Abstract
There is growing evidence that schizophrenia (SZ) and bipolar disorder (BD) overlap significantly in risk factors, neurobiological features, clinical presentations, and outcomes. SZ is characterized by well documented gray matter (GM) abnormalities in multiple frontal, temporal and subcortical structures. Recent voxel-based morphometry (VBM) studies and meta-analyses in BD also report GM reductions in overlapping, albeit less widespread, brain regions. Psychosis, a hallmark of SZ, is also experienced by a significant proportion of BD patients and there is evidence that psychotic BD may be characterized by specific clinical and pathophysiological features. However, there are few studies comparing GM between SZ and psychotic BD. In this study we compared GM volumes in a sample of 58 SZ patients, 28 BD patients experiencing psychotic symptoms and 43 healthy controls using whole-brain voxel-based morphometry. SZ patients had GM reductions in multiple frontal and temporal regions compared to healthy controls and in the subgenual cortex compared to psychotic BD patients. GM volume was increased in the right posterior cerebellum in SZ patients compared to controls. However, psychotic BD patients did not show significant GM deficits compared to healthy controls or SZ patients. We conclude that GM abnormality as measured by VBM analysis is less pronounced in psychotic BD compared to SZ. This may be due to disease-specific factors or medications used more commonly in BD.
Keywords: psychosis, voxel-based morphometry, cerebral cortex, schizoaffective disorder
Introduction
Although schizophrenia (SZ) and bipolar disorder (BD) have been classically conceptualized as dichotomous, these two conditions overlap in their genetic and developmental risk factors, have common clinical presentations and treatments, and display qualitatively similar neuropsychological profiles, suggesting that they may lie on a single disease continuum or may be differential expressions of a common pathology (Craddock and Owen, 2005; van Os, 2009). One of the best characterized brain abnormalities in SZ is gray matter (GM) reductions, consistently reported by numerous morphometric studies using region of interest (ROI) or whole-brain voxel-based morphometry (VBM) analyses. On the other hand, the majority of structural neuroimaging studies in BD are ROI based and this literature consists of a small number of studies examining a limited number of structures with small sample sizes (Kempton et al. 2008). Though VBM studies in BD are increasing in number in recent years, findings remain contradictory (Ellison-Wright and Bullmore, 2010). Nonetheless, two of the three recent meta-analyses of VBM studies in BD revealed GM reductions in the insula and anterior cingulate cortex (ACC) (Bora et al., 2010; Ellison-Wright and Bullmore, 2010). A third meta-analysis reported GM reductions in bilateral frontal cortices, cingulate gyrus, left middle temporal gyrus, and thalamus, and increases in the basal ganglia and right pre/postcentral gyri (Yu et al., 2010). GM reductions are typically more extensive in SZ than in BD patients (Ellison-Wright and Bullmore, 2010) even when SZ studies are selected to match the mean age of onset and illness duration of BD studies (Yu et al., 2010).
