Abstract
Introduction
Small case series suggest tremor occurs frequently in IgM-monoclonal gammopathy of undetermined significance (IgM-MGUS) neuropathy. Epidemiologic study to confirm this association is lacking. Whether the neuropathy or another remote IgM-effect is causal remains unsettled.
Materials and methods
An IgM-MGUS neuropathy case cohort (n=207) was compared to age, gender, and neuropathy impairment score (NIS) matched, other-cause neuropathy controls (n=414). Tremor details were extracted from structured neurologic evaluation. All patients underwent nerve conductions.
Results
Tremor occurrence was significantly higher in IgM-MGUS case cohort (29%) than in control cohort (9.2%) (p=0.001). In IgM-MGUS cases, tremor was associated with worse NIS (p=0.025) and demyelinating nerve conductions (p=0.020), but 11 of 60 (18%) IgM-MGUS cases with tremor had axonal neuropathy. In other-cause neuropathy controls, tremor was associated with axonal nerve conductions (p=0.03) but not with NIS severity (p=0.57). Tremor occurrence associated with older age in controls, (p=0.004) but not in IgM-MGUS cases (p=0.272). Most IgM-MGUS tremor cases (49/60) had a postural-kinetic tremor, 8 had rest tremor, 3 had mixed rest-action. Alternative causes of tremor was identified in 42% of IgM-MGUS cases, the most common type is inherited essential tremor 6/60 (p=0.04).
Conclusions
This first epidemiologic case-control study validates association between IgM-MGUS neuropathy and tremor. Among IgM-MGUS neuropathy cases, severity as well as type of neuropathy (demyelinating over axonal) correlated with tremor occurrence. IgM-MGUS paraproteinemia may increase tremor expression in persons recognized with common other risk factors for tremor.
Keywords: Tremor, Peripheral Neuropathy, IgM-MGUS
1. INTRODUCTION
The characteristics of IgM-MGUS neuropathy typically includes older age, male preponderance, symmetric onset, sensory greater than motor deficits, insidious distal lower extremity progression and demyelinating nerve conductions [1]. Previous small case series have suggested that tremor is frequent in IgM-monoclonal gammopathy of undetermined significance (IgM-MGUS) [1-5]. However, most of these reports focused on neuropathy but not tremor. In the most comprehensive electrophysiological study of tremor there were only 6 IgM-MGUS demyelinating cases and a mistimed peripheral input to a central generator was theorized as causative [5]. No study has included age, sex matched controls for a comprehensive epidemiologic analysis. Population based studies have identified that 3.2% of persons 50 years or older and 5.3% greater than 70 years old have MGUS with 17.2% of those having IgM-MGUS [6]. Additionally essential tremor has been estimated to be 23.7 per 100 000 with a mean age of 58 years [7]. Because IgM-MGUS often occurs in older person who are at risk for tremor by alternative mechanisms, coincidental association needs to be considered. Specifically, most reports have not investigated the possibility of alternative incidental superimposed mechanism(s) for tremor.
In IgM-MGUS neuropathy, a primary peripheral mechanism leading to the loss or abnormal sensory input was speculated as the possible cause for tremor, but a correlation between tremor and the severity of motor or sensory abnormalities was not established [5]. Recent case reports have demonstrated that patients with IgM-MGUS neuropathy and tremor benefited from deep brain stimulation (DBS), suggesting a possible component of central origin or regulation in this type of tremor [8-10]. This DBS experience also mimics the reported success in patients with inherited demyelinating neuropathy with neuropathic tremor [11].
In this report, with special attention to tremor, we conducted the first large case-control study of IgM-MGUS neuropathy by comparing the clinical characteristics of 207 IgM-MGUS neuropathy patients to 414 age, sex matched other-cause neuropathy controls. Other-cause neuropathy controls were chosen to consider whether factors specific to IgM-MGUS were responsible for tremor over neuropathy alone.
2. METHODS
2.1 Standard Protocol Approvals, Registrations, and Patient Consents
Institutional review board approval was obtained by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center, and participants’ written informed consent to be included in research was verified. Patients who had requested exclusion from research were excluded from the study.
