Abstract
The high rate of therapeutic failure in the management of alcohol use disorders (AUDs) underscores the urgent need for novel and effective strategies that can deter ethanol consumption. Recent findings from our group showed that ivermectin (IVM), a broad-spectrum anthelmintic with high tolerability and optimal safety profile in humans and animals, antagonized ethanol-mediated inhibition of P2X4 receptors (P2X4Rs) expressed in Xenopus oocytes. This finding prompted us to hypothesize that IVM may reduce alcohol consumption; thus, in the present study we investigated the effects of this agent on several models of alcohol self-administration in male and female C57BL/6 mice. Overall, IVM (1.25–10 mg/kg, intraperitoneal) significantly reduced 24-h alcohol consumption and intermittent limited access (4-h) binge drinking, and operant alcohol self-administration (1-h). The effects on alcohol intake were dose-dependent with the significant reduction in intake at 9 h after administration corresponding to peak IVM concentrations (Cmax) in the brain. IVM also produced a significant reduction in 24-h saccharin consumption, but did not alter operant sucrose self-administration. Taken together, the findings indicate that IVM reduces alcohol intake across several different models of self-administration and suggest that IVM may be useful in the treatment of AUDs.
1. Introduction
Alcohol (ethanol) use disorders (AUDs) have a major national impact in the United States, affecting nearly 18 million people, causing over 100,000 deaths, and costing upward of $235 billion annually (Bouchery et al., 2011; Grant et al., 2004; Harwood, 2000). Alcohol abuse and misuse also possess significant health risks worldwide where alcohol abuse and misuse is the third leading risk factor for premature death and disabilities (World Health Organization). The few drugs currently approved for AUD management attempt to deter alcohol intake by blocking its metabolism or by targeting the neurochemical systems in the downstream cascades leading to craving and dependence (Colombo et al., 2007; Gewiss et al., 1991; Johnson et al., 2007; Steensland et al., 2007). Unfortunately, these approaches, even when applied in combination with psychological strategies, have resulted in limited success as evidenced by the continued high rates of AUDs. As such, development of effective treatments for AUDs represents an important public health goal (Bouchery et al., 2011; Heilig and Egli, 2006; Johnson, 2010; Johnson et al., 2007; Steensland et al., 2007).
In the quest to identify novel therapeutic targets for AUDs, our group and others have found that the P2X4 receptor (P2X4R), a member of the P2X family of ATP-gated channels abundantly expressed in the CNS (Buell et al., 1996; Soto et al., 1996), plays a role in alcohol-induced behaviors (Asatryan et al., 2011; Kimpel et al., 2007; Tabakoff et al., 2009). P2X4Rs are the most abundant P2X receptor subtype expressed in the CNS (Buell et al., 1996; Soto et al., 1996) and are the most alcohol sensitive P2XR identified to date (Davies et al., 2005). In vitro, P2X4Rs are inhibited by ethanol concentrations as low as 5 mM (Asatryan et al., 2010; Davies et al., 2005; Davies et al., 2002; Popova et al., 2010; Xiong et al., 2005; Xiong et al., 2000; Xiong et al., 1999). This concentration of ethanol is well below the 17 mM legal blood ethanol concentration (BEC) that is considered “legal intoxication” (i.e., 0.08%) in the United States. P2X4Rs are expressed in key brain regions implicated in the reinforcing properties of alcohol and other drugs, such as the striatum (Amadio et al., 2007; Krügel et al., 2003). Taken together, the evidence suggests that P2X4Rs may play a role in alcohol addiction through modulation of P2X4Rs in the mesolimbic dopamine (DA) system.
Additional evidence supporting the hypothesis comes from recent studies reporting that the p2rx4 gene may be linked to alcohol intake and/or preference (Kimpel et al., 2007; Tabakoff et al., 2009). First, alcohol-preferring (P) rats show lower functional expression of the p2rx4 gene compared to alcohol-non-preferring (NP) rats (Kimpel et al., 2007). Second, investigations on the genetic determinants of alcohol consumption report an inverse relationship between the expression of the p2rx4 gene and innate alcohol preference (Tabakoff et al., 2009). Overall, these findings suggest that alcohol intake may be modulated by ethanol acting on P2X4Rs and that pharmacological activation of P2X4Rs may reduce alcohol consumption and preference.
While no selective agonists of P2X4Rs have been developed to date, rich evidence has shown that ivermectin (IVM), a broad-spectrum antiparasitic dihydro avermectin derivative used worldwide in humans and animals (Geary, 2005; Molinari et al., 2010; Richard-Lenoble et al., 2003), acts as a potent and selective positive allosteric modulator of P2X4Rs. IVM’s selectivity affinity (between P2XR subtypes) provides the basis for it to be routinely employed to determine the contribution of P2X4Rs in ATP-mediated processes (Khakh et al., 1999).
IVM is believed to act, in part, in close proximity to positions 331 and 336 in P2X4Rs (Jelinkova et al., 2008; Jelinkova et al., 2006; Silberberg et al., 2007). Recent investigations in our laboratory indicated that positions 331 and 336 are also important as targets for the inhibitory actions of ethanol on P2X4Rs expressed in Xenopus oocytes (Popova et al., 2010). Further studies demonstrated that IVM antagonized the inhibitory effects of ethanol on P2X4Rs. Specifically, mutational studies provided evidence that IVM antagonism of ethanol involved interference in the action of alcohol at a putative pocket at or near position 336 in these receptors (Asatryan et al., 2010). This ability of IVM to antagonize the effects of ethanol on P2X4Rs, taken in context with evidence suggesting that P2X4Rs play a role in ethanol preference [presented above and see (Asatryan et al., 2011)], suggested that IVM may reduce ethanol intake and preference. This notion is supported by recent findings that acute administration of IVM reduced maintenance of alcohol self-administration in rats, but results from this work were inconclusive (Kosten, 2011).
The present study systematically tests the hypothesis that IVM reduces ethanol intake and preference in mice. The findings provide strong evidence that acute and short-term chronic IVM can reduce ethanol intake. Pharmacokinetic analyses provide strong support that the actions of IVM on ethanol intake reflect actions of IVM found in the brain. Collectively, the data indicates that IVM holds promise as a novel therapeutic agent for the treatment of AUDs.
2. Materials
2.1. Drugs
IVM [10 mg/ml] (Norbrook Inc., Lenexa, KS) was diluted in a 0.9% sodium chloride solution (saline) to a concentration that would allow for an injection volume of 0.01 ml/g of body weight. Propylene glycol (1,2 Propanediol – solvent used by the manufacturer to dissolve the IVM), purchased from Alfa Aesar (Ward Hill, MA) was diluted in saline at a concentration equivalent to the amount used in the 10 mg/kg dose of IVM. Ethanol was diluted in tap water using either Everclear, a 150 proof solution (Luxco, St. Louis, MO) or Gold Shield Alcohol, a 200 proof USP solution (Gold Shield Chemical Company, Hayward, CA) to achieve a 10% v/v solution (10E). Sucrose (Sigma-Aldrich, St. Louis, MO) was prepared as a 2% w/v (2S) solution in tap water, whereas saccharin (Sigma-Aldrich, St. Louis, MO) was prepared at a 0.033% w/v solution in tap water.
2.2. Animals
Studies were performed, as described in specific experiments, on drug-naïve, C57BL/6J male mice purchased at 6 weeks of age (Jackson Laboratory, Bar Harbor, ME, USA for studies conducted in Portland, OR) or on male and female mice from our internal breeding colony at the University of Southern California (USC). For the studies conducted at USC, C57BL/6J breeders were obtained from the Jackson Laboratory, and new breeders were replaced every 3 generations. Mice were acclimated to the housing facility for one week and group-housed (2–4 mice per cage) in polycarbonate/polysulfone cages at a 12 h light/dark cycle with lights off at 12:00PM (USC) or 0:600PM (Portland) with ad libitum access to food and water. The holding room was maintained at approximately 22°C. All proc edures in this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and all efforts were made to minimize animal suffering. The USC Institutional Animal Care and Use Committee approved the protocols. The operant self-administration study was conducted in Portland, Oregon, and the Institutional Animal Care and Use Committee at the Portland VA Medical Center approved this work.
3. Methods
3.1 IVM effects on 24-h access ethanol and tastant preference drinking in mice
The 24-h access model [e.g., (Belknap et al., 1993; McClearn, 1959; Middaugh et al., 1999; Rodgers, 1966; Yoneyama et al., 2008) is widely used to assess changes in drinking behaviors. We used a modification of the procedure employed by Yoneyama et al. (2008). Briefly, mice (individually housed 3 days before the start of the study) had 24-h access to two inverted bottles with metal sippers placed on the cage tops. Food was distributed near both bottles to avoid food associated tube preferences.
3.1.1. Alcohol studies
One tube contained tap water and the other a 10E solution in tap water. Mice were given free access to 10E with bottle positions alternated every other day. Fresh fluids were provided twice a week when cages were changed. Body weights were recorded daily. Every morning daily fluid intake (to the nearest 0.1 ml) was recorded from both bottles by measuring the level of the meniscus on the graduated drinking tube. Daily 10E intake was measured until it stabilized (+/− 10% variability from the mean dose of the last 3 days). After establishing stable alcohol drinking levels (usually within one week), mice received daily saline injections (intraperitoneal; i.p.) until 10E intake stabilized (which averaged between 4–5 days of saline injections). In all cases, injections were administered immediately prior to the period of 24 h access to 10E versus tap water, so that the change in drinking over 24 h after IVM administration was measured. Mice then received one injection (i.p.) with either IVM (0.65–10 mg/kg) or saline (control for injection effect, per se) daily for the remainder of the study using a within subjects design. Animals were injected with saline on subsequent days until 10E drinking stabilized at baseline levels, and then mice were injected with another dose of IVM. This pattern of IVM administration continued until all doses of a particular study were complete. Consumption of 10E returned to baseline levels prior to the administration of each subsequent dose of IVM tested. A pilot study determined that the effects of IVM on 10E intake following IVM doses less than 10 mg/kg were not significantly altered within 3 days post IVM administration, suggesting a return to baseline 10E intake.
