compared using Student’s t-test. Differences were considered to be significant at a level of p < 0.05.
3 Results and Discussion
3.1 Characterization of micellar carrier systems
Figure 1 presents the amounts of DEX solubilized in different concentrations of SMM and SSM. With an increase in the concentration of total lipids in the SMM system from 25 mg/mL to 95 mg/mL, the solubility of DEX increased linearly from 0.17 mg/mL to 0.45 mg/mL. According to these data, the concentration of DEX in SMM in the transport experiments was 0.45 mg/mL, which was within the clinical dose range in practice [35]. For SSM, the solubility of DEX in SSM increased from 0.24 mg/mL to 0.61 mg/mL when TA concentration was increased from 29 mg/mL to 95 mg/mL. In SMM, both simple and mixed micelles existed and contributed to the solubilization of DEX. As stated in Section 2.2, the concentration of simple micelles of TA in the SMM was ~28 mg/mL. From the slope in Fig. 1, the amount of DEX solubilized by simple micelles of TA was calculated to be 0.23 mg per 28 mg TA, corresponding to 8.2 μg per mg TA simple micelle. The amount of DEX solubilized by the mixed micelles estimated by subtracting the amount of DEX solubilized by TA simple micelles from the total drug solubilized in SMM was 0.22 mg per 95 mg total lipids. This suggests that ~50% of the drug was solubilized by the simple micelles in SMM. From the y-intercept in Fig. 1, the aqueous solubility of DEX in the absence of micelles was approximately 0.07 mg/mL, not significantly different from the solubility of DEX determined in the control (0.1 mg/mL).
Figure 1.
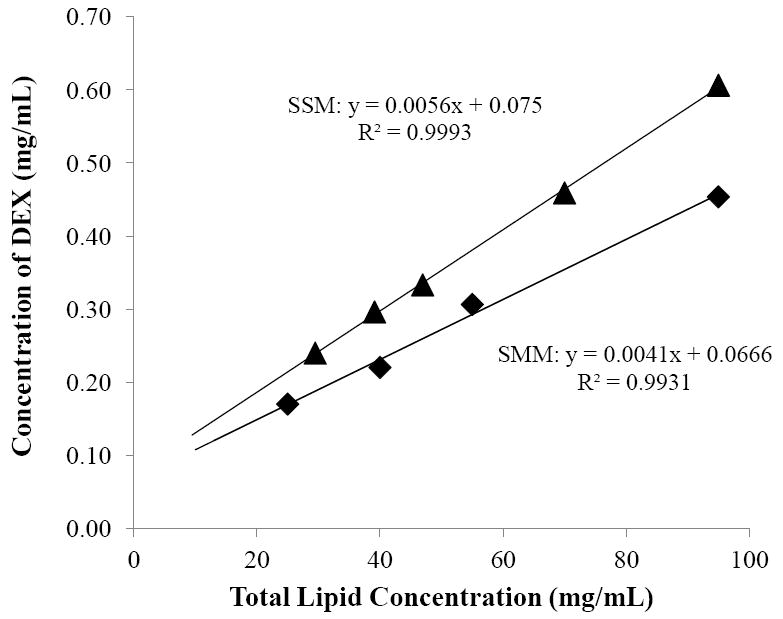
Solubility of DEX as a function of total lipid concentration in the saturated mixed micelle (SMM, closed diamonds) and saturated simple micelle (SSM, closed triangles) systems.
The amount of DEX encapsulated in the UMM system cannot be determined in the DEX solubility study, so transport experiments with dialysis membrane were performed. The passive permeability coefficients of DEX in SMM, UMM, and control solutions across the dialysis membrane were calculated using Eq. 2. The passive permeability coefficient of DEX in the control (2.3 × 10−5 cm/s) was approximately 3 times higher than that of SMM (5.6 × 10 −6 cm/s) and UMM (5.8 × 10 −6 cm/s). The passive permeability coefficient in the control experiment was higher than those of SMM and UMM because DEX loaded in the mixed micelles could not permeate through the MWCO 1000 dialysis membrane [34]. As only free DEX can diffuse across the membrane into the receptor, this allows the determination of the encapsulation efficiencies of the carrier systems using Eq. 3. The encapsulation efficiencies of DEX in SMM and UMM calculated were approximately 75%. These results are consistent with the approximately four times increase in the solubility of DEX in 95 mg/mL SMM from its aqueous solubility (0.1 mg/mL) in the solubility study.
Table 1 summarizes the physical properties of the micellar carrier solutions and the saturated DEX control solution tested in the present study. The physical properties were determined to understand the iontophoretic transport and drug release mechanisms of the micellar carrier systems. The results show that the conductivities of SSM, SMM, and UMM solutions are essentially the same. These results are not significantly different from that of the control despite the relatively high concentration of micelles (28 mg/mL for SSM and 95 mg/mL for SMM and UMM) used in the present study. This can be attributed to the background electrolyte being the main conducting ions in the SMM, SSM, and UMM solutions and the increase in the viscosity of the solutions in the presence of the micelles. The viscosities of SMM and UMM were approximately 1.4 times higher than that of SSM, suggesting that SMM has a larger impact on solution viscosity than SSM. The viscosity of SSM solution was approximately 1.2 times that of the control. The osmolarities of SMM, SSM, and UMM solutions were between 370-420 mOsm, indicating that these micelle solutions were slightly hypertonic.
Table 1.
Properties of the micellar carriers systems and the control in the present study a
| Formulations | Conductivity (mS) | Viscosity (mPa·s) | Osmolarity (mOsm) | Hydrodynamic diameter (nm) | Zeta potential (mV) |
|---|---|---|---|---|---|
| SSM | 15.1 ± 0.2 | 1.18 ± 0.03 | 370 ± 11 | - b | −67.0 ± 1.1 |
| SMM | 13.8 ± 0.6 | 1.64 ± 0.02 | 394 ± 10 | 4.4 ± 0.2 | −61.1 ± 2.0 |
| UMM | 14.1 ± 0.3 | 1.64 ± 0.01 | 420 ± 9 | 4.7 ± 0.2 | −52.0 ± 2.0 |
| Control | 14.8 ± 0.4 | 1.06 ± 0.01 | 303 ± 13 | - b | - b |
Mean ± SD, n ≥ 3.