2.2 Subjects
All IgM-MGUS neuropathy (case cohort) and non-IgM-MGUS, other-cause neuropathy patients (control cohort) were seen by neurologists at Mayo Clinic Rochester between January 1973 and December 2007. All persons with IgM-MGUS neuropathy diagnosis were included without selection for tremor, and all having complete neurologic evaluation including for evaluation of tremor. Some of the IgM-MGUS (N=73) neuropathy patients were subjects in an earlier report comparing bone marrow confirmed IgM-MGUS to Waldenström’s neuropathy [1]. The IgM-MGUS subjects also all underwent hematology consultations, and both the IgM-MGUS patients and non-IgM-MGUS patients had to have completed a comprehensive quantified neurologic examination [12, 13]. Using our standard electrophysiology peripheral neuropathy protocol, motor nerve conductions were reviewed in three motor (peroneal, tibial and ulnar) and two sensory (sural, median) nerves in all persons. Demyelinating and axonal features were determined by standard criteria [14]. Nerve conduction studies were performed within 6 months of a structured evaluation of neuropathy severity by Neuropathy Impairment Score (NIS),[12] and tremor utilizing the Mayo grading scale which includes tremor localization, type, and severity by ordinal numeric scoring, with 0 being normal, 1 slight, 2 moderate, 3 considerable, and 4 maximal [13].
Case cohort-IgM Neuropathy Patients
Inherited and acquired neuropathies but not IgM related were excluded in all cases by standard approach [15, 16].
Control Cohort-Non-IgM-MGUS Neuropathy
Controls were selected from an EMG electronic database of 13,000 available patient charts carrying the diagnosis of neuropathy. IgM-MGUS protein by monoclonal immunofixation studies was negative in all chosen cases. Two controls for every IgM-MGUS neuropathy patient were selected and matched for age, sex, and correlated NIS (mean accepted difference <1 NIS point).
2.3 Data Extraction
Clinical data was extracted from paper records (prior to 1995) and through an electronic medical record database. The NIS data, which includes motor, sensory, and reflex parameters, was extracted from the most recent (up to December 2007) neurology consultation exam sheet and adapted to the neuropathy impairment score sheet (score of 0 = no impairment; maximum possible score is 288 i.e. areflexic, four extremity and bulbar paralysis, desensate) [12]. A NIS score of 25–50 often correlates with interference in daily living and possible gait aids on approaching a score of 50. A NIS score > 50 precludes most trade vocations. Presenting symptoms, sex, and age at diagnosis were recorded from the first neurologic exam for neuropathy. Presence of tremor, family history of tremor (includes essential tremor and Parkinson’s disease), neuropathy type (demyelinating or axonal) were all correlated with neurology examinations in IgM-MGUS subjects.
Furthermore, tremor onset age, neuropathy onset age, whether the patient was additionally seen by a Mayo Neurology Movement Disorders specialist, tremor symmetry, presence of hand rest, postural, and kinetic tremor, rest and action tremor of lower extremity, presence of head or voice tremor, and mixed tremor were abstracted for IgM tremor patients. Moreover, IgM neuropathy tremor patient records were all reviewed by a Mayo Movement Disorders specialist in tremor characterization. Occurrence of tremor, neuropathy type (axonal or demyelinating), and NIS were recorded for non-IgM controls.
2.4 Statistical Analysis
All statistical analyses were performed using JMP®, version 8.0 (SAS institute Inc., Cary, NC). Categorical analysis of neuropathy (axonal or demyelinating), family history of tremor (present or not present) and sex (male or female) were compared against the presence of tremor; a chi-square test of significance was performed to determine the Pearson p-value. For continuous variables, a non-parametric analysis using a Wilcoxon rank-sum test was performed to determine the p-value. Statistical significance was set at 0.05 for all analyses. Analysis was performed to determine if certain variables correlated with tremor incidence in IgM-MGUS and controls: increased age, gender, increased NIS, sensory gait ataxia, proprioception deficit of the upper extremities, demyelination, and family history of tremor.
Sample size and power calculations were performed using nQuery Advisor 7.0. Based on a rate of 29% with tremor in the IgM neuropathy cohort, we matched two controls to each case to have > 90% power to detect odds ratio of 2.0 or greater between cases and controls with respect to the occurrence of tremor, assuming a two-sided chi-square test and alpha = 0.05.
