Abstract
Individuals seeking treatment for their marijuana use rarely achieve sustained abstinence.
Objectives
To determine if THC, a cannabinoid agonist, and lofexidine, an α2-adrenergic receptor agonist, given alone and in combination, decreased symptoms of marijuana withdrawal and relapse, defined as a return to marijuana use after a period of abstinence.
Methods
Nontreatment-seeking, male volunteers (n=8), averaging 12 marijuana cigarettes/day, were maintained on each of four medication conditions for 7 days: placebo, THC (60 mg/day), lofexidine (2.4 mg/day), and THC (60 mg/day) combined with lofexidine (2.4 mg/day); each inpatient phase was separated by an outpatient washout phase. During the first 3 inpatient days, placebo marijuana was available for self-administration (withdrawal). For the next 4 days, active marijuana was available for self-administration (relapse). Participants paid for self-administered marijuana using study earnings. Self-administration, mood, task performance, food intake and sleep were measured.
Results
THC reversed the anorexia and weight loss associated with marijuana withdrawal, and decreased a subset of withdrawal symptoms, but increased sleep onset latency, and did not decrease marijuana relapse. Lofexidine was sedating, worsened abstinence-related anorexia, and did not robustly attenuate withdrawal, but improved sleep and decreased marijuana relapse. The combination of lofexidine and THC produced the most robust improvements in sleep, and decreased marijuana withdrawal, craving and relapse in daily marijuana smokers relative to either medication alone.
Conclusions
These data suggest the combination of lofexidine and THC warrant further testing as a potential treatment for marijuana dependence.
Keywords: Marinol, Britlofex, dependence, withdrawal, treatment, cannabinoid, norepinephrine
Marijuana use is prevalent in American society, and the number of individuals who currently meet DSM-IV criteria for cannabis abuse or dependence has steadily increased since the 1990s (Compton et al., 2004). Although marijuana is less likely to produce dependence than drugs such as nicotine, alcohol or heroin (Anthony et al., 1994), the sheer number of marijuana smokers combined with the increasing potency of marijuana has resulted in a significant number of individuals developing cannabis use disorders, i.e., approximately 1.5% of the U.S. population (Compton et al., 2004).
A number of studies have demonstrated that there is a personal (i.e., not court-mandated) demand for marijuana treatment, particularly when marijuana-specific treatment programs are offered (Roffman et al., 1988; Stephens et al., 1993; Lang et al., 2000). Treatment-seekers report distress about their marijuana use, and repeatedly fail in their attempts to quit, such that rates of marijuana relapse are comparable to those found for other drugs of abuse (Copeland et al., 2001; Stephens et al., 1994, 2000; Moore and Budney, 2003).
One factor that may contribute to high relapse rates is marijuana withdrawal. Both laboratory and clinical studies have demonstrated that over 50% of individuals who smoke marijuana repeatedly throughout the day, 6–7 days per week, experience a time-dependent set of withdrawal symptoms, including decreased food intake, stomach upset, restlessness, irritability, sleep difficulty, and craving. Symptoms of withdrawal typically emerge after 1–2 days of abstinence, and largely resolve within 10 days (Haney et al., 1999b, Boyd et al., 2002; Budney et al., 1998, 2001, 2002, 2004; Kouri and Pope, 2000; Stephens et al., 1993, 2000). In the laboratory, marijuana withdrawal is alleviated by the resumption of marijuana smoking or by THC administration, demonstrating the pharmacological specificity of withdrawal (Haney et al., 1999b, 2004; Hart et al., 2002). Clinically, marijuana smokers report using marijuana to alleviate withdrawal symptoms (Budney et al., 1999), suggesting withdrawal contributes to relapse and the maintenance of marijuana use.
Since pharmacologically treating withdrawal improves treatment outcome for nicotine dependence (see Shiffman et al., 2000) and opiate dependence (Dole, 1988), we have conducted a series of laboratory studies testing the effects of medications on symptoms of marijuana withdrawal. Maintenance on two medications, the antidepressant, bupropion (Haney et al., 2001), and the mood stabilizer, divalproex (Haney et al., 2004), significantly worsened mood during marijuana withdrawal compared to placebo, suggesting that they would not show promise as treatment approaches. Similarly, a clinical pilot trial observed poor compliance and outcome using divalproex to treat marijuana dependence (Levin et al., 2004). The antidepressant, nefazodone, which is sedating compared to the stimulant-like bupropion, decreased a subset of marijuana withdrawal symptoms (Haney et al., 2003), but the medication that most effectively attenuated marijuana withdrawal was THC (dronabinol, Marinol). THC selectively decreased ratings of anxious, miserable, trouble sleeping, chills, and marijuana craving, and reversed the large decreases in food intake during marijuana abstinence as compared to placebo, while producing no cannabinoid intoxication (Haney et al., 2004). These results have recently been replicated in an outpatient setting (Budney et al., 2007).
Yet do medications that decrease withdrawal symptoms also decrease relapse, defined as a return to marijuana use after a period of abstinence? Answering this question in the laboratory is difficult because it is not ethical to offer marijuana to individuals seeking treatment, so relapse must be modeled in marijuana smokers who are not self-motivated to abstain. Thus, prior to testing the effects of medications on relapse, we conducted a preliminary pilot study (described herein) to develop a model of marijuana relapse. We hypothesized that high financial cost would motivate marijuana smokers to abstain, thus modelling (not mimicking) clinical motivation to use less marijuana. The laboratory environment was structured so that a return to marijuana use was costly. This preliminary study tested a range of marijuana cost conditions.
Specifically, nontreatment-seeking, daily marijuana smokers (n=14), were enrolled, and after three days of marijuana abstinence, participants had the opportunity to purchase individual puffs of active marijuana (3.30% THC) throughout the day (maximum of 18 puffs/day). The first puff of marijuana self-administered each day was expensive ($10–$20) regardless of when during the day it occurred. Once the individual had ‘relapsed’ for the day, the cost of subsequent puffs was lower ($2–$4). As predicted, marijuana self-administration decreased markedly as a function of cost: from 10.8 (± 0.7) puffs/day under the $10 cost condition, to 5.5 (± 0.7) puffs/day under the $12 cost condition, to no self-administration under the $15 and 20 cost conditions. These data demonstrate that marijuana self-administration by abstinent, nontreatment-seeking, marijuana smokers varies as a function of marijuana cost.
