Alzheimer’s disease (AD) and Parkinson’s disease are the most common forms of age-related neurodegenerative disorders. The pathogenesis of these and other neurodegenerative diseases remains unclear, and effective treatments are currently lacking. However, recent studies from three diverse disciplines, neuropathology, genetics, and biophysics, have begun to converge on a novel target for therapeutic attack: ordered protein aggregation. Indeed, abnormal protein aggregation characterizes many, if not all, neurodegenerative disorders, not just AD and Parkinson’s disease, but also Creutzfeldt–Jakob disease, motor neuron diseases, the large group of polyglutamine disorders, including Huntington’s disease (1), as well as diseases of peripheral tissue like familial amyloid polyneuropathy (FAP). Many of these deposits were originally identified by their histochemical staining property, hence their designation as amyloid (starch-like). Subsequently, it was learned that amyloid deposits contain extremely insoluble protein fibrils that share similar morphological features (80- to 150-Å fibrils) but comprise many different proteins with no obvious sequence similarity. This review will focus on biophysical studies of protein aggregation in AD and FAP, where mechanistic models connecting pathological and genetic data to clinical disease are beginning to emerge. These two examples illustrate two ends of the biophysical spectrum: in one (AD), a flexible peptide is poised to form fibrils, whereas in the other (FAP), a stable globular tetramer must dissociate and partially unfold before forming a new stable fibril structure.
Disease-Linked Mutations Localize to Fibril-Forming Proteins.
The correlation and colocalization of protein fibrils with tissue degeneration suggests that fibrillization either contributes to cell death or is an inseparable epiphenomenon. Recent genetic studies support the former possibility. Mutations that cause Huntington’s disease, Creutzfeldt–Jakob disease, and FAP are localized to the genes encoding the fibril-forming proteins huntington, prion protein, and transthyretin, respectively. In AD and Parkinson’s disease as well, rare, early-onset forms have been linked to mutations in the fibril-forming proteins [amyloid β-protein (Aβ, see below) or its precursor, amyloid precursor protein and α-synuclein, respectively]. Thus, increasing evidence favors the postulate that protein fibrillization is an early and critical process in all of these diverse diseases (although the fibril itself may not be the culprit). It follows that inhibition of fibril formation could be a viable therapeutic strategy. Therefore, it is critical to develop an understanding of the process of fibril formation at a molecular level.
AD-Associated Amyloid. Amyloid isolated from AD brain tissue consists predominantly of a family of polypeptides designated Aβ to indicate their source (amyloid plaque) and their secondary structure within the plaque-derived fibrils (β-sheet). The 9-aa peptide (Aβ34–42), derived from the C-terminal sequence of the minor component Aβ42, forms unusually stable and highly ordered fibrils (2). These unusual properties suggested that the process of fibril assembly is a highly ordered (entropically expensive) one and that the Aβ C terminus is a critical determinant of its rate. Moreover, it was demonstrated that fibril formation is a nucleation-dependent process, resembling, in some ways, crystallization. Specifically, supersaturated solution of amyloid peptides were metastable, but immediate fibril formation could be “seeded” by addition of a small amount of preformed amyloid fibrils comprising the identical peptide (3). The time required to nucleate Aβ40 fibrils was shown to be significantly longer than that to nucleate Aβ42 fibrils, but the former process could be seeded by preformed Aβ42 fibrils (2).
The importance of the Aβ42 form was confirmed by the finding that selective elevation of Aβ42 level occurs in all three early-onset FAD genes identified to date: amyloid precursor protein, presenilin-1, and presenilin-2 (4). These data provide a satisfying scenario to explain the early-onset forms of AD, but not the common sporadic forms of AD (>95% of cases) where consistent elevation of Aβ42 has not been reported. Interestingly, in a small group of patients with severe head injury, a recognized risk factor for AD, short-lived elevation of Aβ42 levels was noted in the first week after head injury (5). Although transient, it is possible that this effect is sufficient to produce Aβ42 fibril seeds responsible for later disease.
Aβ Protofibrillar Intermediates.