3. RESULTS
3.1 Cases cohort: 207 IgM-MGUS Neuropathy Patients
We identified 207 patients with IgM-MGUS neuropathy diagnosis, 150 males (72%) and 57 (28%) females. Tremor was documented in 60 cases (29%); the median age of neuropathy onset was 63.5 years and the median age for tremor onset was 67.0 years. Gender preponderance for tremor occurrence was not found, as 44 (73%) out of 60 tremor patients are male, which is in accordance with the overall gender proportion. Among these 207 IgM-MGUS neuropathy patients, 145 (70%) had demyelinating polyneuropathy while 62 (30%) had axonal-neuropathy, consistent with the previous reports showing IgM-MGUS is associated with demyelinating neuropathy. Among 60 tremor patients, we found an over-representation of demyelinating neuropathy (49/60; 82%) comparing to axonal-neuropathy (11/60; 18%; p = 0.020); the results are summarized in Figure 1 and Table 1. Well controlled diabetes was identified in 10 of the 60 IgM-MGUS tremor neuropathy persons, but without retinopathy or nephropathy. Thus diabetes is not felt as primary in neuropathy cause.
Figure 1. IgM-MGUS Neuropathy Cases vs. non-IgM-MGUS Neuropathy Controls.
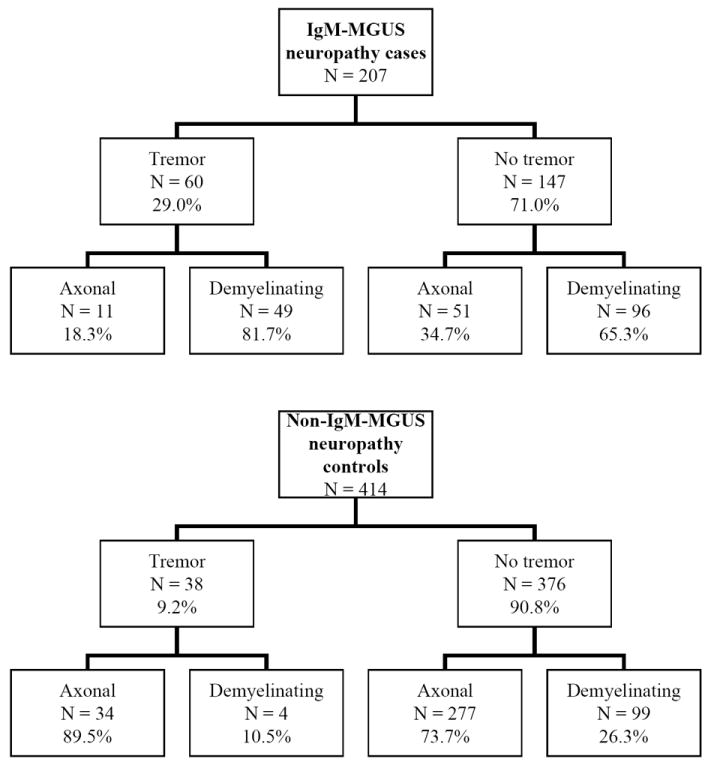
Table 1.
Demographics of IgM-MGUS Cases and Non-IgM-MGUS Neuropathy Controls
| IgM-MGUS Neuropathy Cases | |||
|---|---|---|---|
| Tremor (N=60) | No Tremor (N=147) | p-value | |
| Sex | |||
| Males | 44 (73%) | 106 (72%) | 0.860 |
| Females | 16 (27%) | 41 (28%) | |
| Neuropathy Type | |||
| Demyelinating | 49 (81.7%) | 96 (65.3%) | 0.020* (demyelinating) |
| Axonal | 11 (18.3%) | 51 (34.7%) | |
| Family History Tremor | |||
| Not Present/Unknown | 54 (90%) | 147 (100%) | <0.001* |
| Present | 6.0 (10%) | 0.0 (0.0%) | |
| Median NIS | 26.0 | 20.0 | 0.030* |
| Non-IgM-MGUS Neuropathy Controls** | |||
| Tremor (N=38) | No Tremor (N=376) | p-value | |
| Neuropathy Type | |||
| Demyelinating | 4 (10.5%) | 99 (23.9%) | 0.030* (axonal) |
| Axonal | 34 (89.5%) | 277 (66.9%) | |
| Median NIS | 18.5 | 22.0 | 0.570 |
= Data significant at 0.05 (using Pearson p-values)
= Matched 2:1 to IgM-MGUS Neuropathy Patients for age, sex, and neuropathy impairment score (NIS < 1 point mean accepted difference).
