Abstract
Triple negative breast cancer (TNBC) constitutes 10–25% of patients with breast cancer. TNBC is an aggressive phenotype affecting younger age groups and has poor prognosis. We retrospectively analysed 50 triple negative breast cancer patients attending our outpatient department among 270 breast cancer patients. The incidence of TNBC was 18.5%, and most of them were premenopausal 56% (28/50) with mean age was 46.66 ± 13.87 (Range 28–72 years). Most of them had Invasive ductal cancer 94% (47/50) and were high grade (Grade 3−96%)(48/50). Five patients presented with metastatic disease (2 patients only Skeletal, 1 patient with Skeletal and Lung, 1 patient with Lung and 1 patient with Liver) and 7 patients developed recurrence (all 7 had chest wall recurrence, 3 had supraclavicular lymph node recurrence, 2 had skeletal metastases and 1 had developed brain metastases) during follow up. The mean disease free survival was 15 months (Range 3–58 months) and overall survival was 20.14 months (Range 5–70 months). Fifty six percent (28/50) of patients were premenopausal and mean age of presentation was 46.66 ± 13.87 years (Range 28–72 years). Ten percent (5/50) presented with metastatic disease and 15% (7/45) developed metastases during follow up. Five patients (10%) died during follow up. Hence, Triple negative breast cancer is aggressive, with rapid progression leading to mortality in younger patients.
Keywords: Triple negative breast cancer, Basal like breast cancer, Breast cancer
Introduction
With development of various technologies, and with collaboration of pathology and genetics, breast cancer is now considered as a heterogeneous disease with different morphological features and clinical behaviour. Hence, nowadays Immunohistochemistry, microarray techniques and cytogenetics are necessary for exact diagnosis, better prognostication and for application of newer modalities of treatment. Perou, Surlie et al. classified breast cancer into 5 intrinsic subtypes based on cDNA (complementary DNA) microarray studies: Luminal A, Luminal B, Normal breast like, Her2 and basal like breast cancer (Table 1) [1–4]. But there is no standard classification of these subtypes and newer subtypes are been described recently [5]. These classifications are based on considering that there are two types of epithelial cells in human mammary gland- luminal and basal (and/or myoepithelial) cells. These two cell types can be distinguished immunohistochemistrically that luminal cells express ER , PR receptors and they are positive for keratins 8/18, whereas basal cells are positive for keratins 5/6, and 17 [6, 7].
Table 1.
Microarray classification of breast cancer
| Subtypes | ER, PgR, Her 2 Status | Other IHC features | Cell of origin | Other characteristics |
|---|---|---|---|---|
| Luminal A | ER + or PgR + or both, Her 2- | •Keratin 8/18 + ve | Luminal epithelial cell | •Younger age |
| •Best prognosis | ||||
| •Low rates of recurrence | ||||
| •Higher survival rate | ||||
| Luminal B | ER + or PgR + or both, Her 2+ | •Keratin 8/18 + ve | Luminal epithelial cell | •Higher tumor grade |
| •Poorer prognosis | ||||
| Basal like | ER-, PgR-, Her2-/+ | •Keratin 5/6/17 + ve | Basal/ myoepithelial cell/Bipotent progenitor | •15% |
| •EGFR + ve | •Younger age | |||
| •Associated with hereditary BRCA 1 | ||||
| •Poorer prognosis compared to other types | ||||
| •Spread to axillary nodes, less common to bones | ||||
| Her 2+ | ER-, PgR-, Her2+ | – | Late luminal progenitor | •20–25% |
| •Poorer grade | ||||
| •Lymph nodes positive | ||||
| •Early distant metastases | ||||
| •Poor prognosis | ||||
| •Frequent relapse | ||||
| Normal breast like | Tumors that do not fill into any of these categories | – | Luminal epithelial cell | •6–10% of all breast cancers |
| • Small tumors | ||||
| •Good prognosis | ||||
| •More common in postmenopausal | ||||
| •Associated with fibroadenomas | ||||
| Claudin low | ER-, PR-, Her2- | •Mesenchymal markers | Stem cell | •5–10% of all tumors |
| •Typically Triple negative | ||||
| •Low expression of cell-cell junction proteins (like E-Cadherin) | ||||
| •Lymphocytic infiltrates |
IHC Immunohistochemistry, ER Estrogen Receptor, PgR Progesterone Receptor, + Positive, - Negative
As there is no internationally accepted definition for basal like cancer, the terms “basal like cancer” and “triple negative cancer” have been used interchangeably by many authors. However, they are not synonymous? Approximately 75% of basal like cancers are triple negative but 25% of them may express Her2 or hormone receptors and similarly around 70–75% of tripe negative cancers are basal like cancers [8].