Psychotic symptoms are a hallmark of SZ, but, they are also experienced by a significant proportion of patients with BD (Pope and Lipinski, 1978). There is evidence that BD with psychotic features differs from non-psychotic BD in family history (Potash et al., 2001), clinical course and outcome (Bellivier et al., 2001; Coryell et al., 2001), cognitive functions (Glahn et al., 2007) and biological signature as identified by electrophysiological and neuroimaging studies (Olincy and Martin, 2005; Strasser et al., 2005). Intriguingly, deficits in executive function and working memory, electrophysiological disturbances and white matter deficits in psychotic BD were similar to SZ in some studies, suggesting cross-diagnostic abnormalities shared by psychotic disorders (Bora et al., 2008). On the other hand, there are few morphometric studies comparing psychotic BD patients to healthy controls, and these are mainly ROI based. Majority of these studies report no volumetric abnormalities in the examined regions including the amygdalohippocampal complex (see Velakoulis et al., 1999 for an exception), thalamus, left planum temporale and Heschl’s gyrus, fusiform gyrus, superior temporal gyrus (STG) and insula (Bora et al., 2008). Volume reductions were reported only in the subgenual cingulate cortex (Hirayasu et al., 1999), left temporal lobe (Kasai et al., 2003a) and inferior temporal gyrus (Kuroki et al., 2006) while one study reported increased striatal volume (Getz et al., 2002). However, VBM studies in psychotic BD are sparse and report discrepant findings including no difference (Kubicki et al., 2002; McDonald et al., 2005), decreases (Cui et al., 2010; Tost et al., 2009) or increases (Cui et al., 2010). Furthermore, these studies are characterized by several limitations, including small sample sizes (Farrow et al., 2005), or inclusion of heterogeneous patient groups with affective psychosis (Morgan et al., 2007),
Taken together, the literature suggests that GM volume abnormalities are pronounced and widespread in SZ but inconsistently found in BD with psychotic features. In addition, there are few studies using an unbiased VBM approach and examining patients with both conditions in the same study. Therefore, it is currently not clear whether the negative findings in the BD literature are a result of sample selection and technical issues or represent a true biological pattern. In this study we compared the GM volume of patients with BD currently experiencing psychotic symptoms with a sample of patients with SZ and schizoaffective disorder, and with healthy controls. We hypothesized that the SZ group would show decreased GM compared with controls in a distributed set of brain regions consistent with previous studies. We also hypothesized that the BD patient group would show GM volume reductions in overlapping frontal and temporal regions but these would be in a limited distribution and less significant in magnitude than those seen in SZ.
Materials and Methods
Subjects
Sixty-one patients with DSM-IV diagnosed schizophrenia, 32 patients with bipolar I disorder, and 45 normal control subjects (NC) participated in this study. Subjects were drawn from samples recruited for 3 separate studies, from the Psychotic Disorders Division, McLean Hospital. There were no changes in the software or hardware of the scanner between studies. Controls and SZ patients were recruited from all three studies and BD patients from two of them. Usable data were obtained from 58 SZ, 28 BD and 43 NC subjects. SZ group included 21 schizoaffective (SZA) patients hospitalized for psychotic symptoms who were not in a current mood episode. All patients were experiencing psychotic symptoms and treated with medication at the time of the scan. The BD group consisted primarily of patients in a manic episode (n=18), but also included patients in mixed (n=5) and depressive (n=1) episodes, as well as euthymic patients with residual psychotic features (n=4). Exclusion criteria included: 1) being ≥ 60 or ≤ 18 years old; 2) having contraindication for magnetic resonance (MR) scanning; and 3) having a history of neurological disorder, mental retardation or head trauma.
Subjects were recruited following approval by the McLean Hospital Instutional Review Board. Patients were assessed with the Structured Clinical Interview for DSM-IV (SCID), Positive and Negative Syndrome Scale (PANNS), Young Mania Rating Scale (YMRS), and Montgomery-Asberg Depression Rating Scale (MADRS) on scan day. We did not have the information on YMRS and MADRS scores (n=14) and antipsychotic and anticonvulsant drug doses (n=12) for one set of SZ patients. In addition, handedness information was missing for several participants (n=22). Normal controls were assessed by the SCID and no axis I disorder was identified. The demographic and clinical variables are in Table 1.
Table 1.