3.2 Control cohort: 414 Non-IgM-MGUS Neuropathy Patients
To achieve 2:1 control-to-case ratio, we identified 414 age, gender, and NIS matched, non-IgM neuropathy patients as the control group. Tremor was only found upon examination in 38 subjects (9.2%), while 376 (90.8%) had no tremor at initial examination nor manifested during follow up visits. Among these 414 non-IgM-MGUS neuropathy controls, 103 (25%) had demyelinating polyneuropathy while 311 (75%) had axonal neuropathy. Inherited (n=35) or chronic immune demyelinating polyradiculoneuropathy (n=45) accounted for the majority of demyelinating varieties. In the axonal neuropathies, inherited forms were also common (n=103) but many patients were without a specific identified cause or had suspected miscellaneous causes most commonly diabetic neuropathy.
Interestingly, among 38 tremor cases of the control group, only 10.5% (4/38) had demyelinating neuropathy while 89.5% (34/38) had axonal neuropathy (Figure 1). In contrast to the IgM-MGUS case cohort, axonal neuropathy was found associated with tremor occurrence (34/38; 89.5%) versus non-tremor (277/376; 73.7%) (p = 0.032) (Table1).
3.3. Comparison of Case Cohort and Control Cohort
The occurrence of tremor in IgM-MGUS cases (60/207; 29%) was significantly higher comparing to non-IgM, other-cause neuropathy controls (38/414; 9.2%; p<0.0001) (Table 2), illustrating the association exists even when older age and NIS were taken into consideration. Demyelinating neuropathy is more common in IgM-MGUS cases while axonal neuropathy is more prevalent in other-cause neuropathy controls. Interestingly, tremor patients with non-IgM-MGUS neuropathy are associated with axonal cause while tremor patients in IgM-MGUS cases are more likely to have demyelinating neuropathy. Additionally, Among IgM-MGUS cases, the NIS was found significantly higher (p=0.03) in tremor cases (median NIS=26) than non-tremor cases (median NIS=20), suggesting the severity of neuropathy is associated with the occurrence of tremor in IgM-MGUS neuropathy cases. In contrast, we did not find the NIS is associated with tremor in non-IgM-MGUS control cohort (Table 1). Interestingly, age did not correlate with tremor occurrence in IgM-MGUS cases, but in non-IgM-MGUS controls age significantly correlated with tremor occurrence (p=0.004).
Table 2.
Correlation of Tremor with IgM-MGUS Neuropathy
| Tremor (N=98) | No Tremor (N=523) | p-value | |
|---|---|---|---|
| IgM-MGUS Neuropathy Cases | 60 (29%) | 147 (71.0%) | <0.0001* |
| Non-IgM-MGUS Neuropathy Controls** | 38 (9.2%) | 376 (90.8%) |
= Data significant at 0.05 (using Pearson p-values)
= Matched 2:1 for age, sex, and neuropathy impairment score (NIS < 1 point mean accepted difference).
3.4 Tremor Features
Among the 60 IgM-MGUS neuropathy patients with tremor, 49 (82%) had a postural-kinetic hand component, 8 (13.6%) had a resting hand tremor and 3 (5.0%) had a mixed rest-action hand tremor. A chin, head, or voice tremor was infrequently found in 2 (3.3%). Upper extremity proprioceptive loss was observed in 12 (20%) patients, comparable to the non-tremor IgM-MGUS cases (42/147; 29%, p>0.05). An explanation for hand tremor was identified in 25 (42%), see Table 3, whereas no apparent explanation could be found in 35 (58%). The most commonly found elucidation was familial essential tremor 6/60. Table 3, also shows the associated diseases or medications related to tremor for the IgM patients. We were able to identify the time of onset for both tremor and neuropathy in 22 of 60 IgM-MGUS tremor patients. Among them 18 developed tremor after neuropathy was identified, 4 developed tremor prior to the diagnosis of neuropathy and all were diagnosed as familial essential tremor (median onset before neuropathy=7 yrs, range of 5-17). It is assumed that the extent of tremor was mild in a majority of the IgM-MGUS patients as only 15 out of 60 were either initially seen or referred to a movement disorders specialist for tremor evaluation. Among them, four had major disability with difficulty eating, dressing, and other fine motor skills of the upper extremities. The mildness of tremor was emphasized by low quantitative abnormalities (median=1, range 1-3; scale of 0-4, where 0 = no tremor and 4 = severe tremor) and the small number of IgM tremor patients initially presenting with complaints of tremor versus neuropathy (2 of 60). Tremor was symmetric in the majority of 60 IgM-MGUS patients with tremor (n=47, 78%), and minority were mildly asymmetric (n=13; 22%). Only 1 individual underwent DBS treatment for tremor.