3.1.2. Saccharin studies
Upon conclusion of one IVM/alcohol study, a tastant study was conducted similar to that for ethanol, with the exception that the 10E solution was replaced with a bottle containing a saccharin solution (0.033% w/v). Daily saccharin intake was measured until it stabilized (+/− 10% variability from the mean dose of the last 3 days). After establishing stable drinking levels, mice received daily saline injections (i.p.) until intake stabilized. Mice then received one injection (i.p.) with either IVM (2.5, 5.0 mg/kg) or saline (control for injection effect, per se) daily for the remainder of the study using a within subjects design.
3.2. IVM effects on intermittent limited access drinking in mice
A modified version of drinking in the dark (DID) procedure (Rhodes et al., 2005) was used, following intermittent limited access procedures similar to recent studies reported by others (Neasta et al., 2010). Briefly, mice had 4-h access to one bottle containing 10E every other day. Alcohol drinking sessions began 3 h into the circadian dark on Monday, Wednesday, and Friday with a 48-h (weekend) alcohol deprivation period. A single bottle of water was continuously available between drug access periods. The volume from the alcohol containing bottle was measured immediately prior to the start and immediately following the 4-h drinking period. Following the establishment of stable baseline alcohol drinking levels and habituation to saline injections, a single 10 mg/kg dose of IVM was administered at 6 h prior to the start of the drinking session. Based on our pharmacokinetic analysis of IVM in plasma and brain, initiation of the limited access procedures 6 hours after IVM administration allowed for the evaluation of IVM on 10E intake as IVM approached peak brain concentrations, at peak, and after peak IVM concentrations.
3.3. IVM effects on operant ethanol and sucrose self-administration in mice
Daily sessions were carried out Monday to Friday in 8 modular chambers (21.6 × 17.8 × 12.7 cm) with stainless steel grid floors (Med Associates, Inc., St. Albans, VT), according to published procedures (Ford et al., 2007a; Ford et al., 2009; Ford et al., 2007b; Tanchuck et al., 2011). Each chamber contained a house light, two ultra-sensitive retractable levers, and a retractable sipper apparatus that held a 10 ml graduated pipette with a double ball bearing sipper tube that allowed volume measurements to the nearest 0.05 ml. A lickometer circuit was connected to each metal sipper to monitor lick patters via MED-PC software (Med Associates, Inc.). Each chamber was housed within a wooden cabinet (61 × 38 × 33 cm; Fisher Custom Woodworking, Portland, OR) that contained a fan to minimize outside noise and to facilitate airflow.
The present study utilized the “sipper” model of operant self-administration, which we have described in detail elsewhere (Ford et al., 2009; Ford et al., 2007b). Advantages to the use of the “sipper” model are that the appetitive and consummatory phases of self-administration can be procedurally separated [e.g., (Samson et al., 2000; Samson et al., 1998)] and that C57BL/6 mice exhibit a heightened appetitive drive to acquire ethanol access and more than a two-fold increase in ethanol consumption with the “sipper” procedure than when responding on a fixed ratio schedule (Ford et al., 2007a). Briefly, separate groups of male C57BL/6J mice were trained to respond for access to either a 10E or 2S solution via sucrose fading. Upon completion of training, a single response requirement of 16 presses on the active lever (RR16) resulted in 60 min of continual access to the fluid reinforcer. A 20 min time limit to complete the RR16 schedule was imposed. The first study was conducted in male C57BL/6J mice that had a 6 month history of ethanol or sucrose self-administration and prior drug exposure, and the second study was conducted in C57BL/6J mice that were experimentally naïve.
In the first study, C57BL/6J male mice were 8 weeks old upon purchase and had approximately 6 months of experience with either ethanol (n =7) or sucrose (n = 10) self-administration before testing in the present study. Mice had previously been tested for effects of the synthetic neurosteroid ganaxolone on ethanol and sucrose self-administration in sessions where animals had 30 min of continual access to the reinforcer, and each mouse had the same history of treatment exposure (Ramaker, Ford and Finn, in preparation). Then, mice were maintained on an operant self-administration procedure without any drug injections before the initiation of this study. Mice were then habituated to saline injections (6.5 – 8.5 h pretreatment time was selected to be similar to the 6 h drug pre-treatment in the DID alcohol study and to be compatible with the time to achieve maximal concentration, which was found to be at 8–10 h following IVM administration) and exhibited stable baselines of 10E or 2S with less than 10% variability between sessions. An initial pilot study determined that there were minimal effects of IVM (0.65, 2.5, or 10 mg/kg versus saline, within-subjects design) when animals had 30 min of continual access to 10E or 2S (data not shown). Thus, session length was increased to 60 min of continual reinforcer access (10E or 2S) over a period of approximately 2 weeks, and animals did not receive any injections during this time. Because mice already were habituated to saline injections, they received a single injection of saline and then IVM (10 mg/kg) a few days later during the final week of testing.
In the second study, C57BL/6J male mice were 8 weeks old upon purchase. Mice were trained to respond for access to a 10E solution as described above. Upon completion of training, a single response requirement of 16 presses on the active lever (RR16) resulted in 60 min of continual access to the 10E solution. Once the animals exhibited stable baselines of 10E with less than 10% variability between sessions, mice were then habituated to saline injections (6.25 – 8.5 h pretreatment time) during one week of testing. The following week, mice received a single injection of saline and then IVM (10 mg/kg) a few days later.
3.4. Quantification of IVM using liquid chromatography with tandem mass spectrometry (LC-MS/MS)
Brain tissue and plasma samples were collected from separate groups of animals at 1, 6 and 8 h after being administered with IVM (0, 0.25, 2.5, or 10 mg/kg, i.p.). Samples were extracted, using either 100 mg of brain sample or 50 μL of plasma. The whole brain sample was excised and placed into liquid nitrogen for 1 min to flash freeze. The sample was then pulverized, where 50 μL of 500 ng/mL abamectin (ABM) was added as an internal standard. The entire mixture was extracted using 1 mL ethyl acetate, and the samples vigorously mixed and centrifuged at 5000 rpm for 5 min. This process was performed 4 times, and the supernatants were collected and evaporated to dryness using a steady stream of nitrogen gas.
To 50 μL plasma, 50 μL of 500 ng/mL ABM was added, and the samples were extracted using 4 mL of ethyl acetate and centrifuged at 13,000 rpm for 10 min. The organic layer was then collected and evaporated to dryness using a steady stream of nitrogen. The evaporated residues were all reconstituted in 100 μL of 0.1% formic acid in acetonitrile. A 30 μL aliquot was injected into Agilent 1100 HPLC system linked into an AB Sciex API 3000 turboionspray mass spectrometer. The analytes were separated using an ACE C18 column, with a gradient mobile phase system. The mobile phase consisted of component A, which was 0.1% formic acid, and component B, which was 0.1% formic acid in acetonitrile. The gradient program was set at 50% component B for 1 min, and then a gradient from 50% to 95% component B for the next 5 minutes. The amount of IVM and ABM were quantified using the mass spectrometer set in the positive mode and monitoring the following multiple reaction monitoring (MRM) using parent to transition ions of 892 →307.2 and 867.6→453.3, respectively. The lowest level of detection was 2 ng/mL.
3.5. Pharmacokinetic Analysis
The concentration of IVM in brain and plasma were analyzed using a non-compartment model. Serial blood and tissue IVM quantification was used to calculate the pharmacokinetic parameters such as the maximum IVM concentration (Cmax), time to achieve maximal IVM (Tmax), half-life, elimination constant, and area under the curve (AUC).
3.6. Statistical analyses
For each study, consumption of each solution corrected for body weight (ethanol = g/kg, saccharin = mg/kg, sucrose = g/kg), and ethanol preference ratio (mls ethanol ÷ total mls) were calculated for each IVM/saline dose tested. ANOVA assessed IVM dose effects with IVM dose or time [saline pre-treatment (pre IVM), IVM dose, saline post-treatment (post IVM)] as a repeated measures factor on the dependent variables (ethanol and water intake in mls, ethanol intake in g/kg, and ethanol preference ratio). Separate analyses were conducted for each sex, since males and females were tested in separate studies. For the operant self-administration data, cumulative records of lever and sipper (lick) responding were recorded by MED-PC IV software (MED Associates, Inc.). Measures of appetitive (response rate, latency to first press, latency to first bout) and consummatory (bout frequency, size, duration and lick rate) processes were calculated from these cumulative records via a custom data analysis program (http://www.r-project.org). Based on our previous work, a bout was defined as ≥ 20 licks with < a 60 sec pause between successive licks (Finn et al., 2008; Ford et al., 2007a; Ford et al., 2009; Ford et al., 2007b). ANOVA with repeated measures was used to examine IVM effects on active and inactive lever responses, intakes of 10E and 2S (volume and dose), latencies to reinforcer access, and the bout parameters mentioned above. Since we were predicting a differential effect of IVM on intake of 10E and 2S, planned comparisons were conducted in the absence of a significant interaction. Significant main effects and interactions of the ANOVAs were further investigated with post-hoc tests (i.e., Tukey’s test, t-tests with Bonferroni correction, paired t-tests). For all studies, significance set at p ≤ 0.05.
4. Results
4.1. IVM administration significantly reduced alcohol intake and preference in male mice
4.1.1. Single dose administration of IVM (10 mg/kg) decreased 10E intake and preference
We initiated our investigation by examining the impact of acute administration of IVM (10 mg/kg) on alcohol intake using a 24-h access two-bottle choice paradigm (10E versus tap water). The lower limit of alcohol consumed by mice using this model suggests that this model mimics social drinking (Blednov et al., 2010). We started with the 10 mg/kg dose in order to maximize the chance of observing an effect of treatment. The 10 mg/kg dose was the highest dose that could be given without introducing possible CNS toxicity (Lerchner et al., 2007; Merck et al., 1988).