Demographic and clinical variables of the subjects.
| Normal controls | Bipolar Disorder | Schizophrenia | F/t | P | |
|---|---|---|---|---|---|
| Age | 36.4 (10.5) | 32.9 (11.9) | 38.7 (10.6) | 2.446 | 0.091 |
| Sex (F/M) | 20/38 | 10/17 | 15/28 | 0.973 | |
| Handedness (R/L) | 23/2 | 23/1 | 51/6 | 0.482 | |
| PANSS total | N/A | 56.1 (13.9) | 62.3 (19.1) | 1.504 | 0.136 |
| PANSS positive | N/A | 19.1 (6.6) | 18.1 (7.3) | 0.5638 | 0.574 |
| YMRS | N/A | 22.8 (10.3) | 13.2 (8.3) | 4.345 | < 0.001** |
| MADRS | N/A | 10.2 (5.2) | 10.8 (8.2) | 0.3653 | 0.716 |
| CPZ equivalent | N/A | 301.2 (326.0) | 447.2 (429.5) | 1.854 | 0.068 |
| Lithium | N/A | 13 (48.1 %) | 7 (15.2 %) | 0.006* | |
| Anticonvulsants | N/A | 13 (48.1 %) | 10 (21.7 %) | 0.035* |
The following items had missing data: YMRS and MADRS (n=14), handedness (n=22), antipsychotic and anticonvulsant doses (n=12).
Image Acquisition
All subjects underwent a structural MRI scan in a single Siemens 3 Tesla Trio scanner (Erlangen, Germany) with a quadrature radio frequency (RF) coil (Bioengineering, Inc., Minneapolis, Minnesota) using three dimensional (3-D) inversion-prepared sagittal fast low-angle shot (FLASH) sequence. Echo time/repetition time/time to inversion (TE/TR/TI) were 2.74 msec/2.1 sec/1.1 sec; echo spacing 6.3 msec; 12° flip angle. Field of view (FOV) was 256 mm with 1×1 mm pixels and 1.33 mm slices.
VBM and Statistical Analysis
T1 image files were converted to analyze format using MRicro (http://www.sph.sc.edu/comd/rorden/mricro.html). All images were then visually inspected. Optimized VBM was carried out with FSL-VBM analysis software v1.1 (Good et al. 2001). First, non-brain structures were removed by brain extraction tool (BET) v2.1 (http://www.fmrib.ox.ac.uk/fsl/bet2/index.html). Next, extracted images were segmented into GM, white matter and cerebrospinal fluid and GM images were aligned to the ICBM-152 template via non-linear registration. The registered GM images were averaged to create a study specific template at 2×2×2 mm3 resolution and the native GM images were non-linearly registered to this template. ‘Modulation’ was introduced to compensate for the arising enlargement or contraction of the GM volume during normalization by dividing by the Jacobian of the warp field. Modulated GM images were then smoothed with a full width at half maximum (FWHM) ~12 mm isotropic Gaussian kernel. FWHM ~7 mm kernel was used in repeat analysis for the contrasts with negative results because smaller kernels are better suited to studying abnormalities in smaller structures (Honea et al., 2005).
GM differences were analyzed by voxelwise general linear model implemented in FSL, using an analysis of covariance (ANCOVA) model controlling for age, gender and total GM. Permutation-based non-parametric testing (5000 permutations) FWE-corrected for multiple comparisons was used with threshold-free cluster enhancement (TFCE); statistical significance was set at p < 0.05. Finally, we carried out an additional analysis of the SZ group excluding individuals diagnosed with SZA. The threshold masked images were then visualized on the MNI-152 template.
Between-group differences in demographic and clinical variables were calculated using analysis of variance (ANOVA), t-tests, and chi-square tests as appropriate. Statistical significance was set at p < 0.05, two-tailed.
Results
There were no between-group differences in age, gender and handedness. BD patients had significantly higher YMRS scores (p<0.001) than the SZ and NC groups, and there were more patients in the BD group using lithium (p=0.006) and anticonvulsant drugs (p=0.035). No other clinical variable was different between groups (Table 1).