Table 3.
Alternative potential tremor mechanisms in IgM-MGUS neuropathy Cases (N = 25/60 total tremor patients, 42%)
| IgM-Neuropathy Patients with Tremor | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comorbidity | Familial Essential Tremor | Parkinsonism | Cerebellar Ataxia | Alcohol | CNS Neoplasia | Meds | Multisystem Degeneration | Stroke |
| Frequency | 6 | 4a | 4b | 3d | 3d | 3e | 1 | 1f |
| Percent | 24% | 16% | 16% | 12% | 12% | 12% | 4% | 4% |
Parkinsonian features with rest and action component
Cerebellar findings with tremor onset
Documented alcohol abuse associated with tremor
Tremor onset with CNS neoplasm
Fluphenazine, metoclopramide, and thiothixene induced tremor
Unilateral hand tremor associated with hemiparesis, residuum from ipsilateral stroke
3.5 DBS in one IgM-MGUS Tremor Patient
At age 25 years, the patient developed a postural tremor similar to five of his first degree relatives. At age 42 years, he was identified to have a length-dependent demyelinating peripheral neuropathy not found in his relative. Also, an IgM-MGUS monoclonal protein was identified and he was diagnosed with IgM-MGUS neuropathy. In this context, the tremor markedly worsened, and was much more severe than any other affected relatives. He was eventually refractory to all medications including primidone, propranolol, and lorazepam. At age 66 years, a left thalamic stimulator (into the ventral intermediate thalamus) was implanted (DBS, Medtronic, Minneapolis, MN). Treatment with DBS effectively controlled his tremor, allowing him to use his right hand again for dexterous tasks, including writing.
4. DISCUSSION
In this first epidemiologic case-control study using the largest number of IgM-MGUS neuropathy cases reported to date an association between tremor and IgM-MGUS neuropathy is validated. Specifically, we found that IgM-MGUS neuropathy patients were more likely to have tremor comparing to age, sex, neuropathy severity-matched non-IgM MGUS neuropathy controls (29% vs. 9.2%, p<0.001). Peripheral demyelination was associated with the occurrence of tremor in IgM-MGUS neuropathy (49, 82%, p = 0.02). However, there was common occurrence of tremor in IgM-MGUS patients with axonal neuropathy (11 of 60, 18%). Interestingly, the association between tremor and demyelinating nerve conduction was not found in other-cause neuropathy controls, instead in them tremor was associated with axonal neuropathy (p=0.03). These results would suggest that tremor may more specifically link to IgM paraprotein in IgM-MGUS and not neuropathy type alone. Additionally, older age was associated with tremor occurrence in non-IgM-MGUS neuropathy controls, but not in the IgM-MGUS neuropathy cases, supporting the specific link between tremor and IgM paraprotein.
The frequency of tremor we observed in IgM-MGUS neuropathy is lower than selected case series [2-5], but we did not select based on tremor presence rather on the occurrence of neuropathy and included all IgM-MGUS neuropathy persons. We identified an alternative cause of tremor in 42% tremor cases (25/60) in IgM-MGUS neuropathy. Familial essential tremor was the most common alternative cause identified (6/60, 10%). Because family history can not be validated in many persons with genetic susceptibility to tremor, it is likely or probable that genetic essential tremor is more common in our cohort than we can definitively state. For most IgM-MGUS patients, tremor was incidentally found in the neurologic evaluation of neuropathy. Only 25% (15/60) were referred for a movement disorders consultation and four had major disability related to tremor. Based on our results, it is plausible to believe that IgM-MGUS neuropathy may increase the clinical expression of a genetic essential tremor or other inherent tremor mechanism(s). Comprehensive review for familial, degenerative, and other medical causes of tremor is emphasized in IgM-MGUS neuropathy patients. This is supported by the range of tremor types seen in our IgM-MGUS cohort. Specifically, it seems unlikely that the previously proposed mechanism of a mistimed peripheral input to a central generator [5] by neuropathy could cause such a diversity of identified tremors in our IgM-MGUS tremor neuropathy cohort.