The present objective was to use this model to determine the effect of medications on both marijuana withdrawal and relapse. The $10 cost condition was selected because it resulted in enough marijuana self-administration for a medication effect to be detected. Two medications, THC and the centrally-acting, α2-receptor agonist, lofexidine, administered alone and together, were tested. The rationale for testing lofexidine was based on preclinical data showing that withdrawal from cannabinoids results in noradrenergic hyperactivity (see Hart, 2005), and α2-receptor agonists decrease noradrenergic cell firing and release (Carter, 1997). Clonidine, a nonspecific α2-receptor agonist that attenuates certain opioid withdrawal symptoms (Uhde et al., 1980), also reverses symptoms of precipitated THC withdrawal in mice (Lichtman et al., 2001). Similarly, the decrease in precipitated withdrawal in THC-dependent mice by prostaglandin is hypothesized to be mediated by decreased noradrenergic activity (Anggadiredja et al., 2003). Lofexidine, which is approved for the treatment of opioid withdrawal in the United Kingdom (Strang et al., 1999) and is undergoing clinical trials in the U.S., has been shown to be as effective as clonidine in attenuating opioid withdrawal symptoms, but has a more favorable side effects profile regarding hypotension and sedation (Bearn et al., 1996; Lin et al., 1997; Kahn et al., 1997).
The rationale for testing THC in this model is three-fold. Although frequent administration (5 times/day) of a low THC dose (10 mg) reduces symptoms of marijuana withdrawal without producing intoxication, this dosing regimen would be unrealistic in the clinic. Thus, the first objective was to determine if less frequent administration (3 times/day) of a higher THC dose (20 mg) would be as effective at decreasing marijuana withdrawal. A second objective was to determine if significantly reducing symptoms of marijuana withdrawal decreases relapse. Finally, because lofexidine and THC have distinct mechanisms of action, a third objective was to determine the effect of combining these two medications on marijuana relapse.
Methods
Participants
Eight male research volunteers [4 Black, 3 Hispanic, 1 mixed race: mean age (± SD) = 29 ± 7 years] completed the experiment. Volunteers provided a detailed drug and medical history, received medical and psychiatric evaluations shortly before study onset, and gave written informed consent for all aspects of the study. Participants reported smoking 12.2 (± 8.1) marijuana cigarettes per day, 7.0 (± 0.0) days per week. On average, they had smoked marijuana for 12.1 (± 2.4) years. Five participants reported drinking alcohol weekly (3.8 ± 2.9 drinks/week), and six smoked tobacco cigarettes (9.3 ± 5.9 cigarettes/day), and continued to smoke throughout the experiment. Other drug use was infrequent, and urine drug screens only tested positive for cannabinoids. Participants were within accepted weight ranges for their height [77.9 ± 10.3 kg]. No participant had orthostatic blood pressure [≥ 20 mmHg decrease in SP or ≥ 10 mmHg decrease in DP from sitting to standing (Consensus Committee of the American Autonomic Society, 1996)].
Participants were instructed that the study investigated how medications influence the effects of marijuana. They were told that one of the study medications was lofexidine, and that it was not FDA-approved. Participants were not told that they would receive THC, but were told that they may receive FDA-approved antidepressants, anticonvulsants, medications to increase appetite, or placebo. They were also told that two different strength marijuana cigarettes (“Dose A” and “Dose B”), varying in their concentration of Δ9-THC, would be tested. Prior to discharge, participants were fully informed about the experimental conditions. The New York State Psychiatric Institute's Institutional Review Board approved all procedures.
Laboratory
Participants, in two groups of four, lived in a residential laboratory in the New York State Psychiatric Institute. The laboratory has four private participant rooms, a common recreational area, two single-occupancy bathrooms, two single-occupancy shower rooms, and two vestibules used for exchanging supplies (see Haney et al., 1999a). Output from a video- and audio-monitoring system terminating in an adjacent room allowed for continuous observation of participants (except while in the bathroom or in private dressing areas), but no recordings were made. Each participant’s computer was linked with a computer in the control room, allowing for a continuous on-line interaction between participants and staff, but not between participants.
Procedure
Prior to study onset, participants completed two training sessions (3–4 hr/session) on the tasks. As shown in Table 1, the study comprised four, 8-day inpatient phases, each testing the effect of a different medication maintenance condition. Each inpatient phase was separated by an 8–11 day outpatient phase, which allowed participants to return to their normal pattern of marijuana use, gave time for medication clearance, and decreased the length of continuous time inpatient. During the outpatient phase, participants were instructed to abstain from illicit drugs (excluding marijuana, for which no instructions were given). Urine toxicologies were conducted at each laboratory visit.
TABLE 1.
Representative Study Schedule
| Phase | Outpatient | Inpatient | Outpatient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12–16 |
| Times | EXP | EXP | Self-administration | |||||||||
| MJ 1000: | 0.0 | 3.3 | 3.3 | 0.0 | 0.0 | 0.0 | 3.3 | 3.3 | 3.3 | 3.3 | -- | -- |
| MJ 1130: | 0.0 | 3.3 | 3.3 | 0.0 | 0.0 | 0.0 | 3.3 | 3.3 | 3.3 | 3.3 | -- | -- |
| MJ 1300: | 0.0 | 3.3 | 3.3 | 0.0 | 0.0 | 0.0 | 3.3 | 3.3 | 3.3 | 3.3 | -- | -- |
| MJ 1430: | 0.0 | 3.3 | 3.3 | 0.0 | 0.0 | 0.0 | 3.3 | 3.3 | 3.3 | 3.3 | -- | -- |
| MJ 1600: | -- | -- | 3.3 | 0.0 | 0.0 | 0.0 | 3.3 | 3.3 | 3.3 | 3.3 | -- | -- |
| MJ 2200: | -- | -- | 3.3 | 0.0 | 0.0 | 0.0 | 3.3 | 3.3 | 3.3 | 3.3 | -- | -- |
| Medication (mg/dose) | ||||||||||||
| Placebo | -- | -- | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -- |
| Lofex: | -- | -- | 0 | .4 | .6 | .6 | .6 | .6 | .6 | .4 | .2 | -- |
| THC: | -- | -- | 0 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 10 | -- |
| Lofex+THC: | -- | -- | 0 | .4, 20 | .6, 20 | .6, 20 | .6, 20 | .6, 20 | .6, 20 | .4, 20 | .2,10 | -- |
MJ: marijuana cigarette strength (% THC). EXP: 3 puffs of marijuana were experimenter-administered at each time point for no cost. Self-administration: individual marijuana puffs available for purchase. Lofex = lofexidine, administered at 0830, 1300, 1730, 2200. THC: administered at 0900, 1430, 2200; the order of medication conditions were counter-balanced between and within-groups.