Not applicable.
The effective sizes (i.e., hydrodynamic diameter) of mixed micelles SMM and UMM were 4.4 and 4.7 nm, respectively (Table 1), whereas that of the simple micelle SSM was below the detectable range of the instrument. This is consistent with the general view that simple micelles are smaller than the mixed micelles. In addition, the sizes of the micelles in SMM and UMM were studied upon dilution with PBS. The results show that dilution led to an increase in the size of SMM and UMM (to ~20 nm at 20-fold dilution) followed by a slight decrease in size upon further dilution due to the formation of monodisperse vesicles. This trend is consistent with a previous report that the size of TA/lecithin mixed micelles first increases and then decreases with dilution [36]. The zeta potentials of the micellar carrier systems were also determined, and the results show that the micellar carriers were net negatively charged in PBS under the experimental conditions in the present study. Overall, SMM and SSM differed in their properties including micellar carrier sizes and solution viscosity. These differences could influence transscleral transport, drug release, and scleral loading of DEX of these micellar carrier systems as discussed in the following sections.
3.2 Transport study with SMM and SSM
Figures 2A and 2B show the cumulative amounts of DEX transported across human sclera in the passive and iontophoretic transport experiments with SMM and SSM solutions. Before the discussion of the SMM data, it should be realized that both simple and mixed micelles exist in the SMM solution due to the relatively high concentration of TA compared to LE (4:1 mole ratio) in the micellar system (also see Section 3.1); these simple micelles likely contribute to the behavior of DEX delivery in the passive and iontophoretic transscleral transport experiments. For both SSM and SMM, higher cumulative amounts of DEX transported across the sclera were observed during cathodal iontophoresis as compared to their passive counterparts. These results are consistent with the negative charges and zeta potential of SMM and SSM (Table 1) and demonstrate the direct electric field effect of iontophoresis (i.e., electrorepulsion or Nernst-Planck effect) [14] on these carrier systems. For SMM, the higher cumulative amounts of DEX delivered in the anodal iontophoresis experiment than those in passive delivery were likely attributed to the contribution of electroosmosis. The electroosmosis effect was expected to be larger for the mixed micelles in SMM than for SSM due to the difference in their sizes (Table 1) [37]. Accordingly, the cumulative amounts of DEX in anodal and cathodal iontophoresis of DEX in SMM were not significantly different. In summary, the observed iontophoresis enhancement of both simple and mixed micelle systems suggests that these carrier systems can be efficiently delivered across human sclera using iontophoresis as compared to passive delivery.
Figure 2.
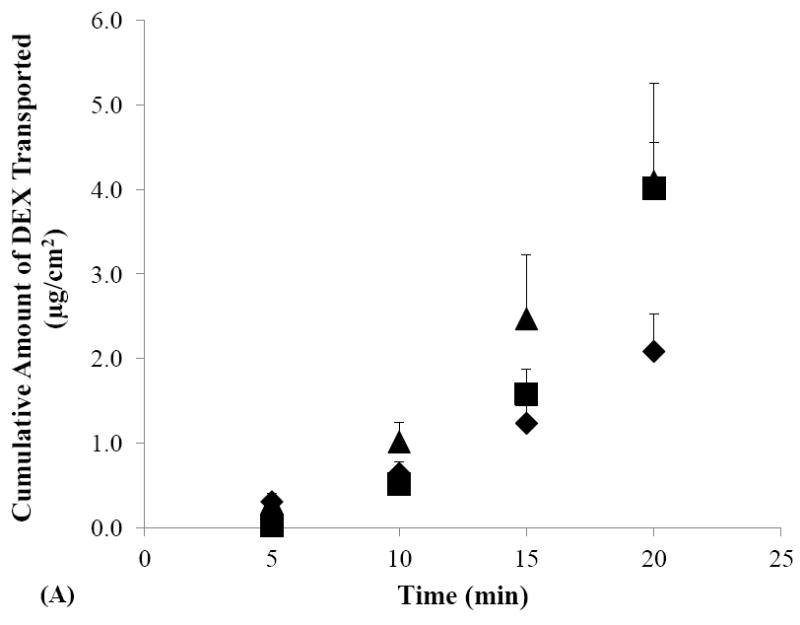
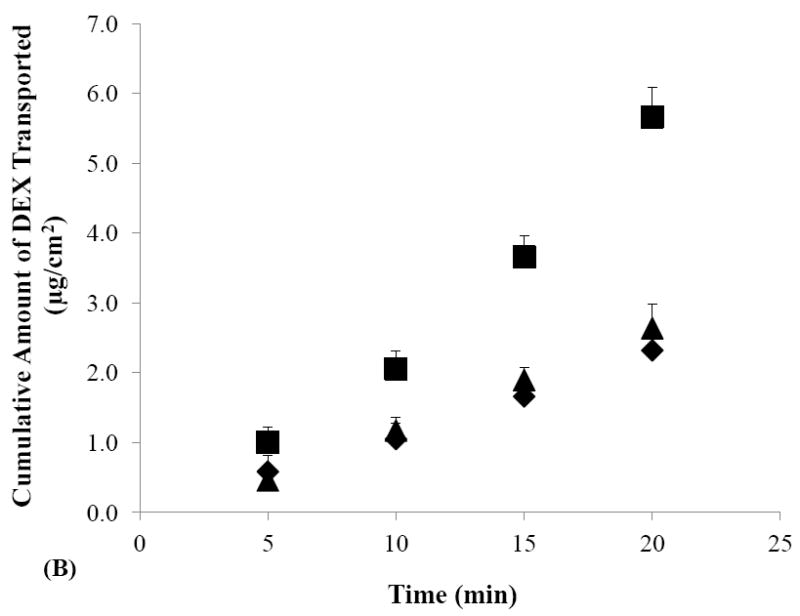
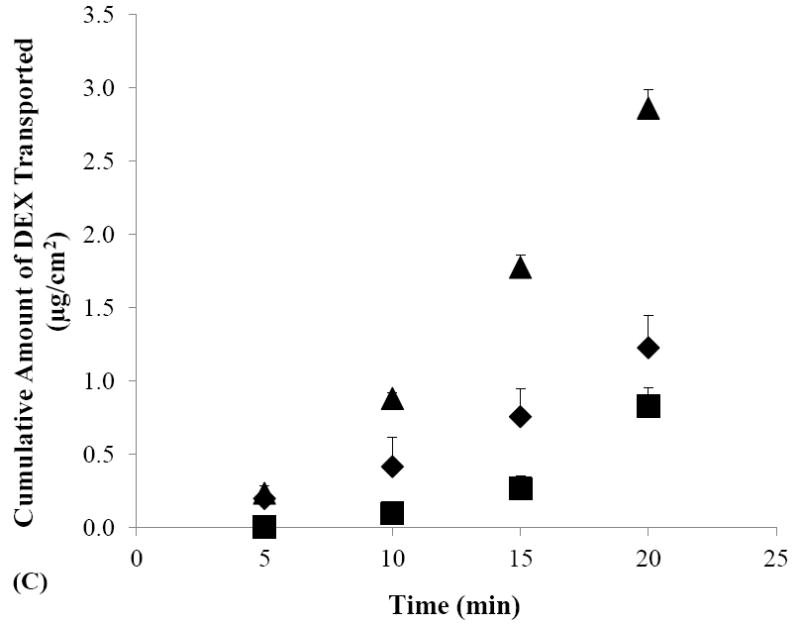