More severe IgM-MGUS neuropathy, as indicated by greater neuropathy impairment score (NIS), is correlated with tremor occurrence in our IgM-MGUS neuropathy cases (p=0.03). This association is not seen in other-cause neuropathy controls, suggesting IgM-MGUS neuropathy may increase the clinical expression of tremor. Previous case studies have not found this correlation [2, 4] possibly due to the small sample size and the different measurement approach used in their studies. In this report, the severity was based on a quantitative summation of all motor sensory reflex deficits [12], all IgM-MGUS cases and controls underwent comprehensive examination and electrophysiologic study.
Reports have demonstrated DBS as an effective treatment of IgM neuropathy-related tremor [8-10]. In our cohort, one person had pre-existing familial essential tremor, along with the diagnosis of IgM-MGUS neuropathy. He underwent DBS and had significant improvement. Although this is a single case in our cohort, the potential role of IgM-MGUS as additive effect of inherent genetic mechanism might be suggested. Para inflammatory mechanism of central tremor is on possibility in cause and not without precedent. Cerebellar tremors as symptoms of parainflammatory cerebellar degeneration are well recognized [17, 18]. We did not see cerebellar or rubral tremor but did find rest tremor in several persons (8; 13.6%) with Parkinsonism. Other reported associations of tremor and paraneoplastic disease include anti-Hu (ANNA1) antibody-associated paraneoplastic disease and orolingual tremors [19] or orthostatic tremor [20].
A prospective study of IgM-MGUS neuropathy-associated tremor will be important in the better understanding of the pathogenic mechanism(s). From this large retrospective case-control study, the association of tremor with IgM-MGUS neuropathy is firmly established. IgM-MGUS paraproteinemia may increase tremor expression in persons recognized with common other risk factors for tremor.
Acknowledgments
This study was supported by the National Institute of Neurologic Disorders and Strokes (NINDS) K08 (NS065007) and the research committee of Mayo Department of Neurology. The authors also wish to thank the Mayo Center for Translational Science Activities for their support (NIH UL1 RR024150).
Author Roles: M.C.A., N.K., M.L.M., C.J.K. were involved in the conception, execution, review and critical edits. M.C.A., N.K., and C.J.K. wrote the manuscript.
Footnotes
Financial Disclosures:
Mr. Matt Ahlskog reports no disclosures.
Dr. Kumar reports no disclosures.
Dr. Mauermann reports no disclosures.
Dr. Klein is on the editorial board of The Journal of Peripheral Nerve Society and is supported by the National Institute of Neurologic Disorders and Strokes (NINDS) K08 (NS065007). The statistical analysis was supported by NIH UL1 RR024150.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein CJ, Moon JS, Mauermann ML, Zeldenrust SR, Wu Y, Dispenzieri A, et al. The neuropathies of Waldenstrom’s macroglobulinemia (WM) and IgM-MGUS. Can J Neurol Sci. 2011 Mar;38(2):289–95. doi: 10.1017/s0317167100011483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith IS. The natural history of chronic demyelinating neuropathy associated with benign IgM paraproteinaemia. A clinical and neurophysiological study. Brain. 1994 Oct;117(Pt 5):949–57. doi: 10.1093/brain/117.5.949. [DOI] [PubMed] [Google Scholar]
- 3.Smith IS, Kahn SN, Lacey BW. Chronic demyelinating neuropathy associated with benign IgM paraproteinaemia. Brain. 1983;106:169–95. doi: 10.1093/brain/106.1.169. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC, Teravainen H, Engel WK. Tremor as a feature of chronic relapsing and dysgammaglobulinemic polyneuropathies. Incidence and management. Arch Neurol. 1984 Jul;41(7):711–4. doi: 10.1001/archneur.1984.04050180033012. [DOI] [PubMed] [Google Scholar]