To better understand this concept triple negative is a phenotype which can include basal like cancers, claudin low cancers, normal breast like tumours and BRCA 1 deficient breast tumours. Basal like cancer is more like a genotype where genetic microarray techniques had been used to better characterise them. Triple negative cancers are defined as tumours that lack ER, PR and HER 2 expression and account for 10–25% of all breast carcinomas [9–11]. Apart from invasive ductal carcinoma, medullary, metaplastic, secretory, pleomorphic lobular carcinomas, adenoid cystic carcinomas etc., also belong to triple negative tumours. The main characteristics of triple negative breast cancers are they are frequent in younger women (<50 years), more frequent in African- American women, present as interval cancers, highly chemosensitive [12, 13], weak association between tumour size and lymph node metastases, more aggressive, higher chance of brain metastases, high chance of recurrence during 1st and 3rd year and shorter survival following first metastatic event when compared to other subtypes.
Here we are presenting a retrospective analysis of 50 patients of triple negative cancers and there clinicopathological features.
Materials and Methods
We retrospectively analysed 50 patients who were triple negative of 270 breast cancer patients who had attended our outpatient department in a single unit from January 2010 to September 2011. The incidence of triple negative breast cancer (TNBC) was 18.5% (50/270). The mean age at presentation was 46.66 years with a range from 28 to 72 years. The general characteristics of these patients has been as follows: (Table 2)
Table 2.
Characteristics of patients
| Characteristics | Number of patients ( Total N = 50) |
|---|---|
| Post menopausal | 22(44%) |
| Pre-menopausal | 28(56%) |
| Age | |
| Mean | 46.66 ± 13.87 |
| Range | 28–72 years |
| Clinical presentation | |
| Lump | 33(66%) |
| Ulcer | 59(10%) |
| Post Surgery (Modified Radical Mastectomy) | 12(Early-7, Locally advanced-5) |
| Histology | |
| Invasive ductal | 47(94%) |
| Lobular | 1(2%) |
| Medullary | 2(4%) |
| Grade | |
| 1 | 0 |
| 2 | 2(4%) |
| 3 | 48(96%) |
| Stage at presentation | |
| Early | 13(26%) |
| Locally advanced | 20(40%) |
| Post surgery | 12(24%) |
| Metastatic | 5(10%) 1 had both bone and lung metastases) |
| Bone | 3 |
| Lung | 2 |
| Liver | 1 |
| Neoadjuvant chemotherapy | 15 |
| Complete Pathological response | 0 |
| Complete clinical response | 4(26.6%) |
| Partial response | 9(60%) |
| No response/ Progressive disease | 2(13.3%) |
| Surgery performed | |
| Post Modified Radical Mastectomy | 12(24%) |
| Breast conservation surgery | 3(6%) |
| Modified Radical Mastectomy | 31(62%) |
| Palliative mastectomy | 2(4%) |
| No surgery | 2(4%) |
| Recurrence | 7 patients(14%) |
| Chest wall recurrence | 7(14%) |
| Supraclavicular lymph node recurrence | 3(6%) |
| Bone metastasis | 2(4%) |
| Brain metastases | 1(2%) |
| Disease free survival | |
| Mean | 15 months |
| Range | 3–58 months |
| Overall survival | |
| Mean | 20.14 months |
| Range | 5–70 months |
Of these 50 patients, 28 were premenopausal and 22 were postmenopausal. There was no family history of breast cancer in any patient. Twelve patients [12] had undergone modified radical mastectomy (MRM) outside and had been referred to our hospital for further management. Five patients had metastatic disease at presentation of which three had bone metastasis (one patient had solitary vertebral metastasis and other two had multiple bony metastases), two had lung metastasis of which one had pleuritis carcinomatosis (one patient had bone metastases also), and one had liver metastases. The patient with solitary bone metastases underwent chemotherapy (Adriamycin and Cylcophosphamide × 4 cycles) followed by MRM, two other patients with bone metastases disease underwent palliative mastectomy. All patients who underwent MRM underwent adjuvant chemotherapy and patients with locally advanced cancer had also received radiotherapy.
