Abstract
Retinal S-antigen and interphotoreceptor retinoid-binding protein-3 play a significant role in the etiopathogenesis of Eales' disease. Protein 3D structures are functionally very important and play a significant role in progression of the disease, hence these 3D structures are better target for further drug designing and relative studies. We developed 3D model structure of retinol-binding protein-3 and retinal S-antigen protein of human involved in Eales' disease. Functional site prediction is a very important and related step; hence, in the current course of analysis, we predicted putative functional site residues in the target proteins. Molecular models of these proteins of Eales' disease as documented in this study may provide a valuable aid for designing an inhibitor or better ligand against Eales' disease and could play a significant role in drug design.
Keywords: Eales' disease, Retinol-binding protein-3, Retinal S-antigen, Hemorrhages, Venoocclusive, Modeling, Functional sites
Introduction
Eales’ disease is an idiopathic, inflammatory, venoocclusive disorder of eye, which is predominantly characterized by recurrent vitreous hemorrhages, induced inflammation with white haze layer around the outer coat of the veins in retina [1]. It eventually leads to sudden appearance of black spots in front of the eye or painless loss of vision [2]. Eales’ disease most commonly affects healthy young adult males and is an important cause of preventable blindness in young adults [3]. The predominant age of onset of symptoms is 20–30 years [4]. Eales’ disease is characterized by retinal periphlebitis, peripheral retinal ischemia, and hemorrhage [5]. Eales’ disease is distinctively characterized both by stage of inflammation as well as stage of proliferation [6].
Retinol S-antigen and interphotoreceptor retinoid-binding protein play a significant role in the etiopathogenesis of this condition. This retinal S-antigen protein has already been purified by chromatographically from whole retina [7]. The 48-kDa protein, a soluble protein found in rod outer segments, is purified through its specific binding to photo excited rhodopsin and is involved in the quenching of light-induced guanosine 3′, 5′-monophosphate-phosphodiesterase activity [8]. A previous study has already proved about the significance of this protein in Eales’ disease [9]. Lymphocyte proliferative responses were tested in vitro against native S-antigen, its uveitopathogenic peptides (peptide M and peptide G), yeast histone H3 peptide and uveitopathogenic fragment of Interphotoreceptor retinoid-binding protein (IRBP; R16) to establish their role in the pathogenesis of Eales' disease [9]. These significant findings prove about the role of these proteins with reference to Eales’ disease.
As it is well known that protein 3D structures is functionally very important and play a significant role in progression of disease, these structures can be used as a better target for further drug designing and relative studies against Eales' disease [10].
Thus, on the basis of functionality of selected proteins involved in Eales’ disease, we developed 3D model structure of retinol-binding protein-3 and retinal S-antigen protein of human. For structure development, we used homology modeling approach as the protein sequences of these proteins are available in GenBank. Further, for obtaining more refined structure, we also determined the putative functional site for the model proteins by using various prediction servers and obtained structure are validated by molecular docking.
Material and Methods
Sequence retrieval
The protein sequence of retinol-binding protein-3 and retinal S-antigen from Homo sapiens was obtained from GenBank (Accession number: NP_002891.1 and NP_000532.2) and furnished as query sequence for comparative modeling.
Physicochemical characterization
Theoretical isoelectric point (pI), molecular weight, number of positive and negatively charged residues, extinction coefficient, instability index, aliphatic index and grand average hydropathicity (GRAVY) were predicted using the Expasy’s protparam server [11] (Table 1).
Table 1.
Physicochemical parameters of retinol-binding protein-3 and retinal S-antigen
| Name of proteins | Isoelectric point (pI) | Molecular Weight (MW) | Negatively charged residues (−R) | Positively charged residues (+R) | Extinction coefficient (EC) | Instability index (II) | Aliphatic index (AI) | GRAVY |
|---|---|---|---|---|---|---|---|---|
| Retinol-binding protein-3 | 4.98 | 135,362.60 | 144 | 85 | 136,640 | 43.45 | 100.31 | −0.041 |
| Retinal S-antigen | 6.14 | 45,119.50 | 55 | 51 | 26,485 | 34.90 | 88.27 | −0.335 |
Comparative modeling
The protein sequence was subject for comparative homology modeling via Swiss model to generate putative 3D model. The Swiss model performs the sequence alignments and searches the putative template protein for generating the 3D model of query sequence [12–14]. The constructed models were energy minimized by CHIMERA [15]. The 3D structures were further verified by VERYFY3D [16, 17]. RMS-Z score for bond angles of modeled protein structures were estimated by QMEAN Server [18]. The generated models are viewed by Chimera tool.