- 5.Bain PG, Britton TC, Jenkins IH, Thompson PD, Rothwell JC, Thomas PK, et al. Tremor associated with benign IgM paraproteinaemic neuropathy. Brain. 1996 Jun;119(Pt 3):789–99. doi: 10.1093/brain/119.3.789. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. The New England journal of medicine. 2006 Mar 30;354(13):1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 7.Rajput AH, Offord KP, Beard CM, Kurland LT. Essential tremor in Rochester, Minnesota: a 45-year study. Journal of neurology, neurosurgery, and psychiatry. 1984 May;47(5):466–70. doi: 10.1136/jnnp.47.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayreuther C, Delmont E, Borg M, Fontaine D. Deep brain stimulation of the ventral intermediate thalamic nucleus for severe tremor in anti-MAG neuropathy. Mov Disord. 2009 Oct 30;24(14):2157–8. doi: 10.1002/mds.22604. [DOI] [PubMed] [Google Scholar]
- 9.McMaster J, Gibson G, Castro-Prado F, Vitali A, Honey CR. Neurosurgical treatment of tremor in anti-myelin-associated glycoprotein neuropathy. Neurology. 2009 Nov 17;73(20):1707–8. doi: 10.1212/WNL.0b013e3181c1de66. [DOI] [PubMed] [Google Scholar]
- 10.Ruzicka E, Jech R, Zarubova K, Roth J, Urgosik D. VIM thalamic stimulation for tremor in a patient with IgM paraproteinaemic demyelinating neuropathy. Mov Disord. 2003 Oct;18(10):1192–5. doi: 10.1002/mds.10510. [DOI] [PubMed] [Google Scholar]
- 11.Breit S, Wachter T, Schols L, Gasser T, Nagele T, Freudenstein D, et al. Effective thalamic deep brain stimulation for neuropathic tremor in a patient with severe demyelinating neuropathy. Journal of neurology, neurosurgery, and psychiatry. 2009 Feb;80(2):235–6. doi: 10.1136/jnnp.2008.145656. [DOI] [PubMed] [Google Scholar]
- 12.Dyck PJ, Sherman WR, Hallcher LM, Service FJ, O’Brien PC, Grina LA, et al. Human diabetic endoneurial sorbital, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980;8:590–6. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- 13.Bastron JAB, Reginald F, Brown JA, Clark EC, Corbin KB, Daly DD, Eaton LM, Goldstein NP, Lambert EH, Millikan CH, Mulder DW, Parker HL, Rooke ED, Rushton JG, Siekert RG, Whisnant JP, editors. Clinical Examinations in Neurology. Philadelphia: Saunders; 1957. [Google Scholar]
- 14.Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) Report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology. 1991 May;41(5):617–8. [PubMed] [Google Scholar]
- 15.Dyck PJ, Dyck PJB, Chalk CH. The 10 P’s: A mnemonic helpful in characterization and differential diagnosis of peripheral neuropathy. Neurology. 1992;42:14–8. doi: 10.1212/wnl.42.1.14. [DOI] [PubMed] [Google Scholar]
- 16.Mauermann ML, Burns TM. Pearls and Oy-sters: Evaluation of peripheral neuropathies. Neurology. 2009 Feb 10;72(6):e28–31. doi: 10.1212/01.wnl.0000342135.27500.df. [DOI] [PubMed] [Google Scholar]
- 17.Bolla L, Palmer RM. Paraneoplastic cerebellar degeneration. Case report and literature review. Archives of Internal Medicine. 1997 Jun 9;157(11):1258–62. doi: 10.1001/archinte.157.11.1258. [DOI] [PubMed] [Google Scholar]
- 18.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurology. 2008;7(4):327–40. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valentino P, Labate A, Pirritano D, Crescibene L, Cascini G, Quattrone A. Orolingual tremor as unusual presentation of anti-Hu paraneoplastic syndrome. Movement Disorders. 2008 Sep 15;23(12):1791–2. doi: 10.1002/mds.22205. [DOI] [PubMed] [Google Scholar]
- 20.Gilhuis HJ, van Ommen HJ, Pannekoek BJM, Sillevis Smitt PAE. Paraneoplastic orthostatic tremor associated with small cell lung cancer. European Neurology. 2005;54(4):225–6. doi: 10.1159/000090715. [DOI] [PubMed] [Google Scholar]