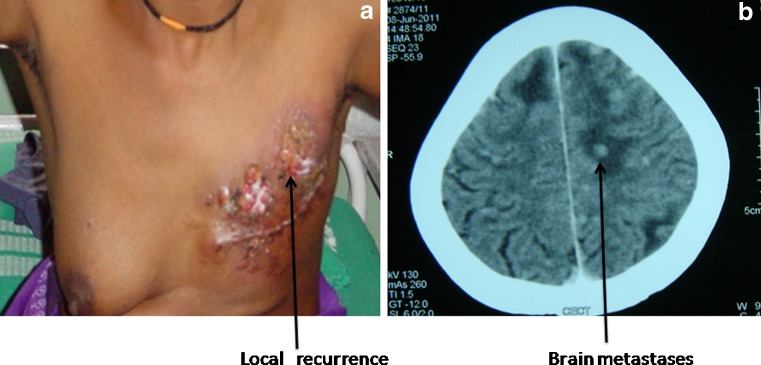
Fifteen patients underwent neoadjuvant chemotherapy (14 with LABC, and one with solitary bone metastases) of which 9(60%) had partial response, 4(26%) had complete clinical response and 2(13%) had no response and the patient with solitary bone metastases had disappearance of the lesion on follow up scan and she underwent MRM. Of the 45 patients who had no distant metastases at presentation, 7 developed recurrence, of which all had local recurrence (15%) had chest wall recurrence and 3(6%) had supraclavicular lymph node recurrence, 2(4%) had bone metastases and one (2%) had brain metastases (Table 3) (Fig. 1). One patient was operated 58 months back in a peripheral centre before she had developed chest wall and supraclavicular recurrence and was referred to us. She was treated with chemotherapy with disappearance of supraclavicular recurrence and later excision of the local recurrence. The mean disease free survival was 15 months with range from 3 months to 58 months. The mean overall survival was 20.14 months with range of 5–70 months. Five patients (10%) expired during follow up, three patients who presented initially with lung and liver metastasis and two patients who had progressed during treatment.
Table 3.
Clinicopathological characterisitics of patients who developed Recurrence
| S.No. | Age (Years) | Menopaus al staus | Treatment given | Type of cancer, Grade | Initial stage | Adjuvant treatment | Recurrence | DFS (Months) | OS (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 48 | Pre | MRM | IDC, 3 | T3N1M0 | CT + RT | LR | 12 | 17 |
| 2. | 55 | Post | MRM | IDC, 3 | T2N2aM0a | CT + RT | LR | 3 | 5 |
| 3. | 48 | Post | NACT➔MRM | IDC, 3 | T2N3aM0 | CT + RT | LR + SCLN + Brain metastases | 6 | 10 (Expired) |
| 4. | 28 | Pre | NACT➔MRM | IDC, 3 | T4bN1M0 | CT + RT | LR + Bone metastases | 9 | 11 (Expired) |
| 5. | 45 | Pre | MRM | IDC, 3 | T2N1M0a | CT + RT | LR + SCLN | 58 | 70 |
| 6. | 54 | Post | NACT➔MRM | IDC, 2 | T4bN2aM0 | CT + RT | LR + Bone metastases | 12 | 14 |
| 7. | 40 | Pre | NACT➔MRM | IDC,3 | T3N1M0 | CT + RT | LR + SCLN | 10 | 14 |
a- Post surgery outside referred to us, Pre Premenopausal, Post Postmenopausal, NACT Neoadjuvant chemotherapy, MRM Modified radical mastectomy, CT Chemotherapy, RT Radiotherapy, LR local recurrence, SCLN supraclavicular Lymph node metastases, IDC Invasive ductal cancer, DFS Disease free survival, OS Overall survival
Fig. 1.
(a) A patient developing local recurrence; 1(b) Contrast enhanced computer tomography showing multiple brain metastases
Discussion
The incidence of triple negative breast cancer was 18.5% which is within the range as world literature of the range of 10–24%. Ram prabhu etal retrospectively analysed 636 patients and the incidence of TNBC was 24.4% [10]. Triple negative breast cancer is a heterogeneous disease with various genotypes within it. These group of patients are associated with grave prognosis and very less is been known about its behaviour from our subcontinent [14, 15]. Basal like and triple negative terminology has been used vice versa without any clear cut demarcations. Basal like cancers cell of origin is thought to be from the basal/ myoepithelial cells and they express high molecular weight basal cytokeratins (CK 5/6, CK 14, CK 17), vimentin, p-cadherin, EGFR, etc., in contrast to luminal cancers which originate from a differentiated luminal precursor cell. Birnbaum et al. performed a study by using 500 gene set to 172 cases of TNBC; in which he found 71.5% of patients who were triple negative expressed markers for basal carcinoma and 23.1% patients who were triple negative failed to express these markers for basal like cancer [8]. Also TNBC, are commonly associated with high nuclear mitotic grade, larger tumour size, more aggressive expression profile with low Bcl-2 expression and high expression of p53, Rb gene mutations and increased Ki67 expression [16, 17].