Immediately prior to each of the four inpatient stays, participants came to the laboratory for two, 5-hour, marijuana sample sessions to familiarize them with Dose A and Dose B. In one sample session, participants smoked an active marijuana cigarette (3.30% THC: labeled “Dose A”) four times, and in the other session, participants smoked a placebo marijuana cigarette (0.00% THC: labeled “Dose B”) four times using smoking procedures described below. They were told that the strength of Dose A and Dose B would not change throughout the study, and that they should pay attention to how each dose made them feel as they would later make decisions regarding their self-administration. Sample sessions were repeated prior to each inpatient phase so that if the medications altered marijuana’s direct effects in one inpatient phase, the decision to self-administer marijuana in subsequent inpatient phases would not be affected.
Participants moved into the laboratory after the second marijuana sample session. Beginning at 0815 each morning after move-in, participants completed a 7-item visual analog scale (VAS) sleep questionnaire (see Haney et al., 2004), and a 44-item VAS measuring a range of moods and physical symptoms, and were then weighed. The first of six, 30-min task batteries, comprising five performance measures (described below) and the VAS began at 0915. Participants completed two task batteries from 1015–1145, each consisting of the same five tasks and the VAS. The recreation area was available from 1215–1245. Four task batteries were completed from 1315–1645, and then the recreation area became available again at 1700. Two films were shown each evening. A 6-item Drug-effect Questionnaire (DEQ: Evans et al., 1995) was completed at 1830 (60 min after the 1730 lofexidine capsule). At 2155, the recreation area was no longer available. At 2330, a second DEQ was completed (90 min after the 2200 THC capsule), and participants were given $50 in ‘play money’ representing a portion of their daily study earnings. Each participant stored their money in their own lockbox and locked cabinet in the vestibule. Lights were turned off by 2400.
Capsule Administration
Lofexidine and THC capsule administration was double-blind and counter-balanced across participants. Both medications were packaged into size 00 opaque capsules with lactose filler by the New York State Psychiatric Institute Research Pharmacy. Capsules were administered 7 times per inpatient day, but the content of the capsules varied across medication conditions. Lofexidine (Britlofex®: 0.0, 0.4, 0.6 mg), provided by US WorldMeds, was administered at 0830, 1300, 1730, 2200. Doses of lofexidine can be initiated without prolonged dose build-up (Bearn et al., 1998; Yu et al., 2000), so participants took 1.6 mg/day on the first day and began 2.4 mg/day dosing on the second day. Blood pressure was taken twice prior to each lofexidine administration: after participants had been seated for 3 min, and then after they had been standing for 1 min. Lofexidine capsules were not administered if: (1) seated SP was under 90 mmHg, (2) SP decreased by ≥ 20 mmHg upon standing, or if (3) DP decreased by ≥ 10 mmHg upon standing. Participants who missed more than two dosings/day on two study days would have been discharged, but none did.
Oral THC (Marinol®: 0, 20 mg) capsules, purchased from Unimed Pharmaceuticals (Marietta, GA), were administered at 0900, 1430 and 2000. In marijuana smokers, 20–30 mg given 4 times/day produced intoxication, while 10 mg given 5 times/day decreased symptoms of marijuana withdrawal without producing intoxication (Haney et al., 1999a, 2004). A study objective was to decrease the number of capsule administrations per day from five to three.
Doses for both medications were titrated down over one day.
Marijuana Administration
Participants each received a marijuana cigarette (provided by the National Institute on Drug Abuse) at each smoking occasion. Marijuana was administered using a cued-smoking procedure (Foltin et al., 1987). Colored lights mounted on the ceiling of the recreation area signaled 'light the cigarette' (30 sec), 'get ready' (5 sec), 'inhale' (5 sec), 'hold smoke in lungs' (10 sec) and 'exhale.' Participants smoked three puffs in this manner, with a 40-sec interval between each puff. Cigarettes were rolled at the ends and were smoked through a cigarette holder so the marijuana was not visible. Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24h prior to use.
Marijuana was either experimenter-administered at no cost, or was available to purchase for self-administration; participants were not informed which condition it was until 0950 each morning. During the first inpatient day (day 3), participants received placebo capsules and smoked experimenter-administered, active marijuana (Dose A) six times throughout the day (Table 1). The purpose of this day was to standardize marijuana exposure prior to the onset of abstinence. On the subsequent three inpatient days (days 4–6), Dose B (placebo marijuana) was available for self-administration, followed by four days (days 7–10) when Dose A was available for self-administration. The three days of Dose B availability enforced marijuana abstinence whether participants chose to self-administer or not. Self-administration of Dose A following these three days of abstinence was the measure of ‘relapse.’
During self-administration days, participants had six opportunities throughout the day to purchase 0, 1, 2, or 3 puffs of the available dose using their study earnings. The cost was $10 for the first puff of the day, and $3 for all subsequent puffs; if all puffs were purchased on a given day, the cost was $61. Individuals who chose to smoke marijuana went to a vestibule alone, took out the appropriate amount of money from their lockbox, then smoked the number of puffs purchased using the cued-puffing procedures. Participants who did not choose to smoke were still required to sit in the vestibule for 2 minutes so that the other participants would not know if they had purchased marijuana.
Task Battery
Each task battery consisted of a 3-min digit-symbol substitution task (DSST), a 3-min repeated acquisition task, a 10-min divided attention task (DAT), a 10-min rapid information task (RIT), an immediate and delayed digit-recall task, and the 44-item VAS. The battery measures aspects of learning, memory, vigilance, and psychomotor ability (see Foltin et al., 1996). Participants were instructed to complete each task as quickly and as accurately as possible, and to complete the items on the VAS based on how they were feeling at that moment.
Social Behavior
A computerized observation program was used to categorically record behavior every 2.5 min during each evening recreation period. Behaviors were divided into two categories: private and social. Private behaviors occurred in each participant’s room or in the bathroom or shower. Social behaviors occurred in the recreation area, and were categorized as being either verbal or nonverbal. Participants were aware that they were being observed during the recreational period.