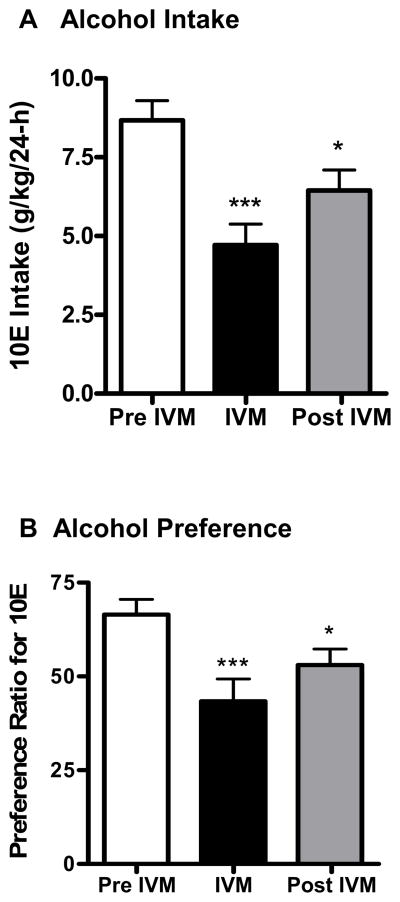
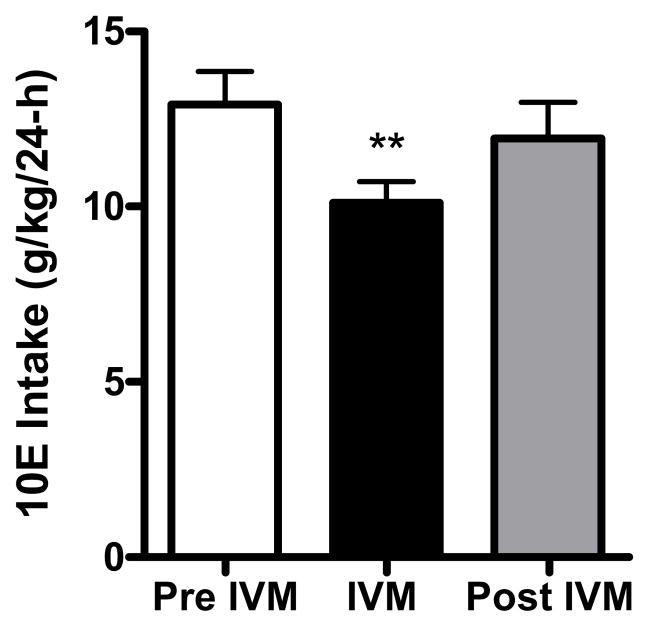
Prior to the initiation of saline injections, baseline 10E intake during the 24-h period was 13.47 ± 0.61 g/kg (n = 11). As illustrated in Fig 1A, 10E intake stabilized at 8.67 g/kg following saline injections. Acute administration of 10 mg/kg IVM was analyzed (pre IVM, IVM, post IVM) and found to significantly reduced alcohol intake in excess of 45%, compared with pre IVM injections (Fig. 1A) [F(2,30) = 16.85, p<0.001], and 10E intake remained significantly lower than saline on the day following IVM treatment (by more than 25%, shown as post IVM injections in Fig. 1A). The reduction in 10E intake induced by IVM was paralleled by a 35% decrease in ethanol preference [F(2,30) = 10.11, p<0.001] (Fig. 1B). This dose of IVM also significantly increased water consumption by about 47%, when compared to pre-IVM injections [F(2,30) = 3.577, p<0.05] but there was no change in total fluid intake (data not shown). Using a separate group of animals, we also tested the effects of the vehicle alone (i.e., propylene-glycol) on 10E intake to ensure that the changes observed in this first study could not be attributed to an action of the vehicle, per se. The vehicle did not significantly alter 10E intake, water intake, fluid intake or preference ratio (data not shown) giving us confidence that the changes in alcohol drinking were attributed to the drug (IVM) and not the vehicle (propylene-glycol). Thus, subsequent studies used saline as the vehicle treatment.
Figure 1.
IVM (10 mg/kg) reduces A) 10% v/v ethanol (10E) intake and B) preference ratio for 10E in male C57BL/6J mice using a 24-h access two-bottle choice paradigm. After attaining stable drinking levels for 3 consecutive days, IVM was administered. Bars represent levels from the day prior to IVM injection (white; Pre IVM), the day of the IVM injection (black; IVM) and the day after the IVM injection (gray; Post IVM). Values represent the mean ±SEM for 11 mice.*P<0.05, ***P<0.001 versus Pre IVM, Tukey multiple comparison post-hoc test.
4.1.2. IVM decreased 10E intake and preference in a dose-dependent manner in male mice
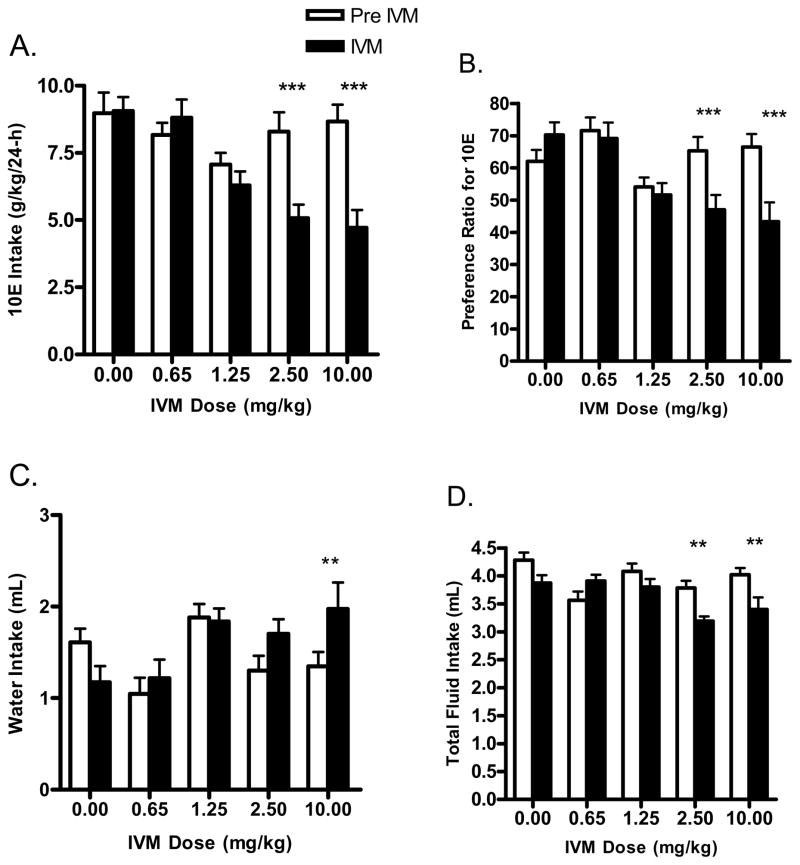
We extended the initial single dose IVM experiment to one where several doses of IVM (0.65 – 10 mg/kg) were tested for their effects on 10E intake. When the analysis was conducted across time (pre-IVM, IVM, post-IVM), there was a significant effect of IVM on ethanol intake [F(2,100) = 14.37, p<0.001]. The analysis of IVM dose indicated that IVM significantly reduced alcohol intake in a dose dependent manner (Fig. 2A) [F(4,100) = 5.51, p<0.001]. The interaction between time and dose was significant [F(8,100) = 5.53, p<0.001]. Bonferroni post-hoc comparisons versus pre-IVM indicated that 2.5 mg/kg IVM was the lowest dose of IVM that significantly reduced alcohol intake in this study [i.e., 2.5 mg/kg ~39% (t=5.025, p<0.001)]. The two lowest doses of IVM tested (i.e., 0.65 mg/kg and 1.25 mg/kg) did not significantly alter 10E intake. In addition, saline injections alone, did not significantly alter 10E intake. For the doses of IVM that significantly reduced 10E intake, we found that 10E intake returned to comparable pre-IVM intake levels by the third day post-IVM injection. The exception was 10 mg/kg IVM. For this dose, we found that 10E intake did not fully return to pre-IVM levels until 4–5 days post-IVM injection (data not shown).
Figure 2.
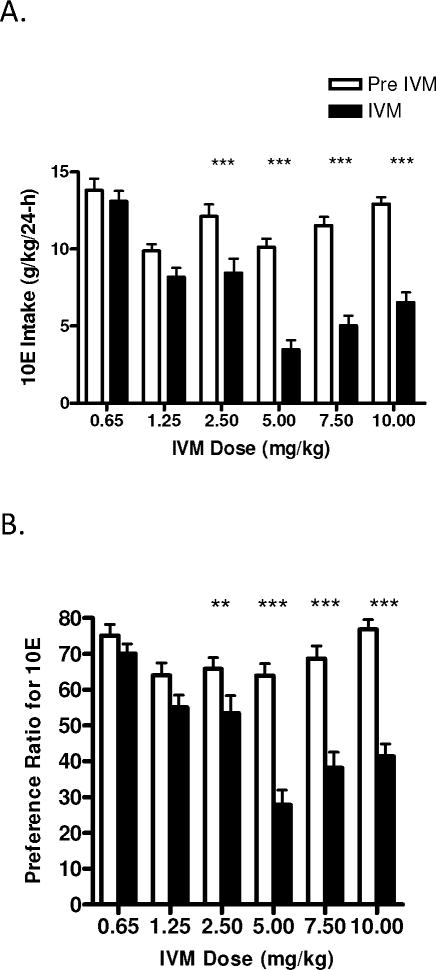
IVM dose response study in male C57BL/6J mice using a 24-h access two bottle choice paradigm. Each dose of IVM was administered after achieving stabilized drinking for 3 consecutive days. Bars represent levels from the day prior to IVM injection (white; Pre IVM) and the day of the IVM injection (black; IVM). A) IVM (2.5 and 10 mg/kg) significantly reduced 10E intake. B) IVM (2.5 and 10 mg/kg) significantly reduced preference ratio for 10E. The effects of IVM on water and total fluid intake are presented in panels C and D, respectively. Values represent the mean ±SEM for 11 mice per dose group. **P<0.01, ***P<0.001 versus respective pre IVM condition, Bonferroni’s post-hoc test.