Cumulative amounts of DEX transported across human sclera versus time in the passive (closed diamonds), cathodal iontophoresis (closed squares), and anodal iontophoresis (closed triangles) transport experiments of (A) SMM, (B) SSM, and (C) control. Data represent the mean and standard deviation, n ≥ 3.
Figure 2C shows the cumulative amounts of DEX transported across human sclera in the passive and iontophoretic transport experiments with the control solution. In these control experiments, significantly higher cumulative amount of DEX transported across the sclera was observed during anodal iontophoresis as compared to those of passive transport and cathodal iontophoresis. The enhancement of DEX transport observed in the anodal iontophoresis control experiment is consistent with the dominant effect of electroosmosis upon DEX transport across the negatively charged human sclera under the condition in the present study.
To compare transscleral transport of SMM and SSM, the apparent permeability coefficients of human sclera for DEX in SMM, SSM, and the control were calculated by normalizing the flux with the donor concentration (Fig. 3). The passive permeability coefficient of DEX in SMM was lower than those of SSM and the control. This is consistent with the larger effective size (hydrodynamic diameter) of SMM as compared to that of SSM (see Section 3.1). DEX in the control experiments had the highest passive permeability coefficient due to its molecular size and diffusion coefficient in the sclera compared to those of the carrier systems. Similarly, the higher cathodal iontophoretic permeability coefficient of DEX in SSM than that of SMM could be partly attributed to the smaller effective sizes of SSM than SMM.
Figure 3.
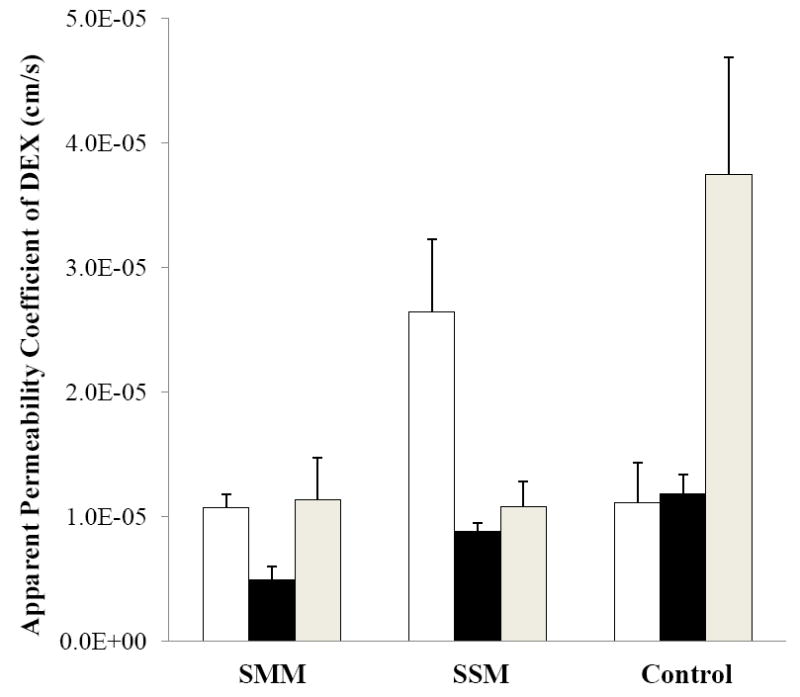
Apparent permeability coefficients of DEX of SMM, SSM, and the control in the cathodal iontophoresis (open bars), passive (black bars), and anodal iontophoresis (gray bars) transport experiments with human sclera. Data represent the mean and standard deviation, n ≥ 3.
It should be pointed out that the conductivity results of SMM, SSM, and control solutions (Table 1) suggest similar voltage drop across the sclera in the present constant current iontophoretic transport experiments. Therefore, DEX iontophoretic transport in the SMM, SSM, and control solutions was likely under similar iontophoretic driving forces (i.e., constant current iontophoresis of constant electrical potential). This allows the direct comparison of the permeation data to understand the interplay of electrophoresis and electroosmosis of the micellar systems upon flux enhancement in the present study. In addition, the osmolarity results in Table 1 suggest that convective solvent-flow driven transport due to the water concentration gradient across the sclera (the difference in osmolarities of the receptor solution and the SMM, SSM, and UMM donor solutions) in the transport experiments was not likely to be significant. The effective sizes and charges of the micellar carriers and their encapsulation capacity are the primary factors influencing iontophoretic transscleral transport in the present study.