Food
At 0815 each morning, participants received a box of food containing meal items, snacks and beverages to be consumed at any time. Frozen meal items were available by request. Additional units of any item were freely available. Participants were instructed to scan custom-designed bar codes whenever they ate or drank, specifying substance and portion. At 2330, participants returned their food box to a staff member. Food was not available between 2330 and 0815.
Sleep
Each night, participants wore the Nightcap® sleep monitoring system (Respironics, Atlanta, GA), consisting of a portable amplifier attached to two leads with adhesive electrodes. One lead attached to the forehead to measure body movement, and the other lead attached to the eyelid to measure eye movement. The Nightcap® was turned on at midnight, when lights were turned off and participants were required to remain in bed. Measures include sleep onset latency and total sleep time. The Nightcap® has been validated using traditional polysomnographic measurement (Ajilore et al., 1995).
Tobacco cigarette smoking
The number of tobacco cigarettes smoked was recorded each evening by counting the remaining cigarette butts in each participant’s ashtray. Participants were instructed not to share cigarettes or to throw out cigarette butts, and were monitored to prevent these events from occurring.
Data Analysis
Repeated measures analyses of variance (ANOVA) with planned comparisons were used to determine the effect of each medication condition on marijuana withdrawal and relapse. Behavioral outcomes included: the amount of money spent to purchase marijuana, mood (peak VAS ratings), capsule effects (peak DEQ ratings), marijuana craving (mean VAS ratings), task performance, social behavior (time spent in private or interacting with other participants), number of cigarettes per day, objective sleep measures (total time sleeping, sleep onset latency), subjective sleep measures (sleep questionnaire), food intake (total energy intake, percent macronutrient), and body weight. There were two within-group factors: medication condition (placebo, THC, lofexidine, THC/lofexidine) and inpatient day. Three planned comparisons assessed if there was a medication effect: (1) during marijuana withdrawal (THC, lofexidine, THC/lofexidine were each compared to placebo during days 2 and 3 of withdrawal), and (2) on relapse (THC, lofexidine, THC/lofexidine were each compared to placebo during the 4 days of active marijuana availability). Results were considered statistically significant at p values < 0.05. Huynh-Feldt corrections were used, when appropriate.
Results
Withdrawal (Inpatient Days 5–6)
Subjective-Effects Rating
Figure 1 and 2, and Table 2, which portray ratings during marijuana abstinence on the VAS and DEQ scales as a function of medication condition, show that all three medication conditions decreased ratings of restless and chills compared to placebo. THC alone significantly increased ratings of good drug effect, mellow, irritable (Fig. 1), and talkative (Table 2). Lofexidine alone increased ratings of sedated (Fig. 1), while decreasing ratings of upset stomach (Table 2) compared to placebo. THC/lofexidine increased ratings of good drug effect and sedated, while decreasing ratings of marijuana craving (Fig. 2), upset stomach, social, talkative, and cigarette craving (Table 2).
Figure 1.
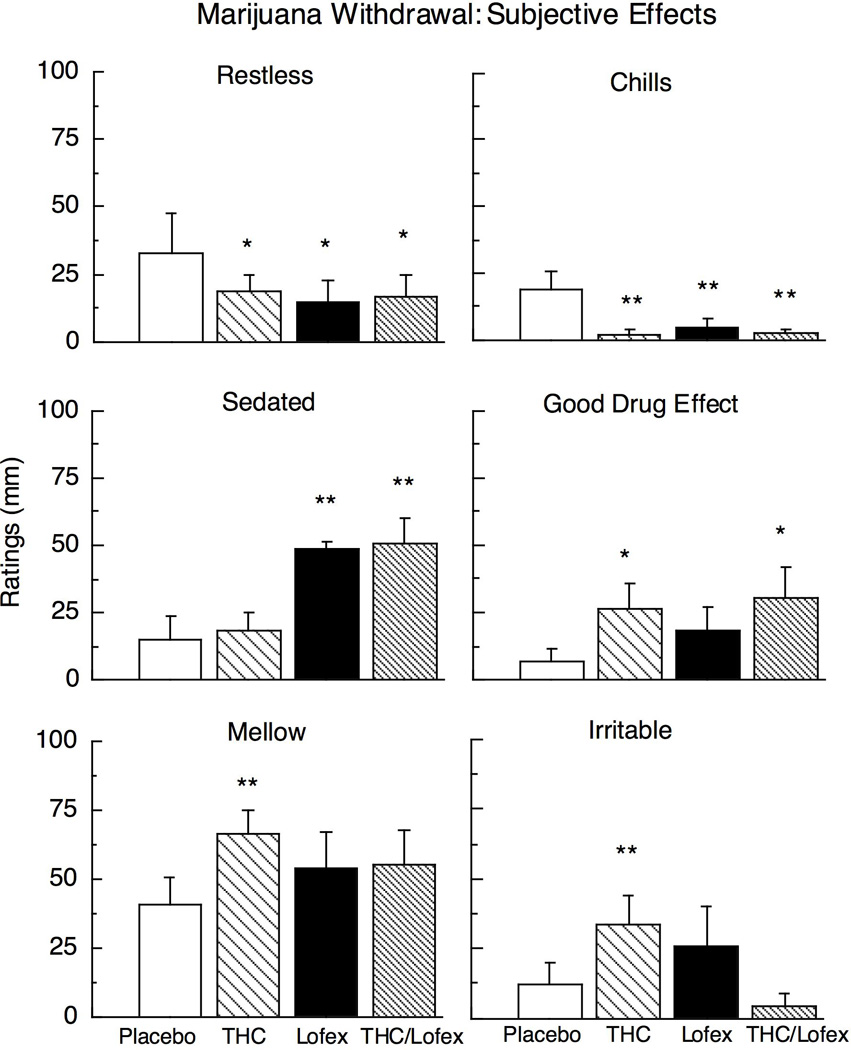
Selected peak subjective-effect ratings as a function of THC (60 mg/day), lofexidine (2.4 mg/day) and the THC (60 mg) and lofexidine (2.4 mg/day) combination during marijuana abstinence (inpatient days 5–6); maximum score = 100 mm. Asterisks indicate a significant difference between medication and placebo (* p < 0.05; ** p < 0.01). Error bars represent ± standard error of the mean (SEM).