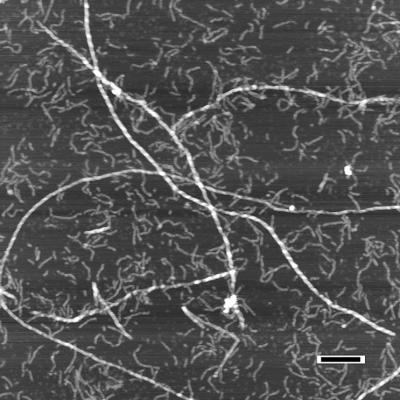
The simplest version of a nucleation-dependent polymerization process involves two stable states, the monomeric protein and the fibril (2). Once the critical concentration of monomer is reached, an equilibrium between monomer and fibril is rapidly established. This simple mechanism has been proposed to explain the polymerization of sickle-cell hemoglobin to produce the intracellular fibrils that lead to erythrocyte deformation (sickling) (6). Early kinetic studies of Aβ fibrillation appeared to be consistent with this simple mechanism, because an intermediate species was not detected (2). However, the situation changed when atomic force microscopy analysis was carried out, a technique providing three-dimensional information about species adsorbed to surfaces without the need for extensive sample preparation. Initial studies using microfabricated silicon tips with 5- to 10-nm resolution demonstrated discrete but transient intermediate Aβ species, designated protofibrils (7). The intermediate protofibril is ≈40% the height of the product fibril (3.5 nm vs. 8 nm). It appears very early in the process and disappears as fibrils appear (7–9). Protofibrils rarely reached lengths >400 nm (far shorter than the shortest observed fibrils), explaining the failure to detect them by classical light-scattering methods (Fig. 1). Under certain conditions (low temperature, low salt, no agitation), protofibril elongation is well behaved. The transition from protofibrils to fibrils, however, was sudden, unpredictable, and accelerated by the addition of small amounts of seed fibrils (8). The fibril morphology strongly suggests that the transition involves the association and winding of protofibrils, possibly accompanied by a conformational change (8). The conformational-change scenario may explain the enhanced stability of fibrils to dilution and chemical denaturation. Aβ42 and Aβ40 fibrils assemble via analogous steps and elongate at comparable rates (10), although Aβ42 protofibrils are populated more rapidly and to a greater extent (7–9). Amyloid protofibrils comprising other proteins have recently been described by several laboratories (11, 12) and have led to the hypothesis that Aβ protofibrils, rather than amyloid fibrils, are the pathogenic species (11, 13).
Figure 1.
Atomic-force microscopy of Aβ1–40 demonstrating assembly of amyloid fibrils from protofibrils. Preincubated Aβ1–40 (100 μM) was seeded with preformed fibrils amounting to ≈1% of the initial Aβ1–40 concentration. The aggregates were analyzed 7 days later. In this image, protofibrils appear as smaller and more flexible aggregates (≈4 nm in height) whereas the amyloid fibrils are substantially longer and more rigid (≈8 nm). (Bar = 200 nm.) [Reproduced with permission from ref. 8 (Copyright 1997).]
Transthyretin and FAP. Transthyretin is a tetrameric protein that is the backup transporter of thyroxine and the main transporter of the retinol-binding protein (carries 20% of vitamin A) (14). In unfortunate individuals, wild-type transthyretin is converted into numerous soluble quaternary structures that ultimately assemble into amyloid fibrils that infiltrate the heart in a disease called senile systemic amyloidosis (cardiomyopathy) (15). In related diseases, more than 60 mutations that are known to make transthyretin more amyloidogenic are associated with diseases collectively referred to as FAP (16). Typically, the familial diseases have a much earlier age of onset (30 vs. 80 yr), and the patients present with peripheral neuropathy and/or organ dysfunction as a result of massive transthyretin amyloid deposition. Transthyretin amyloid diseases are protein-misfolding diseases where the normally folded tetrameric protein dissociates to an alternatively folded monomer that is capable of self-assembly into amyloid fibrils (17, 18). Interestingly, the single-site mutations that predispose transthyretin to aggregation do so by destabilizing transthyretin in both a thermodynamic and kinetic sense. The kinetic destabilization refers to lowering the activation free energy for tetramer dissociation to the alternatively folded monomer, which appears to be the rate-determining step in transthyretin amyloid fibril formation. The alternatively folded transthyretin monomer self-assembles through numerous oligomeric intermediates that combine to form oligomers of increasing complexity, which ultimately become amyloid fibrils (12). It is the oligomeric intermediates that are currently the key suspect in the etiology of neurodegenerative diseases.