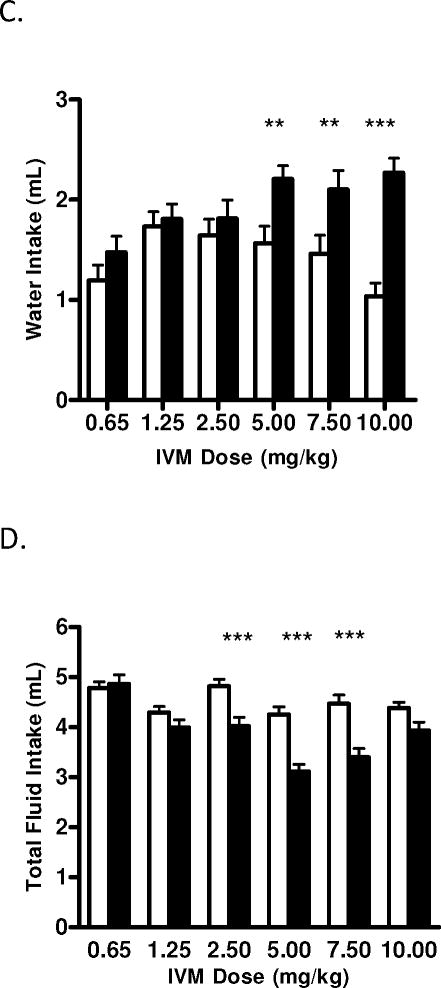
The investigation also found that the reductions in ethanol intake induced by IVM were accompanied by decrease in ethanol preference, which was revealed when the analysis was conducted across time [F(2,100) = 8.92, p<0.001]. Moreover, the analysis of dose indicated that IVM significantly reduced alcohol intake in a dose dependent manner [F(4,100) = 3.69, p<0.05]. The interaction between time and IVM dose was significant [F(8,100) = 7.40, p<0.001], with Bonferroni post-hoc tests confirming a significant decrease in ethanol preference ratio following doses of 2.5 mg/kg (t=4.527, p<0.001) and 10 mg/kg (t=5.715, p<0.001) IVM (Fig. 2B). While there was no main effect of IVM to significantly alter water intake when the analyses were conducted across time or dose, there was a significant interaction between time and IVM dose [F(8,100) = 7.09, p<0.001]. Post-hoc comparisons confirmed that water intake was increased significantly with the IVM dose of 10 mg/kg (t=3.430 p<0.01; Fig. 2C). Investigating the effects of IVM on total fluid intake (Fig. 2D) across time we found a significant reduction [F(2,100) = 13.66, p<0.001]. The analysis of IVM dose indicated that dose also had a significant effect on fluid intake [F(4,100) = 3.50, p<0.05]. Furthermore, there was a significant interaction between time and dose [F(8,100) = 5.40, p<0.001], with post-hoc comparisons confirming that fluid intake decreased after IVM doses of 2.5 mg/kg (t=3.526, p<0.01) and 10 mg/kg (t=3.689, p<0.01).
4.1.3. Time-course of IVM’s effect on alcohol intake in male mice
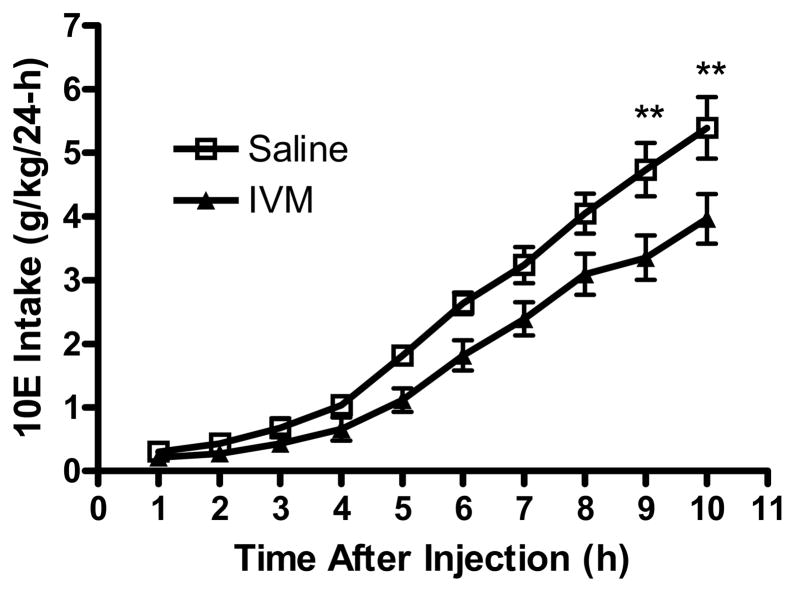
IVM has been previously reported to have slow absorption, with a wide distribution. The clearance of IVM is slow, which may be attributed to a low level of metabolism (Merck et al., 1988). The normal anti-parasitic dosage is 200 μg/kg. However, for this study we evaluated the time course of IVM’s effect on hourly ethanol intake (10E versus water) when used at a dose of 10 mg/kg. IVM or saline was administered 1h before the first reading. As expected, 10E intake increased significantly across time [F(9,190) = 80.89, p<0.001] and was significantly reduced following IVM pre-treatment [F(1,190) = 37.33, p<0.001]. The interaction between time and IVM dose was not significant. We conducted planned comparisons, which confirmed that ethanol consumption was significantly decreased at 9 and 10 h after the administration of IVM (Fig. 3). This correlated well with the time to achieve maximal concentration or Tmax, which was found to be at 8–10 h following IVM administration.
Figure 3.
IVM (10 mg/kg) administered to male C57BL/6J mice significantly reduced 10E intake approximately 9 hours after IVM administration. The intake was measured on an hourly basis, up to 10 hours following IVM (closed) or saline (open) administration. Values represent the mean ±SEM cumulative intake for 10–11 male mice per treatment group. **P<0.01 versus saline-treated group, Bonferroni’s post-hoc test.
4.2. Pharmacokinetics of IVM in plasma and brain
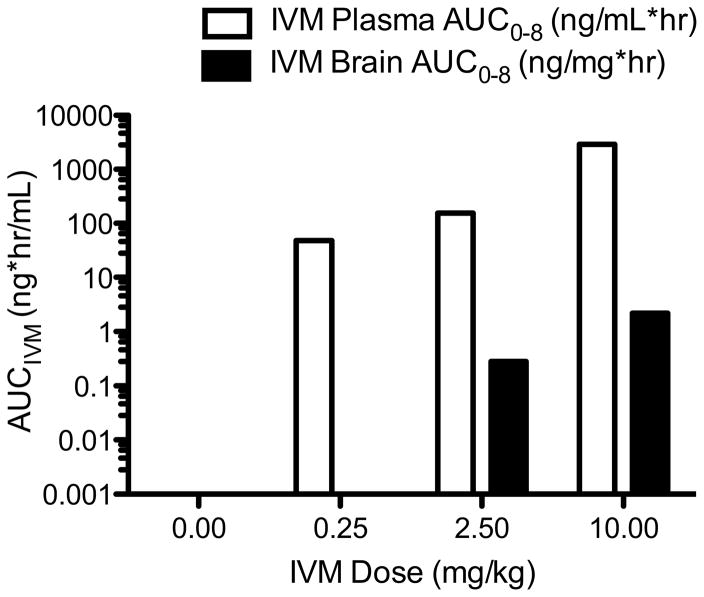
To determine a pharmacokinetic-pharmacodynamics (PK/PD) relationship, we conducted an optimal dose finding study, where six mice were assigned to each dosage group (0, 0.25, 2.5 and 10 mg/kg). The AUC for plasma and brain IVM was dosage proportional (Fig 4). Plasma and brain IVM AUC also correlated with the percentage reduction of ethanol consumption (Table 1). Specifically, the lowest IVM dosage leading to detectable IVM in the brain was found at 2.5 mg/kg, which corresponded to lowest IVM dosage to reduce ethanol consumption. At this dosage, the IVM AUC in plasma and brain was 155 ng*hr/mL and 0.28 ng*hr/mg of tissue, respectively.
Figure 4.
IVM AUC in plasma and brain tissue was determined following injection of various IVM dose groups. A dose-dependent IVM AUC was found in the plasma (open bars) and brain tissue (black bars).
Table 1.
Three doses of IVM were evaluated to determine the IVM disposition in both plasma and brain tissue. IVM produced a dose-dependent increase in detectable IVM levels in plasma and brain tissue.
| IVM Dose (mg/kg) | % Reduction of Ethanol Consumption | IVM Plasma AUC0-8 (ng*hr/mL) | IVM Brain AUC (ng*hr/mg) |
|---|---|---|---|
| 0.25 | No Effect | 48.91 | 9.6×10−05 |
| 2.5 | 32 % | 155.56 | 2.8×10−01 |
| 10 | > 50% | 2928.31 | 2.20×1000 |
4.3. IVM administration reduced alcohol intake and preference in female mice
The effects of IVM on alcohol intake and preference were also examined in female mice, as they typically drink higher levels of alcohol than that of males (Finn et al., 2004; Yoneyama et al., 2008). In agreement with previous investigations (Finn et al., 2004; Yoneyama et al., 2008), the degree of 10E intake in female mice was greater than what we observed in male mice (females ~15 g/kg; males ~10 g/kg). As presented in Fig. 5A, IVM significantly reduced ethanol intake in a dose dependent manner [F(6,252) = 20.16, p<0.001]. When the analysis was conducted across time (pre-IVM, IVM, post-IVM), there was a significant effect of IVM on ethanol intake [F(2,252) = 79.53, p<0.001]. The interaction between time and dose was significant [F(12,252) = 7.50, p<0.001]. Bonferroni post-hoc comparisons versus pre-IVM indicated that the highest dose of IVM tested (10 mg/kg) significantly reduced ethanol intake by approximately 50% (t=8.214, p<0.001). Lower doses of IVM also significantly reduced ethanol intake (i.e., 2.5 mg/kg ~30% (t=4.735, p<0.001), 5.0 mg/kg ~66% (t=8.536, p<0.001), 7.5 mg/kg ~56% (t=8.318, p<0.001). Intake of 10E remained significantly lower than pre IVM levels on the day after IVM injection of the 5.0, 7.5 and 10 mg/kg doses (data not shown). However, over the following 3-day period (post IVM), 10E intake in all groups of animals was similar to their pre IVM values (pre IVM exposure), and was significantly higher than 10E intake in presence of IVM (data not shown).
Figure 5.