In the study using the three-stage transport protocol of passive transport followed by cathodal iontophoresis and then second passive transport, no significant difference was observed in the passive permeability coefficients of SMM before and after iontophoresis (4.5 ± 0.8 × 10−6 and 6.2 ± 1.1× 10−6 cm/s, mean ± SD, n = 4, respectively). The essentially same permeability coefficients in the first and second passive stages suggest no irreversible electropermeabilization effect on the barrier properties of human sclera for micellar transport under the iontophoretic conditions in the present study.
3.3 Drug release study with SMM and SSM
The effects of iontophoretic delivery on drug release from the sclera were first investigated. As shown in Figs. 4A and 4B, drug release from the sclera in the SMM and SSM experiments was enhanced after cathodal iontophoresis as compared to those after anodal iontophoresis and passive transport. These results are consistent with the findings in the transport study (Section 3.2) that cathodal iontophoresis can effectively enhance transscleral delivery of these micellar carrier systems. Conversely, in the control experiment without the carrier systems (Fig. 4C), drug release was enhanced after anodal iontophoresis as compared to those after passive and cathodal iontophoretic transport, consistent with electroosmotic enhancement of DEX delivery during anodal iontophoresis. Both these trends can be attributed to the ability of iontophoresis in enhancing drug loading into the tissue compared to that of passive delivery, and hence providing better sustained drug delivery from the sclera. The effects of iontophoresis upon drug loading will be discussed in the next section (Section 3.4).
Figure 4.
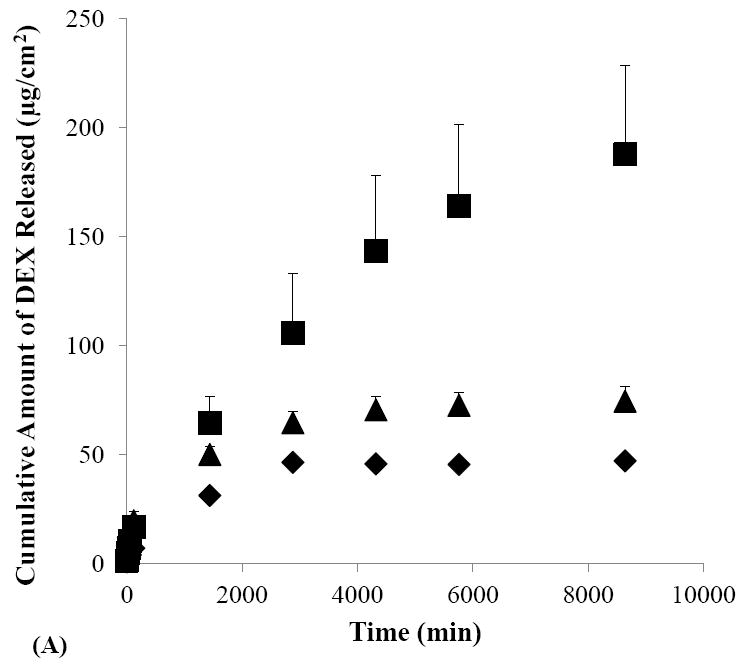
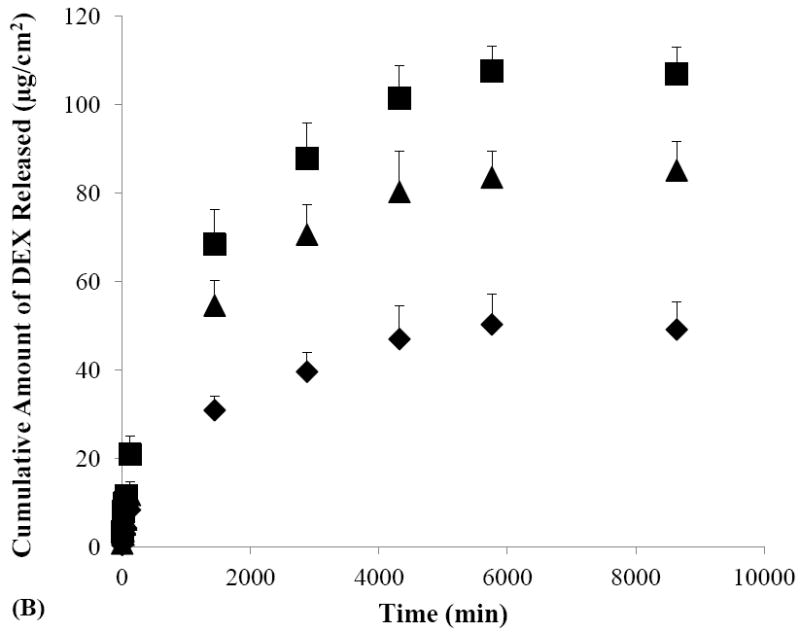
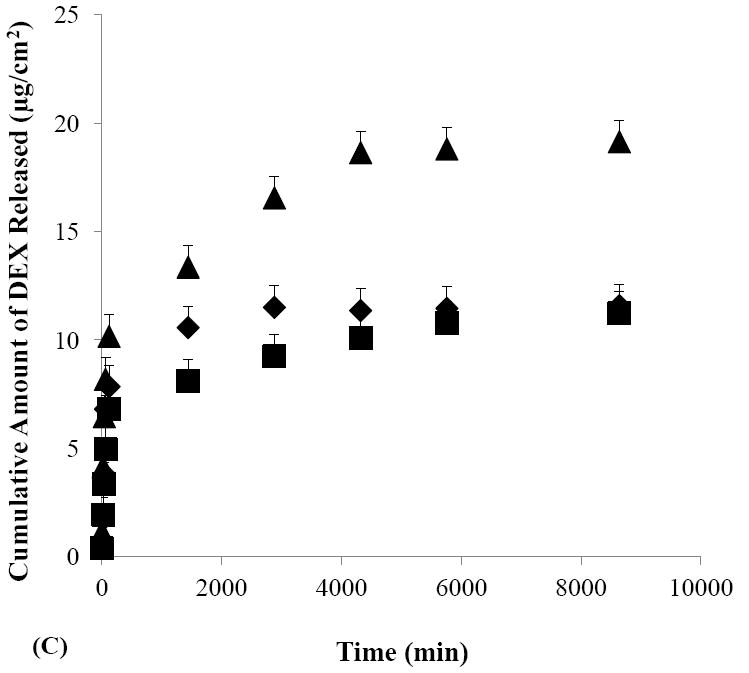
Cumulative amounts of DEX released from human sclera versus time in the release studies performed after the passive (closed diamonds), cathodal iontophoresis (closed squares), and anodal iontophoresis (closed triangles) transport experiments of (A) SMM, (B) SSM, and (C) control. Data represent the mean and standard deviation, n ≥ 3.