The extensive biophysical studies carried out thus far on transthyretin have significantly increased our understanding of the mechanism by which this protein is converted from its normally soluble state into amyloid fibrils (18). This information makes it possible to envision novel therapeutic strategies to intervene in transthyretin and related amyloid fibril formation processes and importantly to critically test the amyloid hypothesis, which implies that amyloid fibril formation causes these diseases. Knowing that the rate-determining step of transthyretin amyloid fibril formation is tetramer dissociation, we explored the possibility that small-molecule inhibitors that bound to the normally folded tetramer by using the unoccupied thyroxine binding site would make both the kinetics and thermodynamics less favorable for dissociation to the alternatively folded monomer (19–21). In in vitro systems, this strategy is effective for both the wild-type protein and all of the FAP variants evaluated thus far. In addition, biophysical studies suggest that small molecules binding to and intercepting the monomeric amyloidogenic intermediate could also arrest the amyloid fibril formation process, although active compounds have yet to be discovered.
In summary, recent biophysical studies have provided important insights into the mechanisms of amyloid fibril formation. In turn, this information may prove to be essential in the development of new therapeutic strategies that will allow us to critically test the amyloid hypothesis in both animal models and in human neurodegenerative diseases.
ABBREVIATIONS
- AD
Alzheimer’s disease
- FAP
familial amyloid polyneuropathy
- Aβ
amyloid β-protein.
References
- 1.Trojanowski J Q, Goedert M, Iwatsubo T, Lee V M Y. Cell Death Diff. 1998;5:832–837. doi: 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- 2.Harper J D, Lansbury P T., Jr Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 3.Jarrett J T, Lansbury P T., Jr Biochemistry. 1992;15:12345–12352. doi: 10.1021/bi00164a008. [DOI] [PubMed] [Google Scholar]
- 4.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 5.Raby C A, Morganti-Kossmann M C, Kossmann T, Stahel P F, Watson M D, Evans L M, Mehta P D, Spiegel K, Kuo Y M, Roher A E, Emmerling M R, et al. J Neurochem. 1998;71:2505–2509. doi: 10.1046/j.1471-4159.1998.71062505.x. [DOI] [PubMed] [Google Scholar]
- 6.Eaton W A, Hofrichter J. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 7.Harper J D, Wong S S, Lieber C M, Lansbury P T., Jr Chem Biol. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 8.Harper J D, Lieber C M, Lansbury P T., Jr Chem Biol. 1997;4:951–959. doi: 10.1016/s1074-5521(97)90303-3. [DOI] [PubMed] [Google Scholar]
- 9.Walsh D M, Lomakin A, Benedek G B, Condron M M, Teplow D B. J Biol Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 10.Harper J D, Wong S S, Lieber C M, Lansbury P T., Jr Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- 11.Lansbury P T., Jr Proc Natl Acad Sci USA. 1999;96:3342–3344. doi: 10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lashuel H, Lai Z, Kelly J W. Biochemistry. 1998;37:17851–17864. doi: 10.1021/bi981876+. [DOI] [PubMed] [Google Scholar]
- 13.Lambert M P, Barlow A K, Chromy B A, Edwards C, Freed R, Liosatos M, Morgan T E, Rozovsky I, Trommer B, Viola K L, et al. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake C C F, Geisow M J, Oatley S J. J Mol Biol. 1978;121:339–356.12. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 15.Cornwell G C, Sletten K, Johansson B, Westermark P. Biochem Biophys Res Comm. 1988;154:648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson D R, Buxbaum J N. Adv Hum Genet. 1991;20:69–123. doi: 10.1007/978-1-4684-5958-6_2. [DOI] [PubMed] [Google Scholar]
- 17.Kelly J W, Colon W, Lai Z, Lashuel H A, McCulloch J, McCutchen S L, Miroy G J, Peterson S A. Adv Protein Chem. 1997;50:161–181. doi: 10.1016/s0065-3233(08)60321-6. [DOI] [PubMed] [Google Scholar]
- 18.Kelly J W. Curr Opin Struct Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 19.Miroy G J, Lai Z, Lashuel H, Peterson S A, Strang C, Kelly J W. Proc Natl Acad Sci USA. 1996;93:15051–15056. doi: 10.1073/pnas.93.26.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson S A, Klabunde T, Lashuel H A, Purkey H, Sacchettini J C, Kelly J W. Proc Natl Acad Sci USA. 1998;95:13407–13412. doi: 10.1073/pnas.95.22.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baures P W, Peterson S A, Kelly J W. Bioorg Med Chem. 1998;6:1389–1401. doi: 10.1016/s0968-0896(98)00130-8. [DOI] [PubMed] [Google Scholar]