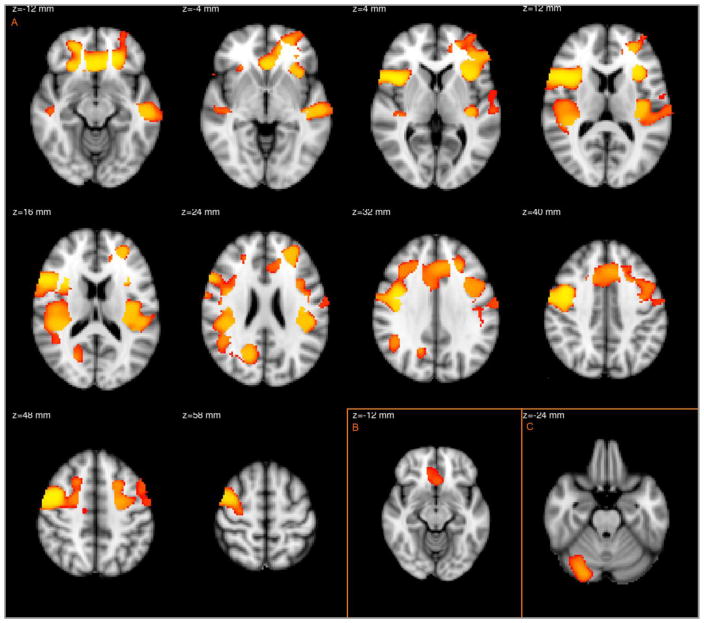
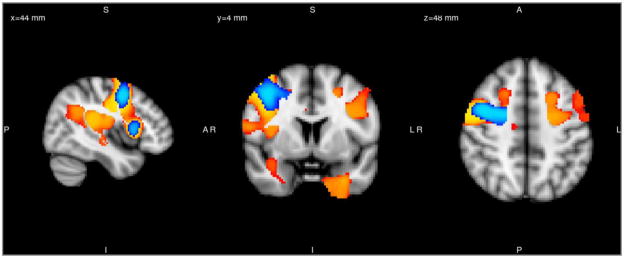
The voxelwise comparison between SZ and NC revealed decreased GM volume in multiple prefrontal and temporal regions distributed in three clusters (Table 2, Figure 1A). The regions included bilateral ACC (dorsal, ventral and subgenual), bilateral orbitofrontal cortex, bilateral inferior frontal gyri, bilateral insula, bilateral precentral gyrus extending to middle frontal gyrus, bilateral planum temporale with parietal operculum cortex, right STG, left superior, middle and inferior temporal gyri and right precuneal cortex. SZ patients had increased GM volume in a single brain region, the right cerebellum, posterior lobe (Figure 1C). When compared with BD patients, SZ patients had decreased GM in bilateral subgenual cortex (Figure 1B). Excluding SZA patients revealed decreased GM in overlapping regions in the right precentral gyrus with middle frontal gyrus and right frontal operculum with inferior frontal gyrus in the Controls>SZ contrast. (Figure 2).
Table 2.
Clusters and local maxima with significant gray matter differences in group comparisons
| Contrast | Cluster size (voxels) | MNI coordinates of local maxima(mm) | Anatomical region | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 24162 | 46 | 14 | 4 | R frontal operculum cortex | |
| Control>SZ | |||||
| 318 | 38 | 2 | −20 | R insula | |
| 19 | 14 | −14 | 48 | R precentral gyrus | |
| BD>SZ | 405 | −2 | 24 | −16 | L subgenual cortex |
| SZ>Control | 929 | 28 | −82 | −26 | R cerebellum, posterior lobe |
Only the anatomical regions corresponding to the local maxima of the clusters are listed. Some clusters cover more than one anatomical region and the full list of regions can be found in the Results section.
Figure 1.
Regions with decreased GM in schizophrenia patients for controls>SZ (A) and BD>SZ (B) contrasts and increased GM for SZ>controls (C) contrast.
Figure 2.
Regions with significant gray matter reductions in the schizophrenia group after excluding schizoaffective patients are displayed (blue) and superposed on the analysis with the original schizophrenia patient group (yellow).
By contrast, the BD patients did not show any significant GM volume differences when compared with controls or SZ patients.