In our study at presentation, 5 patients had metastatic disease, of which three had bone metastases (one solitary, one multiple and one with multiple bone and lung metastases), one had only lung metastases and other with only liver metastases. Visceral metastases are thought to be more common than bone metastases, but we had 3 bone and 3 visceral metastases. In our analysis the predominant group were premenopausal patients, and most of them presented in locally advanced stage. Of 15 patients who had received anthracycline based neoadjuvant chemotherapy, 9 patients (60%) had partial response, 4(26%) had complete clinical response, 2(13%) had no response and none had complete pathological response. Seven patients had chest wall recurrence and the mean disease free survival was 15 months. This supports the literature, that TNBC recur usually within first 3 years after initial treatment, and beyond 5 years of diagnosis recurrence decreases by 50% compared to Hormone receptor positive disease [14, 18, 19].
Brain metastases has been thought to be more common with TNBC patients risk being 6–46% [20–22]of those experiencing metastatic disease, we had one patient with multiple brain metastases who received palliative radiotherapy but later succumbed to death within 4 months of diagnosing brain metastases.
Five patients (10%) expired during the follow up of which three had metastatic disease at presentation and the other two who had developed metastases during the follow up. It’s too short time to comment on overall survival, the mean Overall survival was 20.14 months (Range 5–70 months). Various randomized trials had shown decreased survival in TNBC patients compared with other tumour types. But these differences wear off at 10 years of follow up among TNBC and non – TNBC patients.
Conclusion
Triple negative breast cancer occurs in younger patients, they are high grade, have aggressive behaviour and should be considered as prognostic factor in patients with breast cancer. Most of the recurrences occur early within first 3 years of surgery and they progress rapidly.
References
- 1.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prat A, Perou CM. Mammary development meets cancer genomics. Nat Med. 2009;15:842–844. doi: 10.1038/nm0809-842. [DOI] [PubMed] [Google Scholar]
- 5.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakha EA, Putti TC, Abd El-Rehim DM, et al. Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol. 2006;208:495–506. doi: 10.1002/path.1916. [DOI] [PubMed] [Google Scholar]
- 7.Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161:1991–1996. doi: 10.1016/S0002-9440(10)64476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertucci F, Finetti P, Cervera N, et al. How basal are triple-negative breast cancers ? Int J Cancer. 2008;123:23 6–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 9.Cheang MCU, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 10.Ram Prabu MP, Raina V, Shukla NK, Mohanti BK, Deo SVS (2011) A study of triple-negative breast cancer at a cancer institute in India. J Clin Oncol 29 :(suppl; abstr e11548)
- 11.Patil VW, Singhai R, Patil AV et al (2011) Triple-negative (ER, PgR, HER-2/neu) breast cancer in Indian women. Breast Canc Targ Ther 3:9–19 [DOI] [PMC free article] [PubMed] [Retracted]
- 12.Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin–cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 13.Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 14.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 15.Harris L, Broadwater G, Lin N, et al. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342. Breast Canc Res. 2006;8:R66. doi: 10.1186/bcr1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulkes WD, Brunet JS, Stefansson IM, Straume O, Chappuis PO, Begin LR, Hamel N, Goffin JR, Wong N, Trudel M, Kapusta L, Porter P, Akslen LA. The prognostic implication of the basal-like (cyclin E high/p27 low/p53?/glomeruloid- microvascular-proliferation?) phenotype of BRCA1-related breast cancer. Cancer Res. 2004;64:830–835. doi: 10.1158/0008-5472.CAN-03-2970. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Pinilla SM, Sarrio D, Honrado E, Moreno-Bueno G, Hardisson D, Calero F, Benitez J, Palacios J. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J Clin Pathol. 2007;60:1006–1012. doi: 10.1136/jcp.2006.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tischkowitz M, Brunet J-S, Begin L, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nofech-Mozes S, Trudeau M, Kahn H, et al. Patterns of recurrence in the basal and non-basal subtypes of triple-negative breast cancers. Breast Canc Res Treat. 2009;118:131–137. doi: 10.1007/s10549-008-0295-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin NU, Claus E, Sohl J et al (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer. Cancer 113:2638–2645 [DOI] [PMC free article] [PubMed]
- 21.Heitz F, Harter P, Traut A et al (2008) Cerebral metastases (CM) in breast cancer (BC) with focus on triple-negative tumors. J Clin Oncol (Meeting Abstracts); 26: (Abstr 1010)
- 22.Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20:621–627. doi: 10.1093/annonc/mdn682. [DOI] [PMC free article] [PubMed] [Google Scholar]