Figure 2.
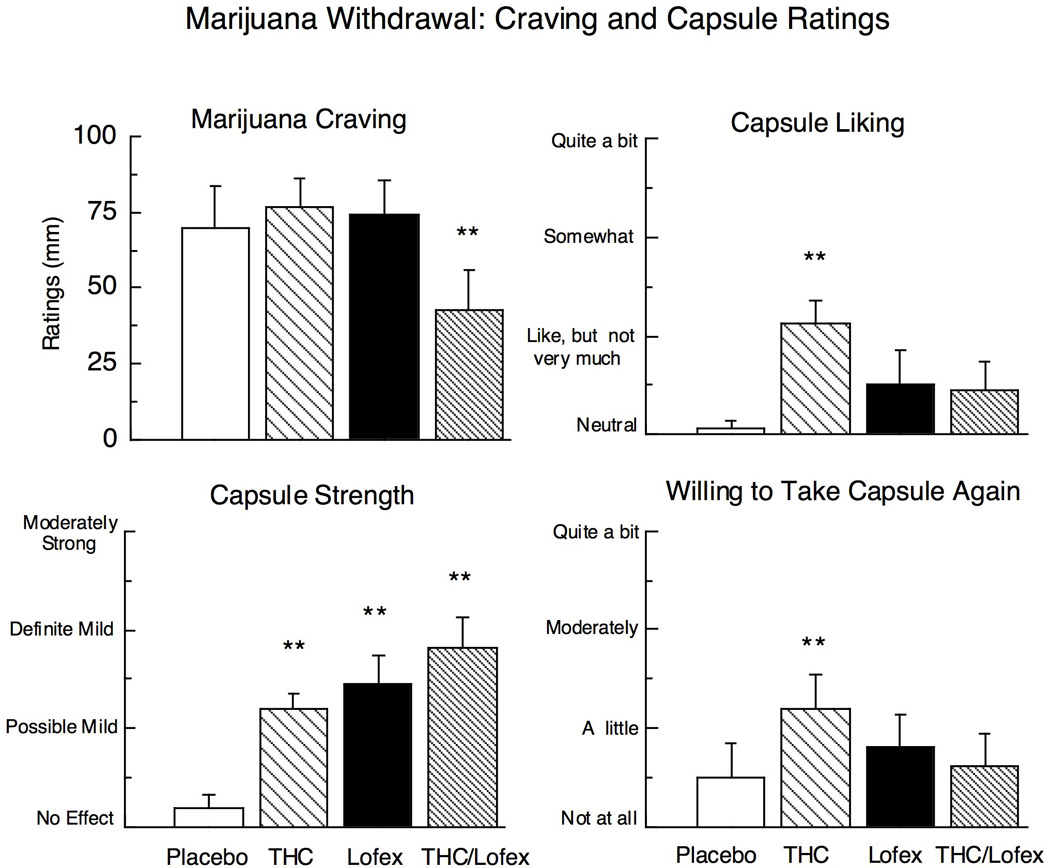
Selected peak ratings on the Drug-effects Questionnaire and ratings of marijuana craving averaged across the session as a function of THC (60 mg/day), lofexidine (2.4 mg/day) and the THC (60 mg) and lofexidine (2.4 mg/day) combination during marijuana abstinence (inpatient days 5–6). Asterisks indicate a significant difference between medication and placebo (* p < 0.05; ** p < 0.01). Error bars represent ± standard error of the mean (SEM).
TABLE 2.
Selected mean (± SEM) ratings during marijuana abstinence as a function of medication condition
| Placebo | THC | Lofexidine | THC/Lofexidine | ||
|---|---|---|---|---|---|
| Subjective Effects (VAS) | |||||
| Upset Stomach | 10 (7) | 5 (5) | ⇓ 0 (0)* | ⇓ 1 (1)* | |
| Social | 74 (8) | 75 (5) | 62 (12) | ⇓ 48 (14)** | |
| Talkative | 51 (13) | ⇑62 (7)* | 53 (14) | ⇓ 39 (15)* | |
| Cigarette Craving | 65 (12) | 70 (10) | 57 (11) | ⇓ 47 (10)* | |
| Performance Tasks | |||||
| RIT (hits/target) | 84.4 (6.1) | ⇑ 91.7 (2.6)** | ⇑ 92.0 (2.7)** | ⇑ 89.8 (3.3)* | |
| DAT: False Alarms | 4.4 (1.3) | 5.9 (3.8) | ⇑ 9.2 (5.9)* | 5.4 (2.2) | |
| RA: Total Errors | 50.9 (8.5) | 53.2 (7.5) | ⇑ 58.5 (9.3)* | 47.9 (8.1) | |
| Social Behavior | |||||
| Time spent talking (min) | 106.9 (22.7) | 22.7) ⇓ 47.8 (5.8)** | ⇓ 79.7 (23.1)* | ⇓ 26.1 (8.3)** | |
| Blood Pressure | |||||
| Diastolic (seated) | 78.7 (4.3) | ⇓ 75.3 (3.0)** | ⇓ 69.9 (3.2)** | ⇓ 65.8 (2.7)** | |
| Diastolic (standing) | 85.8 (3.8) | ⇓ 81.9 (3.0)** | ⇓ 75.4 (3.3)** | ⇓ 71.9 (2.9)** | |
| Systolic (seated) | 130.4 (3.0) | ⇓ 126.1 (2.1)** | ⇓ 119.7 (3.2)** | ⇓ 14.8 (2.2)** | |
| Systolic (standing) | 135.6 (3.0) | ⇓ 129.1 (2.4)** | ⇓ 20.6 (3.4)** | ⇓ 116.3 (3.8)** | |
Note Peak ratings (0–100 mm, visual analog scale) averaged over 2–3 days of marijuana abstinence. Arrows indicate the direction of the drug effect.
Asterisks represent significant differences between placebo and medication dose condition; * p < 0.05, ** p < 0.01.
Peak ratings of capsule strength were significantly increased by all three medication conditions. THC alone also increased ratings of capsule liking and willingness to take the capsule again as compared to placebo (Fig. 2).
Observer Ratings
There were no significant medication effects on objective ratings of marijuana withdrawal.