No brain region in either patient group showed a correlation between GM volume and PANSS positive scores, or chlorpromazine dose equivalents of antipsychotic medications.
Finally, whole-brain GM was compared between lithium users (n=18) and non-users (n=22) in the subsample of patients with BD and schizoaffective disorder bipolar subtype. These two groups matched in all clinical and demographic variables. No significant GM differences were found in this analysis.
Discussion
Using a whole-brain VBM approach in a cross-diagnostic sample of patients with psychotic disorders, we found that SZ patients showed GM volume reductions in multiple cortical regions compared to controls and BD patients, whereas psychotic BD patients did not. All of the regions with decreased GM compared to controls were previously highlighted by several VBM studies according to a pooled data meta-analysis of VBM studies in SZ (Honea et al., 2005). Our findings also match the results in the recent coordinate-based meta-analyses (Chan et al., 2009; Ellison-Wright and Bullmore, 2010; Ellison-Wright et al., 2008; Glahn et al., 2008; Yu et al., 2010). We did not find abnormalities in some of the regions reported in previous studies (e.g. hippocampus) possibly due to differences in sample characteristics and methodological variances in this literature.
SZ patients had decreased GM only in the subgenual cortex compared to BD patients. This result is in line with an ROI study that found a trend level decrease in the GM in the subgenual cortex of first episode SZ patients compared to first episode affective psychosis patients (predominantly manic) (Hirayasu et al., 1999). Other studies report GM deficits in SZ compared to affective psychosis patients in several frontal, temporal, parietal and subcortical structures by VBM or ROI analysis (Cui et al., 2010; Hirayasu et al., 2000; Kasai et al., 2003b; Kasai et al., 2003c; Kubicki et al., 2002; Lee et al., 2002; McCarley et al., 2002; McDonald et al., 2005), no difference in the ACC (Koo et al., 2008). However, except the report by Cui et al. all of these studies were in first episode samples and given the differences in the progress of the GM abnormalities in these disorders (Kasai et al., 2003b) comparisons in first episode samples may differ from chronic samples. Our patients were primarily chroncially ill, but unlike the Cui et al. study we did not recruit only patients with a family history of SZ or BD, and the threshold and the correction methods were also different in our analysis which may explain the discrepant findings.
We also report higher GM volume in the right cerebellum in the SZ group compared to controls. There are now several lines of evidence supporting that cerebellar pathology contributes to the cognitive, affective, perceptual and motor deficits seen in SZ (Picard et al., 2008). However, morphometric studies measuring the volume or GM volume of this region have given conflicting results, showing a decrease (Chua et al., 2007; Tanskanen et al., 2008), an increase (Ha et al., 2004; Suzuki et al., 2002), or no difference (Hulshoff Pol et al., 2002; James et al., 2004) in SZ patients. In one particular study reporting increased GM in the cerebellum (Ha et al., 2004), only patients with paranoid SZ were included. Although we did not restrict our sample to any subtype, the majority of our patients were of the paranoid subtype. Further studies are needed to clarify the determinants of GM volume in the cerebellum in SZ.