To examine the effects of micellar carrier systems on the rate of DEX released from the sclera, the results in Figs. 4A and 4B were compared with those in Fig. 4C. Figure 4C shows that more than 60% of DEX was released within two hours after passive delivery whereas Figs. 4A and 4B shows only 14% and 16% of DEX were released in the same time period after passive delivery of SMM and SSM, respectively. Similarly, drug release after iontophoretic transport of the micellar carrier systems was generally slower as compared to that of the control after iontophoresis. For example, approximately 33% of DEX was released in one day after cathodal iontophoresis of SMM while the release of DEX was 63% in one day after anodal iontophoresis of the control. These results suggest that SMM and SSM carrier systems exhibit sustained release properties by providing slower DEX release than the control. SMM and SSM also have different sustained drug delivery profiles. A comparison of drug release from the sclera after cathodal iontophoresis of SMM with that of SSM shows that approximately 55% of DEX was released in two days with SMM, which was less than the approximately 80–90% of DEX released with SSM in the same time period. These results suggest that SMM provides better sustain release properties than SSM.
3.4 Drug extraction study with SMM and SSM
Figure 5 presents the total amounts of DEX loaded in the sclera at the end of the transport experiments. The results show that the amounts of DEX loaded in the sclera after cathodal iontophoretic transport of SMM and SSM were significantly larger than those after passive transport of SMM and SSM. The two- to four-fold higher drug loading into the sclera after iontophoretic transport than passive delivery supports the hypothesis that iontophoresis enhances the loading of charged micellar carrier systems into the sclera and hence provides enhanced sustained transscleral drug delivery after iontophoresis. This hypothesis is also consistent with the sclera transport data observed in Section 3.2 in which higher apparent permeability coefficients were observed during cathodal iontophoresis (SMM and SSM) as compared to their permeability coefficients in passive delivery. On further comparison of enhanced drug loading due to iontophoresis, the enhancement of drug loading was larger for SMM than for SSM after cathodal iontophoresis. This might be related to the higher DEX concentration in the donor in the transport experiment of SMM than that of SSM (0.45 mg/mL versus 0.23 mg/mL, respectively).
Figure 5.
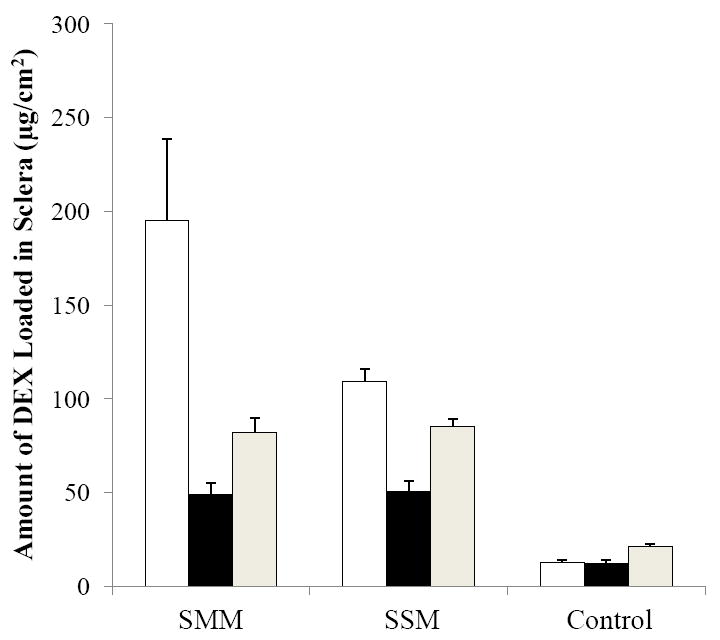
Amounts of DEX loaded in human sclera after cathodal iontophoresis (open bars), passive (black bars), and anodal iontophoresis (gray bars) transport experiments with SMM, SSM, and control. Data represent the mean and standard deviation, n ≥ 3.
To investigate the effects of the micellar carriers on scleral drug loading, the amounts of DEX loaded in the sclera after SMM and SSM passive delivery were compared with that of the control. The amount of DEX loaded into the sclera after passive drug delivery with the control was significantly smaller than those in SMM and SSM. This suggests that the improved aqueous solubility of DEX in the micellar carrier systems enhanced drug loading into the sclera in passive transscleral delivery. Similar findings of the micellar carrier systems being more effective in loading the drug into the sclera in iontophoretic delivery as compared to iontophoretic delivery of the control were also observed. Together, these results demonstrate the advantages of iontophoretic delivery and the micellar systems for sustained ocular drug delivery. It should be pointed out that the amounts of drug loaded into the sclera from iontophoresis correspond to tissue concentration comparable to or higher than the drug concentration in the donor chamber of the transport study within the uncertainties of the dimensions of the sclera mounted on the diffusion cells. This is probably due to iontophoretic enhanced drug loading into the membrane [38] saturating the sclera under these conditions. In addition, drug delivery from the small amount of residual donor solution on the surface of the sclera after the removal of the donor solution in the release study could also contribute to this observation.