Objective and Subjective Sleep Measures
Figure 3 portrays objective and subjective sleep data during marijuana abstinence as a function of medication condition. Compared to placebo, lofexidine and THC/lofexidine significantly increased the percentage of time participants spent sleeping and increased ratings of “Fell asleep easily.” Objective latency to fall asleep was significantly increased by THC alone and decreased by THC/lofexidine. THC/lofexidine also significantly increased ratings of sleep satisfaction compared to placebo.
Figure 3.

Objective and subjective measures of sleep, and daily caloric intake during marijuana abstinence as a function of THC (60 mg/day), lofexidine (2.4 mg/day) and the THC (60 mg) and lofexidine (2.4 mg/day) combination (inpatient days 5–6). Asterisks indicate a significant difference between medication and placebo (* p < 0.05; ** p < 0.01). Error bars represent ± standard error of the mean (SEM).
Food Intake and Body weight
Total daily caloric intake during marijuana abstinence, shown in Figure 3, was significantly increased by THC, and significantly decreased by lofexidine as compared to placebo. Lofexidine also altered the pattern of macronutrient intake. Under lofexidine conditions, participants derived a significantly lower proportion of daily calories from fat (27.8 ± 1.9%) compared to placebo medication (31.3 ± 2.0%), while consuming a higher proportion of carbohydrates (placebo: 55.4 ± 2.2%, lofexidine: 59.3 ± 1.9%).
The medication effects on caloric intake were reflected by changes in body weight during marijuana abstinence. For each medication condition, body weight on the morning of the first day of marijuana abstinence (prior to active medication administration), was compared to body weight after three days of marijuana abstinence. After three days of abstinence, participants lost 1.8 kg under placebo conditions (p < 0.001) and 1.2 kg under lofexidine conditions (p < .002). Weight did not significantly change when participants were maintained on THC or THC/lofexidine.
Performance Effects
Table 2 demonstrates that each medication condition improved performance on the rapid information task during marijuana abstinence by increasing the number of responses for three consecutive odd or even numbers. There were few other effects of medication condition on task performance overall. Lofexidine worsened performance on the DAT (divided attention task) by increasing responding for targets that were not there. Lofexidine also worsened performance on the Repeated Acquisition task by increasing the number of sequences entered incorrectly.
Tobacco cigarette smoking
Under placebo medication conditions, the six participants who smoked tobacco cigarettes averaged 9.6 ± 1.3 cigarettes per day during marijuana abstinence. The THC/lofexidine condition significantly decreased the number of cigarettes smoked per day to 7.4 ± 1.0.
Social Behavior
The amount of time participants spent in private or with at least one other person in the recreation area did not vary as a function of medication condition during marijuana abstinence. However, Table 2 demonstrates that the mean amount of time participants spent talking in the recreation area during the 300-minute recreation period was significantly decreased by each medication condition compared to placebo.
Blood pressure
Table 2 shows that all three medication conditions significantly decreased systolic and diastolic blood pressure (both while participants were seated, and then after standing for 1 minute) during marijuana abstinence as compared to placebo. Three participants missed 1–2 capsule administrations over the course of the study. Two participants had orthostatic hypotension on 1–2 occasions, and one participant had elevated systolic pressure on 2 occasions.
Relapse (Inpatient Days 7–10)
Figure 4 portrays the mean amount of money participants spent to self-administer marijuana following the 3-day period of marijuana abstinence. Both lofexidine alone (p < 0.03) and THC/lofexidine (p < 0.004) significantly decreased marijuana self-administration compared to placebo. The percentage of volunteers who went four consecutive days without relapsing to any marijuana use was 25% under placebo, THC, and lofexidine conditions but doubled to 50% during THC/lofexidine maintenance. Self-administration of marijuana Dose B (0.00% THC) was infrequent and did not vary significantly as a function of medication condition.
Figure 4.

Average amount of money spent on marijuana self-administration during the four days following a 3-day period of marijuana abstinence (inpatient days 7–10) as a function of THC (60 mg/day), lofexidine (2.4 mg/day) and the THC (60 mg) and lofexidine (2.4 mg/day) combination. Asterisks indicate a significant difference between medication and placebo (* p < 0.05; ** p < 0.01). Error bars represent ± standard error of the mean (SEM).
Discussion
This study evaluated the effects of three medication regimens on a range of behaviors during abstinence from marijuana (withdrawal), followed by a period in which marijuana was available for self-administration (relapse) in marijuana-dependent research volunteers. The results, summarized in Table 3, demonstrate that this dose regimen of THC (20 mg tid) reversed the anorexia and weight loss associated with marijuana withdrawal, but only decreased a small subset of marijuana withdrawal symptoms. THC also increased the latency to fall asleep, and did not decrease marijuana relapse. The lofexidine dose regimen (0.6 mg qid) was sedating, worsened abstinence-related anorexia and weight loss, and also did not robustly attenuate the mood symptoms of marijuana withdrawal. Lofexidine did, however, significantly improve objective and subjective sleep measures during withdrawal, and decreased marijuana relapse compared to placebo. The combination of THC and lofexidine was also sedating, but decreased a broad range of withdrawal symptoms, including marijuana craving. This medication combination also improved objective and subjective sleep measures during marijuana abstinence, and decreased marijuana relapse compared to placebo. In fact, half the participants went all 4 days without smoking active marijuana when maintained on THC and lofexidine (compared to 25% of participants under placebo conditions). Overall, the combination of lofexidine and THC produced the most robust medication effects on sleep, marijuana craving and relapse relative to either medication alone.
TABLE 3.
Summary of Results
| THC | Lofexidine | THC/Lofexidine | |
|---|---|---|---|
| Marijuana Withdrawal |
|
|
|
|
|
|
|
| |||
| Marijuana Relapse | No Change | Decrease | Decrease |
Based on the literature (Haney et al., 2004; Budney et al., 2007), we had predicted that THC would decrease relapse by reducing the negative reinforcing effects of marijuana, i.e., a return to marijuana use to alleviate symptoms of withdrawal. However, under the present dose regimen, THC instead produced a mild but significant intoxication (good drug effect, mellow, capsule liking, increased willingness to take capsule again) without reducing marijuana craving, or the mood and sleep symptoms associated with marijuana withdrawal. Correspondingly, THC did not decrease marijuana relapse. Our earlier study, testing a lower THC dose (10 mg) administered frequently (5 times) throughout the day, produced no intoxication and attenuated a range of withdrawal symptoms, including marijuana craving (Haney et al., 2004). Although this might suggest that lower, more frequent doses of THC would be most effective, others have shown that in an outpatient setting, among participants who were selected because they manifested symptoms of marijuana withdrawal, higher doses of THC (30 mg tid) decreased marijuana withdrawal symptoms during marijuana abstinence (Budney et al., 2007). Thus, further research is needed to replicate the current findings, and to characterize the factors producing the most robust attenuation of marijuana withdrawal by THC, e.g., dose, frequency of dose administration, participant characteristics.