IVM dose response study in female C57BL/6J mice using a 24-h access two bottle choice paradigm. Each dose of IVM was administered after achieving stabilized drinking for 3 consecutive days. Bars represent levels from the day prior to IVM injection (white; Pre IVM) and the day of the IVM injection (black; IVM). A) IVM (2.5–10 mg/kg) significantly reduced 10E intake and B) preference ratio for 10E. The effects of IVM on water and total fluid intake are presented in panels C and D, respectively. Values represented the mean ±SEM for 19 mice per dose group. **P<0.01, ***P<0.001 pre IVM condition, Bonferroni’s post-hoc test.
In addition, we found that IVM decreased 10E preference. Analysis of the data across time revealed a significant decrease in preference for ethanol [F(2,252) = 77.80, p<0.001]. Moreover, the analysis of IVM dose indicated that IVM significantly reduced alcohol preference in a dose dependent manner [F(6,252) = 10.45, p<0.001]. There was a significant interaction between time and IVM dose [F(12,252) = 10.11, p<0.001], with Bonferroni post-hoc tests confirming a significant decrease in ethanol preference ratio following IVM doses of 2.5 mg/kg (t=3.323, p<0.01), 5.0 (t=9.650, p<0.001), 7.5 (t=8.154, p<0.001) and 10 mg/kg (t=9.470, p<0.001) (Fig. 5B). With regard to the effects of IVM on water intake, conducting the analyses across IVM dose, [F(6,252) = 3.27, p<0.01] and time [F(2,252) = 17.80, p<0.001] revealed significant effects of IVM on water intake. The interaction between time and IVM dose also was significant [F(12,252) = 6.55, p<0.001]. Post hoc tests revealed a significant difference in water intake following IVM doses of 5.0 mg/kg (t=3.752, p<0.01), 7.5 mg/kg (t=3.764, p<0.01) and 10 mg/kg (t=7.196, p<0.001) (Fig. 5C). Finally, when investigating the effects of IVM on total fluid intake (Fig. 2D) across time, we found a significant reduction [F(2,252) = 35.34, p<0.001]. The analysis of IVM dose also confirmed a significant effect of IVM on fluid intake [F(6,252) = 8.13, p<0.001]. Furthermore, there was a significant interaction between time and dose [F(12,252) = 4.78, p<0.001], with post-hoc comparisons confirming that fluid intake decreased after IVM doses of 2.5 mg/kg (t=3.988, p<0.001), 5.0 mg/kg (t=5.694, p<0.001), and 7.5 mg/kg (t=5.342, p<0.001) (Fig. 5D).
4.4. Multiple dosing of IVM administration reduced alcohol intake and preference in female mice
The aforementioned studies focused on acute administration of IVM (i.e., a single administration of individual IVM doses). However, alcoholism is a chronic disorder, and as such, treatment strategies most likely would need to be long lasting. To begin to investigate the utility of IVM for use as an anti-alcohol agent, we tested the effects of IVM (1.25 mg/kg/day) administered daily for 7 days (i.p.) on 10E intake and preference. As presented in Fig. 6, 7 daily administrations of IVM significantly reduced alcohol intake, when average 10E intake across the 7 days was analyzed (pre IVM, IVM, post IVM) [F(2,27) = 7.974, p<0.01]. The degree of reduced ethanol drinking over the 7 day period of IVM administration was somewhat varied, ranging from approximately 11–27%, but did not significantly differ between days and no overt changes in behavior, food intake or water intake were observed (data not shown). IVM (1.25 mg/kg), however, did not significantly reduce 10E preference (data not shown). The reduction in alcohol intake was accompanied by increases in average water consumption that ranged from no change to 26%, but these changes were not significantly different from pre IVM levels (data not shown). There was a significant change in average total fluid intake [F(2,27) = 8.862, p<0.001], with a degree of day to day variability ranging from a 20% decrease to a 5% increase over the 7 day testing period (data not shown).
Figure 6.
Daily administration of IVM (1.25 mg/kg/day X 7 days) reduced 10E intake in female C57BL/6J mice using a 24-h access two bottle choice paradigm. After achieving stable drinking levels for 3 consecutive days, IVM was administered for 7 consecutive days. Bars represent levels from the day prior to IVM injections (white; Pre IVM), the 7 day average of the IVM injection (black; IVM) and the day after the IVM injections (gray; Post IVM). Values represented the mean ±SEM for 11 mice. **P<0.01 versus Pre-IVM, Tukey multiple comparison post-hoc test.
Figure 7.
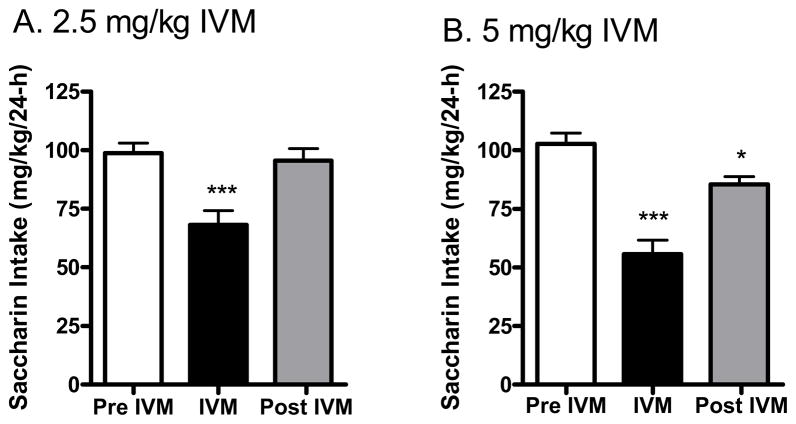
IVM administration reduced saccharin (0.033% w/v) intake in female C57BL/6J mice using a 24-h access two bottle choice paradigm following doses of A) 2.5 mg/kg and B) 5 mg/kg. After achieving stable drinking levels for 3 consecutive days, IVM was administered (separate studies for each dose). Bars represent levels from the day prior to IVM injection (white; Pre IVM), the day of the IVM injection (black; IVM) and the day after the IVM injection (gray; Post IVM). Values represent the mean ±SEM for 19 mice per dose group. ***P<0.001 versus Pre IVM, Tukey multiple comparison post-hoc test.
4.5. IVM reduced saccharin consumption in female mice
To begin to determine whether the effects of IVM were selective for alcohol or could be generalized to other potential reinforcers, we investigated the ability of IVM to reduce saccharin (0.033%) intake and preference. As presented in Fig. 7A, we found that 2.5 mg/kg IVM resulted in a significant reduction in saccharin consumption by approximately 31% [F(2,54) = 20.82, p<0.001]. In contrast, 2.5 mg/kg IVM did not significantly decrease saccharin preference (data not shown). This dose of IVM did not significantly affect water intake (data not shown), but did significantly reduce total fluid intake by approximately 29% [F(2,54) = 24.82, p<0.001]. A second study extended our investigation by testing the effects of 5 mg/kg of IVM on saccharin intake. We found that 5 mg/kg IVM significantly reduced saccharin consumption by more than 45% [F(2,54) = 52.83, p<0.001] (Fig. 7B). In addition, preference for the saccharin solution was significantly decreased by about 7% [F(2,54) = 4.761, p<0.05] (data not shown). In agreement with our 2.5 mg/kg saccharin intake study, 5.0 mg/kg IVM did not significantly affect water intake (data not shown), but did significantly reduce total fluid intake by approximately 42% [F(2,54) = 52.70, p<0.001)]. IVM did not significantly alter animal weight or food intake as compared to baseline (data not shown).
4.6. IVM decreased alcohol intake by 50% in an intermittent limited access paradigm in female mice
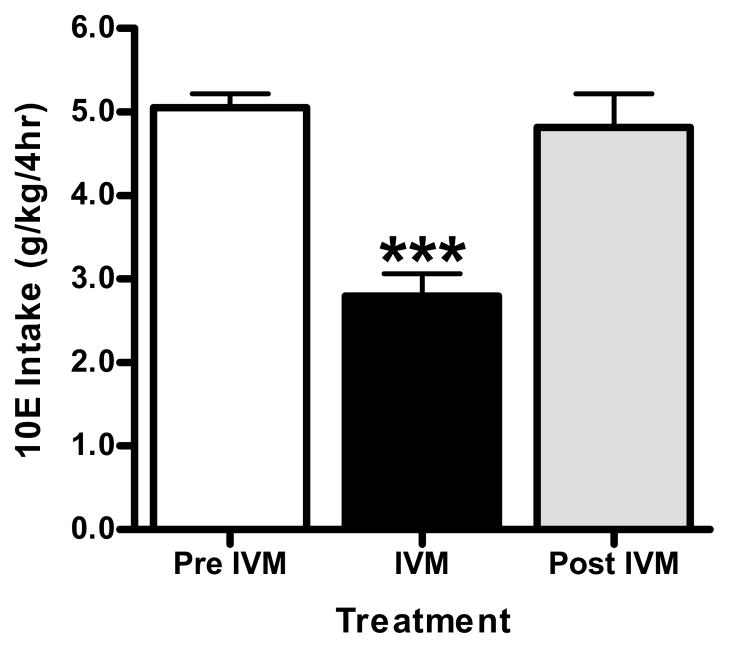
We extended our investigation to a second model of alcohol intake with the use of an intermittent limited access paradigm. Using this model, the animals have access to ethanol for 4 h during the circadian dark phase (Lowery et al., 2010; Neasta et al., 2010; Rhodes et al., 2005), which results in the mice reaching high, pharmacologically active blood ethanol concentrations (BECs) in a short period of time as found in binge-like drinking behavior (i.e., >80 mg%). We utilized the 10 mg/kg dose, since it was the most effective dose in the 24 h two-bottle choice studies, and it was the highest dose that could be given without introducing possible CNS toxicity (Lerchner et al., 2007; Merck et al., 1988).
Prior to the initiation of saline injections, baseline 10E intake during the 4h session was 5.12 ± 0.22 g/kg in the female mice (n = 8). Control, saline injections did not significantly alter baseline alcohol intake in these mice (Fig. 8). Acute administration of 10 mg/kg IVM (administered 6-h prior to the start of the alcohol drinking session) was analyzed (pre IVM, IVM, post IVM) and found to significantly reduced alcohol intake in excess of 45%, when compared with pre IVM injections [F(2,23) = 17.64, p<0.001] (Fig. 8). We found that the mice resumed drinking 10E at levels comparable with their pre IVM drinking levels within 1 drinking session post IVM treatment. This contrasts with our findings from the above 24 h access studies, where the drinking levels did not return to pre IVM levels until 4–5 days post IVM treatment, and suggests that there may be differences in the persistence of IVM’s suppression in ethanol intake with continuous versus intermittent and limited access procedures.