3.5 Transport and release studies with UMM
UMM that contained the same amount of DEX as the control (0.1 mg/mL) was used to study the effects of micellar DEX concentration upon transscleral drug transport and drug release from the sclera. As shown in Table 1 and Section 3.1, the physical properties and encapsulation efficiency of UMM and those of SMM are not significantly different, suggesting that the encapsulation of DEX into the micelles did not significantly alter the physical properties of the micelles. The encapsulation of DEX into the micelles also did not significantly impact transscleral transport of DEX in the micellar carrier systems. For example, the passive (5.7 × 10−6 cm/s) and cathodal iontophoretic (1.3 × 10−5 cm/s) permeability coefficients of sclera for DEX in UMM are essentially the same as those in SMM, respectively.
A comparison of the UMM results with those of the control (saturated DEX solution without the micelles) shows that the passive and iontophoretic permeability coefficients of DEX in UMM are significantly lower than those of the control. This is consistent with UMM being larger in hydrodynamic size than free DEX. Despite the lower permeability coefficients of DEX in UMM relative to the control, UMM shows higher sclera loading than those of the control after passive and iontophoretic delivery.
Figure 6 compares the results of drug release from the sclera in UMM with those of the control (results from Fig. 4). From the release profiles in the figure, approximately 25% of DEX was released from the sclera within 2 hours after passive delivery of UMM, which is significantly smaller than the more than 60% DEX released within the same period after passive delivery in the control experiment. This suggests interactions between the micellar carrier system and the sclera that contributed to the observed slower drug release. Figure 6 also demonstrates the effect of cathodal iontophoresis upon the drug release profiles of UMM. Iontophoresis of UMM enhanced the total amount of DEX released from the sclera over that after passive delivery.
Figure 6.
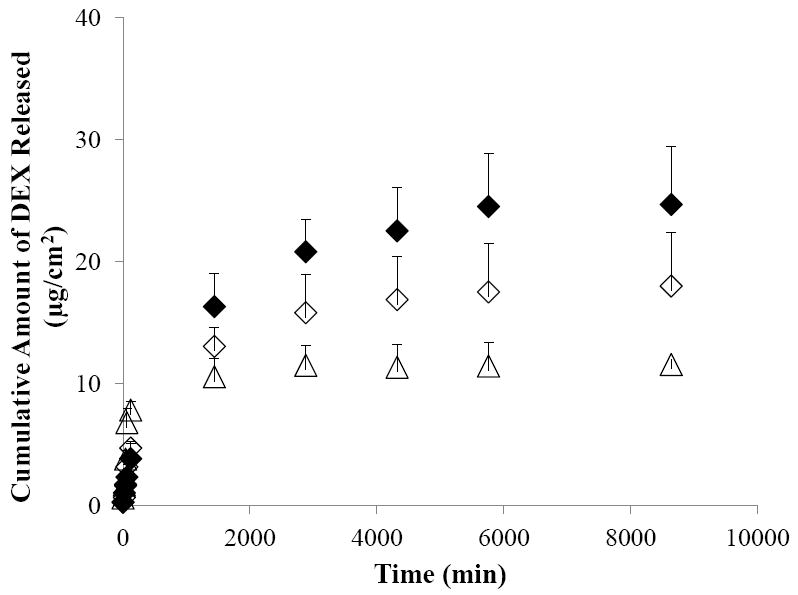
Cumulative amounts of DEX released from human sclera in the release studies performed after the passive (open diamonds) and cathodal iontophoretic (closed diamonds) transport experiments of UMM. The results of the control after passive delivery (open triangles) are presented again for comparison. Data represent the mean and standard deviation, n ≥ 3.
3.6 Mechanisms of micellar carrier sustained delivery
Micellar carrier-sustained DEX delivery could be related to two main factors: improved sclera loading and delayed drug release from the sclera. The increased DEX loading in the sclera due to higher aqueous solubility of DEX in the micellar carrier systems and enhanced delivery of DEX into the sclera by iontophoresis can result in an increase in the total amount of DEX released from the sclera compared with the control. Particularly, the effect of drug loading upon sustained drug delivery is demonstrated in the iontophoresis experiments. After cathodal and anodal iontophoresis that enhanced drug loading, the amounts of DEX released were generally larger and slower than those of passive delivery.
For delayed drug release, the mechanisms can be through carrier and drug interactions with the sclera and slower micellar carrier diffusion than that of free DEX in the sclera. For instance, interactions between these micellar carrier systems and the sclera such as micelle binding to the tissue can result in delayed drug release from the sclera. In addition, the larger effective sizes of the micellar carriers in SMM and SSM and the resultant slower diffusion of these carriers in the sclera relative to those of free DEX would lead to delayed drug release. As evidenced in the results from the UMM studies (Fig. 6), such delayed drug release contributed to the sustained release properties observed in the present study. However, this effect is probably not as significant as sclera loading effect (Figs. 4 and 6). Enhanced sclera loading due to the increase of DEX solubility in SMM and SSM is believed to be the main mechanism for the differences observed between the release profiles of SMM and SSM versus those of the control. Another factor that is worth mentioning is the higher viscosity of SMM and UMM than SSM and the control (Table 1) that can also contribute to the different sustained release profiles of DEX in SMM compared to SSM and the control.