Lofexidine, when given alone, also did not decrease marijuana craving, or most mood symptoms of marijuana withdrawal but lofexidine did improve sleep during marijuana abstinence and decreased marijuana relapse. Disrupted sleep is a consistent symptom of marijuana withdrawal (Haney et al., 2003, 2004; Budney et al., 2001), and lofexidine significantly increased how long participants slept and how easily they perceived falling asleep during abstinence. Combining THC and lofexidine further improved sleep and decreased relapse. In opioid-dependent patients undergoing withdrawal, lofexidine improved self-report ratings of sleep initiation and maintenance compared to methadone, and improvements in sleep appeared to predict retention in treatment (Bestwick et al., 2003). Similarly, in alcohol-dependent patients, sleep onset latency predicted relapse to alcohol use (Brower et al., 1998). Thus, it may be that improvements in sleep are essential to decreasing relapse to marijuana use.
The mechanism for lofexidine’s utility for the treatment of opioid withdrawal is presumed to involve decreased norephinephrine release. Opioid withdrawal is associated with noradrenergic hyperactivity, and alpha-2-adrenergic receptor agonists decrease both norepinephrine release and the primarily physical symptoms of opioid withdrawal (see Herman and O’Brien, 1997). Recently, a clinical study with opioid-dependent patients maintained on naltrexone showed that lofexidine (at twice the daily dose used presently) decreased laboratory measures of stress- and drug cue-induced craving and decreased opioid relapse compared to placebo (Sinha et al., 2007). There is also an extensive preclinical literature showing that lofexidine decreases stress-induced drug-seeking behavior (see Shaham et al., 2003). Given that preclinical studies show that cannabis withdrawal, like opioid withdrawal, is associated with noradrenergic hyperactivity (see Introduction), lofexidine may decrease marijuana relapse in the laboratory by the same mechanism as it decreases opioid relapse: by reducing withdrawal-related noradrenergic hyperactivity.
Similar to its effects on marijuana craving and relapse, the combination of THC and lofexidine also decreased cigarette craving and cigarette smoking during marijuana withdrawal. THC/lofexidine had this effect even though participants were not trying to cut down on cigarettes, and when there were no contingencies to discourage cigarette smoking. Neither medication alone had this effect. Although THC decreases nicotine withdrawal in mice (Balerio et al., 2004), smoking behavior during marijuana abstinence was not decreased by THC either in this study or in our earlier study (Haney et al., 2004). Clonidine appears to decrease smoking in clinical trials compared to placebo, but side effects decrease clonidine’s potential to treat nicotine dependence (see Covey et al., 2000). These data suggest that lofexidine might be further explored for smoking cessation.
Lofexidine, either alone or with THC, decreased blood pressure, but rarely produced clinically significant hypotension, consistent with findings in opioid-dependent patients (Bearn et al., 1998). Lofexidine, alone or in combination with THC, also substantially increased ratings of sedation, but again, the effect did not appear to be aversive. Participants reported that lofexidine produced a mild effect, which they did not particularly like or dislike. In fact, certain positive ratings of THC capsules were reduced when lofexidine was combined with THC. Lofexidine alone also decreased food intake in abstinent marijuana smokers, and shifted the balance of macronutrient intake from fat to carbohydrates.
There were few significant medication effects on psychomotor task performance. All three medication conditions improved performance on the rapid information task compared to placebo. Lofexidine alone significantly increased the number of errors made on a repeated acquisition and divided attention task. These impairments did not occur if lofexidine was given in combination with THC.
There are several issues to consider with the present design. First, given the study length and range of conditions, only one dose of each medication was assessed, limiting our conclusions to the doses selected. Second, the sample was small and not broadly diverse, as there were no women or non-Hispanic Caucasians enrolled, further limiting the generalizability of the conclusions. Third, the study objective was to compare medication effects, so there was no baseline condition to compare to marijuana abstinence to demonstrate withdrawal. Not all marijuana smokers endorse symptoms of withdrawal, and in fact only half of those currently enrolled demonstrated the time-dependent increase in ratings of irritability and restlessness that define marijuana withdrawal. Some studies have dealt with this variability by only analyzing medication effects in participants who demonstrate withdrawal symptoms (e.g., Budney et al., 2001, 2007). An alternative strategy, adopted herein, is to include all participants. A recent study reported that a large percentage (60%) of daily cigarette smokers (≥ 10 cigarettes/day) also do not report experiencing symptoms of nicotine withdrawal (Donny and Dierker, 2007). Yet studies developing medications to treat nicotine dependence do not typically distinguish among those who do and those who do not experience withdrawal. Thus, our conclusions about the effects of THC and lofexidine during marijuana abstinence are based on daily marijuana smokers whether they report withdrawal or not. Importantly, the overall pattern of medication effects described above was the same when only those endorsing withdrawal were analyzed, e.g., THC increased irritability in those undergoing withdrawal as it did for the group as a whole.
To conclude, we have developed a laboratory procedure to assess the effects of medications on factors that likely contribute to marijuana relapse. Medications with distinct mechanisms of action were tested: a cannabinoid agonist and a medication to decrease norepinephrine release. The combination of THC and lofexidine was most effective in reducing symptoms of marijuana withdrawal and shifting choice away from marijuana and toward saving money when the opportunity to relapse was presented. Lofexidine alone decreased relapse, but without an apparent improvement in mood during marijuana abstinence. Lofexidine did, however, improve sleep during marijuana abstinence, suggesting that improving sleep, and perhaps decreasing a stress response during marijuana abstinence decreases relapse. Although the model overall cannot be validated in lieu of clinical data with these medications in treatment seekers, the present results suggest that a future dose-ranging study of THC and lofexidine combinations is warranted.