Functional site prediction
The Profunc and Q site finder software’s were used for structure-based functional site prediction [19–21].
Results
Molecular Modeling
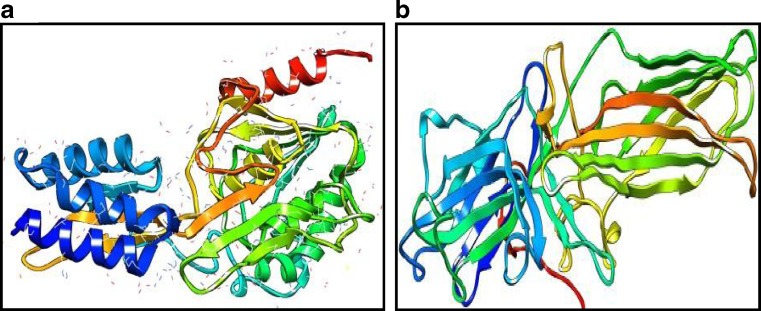
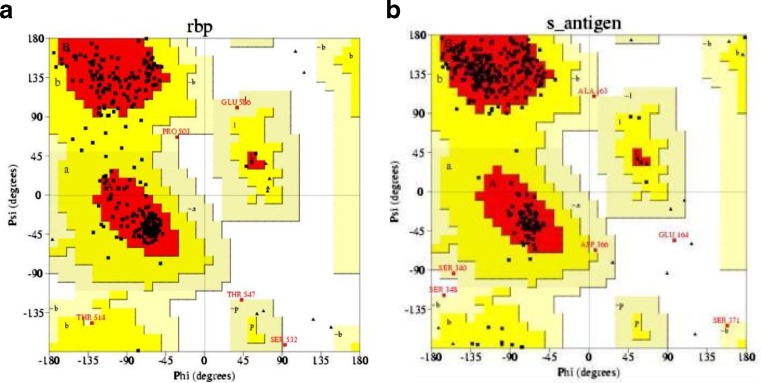
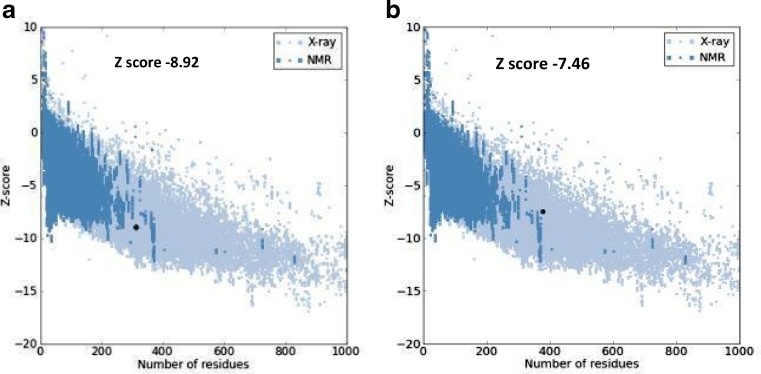
Revealing the structure of retinol-binding protein-3 and retinal S-antigen following parameters of modeled proteins structure by using Swiss model software were identified. The Swiss model software generated the 3D model structures of query proteins NP_002891.1 and NP_000532.2 at resolution of 1.80 and 2.90 Å. Based on template protein 1J7X(A) and 1JSY(A) with sequence identity of 53.67% and 55.20% , E value of 0.00 and 0.00e−1 (Fig.1). The Ramachandran plot analysis (by PROCHECK) of the retinol-binding protein-3 and retinal S-antigen model structures showed that of the residues 98.50% and 98.20% are in favored and additional allowed regions respectively (Fig. 2). Quality assessment of the model via ProSA revealed that the both models matched with NMR region of the plot with a Z score of −8.92 and −7.46 (Fig. 3). This score gives authenticity about good quality of models generated in this study. Energy minimization was performed by Chimera which optimized the model structure RBP from initial energy −8,307.710 kJ/mol to final energy of −9714.836 kJ/mol and for retinal S-antigen initial energy is −23,173.338 kJ/mol to final energy −25,732.042 kJ/mol.