3.7 Potential application of transscleral iontophoresis of the micellar carriers
Sustained release drug delivery systems can be a therapeutic modality for the treatment of chronic ocular disorders such as chronic inflammatory eye diseases [39]. Micellar carrier systems are generally considered as safe [40], can be tailored in a variety of ways to enhance the aqueous solubility of lipophilic drugs, and provide a sustained drug delivery effect. Physical enhancement methods such as iontophoresis have been employed in ocular drug delivery. For example, transscleral iontophoresis was shown to have the potential to enhance the transport of small and macromolecules across the sclera [16]. The results in the present study suggest that the studied micellar carrier systems can be delivered across the sclera by iontophoresis. Particularly, transscleral iontophoresis of the micellar carrier systems was shown to enhance drug loading into the sclera and prolong drug delivery from the sclera. The combination of micellar nanocarriers and transscleral iontophoresis could therefore maintain higher drug concentrations at the site of application, provide sustained release from this site to the site of drug action in the eye, and overcome the need of frequent drug dosing and injections in the eye for the desired therapeutic effect. This drug delivery platform can be a promising noninvasive strategy in the treatment of posterior eye diseases. It could be more cost effective than frequent intravitreal injections, a procedure that generally requires the involvement of a skilled ophthalmologist or retina specialist.
It should be pointed out that the present study was conducted under the in vitro setting. Cautions must be exercised in the interpretation of the results in the present study as it may not predict transscleral drug delivery in practice in vivo due to the absence of blood vasculature and lymphatic clearance in the present experiments in vitro. Further pharmacokinetic studies of iontophoresis and micellar carriers are required to demonstrate the feasibility of these systems in vivo. In addition, the safety and toxicity of the mixed micelles used and those of the combination use of micelles and iontophoresis have not been evaluated in the present in vitro study. Although various studies have demonstrated the safety of mixed micellar carriers in cell lines [41-42] in the concentration range and LE to TA molar ratio similar to those in the present study, it would be difficult to predict their effects in the eye. In a preliminary study to assess the safety of the mixed micelles in the subconjunctival space using mouse eyes, no irritation and toxicity were observed for one week after dosing (unpublished data). Despite these results, future studies are required to fully evaluate the safety of the proposed micellar carrier systems for transscleral iontophoretic delivery.
Acknowledgments
This study was supported by grant EY 015181 from the National Eye Institute (NEI) at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of NEI or NIH. The authors acknowledge the use of tissues procured by the National Disease Research Interchange (NDRI) with support of NIH grant 5 U42 RR006042.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Michel SS, Ekong A, Baltatzis S, Foster CS. Multifocal choroiditis and panuveitis — immunomodulatory therapy. Ophthalmology. 2002;109:378–383. doi: 10.1016/s0161-6420(01)00901-0. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni P. Review: Uveitis and immunosuppressive drugs. J Ocul Pharmacol Th. 2001;17:181–187. doi: 10.1089/10807680151125537. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmol. 2000;107:2024–2033. doi: 10.1016/s0161-6420(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 5.Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW. Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmol. 2010;117:567–575. doi: 10.1016/j.ophtha.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed I, Gokhale RD, Shah MV, Patton TF. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J Pharm Sci. 1987;76:583–586. doi: 10.1002/jps.2600760802. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26:584–587. [PubMed] [Google Scholar]
- 8.Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur J Pharm Biopharm. 1997;44:71–83. [Google Scholar]
- 9.Maurice D. Drug delivery to the posterior segment from drops. Surv Ophthalmol. 2002;47:S41–52. doi: 10.1016/s0039-6257(02)00326-0. [DOI] [PubMed] [Google Scholar]
- 10.Jager RD, Aiello LP, Patel SC, Cunningham ET. Risks of intravitreous injection: A comprehensive review. Retina-the Journal of Retinal and Vitreous Diseases. 2004;24:676–698. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Greaves JL, Wilson CG. Treatment of diseases of the eye with mucoadhesive delivery systems. Adv Drug Deliv Rev. 1993;11:349–383. [Google Scholar]
- 12.Gurtler F, Gurny R. Patent literature-review of ophthalmic inserts. Drug Dev Ind Pharm. 1995;21:1–18. [Google Scholar]
- 13.Gungor S, Delgado-Charro MB, Ruiz-Perez B, Schubert W, Isom P, Moslemy P, Patane MA, Guy RH. Trans-scleral iontophoretic delivery of low molecular weight therapeutics. J Control Release. 2010;147:225–231. doi: 10.1016/j.jconrel.2010.07.107. [DOI] [PubMed] [Google Scholar]
- 14.Eljarrat-Binstock E, Domb AJ. Iontophoresis: A non-invasive ocular drug delivery. J Control Release. 2006;110:479–489. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Nicoli S, Ferrari G, Quarta M, Macaluso C, Santi P. In vitro transscleral iontophoresis of high molecular weight neutral compounds. Eur J Pharm Sci. 2009;36:486–492. doi: 10.1016/j.ejps.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57:2063–2079. doi: 10.1016/j.addr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Behar-Cohen F, El Aouni A, Gautier S, David G, Davis J, Chapon P, Parel JM. Transscleral coulomb-controlled iontophoresis of methyl prednisolone into the rabbit eye: Influence of duration of treatment, current intensity and drug concentration on ocular tissue and fluid levels. Exp Eye Res. 2002;74:51–59. doi: 10.1006/exer.2001.1098. [DOI] [PubMed] [Google Scholar]