Acknowledgment
The U.S. National Institute on Drug Abuse (NIDA) supported this research (DA19239). We also thank NIDA for supplying the marijuana cigarettes and US World Meds for supplying the lofexidine. We are grateful to Brooke Roe, Diana Paksarian, Michael Rubin, and Liah Barnett for their superb assistance in data collection.
References
- Ajilore O, Stickgold R, Rittenhouse CD, Hobson JA. Nightcap: laboratory and homebased evaluation of a portable sleep monitor. Psychophysiology. 1995;32:92–98. doi: 10.1111/j.1469-8986.1995.tb03410.x. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Yamaguchi T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Prostaglandin E2 attenuates SR141716A-precipitated withdrawal in tetrahydrocannabinol-dependent mice. Brain Res. 2003;966:47–53. doi: 10.1016/s0006-8993(02)04169-0. [DOI] [PubMed] [Google Scholar]
- Anthony JC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- Bearn J, Gossop M, Srang J. Accelerated lofexidine treatment regimen compared with conventional lofexidine and methadone treatment for inpatient opiate detoxification. Drug Alcohol Depend. 1998;50:227–232. doi: 10.1016/s0376-8716(98)00030-1. [DOI] [PubMed] [Google Scholar]
- Bearn J, Gossop M, Strang J. Randomized double-blind comparison of lofexidine and methadone in the in-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1996;43:87–91. doi: 10.1016/s0376-8716(96)01289-6. [DOI] [PubMed] [Google Scholar]
- Beswick T, Best D, Rees S, Bearn J, Gossop M, Strang J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: differences between methadone and lofexidine detoxification treatments. Addict Biol. 2003;8:49–57. doi: 10.1080/1355621031000069882. [DOI] [PubMed] [Google Scholar]
- Boyd S, Gorelick D, Huestis M, Heishman S, Dermand JC, Nides MA, et al. Prevalence and persistence of withdrawal symptoms reported by a non-treatment sample of marijuana smokers. Drug Alcohol Depend. 2002;66:S19. [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RA, Hughes JR. Onset, magnitude, and duration of abstinence effects following heavy marijuana use. Drug Alcohol Depend. 2002;66:S23. [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1321. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. Adults seeking treatment for marijuana dependence: A comparison with cocaine-dependent treatment seekers. Exp Clin Psychopharmacology. 1998;6:419–426. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9 tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Carter AJ. Hippocampal noradrenaline release in awake, freely moving rats is regulated by alpha-2 adrenoceptors but not by adenosine receptors. J Pharmacol Exp Ther. 1997;281:648–654. [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treatment. 2001;21:55–64. doi: 10.1016/s0740-5472(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Covey LS, Sullivan MA, Johnston JA, Glassman AH, Robinson MD, Adams DP. Advances in non-nicotine pharmacotherapy for smoking cessation. Drugs. 2000;59:17–31. doi: 10.2165/00003495-200059010-00003. [DOI] [PubMed] [Google Scholar]
- Dole VP. Implications of methadone maintenance on theories of narcotic addiction. JAMA. 1988;260:3025–3029. [PubMed] [Google Scholar]
- Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug Alcohol Depend. 2007;89:93–96. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, Levin FR, Fischman MW. Behavioral and subjective effects of DN-2327 (pazinaclone) and alprazolam in normal volunteers. Behav Pharmcol. 1995;6:176–186. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behavior. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Comer SD, Fischman MW. Effects of fenfluramine in food intake, mood, and performance of humans living in a residential laboratory. Physiol Behav. 1996;59:295–305. doi: 10.1016/0031-9384(95)02098-5. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or Divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology. 2003;165:157–165. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999a;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999b;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Hart CL. Increasing treatment options for cannabis dependence: a review of potential pharmacotherapies. Drug Alcohol Depend. 2005;80:147–159. doi: 10.1016/j.drugalcdep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Hart C, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral d9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Herman BH, O’Brien CP. Clinical medications development for opiate addiction: focus on nonopioids and opioid antagonists for the amelioration of opiate withdrawal symptoms and relapse prevention. Semin Neurosci. 1997;9:158–172. [Google Scholar]
- Kahn A, Mumford JP, Rogers GA, Beckford H. Double-blind study of lofexidine and clonidine in the detoxification of opiate addicts in hospital. Drug Alcohol Depend. 1997;44:57–61. doi: 10.1016/s0376-8716(96)01316-6. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharm. 2000;8:483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Lang E, Engelander M, Brooke T. Report of an integrated brief intervention with self-defined problem cannabis users. J Subst Abuse Treat. 2000;19:111–116. doi: 10.1016/s0740-5472(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, et al. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav. 2001;69:181–188. doi: 10.1016/s0091-3057(01)00514-7. [DOI] [PubMed] [Google Scholar]
- Lin SK, Strang J, Su LW, Tsai CJ, Hu WH. Double-blind randomised controlled trial of lofexidine versus clonidine in the treatment of heroin withdrawal. Drug Alcohol Depend. 1997;48:127–133. doi: 10.1016/s0376-8716(97)00116-6. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. J Subst Abuse Treat. 2003;25:85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Roffman RA, Stephens RS, Simpson EE, Whitaker DL. Treatment of marijuana dependence: Preliminary results. J Psychoactive Drugs. 1988;20:129–137. doi: 10.1080/02791072.1988.10524382. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Extended versus brief treatment for marijuana use. J Consult Clin Psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA and Simpson EE. Adult marijuana users seeking treatment. J Consult Clin Psychology. 1993;61:1100–1104. doi: 10.1037//0022-006x.61.6.1100. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychology. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Strang J, Bearn J, Gossop M. Lofexidine for opiate detoxification: review of recent randomized and open controlled trials. Am J Addict. 1999;8:337–348. doi: 10.1080/105504999305749. [DOI] [PubMed] [Google Scholar]
- Uhde TW, Redmond DE, Jr, Kleber HD. Clonidine suppresses the opioid abstinence syndrome without clonidine-withdrawal symptoms: a blind inpatient study. Psychiatry Res. 1980;2:37–47. doi: 10.1016/0165-1781(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Yu E, Herman BH, Miotto K, Montgomery A, Fudala PJ, Fisher C, et al. In-patient safety evaluation of lofexidine, an alpha-2 adrenergic agonist, as a medication for opiate withdrawal. NIDA Research Monograph. 2000;180:227. [Google Scholar]