We did not find significant GM volume abnormalities in bipolar patients with psychotic symptoms. This is consistent with the majority of the morphometric studies examining medial and lateral temporal lobe structures, insula and thalamus in psychotic BD (summarized in the introduction section) and some of the earlier VBM studies in BD with or without psychotic features (Bruno et al., 2004; Kubicki et al., 2002; McDonald et al., 2005; Scherk et al., 2008). However, several other VBM studies have reported decreased (Almeida et al., 2009; Ha et al., 2009; Haldane et al., 2008; Lyoo et al., 2004; McIntosh et al., 2004; Nugent et al., 2006; Stanfield et al., 2009; Tost et al., 2009) and increased (Adler et al., 2007; Adler et al., 2005; Haldane et al., 2008; Kempton et al., 2008) GM in a variety of brain regions in BD. Unlike most of the above mentioned VBM studies, our sample consisted exclusively of psychotic BD I patients, with the majority of patients in a manic or mixed episode. To our knowledge, there are six previous VBM studies that included bipolar patients with psychotic features and compared the GM volume with healthy controls. One of these studies included only 8 bipolar patients (Farrow et al., 2005) and another did not separately report the results for bipolar patients included in the affective psychosis patient group (Morgan et al., 2007). Consistent with our results, in a sample of 25 SZ and 37 BD I patients with a history of psychotic symptoms, McDonald et al. found no regions of GM abnormality in the BD group, while SZ patients had widespread reductions in the frontotemporal neocortex, insula, medial temporal lobe and thalamus (McDonald et al., 2005). In a first episode psychosis study, affective psychosis patients who were predominantly in a mixed or manic episode (n=14/16) had no significant GM alterations in the whole-brain VBM analysis, while first episode SZ patients had widespread reductions in frontal, temporal and parietal areas (Kubicki et al., 2002). In the other two studies, reductions were found in the middle frontal gyrus, lateral prefrontal cortex, cingulate cortex, middle and superior temporal gyri, occipital cortex and caudate nuclei (Cui et al., 2010; Tost et al., 2009), and in one study, GM was increased in the cerebellum and right putamen (Cui et al., 2010).
There are several limitations associated with this study. First, all except one bipolar and four SZ patients were using antipsychotic medications at scan time. However, it is unlikely that our findings can be explained by antipsychotic effects because the patient groups did not differ in chlorpromazine equivalents of antipsychotics used and there were no correlations between GM volume and antipsychotic dose. By contrast, almost half of the BD patients (13/28) were using lithium and/or anticonvulsants. We compared the whole-brain GM between lithium users and non-users in a subsample composed of BD patients and SZA disorder bipolar subtype patients and found no difference. Although the size of the subsample was small, this result implies that lithium effects do not explain our BD findings. Nevertheless, we cannot exclude the effect of mood stabilizers on our results as lithium and anticonvulsants have documented trophic effects on GM (Manji et al., 2000). Moreover, our study did not account for any potential effects of chronic antipsychotic use on GM volume, which have been recently reported (Ho et al., 2011). A second limitation was that the SZ patients were grouped with SZA patients (n=21). When we excluded SZA patients significant GM reductions were found in an overlapping but more limited distribution (Figure 2). The presence of patients with affective syndromes would be expected to make the results more similar to those in the BD group. The absence of such an effect suggests that our findings were not obscured by the addition of SZA patients. Finally, our sample size for the BD group was at the cusp of what is acceptable for VBM analyses and it is possible that the sample size was not large enough to detect clinically relevant GM abnormalities. For example, the BD group had no significant differences except higher GM volume in subgenual cortex compared to SZ patients. This may suggest that the BD group has reduced GM volume, but not as severe as that seen in SZ. Nonetheless, recruiting psychotic BD patients for neuroimaging studies is challenging and our BD sample size was larger than that of most published morphometric studies with this population.
Recruiting patients all of whom were experiencing active psychotic symptoms, we uncovered discrepant patterns in GM between psychotic BD and SZ suggesting that GM volume alterations are a more prominent feature of SZ. The lack of GM abnormalities in psychotic BD may reflect disease-specific pathophysiology but we cannot rule out the effects of mood-stabilizing medications. A key difference between BD and SZ is the disease course, with BD characterized by non-psychotic baseline between exacerbations. The lack of GM differences in BD could be a reflection of the lesser cumulative burden on an underlying circuit that is selectively taxed during psychotic episodes. Alternatively, the absence of observed GM abnormalities in BD may be causally related to illness course, with an intact circuit functioning to protect the individual from a persistent psychotic state.
Acknowledgments
Role of Funding Source
This study was funded by grants 5K23MH079982 and 1R01MH094594 (Dr. Dost Öngür).