- 18.Eljarrat-Binstock E, Raiskup F, Frucht-Pery J, Domb AJ. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J Control Release. 2005;106:386–390. doi: 10.1016/j.jconrel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Behar-Cohen FF, Parel JM, Pouliquen Y, Thillaye-Goldenberg B, Goureau O, Heydolph S, Courtois Y, De Kozak Y. Iontophoresis of dexamethasone in the treatment of endotoxin-induced-uveitis in rats. Exp Eye Res. 1997;65:533–545. doi: 10.1006/exer.1997.0364. [DOI] [PubMed] [Google Scholar]
- 20.Molokhia SA, Jeong EK, Higuchi WI, Li SK. Examination of penetration routes and distribution of ionic permeants during and after transscleral iontophoresis with magnetic resonance imaging. Int J Pharm. 2007;335:46–53. doi: 10.1016/j.ijpharm.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranta VP, Mannermaa E, Lummepuro K, Subrizi A, Laukkanen A, Antopolsky M, Murtomaki L, Hornof M, Urtti A. Barrier analysis of periocular drug delivery to the posterior segment. J Control Release. 2010;148:42–48. doi: 10.1016/j.jconrel.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Brodin A, Nyqvist-Mayer A. In vitro release studies on lidocaine aqueous solutions, micellar solutions, and o/w emulsions. Acta Pharm Suec. 1982;19:267–284. [PubMed] [Google Scholar]
- 23.Miyazaki S, Yamahira T, Inoue H, Nadai T. Interaction of drugs with bile components. II. Effect of bile on the absorption of indomethacin and phenylbutazine in rats. Chem Pharm Bull (Tokyo) 1980;28:323–326. doi: 10.1248/cpb.28.323. [DOI] [PubMed] [Google Scholar]
- 24.Francis MF, Piredda M, Winnik FM. Solubilization of poorly water soluble drugs in micelles of hydrophobically modified hydroxypropylcellulose copolymers. J Control Release. 2003;93:59–68. doi: 10.1016/j.jconrel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Hendradi E, Obata Y, Isowa K, Nagai T, Takayama K. Effect of mixed micelle formulations including terpenes on the transdermal delivery of diclofenac. Biol Pharm Bull. 2003;26:1739–1743. doi: 10.1248/bpb.26.1739. [DOI] [PubMed] [Google Scholar]
- 26.Mrestani Y, Behbood L, Härtl A, Neubert RHH. Microemulsion and mixed micelle for oral administration as new drug formulations for highly hydrophilic drugs. Eur J Pharm Biopharm. 2010;74:219–222. doi: 10.1016/j.ejpb.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Sznitowska M, Klunder M, Placzek M. Paclitaxel solubility in aqueous dispersions and mixed micellar solutions of lecithin. Chem Pharm Bull (Tokyo) 2008;56:70–74. doi: 10.1248/cpb.56.70. [DOI] [PubMed] [Google Scholar]
- 28.Pepic I, Hafner A, Lovric J, Pirkic B, Filipovic-Grcic J. A nonionic surfactant/chitosan micelle system in an innovative eye drop formulation. J Pharm Sci. 2010;99:4317–4325. doi: 10.1002/jps.22137. [DOI] [PubMed] [Google Scholar]
- 29.Hagigit T, Abdulrazik M, Valamanesh F, Behar-Cohen F, Benita S. Ocular antisense oligonucleotide delivery by cationic nanoemulsion for improved treatment of ocular neovascularization: An in-vivo study in rats and mice. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.11.022. In press. [DOI] [PubMed] [Google Scholar]
- 30.Tong YC, Chang SF, Kao WW, Liu CY, Liaw J. Polymeric micelle gene delivery of bcl-xL via eye drop reduced corneal apoptosis following epithelial debridement. J Control Release. 2010;147:76–83. doi: 10.1016/j.jconrel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Baba K, Tanaka Y, Kubota A, Kasai H, Yokokura S, Nakanishi H, Nishida K. A method for enhancing the ocular penetration of eye drops using nanoparticles of hydrolyzable dye. J Control Release. 2011;153:278–287. doi: 10.1016/j.jconrel.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Enoch HG, Strittmatter P. Formation and properties of 1000-A-diameter, single-bilayer phospholipid vesicles. Proc Natl Acad Sci U S A. 1979;76:145–149. doi: 10.1073/pnas.76.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ollivon M, Lesieur S, Grabielle-Madelmont C, Paternostre M. Vesicle reconstitution from lipid-detergent mixed micelles. Biochim Biophys Acta. 2000;1508:34–50. doi: 10.1016/s0304-4157(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 34.Naganuma H, Liu C-L, Jain UK, Adachi Y, Higuchi WI. Quantitative examination of the simple micelle-mixed micelle coexistence for the taurocholatelecithin system by GP-HPLC. J Colloid Interface Sci. 1997;189:17–22. [Google Scholar]
- 35.Kiernan DF, Mieler WF. The use of intraocular corticosteroids. Expert Opin Pharmacother. 2009;10:2511–2525. doi: 10.1517/14656560903160671. [DOI] [PubMed] [Google Scholar]
- 36.Schurtenberger P, Mazer N, Kanzig W. Micelle to vesicle transition in aqueous solutions of bile-salt and lecithin. J Phys Chem. 1985;89:1042–1049. [Google Scholar]
- 37.Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev. 1992;9:201–237. doi: 10.1016/s0169-409x(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 38.Kasting GB. Theoretical models for iontophoretic delivery. Adv Drug Deliv Rev. 1992;9:177–199. [Google Scholar]
- 39.Booth BA, Vidal Denham L, Bouhanik S, Jacob JT, Hill JM. Sustained-release ophthalmic drug delivery systems for treatment of macular disorders: present and future applications. Drugs Aging. 2007;24:581–602. doi: 10.2165/00002512-200724070-00006. [DOI] [PubMed] [Google Scholar]
- 40.Meaney CM, O’Driscoll CM. A comparison of the permeation enhancement potential of simple bile salt and mixed bile salt:fatty acid micellar systems using the CaCo-2 cell culture model. Int J Pharm. 2000;207:21–30. doi: 10.1016/s0378-5173(00)00526-3. [DOI] [PubMed] [Google Scholar]
- 41.Teelmann K, Schlappi B, Schupbach M, Kistler A. Preclinical safety evaluation of intravenously administered mixed micelles. Arzneimittelforschung. 1984;34:1517–1523. [PubMed] [Google Scholar]
- 42.Alkan-Onyuksel H, Ramakrishnan S, Chai HB, Pezzuto JM. A mixed micellar formulation suitable for the parenteral administration of taxol. Pharm Res. 1994;11:206–212. doi: 10.1023/a:1018943021705. [DOI] [PubMed] [Google Scholar]