Fig. 1.
Modeled 3D structure of a retinol-binding protein-3, b retinal S-antigen using Swiss model
Fig 2.
Ramachandran plot of a retinol-binding protein-3, b retinal S-antigen model generated using Swiss model. The Ramachandran plot calculations on 3D structure of retinol-binding protein-3 and Retinal S-antigen were computed using PROCHECK in SAVS server
Fig 3.
ProSA analysis of modeled protein structure of retinol-binding protein-3 and retinal S-antigen
Functional site residues
Functional site prediction is a very important and related step for such kind of work. Servers such as Profunc and Q site finder which enumerated the putative active site residues in the model structure are used for this analysis. These servers analysis resulted in the following putative functional site residues of the target protein viz: For retinol-binding protein-3 it is PRO409, GLY410, LEU415, VAL429, PHE430, SER446, ALA448, TRP465, TYR495 and PRO527 and for retinal S-antigen ASP37, HIS38, VAL39, ASP128, TYR129, LEU130, PRO131, CYS132, SER133, ARG179, LYS180, GLN182, ASN309, ALA311, THR314, ILE315, and MET360.
Discussion
Thus, on the basis of significant importance at the level of functionality as well as their involvement in progression of in Eales’ disease, we developed a 3D model structure of retinol-binding protein-3 and retinal S-antigen protein of human by in silico modeling approach. As it is well accepted that Swiss model software develops more accurate and authentic protein structure model, for the above reason, we selected this software for our query sequence. It is further added to the credit of our modeled structures that they are also verified and validated by PROCHECK and ProSA analysis softwares. These methods confirm the accuracy of Swiss model-generated protein 3D structures.
Following the very basic assumption of protein biology, i.e., structure to function analysis these structural information can give us idea about the functionality of proteins. This structural-based functional site prediction method enumerates the putative amino acid residues from our protein models of retinol-binding protein-3 and retinal S-antigen. It is also illustrative that these above-mentioned identified residues are involved in making ligand binding site pockets.
The sequence and functional site prediction server gives conformity about the query sequence of RBP-3 and retinal S-antigen protein is putative. Model development and functional site prediction of these proteins will give great focus in understanding the role of amino acids and helps in deciphering the involvement of these residues during mechanism of protein ligand interaction. Molecular models of retinol-binding protein-3 and retinal S-antigen of Eales’ disease as documented in this study may provide a valuable aid in designing an inhibitor or better ligand molecule against Eales’ disease and could play a significant role in drug design. This predictive approach may become applicable for novel drug developmental studies against this idiopathic disorder as there is no proper drug for the cure of Eales' disease.
Acknowledgments
We wish to express our acknowledgement to Mr. Aseem Chauhan, Additional President, RBEF parent organization of Amity University Uttar Pradesh (AUUP) and chairman of Amity University Lucknow Campus; Maj. Gen. K.K Ohri, AVSM (Retd.), Director General of AUUP- Lucknow Campus, Asst. Professor Dr. Rajesh K. Tiwari, Deputy Director and Head, Amity Institute of Biotechnology, Lucknow and Professor Shally Awasthi, Dept. of Pediatrics, CSM Medical university, Lucknow, UP for providing their valuable support and encouragement throughout the work.