Figure 8.
10 mg/kg IVM administration reduced ethanol (10% v/v) intake in female C57BL/6 mice using an intermittent, limited (4-h) access paradigm. After attaining stable drinking levels, IVM was administered. Bars represent levels from the day prior to IVM injection (white; Pre IVM), the day of the IVM injection (black; IVM) and the day after the IVM injection (gray; Post IVM). Values represent the mean ± SEM for 8 mice. ***P<0.001 versus Pre IVM, Tukey multiple comparison post-hoc test.
4.7. Significant reduction in ethanol but not sucrose operant self-administration following IVM in male mice
Prior to the initiation of saline injections, baseline intake during the 30 min session was 1.00 ± 0.14 g/kg for the 10E reinforced mice (n=7) and was 0.49 ± 0.06 g/kg for the 2S reinforced mice (n=10). Pretreatment with IVM did not significantly alter responding on the active lever, as all animals were able to complete the RR16 and gain access to the reinforcer. However, IVM did not alter intake of the 2S or 10E solutions (data not shown) during the 30 min sessions. Analysis of first bout dynamics revealed no effect of IVM on any bout parameters examined (not shown).
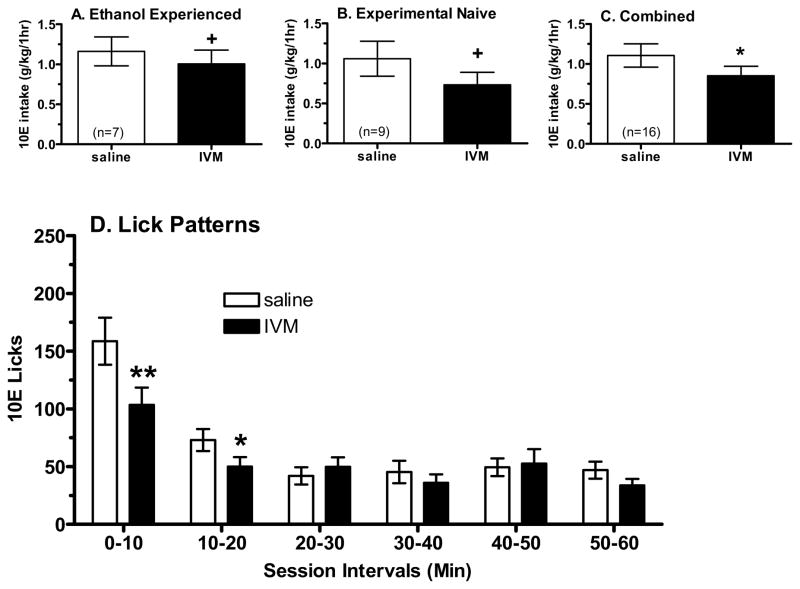
One advantage of the 30 min operant sessions (following access to the reinforcer) is the ability to capture changes in ethanol intake when using drugs with a short half-life. Because this was not a concern with IVM and because we thought that that the low intake and short time frame inherent in a 30 min session might decrease the ability to detect a change in self-administration following IVM, we tested the effects of the 10 mg/kg IVM dose (most effective dose in other models) in a 60 min operant self-administration session in the same animals.
Mice rapidly adjusted to the 60 min operant sessions, and baseline intake was 1.16 ± 0.18 g/kg for the ethanol-reinforced mice (n=7) and was 0.73 ± 0.07 g/kg for the sucrose-reinforced mice (n=8). Pretreatment with IVM did not significantly alter responding on the active lever, as all animals were able to complete the RR16 and gain access to the reinforcer. Planned comparisons revealed a trend for IVM to decrease intake of 10E by 13% (p=0.06, Fig. 9A), while having no effect on sucrose intake (data not shown). Analysis of first bout dynamics revealed that IVM differentially affected latency to reinforcer in the 10E- versus the 2S-reinforced mice (a significant interaction between group and IVM [F(1,12) = 5.97, p<0.05]; not shown). Subsequent analyses revealed that IVM produced a significant 30% decrease in latency to reinforcer only in the 10E reinforced mice (p<0.05; not shown). Similar results were seen with analysis of latency to first bout (not shown). However, first bout size was not significantly altered by IVM, nor was there a significant alteration in the average bout size, bout frequency, or lick rate (not shown). Finally, bin analysis was conducted to examine how IVM was affecting the pattern of reinforcer consumption across 10 min bins (not shown). Importantly, the correlation between licks and intake (g/kg; across the two days when animals received saline or 10 mg/kg IVM) was highly correlated for the 10E- (r=0.93, p=0.003, n=7) and 2S-reinforced (r=0.85, p=0.007, n=8). The high correlations confirmed that the analysis of lick behavior was highly predictive of reinforcer consumption. Bin analysis revealed that IVM altered 10E licks in a consistent (no bin by dose interaction) and modest manner (trend for effect of IVM dose = F(1,6) = 3.75, p=0.10) across the 10 min bins. There was no main effect of IVM or interaction between IVM and bin on 2S licks (not shown).
Figure 9.
Effect of IVM on operant self-administration of 10% ethanol (10E) in male C57BL/6J mice during 60 minute sessions. IVM tended to decrease 10E intake in animals with a long history of ethanol self-administration (panel A) or in animals that were experimentally naïve (panel B). Importantly, the data from the two cohorts did not differ, so the combined data indicated that IVM significantly decreased 10E intake (panel C). Panel D depicts the effect of IVM injection on the temporal distribution of 10E licks in 10 min intervals across the 60 min session in the combined data from the two cohorts (n=16). Values represent the mean ± SEM for the numbers of animals in parentheses for panels A–C. +p ≤ 0.10, *p < 0.05 versus saline (0 mg/kg), paired t-test.
Due to concern that the prolonged ethanol history in the above cohort may have blunted the effects of IVM, a second study examined the effect of the 10 mg/kg IVM dose (or saline) on self-administration of 10E during a 60 min session in experimentally naïve mice. Baseline intake was 1.06 ± 0.22 g/kg (n = 9), and pretreatment with IVM did not significantly alter responding on the active lever. All mice were able to complete the RR16 and gain access to the 10E solution. IVM reduced 10E intake by 31% [F(1,8) = 3.30, p=0.10] (Fig. 9B). No bout parameters reached statistical significance with the exception of a trend for a decrease in first bout size (p<0.10). Bin analysis (not shown) revealed a bin by dose interaction [F(5,40) = 9.14, p=0.004], where 10 mg/kg IVM decreased intake primarily during bin 1 (p=0.025) and bin 2 (p=0.005).
Importantly, the difference in ethanol history between the 2 cohorts did not appear to significantly alter the effect of IVM on 10E intake. This conclusion is supported by analyses that were conducted across studies (referred to as “pass”) to determine whether the data could be collapsed. There was no dose by pass interaction [F(1,14) = 0.666, p=0.43] or a main effect of pass (p = 0.46) on g/kg intake. Similarly, bin analysis of the effect of pass revealed no dose by bin by pass interaction [F(5,70) = 0.80, p=0.554] or a main effect of pass (p=0.91). Analysis on the collapsed data revealed that IVM significantly decreased 10E intake by 23% [F(1,15) = 5.71, p=0.03] (Fig. 9C). Additionally, there was a significant dose by bin interaction (Fig. 9D), with post hoc tests indicating that 10 mg/kg IVM significantly decreased licks during bin1 (p=0.003) and bin 2 (p=0.05).
Discussion
The present study tested the hypothesis that IVM reduces ethanol intake and preference in mice and used several animal models of ethanol self-administration. In support of the hypothesis, we found that IVM significantly reduced alcohol intake following acute IVM administration using 24-h access and intermittent limited access procedures as well as in an operant self-administration procedure. Interestingly, administration of a sub-threshold IVM dose daily for 7 days also significantly decreased 24 h ethanol intake. Pharmacokinetic studies demonstrated that IVM was detected in brain tissues. Brain IVM was correlated to the anti-alcohol intake effects of IVM at a dosage of 2.5 mg/kg and the effects of IVM on ethanol intake correlated with the presence of drug. Taken together, the findings indicate that IVM reduces alcohol intake across several different models of self-administration, albeit with differences in efficacy, and support the notion that IVM may be useful in the treatment of AUDs.
Acute administration of IVM decreased 24-h ethanol intake and preference in male and female mice in a dose-dependent manner. A separate study confirmed that the reduction in alcohol intake reached statistical significance approximately 9 h after IVM administration, which corresponded with the Tmax (8–10 h) in both the plasma and brain compartments. Moreover, IVM plasma half-life of 8–12 h was compatible with the duration of the decrease in ethanol consumption, because ethanol intake did not return to baseline levels until approximately 72 h post-IVM dosage. At this time point, IVM is expected to be over 99% eliminated (Merck et al., 1988).
Acute administration of IVM also significantly reduced higher levels of alcohol drinking when tested using an intermittent limited-access model. This second model results in BECs that mimic excessive or high levels of alcohol drinking. For instance, 4 h ethanol intake of 7 ± 2 g/kg in male C57BL/6J mice was associated with a BEC of 97 ± 9 mg% (Neasta et al., 2010) in one recent study. Similar results were reported by a different group in C57BL/6J male mice. In this study 4 h ethanol intake resulted in approximately 5–6 g/kg and was associated with BECs of approximately 100–125 mg% (Lowery et al., 2010). In female C57BL/6J mice, 4 h ethanol intake of 7.3 ± 0.6 g/kg was associated with a BEC of 92 ± 16 mg% (Rhodes et al., 2007). Since we did not measure BECs in the present study, we do not know if the IVM-induced reduction in high alcohol intake corresponded to a decrease in BEC from binge to non-binge levels. Future studies will be necessary to examine the therapeutic potential of IVM to reduce binge drinking, based on a reduction in BEC to non-binge levels.