We would like to thank our patients for their participation in this study.
Footnotes
Conflict of Interest
Dr. Öngür has received study drug free of charge from Sano -Aventis. He is PI on a research contract with Rules Based Medicine Inc.. Dr Perry Renshaw is a consultant for Ridge Diagnostics and Kyowa Hakko Kirin. He owns stocks in Ridge Diagnostics. Other authors declare that they have no conflict of interest.
Contributors
Dr. Cagri Yüksel and Dr. Dost Öngür designed the study with contribution from Dr. Stephan Heckers and Dr. Perry Renshaw. Julie McCarthy, Danielle Pfaff and Dr. Ann Shinn recruited patients. Julie McCarthy and Danielle Pfaff collected clinical information. Dr. Cagri Yüksel ran the analysis with asistance from Dr. Ann Shinn. Dr. Cagri Yüksel and Dr. Dost Öngür wrote the manuscript with contribution from other co-authors. Dr. Justin Baker contributed to the revision of the manuscript. All authors approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry. 2007;61(6):776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Adler CM, Levine AD, DelBello MP, Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58(2):151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, Kupfer DJ, Phillips ML. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res. 2009;171(1):54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellivier F, Golmard JL, Henry C, Leboyer M, Schurhoff F. Admixture analysis of age at onset in bipolar I affective disorder. Arch Gen Psychiatry. 2001;58(5):510–512. doi: 10.1001/archpsyc.58.5.510. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yucel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67(11):1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Fornito A, Berk M, Pantelis C. Major psychoses with mixed psychotic and mood symptoms: are mixed psychoses associated with different neurobiological markers? Acta Psychiatr Scand. 2008;118(3):172–187. doi: 10.1111/j.1600-0447.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- Bruno SD, Barker GJ, Cercignani M, Symms M, Ron MA. A study of bipolar disorder using magnetization transfer imaging and voxel-based morphometry. Brain. 2004;127(Pt 11):2433–2440. doi: 10.1093/brain/awh274. [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2009;37(1):177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, Cheung JP, Yip L, Tai KS, Suckling J, McAlonan GM. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89(1–3):12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Coryell W, Leon AC, Turvey C, Akiskal HS, Mueller T, Endicott J. The significance of psychotic features in manic episodes: a report from the NIMH collaborative study. J Affect Disord. 2001;67(1–3):79–88. doi: 10.1016/s0165-0327(99)00024-5. [DOI] [PubMed] [Google Scholar]
- Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- Cui L, Li M, Deng W, Guo W, Ma X, Huang C, Jiang L, Wang Y, Collier DA, Gong Q, Li T. Overlapping clusters of gray matter deficits in paranoid schizophrenia and psychotic bipolar mania with family history. Neurosci Lett. 2010;489(2):94–98. doi: 10.1016/j.neulet.2010.11.073. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58(9):713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Getz GE, DelBello MP, Fleck DE, Zimmerman ME, Schwiers ML, Strakowski SM. Neuroanatomic characterization of schizoaffective disorder using MRI: a pilot study. Schizophr Res. 2002;55(1–2):55–59. doi: 10.1016/s0920-9964(01)00210-9. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TH, Ha K, Kim JH, Choi JE. Regional brain gray matter abnormalities in patients with bipolar II disorder: a comparison study with bipolar I patients and healthy controls. Neurosci Lett. 2009;456(1):44–48. doi: 10.1016/j.neulet.2009.03.077. [DOI] [PubMed] [Google Scholar]
- Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY, Kim SI, Kwon JS. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132(3):251–260. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Haldane M, Cunningham G, Androutsos C, Frangou S. Structural brain correlates of response inhibition in Bipolar Disorder I. J Psychopharmacol. 2008;22(2):138–143. doi: 10.1177/0269881107082955. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57(7):692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156(7):1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Bertens MG, van Haren NE, van der Tweel I, Staal WG, Baare WF, Kahn RS. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159(2):244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- James AC, James S, Smith DM, Javaloyes A. Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent-onset schizophrenia. Am J Psychiatry. 2004;161(6):1023–1029. doi: 10.1176/appi.ajp.161.6.1023. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003a;160(1):156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd DA, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003b;60(8):766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003c;60(11):1069–1077. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008 Sep;65(9):1017–32. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65(7):746–760. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17(4):1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki N, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Ersner-Hershfield H, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. 2006;163(12):2103–2110. doi: 10.1176/appi.ajp.163.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59(9):775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55(6):648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000;48(8):740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, Walshe M, Murray RM. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56(8):544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Orr KG, Hutchinson G, Chitnis X, Suckling J, Lythgoe D, Pollock SJ, Rossell S, Shapleske J, Fearon P, Morgan C, David A, McGuire PK, Jones PB, Leff J, Murray RM. Grey matter abnormalities in first-episode schizophrenia and affective psychosis. Br J Psychiatry Suppl. 2007;51:s111–116. doi: 10.1192/bjp.191.51.s111. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30(2):485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Am J Psychiatry. 2005;162(1):43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34(1):155–172. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Lipinski JF., Jr Diagnosis in schizophrenia and manic-depressive illness: a reassessment of the specificity of ‘schizophrenic’ symptoms in the light of current research. Arch Gen Psychiatry. 1978;35(7):811–828. doi: 10.1001/archpsyc.1978.01770310017001. [DOI] [PubMed] [Google Scholar]
- Potash JB, Willour VL, Chiu YF, Simpson SG, MacKinnon DF, Pearlson GD, DePaulo JR, Jr, McInnis MG. The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry. 2001;158(8):1258–1264. doi: 10.1176/appi.ajp.158.8.1258. [DOI] [PubMed] [Google Scholar]
- Scherk H, Kemmer C, Usher J, Reith W, Falkai P, Gruber O. No change to grey and white matter volumes in bipolar I disorder patients. Eur Arch Psychiatry Clin Neurosci. 2008;258(6):345–349. doi: 10.1007/s00406-007-0801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield AC, Moorhead TW, Job DE, McKirdy J, Sussmann JE, Hall J, Giles S, Johnstone EC, Lawrie SM, McIntosh AM. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord. 2009;11(2):135–144. doi: 10.1111/j.1399-5618.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57(6):633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M. Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res. 2002;55(1–2):41–54. doi: 10.1016/s0920-9964(01)00224-9. [DOI] [PubMed] [Google Scholar]
- Tanskanen P, Ridler K, Murray GK, Haapea M, Veijola JM, Jaaskelainen E, Miettunen J, Jones PB, Bullmore ET, Isohanni MK. Morphometric brain abnormalities in schizophrenia in a population-based sample: relationship to duration of illness. Schizophr Bull. 2008;36(4):766–777. doi: 10.1093/schbul/sbn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Ruf M, Schmal C, Schulze TG, Knorr C, Vollmert C, Bosshenz K, Ende G, Meyer-Lindenberg A, Henn FA, Rietschel M. Prefrontal-temporal gray matter deficits in bipolar disorder patients with persecutory delusions. J Affect Disord. 2009;120(1–3):54–61. doi: 10.1016/j.jad.2009.04.009. [DOI] [PubMed] [Google Scholar]
- van Os J. ‘Salience syndrome’ replaces ‘schizophrenia’ in DSM-V and ICD-11: psychiatry’s evidence-based entry into the 21st century? Acta Psychiatr Scand. 2009;120(5):363–372. doi: 10.1111/j.1600-0447.2009.01456.x. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56(2):133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are Bipolar Disorder and Schizophrenia Neuroanatomically Distinct? An Anatomical Likelihood Meta-analysis. Front Hum Neurosci. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]