References
- 1.Sen A, Paine SK, Chowdhury IH, Mukherjee A, Choudhury S, Mandal LK, Bhattacharya B. Assessment of gelatinase and tumor necrosis factor-a level in the vitreous and serum of patients with Eales disease: Role of Inflammation-Mediated Angiogenesis in the Pathogenesis of Eales Disease .Retina. 2011. doi: 10.1097/IAE.0b013e318203c199. [DOI] [PubMed]
- 2.Saxena S, Kumar D, Singh VK, Rajasingh J. Immunological studies in Eales disease: a review. Afro-Asian J Ophthalmol. 1995;13:19–22. [Google Scholar]
- 3.Therese KL, Deepa P, Therese J, Bagyalakshmi R, Biswas J, Madhavan HN. Association of mycobacteria with Eales’ disease. Indian J Med Res. 2007;126:56–62. [PubMed] [Google Scholar]
- 4.Narayanasamy A, Radhakrishnan S, Rishi P, Jyotirmoy B, Shah J, Joyce T. Ratio of the vitreous vascular endothelial growth factor and pigment epithelial-derived factor in Eales disease. J Ocular Biol Dis Informat. 2009;2:20–28. doi: 10.1007/s12177-009-9017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das T, Biswas J, Kumar A, et al. Eales’ disease. Indian J Ophthalmol. 1994;42:3–18. [PubMed] [Google Scholar]
- 6.Das T, Pathengay A, Hussain N, Biswas J. Eales’ disease: diagnosis and management. Eye. 2010;24:472–482. doi: 10.1038/eye.2009.315. [DOI] [PubMed] [Google Scholar]
- 7.Saxena S, Pant AB, Khanna VK, Singh K, Shukla RK, Meyer CH, Singh VK. Tumor necrosis factor-a-mediated severity of idiopathic retinal periphlebitis in young adults (Eales’ disease): implication for anti-TNF-a therapy. J Ocular Biol Dis Informat. 2010;3:35–38. doi: 10.1007/s12177-010-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfister C, Chabre M, Plouet J, Tuyen VV, Kozak Y, Faure JP, Kuhn H. Retinal S antigen identified as the 48 K protein regulating light-dependent phosphodiesterase in rods. Science. 1985;228:891–893. doi: 10.1126/science.2988124. [DOI] [PubMed] [Google Scholar]
- 9.Saxena S, Rajasingh J, Biswas S, Kumar D, Shinohara T, Singh VK. Cellular immune response to retinal S-antigen and interphotoreceptor retinoid-binding protein fragments in Eales' disease patients. Pathobiology. 1999;67:39–44. doi: 10.1159/000028049. [DOI] [PubMed] [Google Scholar]
- 10.Pathan S, Chintan C, Sriram S. Intrinsically unstructured proteins: potential targets for drug discovery. Am J Infect Dis. 2009;5:133–141. doi: 10.3844/ajidsp.2009.133.141. [DOI] [Google Scholar]
- 11.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy Server; John M. Walker, editors: The Proteomics Protocols Handbook, Humana. 2005. p. 571–607.
- 12.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 13.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:387–392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peitsch MC. Protein modeling by e-mail bio/technology. Nature. 1995;13:658–660. [Google Scholar]
- 15.Thomas DG, Conrad CH, Thomas EF. Software extensions to UCSF Chimera for interactive visualization of large molecular assemblies. Structure. 2005;13:473–482. doi: 10.1016/j.str.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Bowie JU, Lüthy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991;253:164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- 17.Lüthy R, Bowie JU, Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992;356:83–85. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- 18.Benkert P, Künzli M, Schwede T. QMEAN server for protein model quality estimation. Nucleic Acids Res. 2009;37:510–514. doi: 10.1093/nar/gkp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskowski RA, Watson JD, Thornton JM. ProFunc: a server for predicting protein function from 3D structure. Nucleic Acids Res. 2005;33:89–93. doi: 10.1093/nar/gki414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alasdair TR, Richard M. Q-Site Finder: an energy-based method for the prediction of protein–ligand binding sites. Bioinformatics. 2005;21:1908–1916. doi: 10.1093/bioinformatics/bti315. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava P, Tiwari A, Trivedi AC. Computational prediction of 3D structure for the matrix protein 2 (BM2) of the influenza B virus. Intl Med Technol Univ Med J. 2010;1:22–36. [Google Scholar]