IVM significantly reduced alcohol-drinking behavior with similar efficacy in male and female mice. This lack of difference suggests that sex-related hormonal differences do not influence IVM’s reduction in alcohol drinking. We did not monitor the stage of the estrous cycle in the female mice during testing. We based this decision on previous studies in which we did not observe systematic changes in ethanol intake across weeks of baseline consumption or following repeated vehicle injections in female C57BL/6 mice [e.g., (Ford et al., 2008)] and on earlier reports that estrous cycle-related changes in operant ethanol self-administration were not observed in female rats that were allowed to cycle freely (Roberts et al., 1998). Thus, it is unlikely that an estrous cycle-related change in ethanol intake in the control conditions confounded interpretations of the data following IVM pretreatment. More important, the findings suggest that estrous cycle may not influence the clinical application of IVM for treatment of AUDs.
IVM significantly reduced ethanol intake in the operant self-administration paradigm, when the data were collapsed across studies. Importantly, the difference in ethanol history between the 2 cohorts did not appear to significantly alter the effect of IVM on 10E intake. However, the reduction in ethanol self-administration was not of the same magnitude as was seen with other measures of ethanol intake where fluid availability was not behaviorally contingent. We believe that these differences reflect the short duration of the operant sessions, rather than whether (or not) instrumental responding was required by the animal to gain fluid access. That is, the operant self-administration procedure resulted in 1-h of fluid access versus the home cage drinking with 24-h or intermittent (4-h) access procedures. This is supported by the bin analysis, which showed a decrease in ethanol self-administration that was still suppressed at 60 min. Further, this modest effect of a 23% decrease in ethanol intake was consistent with recent work in rats where the 10 mg/kg IVM dose produced a 17.8% decrease in the number of sweetened ethanol reinforcers (10E+2S; but intake not reported) earned on a progressive ratio schedule when animals were responding in 3 h sessions (Kosten, 2011). In the present 1-h sessions, all animals were able to complete the response requirement to gain access to the reinforcer following the 10 mg/kg IVM dose, suggesting that IVM was not exerting a non-specific effect on motor activity with the 6 – 8 h pretreatment time. Taken in conjunction with the lengths of the single published operant self-administration study [3-h; (Kosten, 2011)] and our current findings with two drinking models (4-h and 24-h), it is possible that IVM would have exerted a greater reduction in operant ethanol self-administration during a longer session.
Another consideration with regard to oral consumption of ethanol in animals is that sensory modalities such as taste, olfaction, and chemosensory irritation play an important role in the acceptance or rejection of the alcohol solution [e.g.,(Bachmanov et al., 2003)]. Introducing ethanol into a sweet solution facilitated the initiation of reliable operant ethanol self-administration in rodents in the absence of factors such as food or fluid restriction or post-prandial sessions [e.g., (Middaugh and Kelley, 1999; Samson, 1986) and the present study] and increased voluntary ethanol consumption in alcohol-avoiding mouse strains [e.g., (Belknap et al., 1993; Yoneyama et al., 2008)]. Additionally, mutant mice with a deletion in a gene critical in taste transduction significantly decreased preference for alcohol and saccharin solutions without altering consumption of sodium chloride (Blednov et al., 2008). These findings are consistent with studies demonstrating a strong association between consumption of ethanol and sweetened fluids [e.g., (Belknap et al., 1993; Kampov-Polevoy et al., 1999)]. Related to this point, we found that the regimens of IVM that attenuated alcohol consumption also significantly decreased saccharin intake. These data suggest that the ability of IVM to reduce alcohol intake may partially reflect IVM’s ability to negatively modulate the reinforcing and/or chemosensory properties of several rewarding stimuli. This possibility is strengthened by the finding that water intake was significantly increased by IVM, which rules out that the observed phenomena may stem from a generalized hypodipsia. Nonetheless, a future study that examines the manner in which the 10 mg/kg IVM dose alters the dose-response function for ethanol and saccharin by testing across different concentrations of these fluids would provide insight regarding the interaction between IVM and taste sensitivity.
The mesolimbic DA reward system plays a critical role in directing reward seeking and motivational behavior through the regulated release of DA (Di Chiara and Imperato, 1988; Gonzales et al., 2004; Koob, 2009; Weiss et al., 1993). And, recent preclinical studies clinical trials have begun focusing on pharmacotherapies that exert their effects by cortico-mesolimbic DA system modulation [for review see (Johnson, 2010)]. P2X4Rs are expressed in the cortico-mesolimbic DA system (Amadio et al., 2007; Krügel et al., 2003) and are sensitive to physiologically relevant concentrations of ethanol (Ostrovskaya et al., 2011; Xiao et al., 2008). As such, it is plausible to propose that P2X4Rs play a role in the signaling cascades involved in alcohol consumption and addiction. In support of this contention, we recently reported that P2XRs can modulate ethanol’s effect on GABAergic synaptic transmission of DA neurons in the VTA (Xiao et al., 2008). Additional evidence supporting the importance of P2X4Rs comes from recent studies reporting that low functional expression of p2rx4 gene is associated with high alcohol preference (Kimpel et al., 2007; Tabakoff et al., 2009). This concept appears to be supported by our preliminary findings on male p2rx4 knockout (KO) mice, which exhibit significant increases in alcohol intake (unpublished data). Taken together, the findings suggest that the efficacy of IVM in reducing alcohol intake involves, in part, its ability to block the action of ethanol on P2X4Rs.
In mammals, IVM is also purported to act on GABAA and glycine receptors [e.g., see (Dawson et al., 2000; Shan et al., 2001; Spinosa et al., 2002)] and nicotinic acetylcholine receptors (nAChRs) (Krause et al., 1998; Sattelle et al., 2009). All of these receptor families have been linked to the behavioral effects of ethanol (Asatryan et al., 2011; Davies, 2003; Kimpel et al., 2007; Perkins et al., 2010; Vengeliene et al., 2008). Although the current findings do not shed light on the role that other receptors may play in causing the IVM-mediated anti-alcohol effects, it is possible that the reduction in alcohol intake may reflect a combined effect of IVM on several receptors, including GABAARs, nAChRs and P2X4Rs. Additional studies are necessary before definitive conclusions can be drawn.
Collectively, the present findings indicate that IVM might be useful in the treatment of AUDs. The potential translation of the present findings and repurposing of IVM for use in this manner is aided by the twenty plus year history of IVM’s use to treat parasitic diseases in millions of humans (Burkhart, 2000; Guzzo et al., 2002; Omura, 2008). IVM has an excellent safety profile when used as an anthelmintic (Burkhart, 2000; Davis et al., 1999; Omura, 2008). Accordingly, the literature on IVM attests to the safety and relative lack of toxicity of IVM over this period during which billions of doses of IVM-containing products have been used worldwide (Boxall and Long, 2005). Mild to moderate CNS adverse events have been associated with IVM. However, the incidences of these adverse events are rare and appear to be linked to alteration of p-glycoprotein (Pgp) expression, which is found abundantly in the blood brain barrier (BBB) (Edwards, 2003; Geyer et al., 2009; Lespine et al., 2009; Lespine et al., 2007; Sun et al., 2010).
The lack of adverse events with IVM in its current use as an anti-parasitic may be attributed to the manner in which it is dosed—200 mg once yearly or intermittently. However, doses up to 10X the recommended dosage (i.e., 2.0 mg/kg/day) have been safely tested in clinical trials (Guzzo et al., 2002). In rodents, doses less than 10 mg/kg IVM IV did not cause visible CNS depression (Trailovic and Trailovic, 2010), whereas lethality (i.e., LD50) is reported to be between 25–50 mg/kg depending on the sex and species tested (Merck et al., 1988).
The current studies tested doses more like the non-toxic rodent studies described in the previous paragraph. Importantly, we did not observe behavioral changes or other overt signs of toxicity in our studies in which multiple day (7 d) dosing of 1.25 mg/kg IVM elicited a continued reduction in drinking levels. This initial finding, combined with the safety record and other studies of IVM described above suggests that the longer term IVM dosing as would be expected for treating AUDs, would not produce significant adverse effects. However, this issue requires further investigation.
6. Conclusion
The present findings indicate that IVM reduces alcohol intake across several different models of self-administration in both male and female mice. This suggests that IVM may be useful in the treatment of AUDs. The ongoing widespread commercial use of IVM treatments for parasitic infections has led recent efforts to expand the approved uses of IVM in humans as noted by searching the Clinicaltrials.gov Website. However, it is important to point out that these efforts are focusing on using IVM for new indications and new dosing strategies (including multiple day dosing strategies) linked to various antiparasitic actions and not for affecting alcohol consumption.
In view of the current widespread use of IVM as an anthelmintic drug and recent efforts to expand the use of IVM for other indications, the development of IVM as an anti-alcohol agent may represent a fast and economically advantageous approach. In addition, our results highlight IVM as a novel lead structure for the development of novel anti-alcohol agents. Future preclinical and clinical research will be necessary to elucidate the significance of IVM-mediated effects and the potential clinical use of this drug in alcohol use.
Acute ivermectin (IVM) reduced 24 h alcohol intake in male and female mice.
Acute IVM reduced alcohol intake using binge and operant conditioning models.
Repeated IVM persistently reduced 24 h alcohol intake.
IVM’s anti-alcohol intake effects are linked to IVM concentration in the brain.
The findings support development of IVM for treatment of alcohol use disorders.
Acknowledgments
We thank the two anonymous reviewers for their critical comments and helpful suggestions, which greatly improved the manuscript. We also thank Ayeetin Azah, Daniel Kuo, Christina Minh and Vanessa Fimreite for technical assistance. This work was supported, in whole or in part, by the American Foundation for Pharmaceutical Education Fellowship (MY); and National Institutes of Health (NIH), NIAAA, Grants F31AA018926 (LW), KO-1-AA017243 (LA), AA013922 (DLD), Integrative Neurosciences Initiative on Alcoholism [Pilot project AA013517 (DLD)], USC School of Pharmacy (RA and DLD), USC Undergraduate Research Associates Program (DLD, MB, CM) and Rose Hills Foundation Science and Engineering Fellowships (DLD and VF). This work was conducted as partial fulfillment of the requirements for the Ph.D. degree in Molecular Pharmacology and Toxicology, University of Southern California (MY and LW).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadio S, Montilli C, Picconi B, Calabrei P, Volont C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic Signal. 2007;3:389–398. doi: 10.1007/s11302-007-9069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Nam HW, Lee MR, Thakkar MM, Dar MS, Davies DL, Choi DS. Implication of the purinergic system in alcohol use disorders. Alcoholism, clinical and experimental research. 2011;35:584–594. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins DI, Trudell JR, Alkana RL, Davies DL. Ivermectin antagonizes ethanol inhibition in P2X4 receptors. Journal of Pharmacology and Experimental Therapeutics. 2010;334:720–728. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcoholism, clinical and experimental research. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice as genetic models of high alchol consumption. Behav Genet. 2010;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic Costs of Excessive Alcohol Consumption in the U.S., 2006. American journal of preventive medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Boxall A, Long C. Veterinary medicines and the environment. Environ Toxicol Chem. 2005;24:759–760. [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Suprenant A. An antagonist insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burkhart CN. Ivermectin: an assessment of its pharmacology, microbiology and safety. Vet Hum Toxicol. 2000;42:30–35. [PubMed] [Google Scholar]
- Colombo G, Orr— A, Lai P, Cabras C, Maccioni P, Rubio M, Gessa GL, Carai MA. The cannabinoid CB1 receptor antagonist, rimonabant, as a promising pharmacotherapy for alcohol dependence: preclinical evidence. Mol Neurobiol. 2007;36:102–112. doi: 10.1007/s12035-007-0017-y. [DOI] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology. 2005;49:243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcoholism, clinical and experimental research. 2002;26:773–778. [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. Journal of psychiatry & neuroscience : JPN. 2003;28:263–274. [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Paylor R, McDonald MP, Libbey M, Ligler A, Bryant K, Crawley JN. Behavioral effects of ivermectin in mice. Lab Anim Sci. 1999;49:288–296. [PubMed] [Google Scholar]
- Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid A receptor. Journal of Pharmacology and Experimental Therapeutics. 2000;295:1051–1060. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J. 2003;24:1–8. doi: 10.1186/1475-2883-2-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Mark GP, Fretwell AM, Gililland-Kaufman KR, Strong MN, Ford MM. Reinstatement of ethanol and sucrose seeking by the neurosteroid allopregnanolone in C57BL/6 mice. Psychopharmacology. 2008;201:423–433. doi: 10.1007/s00213-008-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Ford MM, Beckley EH, Nickel JD, Eddy S, Finn DA. Ethanol intake patterns in female mice: influence of allopregnanolone and the inhibition of its synthesis. Drug Alcohol Depend. 2008;97:73–85. doi: 10.1016/j.drugalcdep.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Mark GP, Finn DA. Influence of reinforcement schedule on ethanol consumption patterns in non-food restricted male C57BL/6J mice. Alcohol. 2007a;41:21–29. doi: 10.1016/j.alcohol.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Nickel JD, Mark GP, Strong MN, Yoneyama N, Finn DA. The influence of mecamylamine on ethanol and sucrose self-administration. Neuropharmacology. 2009;57:250–258. doi: 10.1016/j.neuropharm.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behavioural Brain Research. 2007b;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends in Parasitology. 2005;21:530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gewiss M, Heidbreder C, Opsomer L, Durbin P, De Witte P. Acamprosate and diazepam differentially modulate alcohol-induced behavioural and cortical alterations in rats following chronic inhalation of ethanol vapour. Alcohol Alcohol. 1991;26:129–137. doi: 10.1093/oxfordjournals.alcalc.a045093. [DOI] [PubMed] [Google Scholar]
- Geyer J, Gavrilova O, Petzinger E. Brain penetration of ivermectin and selamectin in mdr1a,b P-glycoprotein- and bcrp-deficient knockout mice. J Vet Pharmacol Ther. 2009;32:87–96. doi: 10.1111/j.1365-2885.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacology & Therapeutics. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou P, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and alcohol dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, Hsieh JY, Lasseter KC. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. The Journal of Clinical Pharmacology. 2002;42:1122–1133. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- Harwood HJ. Updating estimates of the economic costs of alcohol abuse in the United States: Estimates, update methods, and data. In: The Lewin G, editor. National Institute on Alcohol Abuse and Alcoholism. 2000. [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jelinkova I, Vavra V, Jindrichova M, Obsil T, Zemkova HW, Zemkova H, Stojilkovic SS. Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflugers Arch. 2008;456:939–950. doi: 10.1007/s00424-008-0450-4. [DOI] [PubMed] [Google Scholar]
- Jelinkova I, Yan Z, Liang Z, Moonat S, Teisinger J, Stojilkovic SS, Zemkova H. Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem Biophys Res Commun. 2006;349:619–625. doi: 10.1016/j.bbrc.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Johnson B. Medication treatment of different types of alcoholism. Am J Psychiatry. 2010;167:630–639. doi: 10.1176/appi.ajp.2010.08101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift R. Topiramate for treating alcohol dependence: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol and Alcoholism. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neuroscience. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, Edenberg HJ, McBride WJ. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Dynamics of Neuronal Circuits in Addiction: Reward, Antireward, and Emotional Memory. Pharmacopsychiatry. 2009;42:S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA. Pharmacologically targeting the P2rx4 gene on maintenance and reinstatement of alcohol self-administration in rats. Pharmacology Biochemistry and Behavior. 2011;98:533–538. doi: 10.1016/j.pbb.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: A positive allosteric effector of the alpha 7 meuronal nicotinic acetylcholine receptor. Molecular pharmacology. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Krügel U, Kittner H, Franke H, Illes P. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse. 2003;47:134–142. doi: 10.1002/syn.10162. [DOI] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Lespine A, Dupuy J, Alvinerie M, Comera C, Nagy T, Krajcsi P, Orlowski S. Interaction of macrocyclic lactones with the multidrug transporters: the bases of the pharmacokinetics of lipid-like drugs. Curr Drug Metab. 2009;10:272–288. doi: 10.2174/138920009787846297. [DOI] [PubMed] [Google Scholar]
- Lespine A, Martin S, Dupuy J, Roulet A, Pineau T, Orlowski S, Alvinerie M. Interaction of macrocyclic lactones with P-glycoprotein: Structure-affinity relationship. Eur J Pharm Sci. 2007;30:84–94. doi: 10.1016/j.ejps.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE. The genetics of mouse behavior in novel situations. Journal of comparative and physiological psychology. 1959;52:62–67. doi: 10.1037/h0044664. [DOI] [PubMed] [Google Scholar]
- Merck, Sharp, Dohme . Poision control monograph Ivermectin. Div of Merck & Co Ltd; W. P., Pennsylvania: 1988. [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Molinari G, Soloneski S, Larramendy ML. New ventures in the genotoxic and cytotoxic effects of macrocyclic lactones, Abamectin and Ivermectin. Cyogenet Genome Res. 2010;128:37–45. doi: 10.1159/000293923. [DOI] [PubMed] [Google Scholar]
- Neasta J, Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci USA. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S. Ivermectin: 25 years and still going strong. Int J Antimicrobial Agents. 2008;31:91–98. doi: 10.1016/j.ijantimicag.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, Peoples R, Alkana R, Davies D. Ethanol is a fast channel inhibitor of purinergic P2X4 receptors. J Pharm Exp Ther. 2011;337:171–179. doi: 10.1124/jpet.110.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacology & Therapeutics. 2010;127:53–65. doi: 10.1016/j.pharmthera.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt RL, Li K, Alkana RL, Davies DL. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem. 2010;112:307–317. doi: 10.1111/j.1471-4159.2009.06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Richard-Lenoble D, Chandenier J, Gaxotte P. Ivermectin and filariasis. Fundam Clin Pharmacol. 2003;17:199–203. doi: 10.1046/j.1472-8206.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcoholism, clinical and experimental research. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Rodgers DA. Research activities related to treatment of alcoholism. Comprehensive psychiatry. 1966;7:57–67. doi: 10.1016/s0010-440x(66)80007-x. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism, clinical and experimental research. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcoholism, clinical and experimental research. 2000;24:766–773. [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcoholism, clinical and experimental research. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Sattelle DB, Buckingham SD, Akamatsu M, Matsuda K, Pienaar I, Jones AK, Sattelle BM, Almond A, Blundell CD. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human [alpha]7 nicotinic acetylcholine receptors. Biochemical Pharmacology. 2009;78:836–843. doi: 10.1016/j.bcp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Silberberg SD, Li M, Swartz KJ. Ivermectin interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron. 2007;54:263–274. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor clonned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinosa HS, Stilck SRAN, Bernardi MM. Possible anxiolytic effects of ivermectin in rats. Vet Res Commun. 2002;26:309–321. doi: 10.1023/a:1016094726033. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an {alpha}4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YJ, Long DX, Li W, Hou WY, Wu YJ, Shen JZ. Effects of avermectins on neurite outgrowth in differentiating mouse neuroblastoma N2a cells. Toxicology Letters. 2010;192:206–211. doi: 10.1016/j.toxlet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson H, Kechris K, Bell RL, Hübner N, Heinig M, Pravenec M, Mangion M, Legault L, Dongier M, Conigrave KM, Whitfield JB, Saunders JB, Grant B, Hoffman PL. Genetical genomic determinants of alcohol consumption in rats and humans BMC Biology. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchuck MA, Yoneyama N, Ford MM, Fretwell AM, Finn DA. Assessment of GABA-B, metabotropic glutamate, and opioid receptor involvement in an animal model of binge drinking. Alcohol. 2011;45:33–44. doi: 10.1016/j.alcohol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trailovic AM, Trailovic N. Central and Peripheral Neurotoxic effects of ivermectin in rats. J Met Med Sci. 2010;73:591–599. doi: 10.1292/jvms.10-0424. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. British journal of pharmacology. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. Journal of Pharmacology and Experimental Therapeutics. 1993;267:250–258. [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. Journal of Pharmacology and Experimental Therapeutics. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Hu XQ, Stewart RR, Weight FF, Li C. The mechanism by which ethanol inhibits rat P2X4 receptors is altered by mutation of histidine 241. Br J Pharmacol. 2005;145:576–586. doi: 10.1038/sj.bjp.0706192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. Inhibition by ethanol of rat P2X 4 receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;130:1394–1398. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X 2 and P2X 4 receptor function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]