Abstract
Bicoid (Bcd) is a Drosophila morphogenetic protein and a transcriptional activator. Genetic studies have suggested a role of sumoylation in Bcd function, but it is unknown how Bcd activity is affected specifically by its own sumoylation status. Here we show that Bcd is sumoylated in Drosophila cells. We identify a lysine residue of Bcd as the primary sumoylation site. Using a Bcd mutant defective in being sumoylated, we show that sumoylation of Bcd is inhibitory to its ability to activate transcription. We provide evidence suggesting that the SUMO moiety has an intrinsic inhibitory activity for the activator function of Bcd.
Keywords: Morphogen, Sumoylation, Transcription repression, Bicoid
Introduction
Post-translational modifications play regulatory roles that impact virtually every biological process. Understanding the molecular mechanisms of these regulatory roles is essential to our knowledge about the basic operations and connectivity within cellular and developmental systems. Conjugation of the small ubiquitin-related modifier (SUMO) to substrate proteins is a post-translational modification process referred to as sumoylation [1,2]. Similar to ubiquitination, sumoylation also requires three stepwise enzymatic reactions (E1, E2 and E3) [3,4]. This post-translational modification has been suggested to play regulatory roles in many molecular and cellular processes such as signal transduction, transcription and intracellular trafficking of proteins [5]. Because of the wide spectrum of molecular and cellular activities impacted by sumoylation, it is important to dissect the specificity of sumoylation-mediated regulatory mechanisms.
Morphogens are molecules that form concentration gradients in a developing tissue or embryo to induce distinct cell fates [6–10]. Bicoid (Bcd) is a morphogenetic protein that forms a concentration gradient along the anterior-posterior (AP) axis in early Drosophila embryos [11,12]. It is a homeodomain-containing transcriptional activator and controls AP patterning by activating its target genes in a concentration-dependent manner [13–15]. One of its target genes is hunchback (hb), which, in response to a concentration threshold of the Bcd gradient, is expressed in the anterior half of the embryo [16]. A Bcd-responsible hb enhancer element, which is located upstream of the hb gene, contains multiple Bcd binding sites [16]. This ~250bp enhancer element is sufficient to confer Bcd concentration-dependent responses to reporter genes in both Drosophila embryos and cells [16,17]. Our recent studies have shown that Bcd is subject to ubiquitination, a process that affects the shape of the Bcd gradient [18]. Here we perform molecular studies to investigate the regulatory role and specificity of another post-translational modification, namely, sumoylation.
Previous genetic studies have revealed a critical role of lesswright (lwr), which encodes Ubc9 (the E2 conjugating enzyme in sumoylation), in development. It was shown that mutations in lwr (also known as semushi) cause a defect in the nuclear import of Bcd and, consequently, a reduction in hb expression and AP patterning defects [19]. These results suggest that Bcd is subject to regulation by the sumoylation pathway during development. However, since lwr mutations disrupt globally this post-translational process affecting many relevant substrates in the embryo, precisely how the function of Bcd is regulated by its own sumoylation status remains unclear. In this report, we show that the 489aa Bcd protein is sumoylated in Drosophila cells. Among the three potential sumoylation sites, we identify lysine at position 308 as a primary sumoylation site for Bcd. Our analysis of Bcd-responsive reporter activities suggests that the sumoylation status of Bcd is inhibitory to its function as a transcriptional activator. In addition, a single SUMO moiety covalently attached to Bcd makes this protein almost completely non-functional as an activator. Our study thus provides a first molecular documentation of a specific regulatory role of sumoylation on Bcd activity through the modification of this activator protein as a direct substrate.
Materials and Methods
Plasmids and S2 cells
For expressing proteins in Drosophila S2 cells, the corresponding cDNA sequences were cloned into the pAc5.1/V5-HisC vector (Invitrogen). All plasmids used in this study were generated by standard cloning methods and site-directed mutagenesis was performed using a standard PCR-based method. Drosophila S2 cells were cultured in the SFX insect medium (Hyclone) for all experiments except for those involving dsRNAi treatment, where the Schneider’s Drosophila medium (Invitrogen) containing 10% (v/v) fetal bovine serum (Invitrogen) and 1× antibiotic-antimycotic (Invitrogen) was used.
dsRNAi generation and treatment in Drosophila S2 cells
The protocols for dsRNAi generation and treatment in Drosophila S2 cells were described previously [18]. PCR primers used for targeting smt3 are 5’-TAA TAC GAC TCA CTA TAG GGA TGT CTG ACG AAA AGA AGG G and 5’-TAA TAC GAC TCA CTA TAG GGT TAT GGA GCG CCA CCA GTC TG. Two days after dsRNAi treatment, S2 cells were transfected with a plasmid expressing HA-Bcd. After 40 hours of incubation, cells were then directly boiled in 1× SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) loading buffer and subjected to Western blotting by the anti-HA antibody (Convance) as described previously [18]. Loading control and the efficiency of smt3 knockdown shown in Fig. 1B were detected by anti-β-actin antibody (Abcam) and anti-Smt3 antibodies [20] (a kind gift from Dr. Anne Dejean), respectively, in Western blotting.
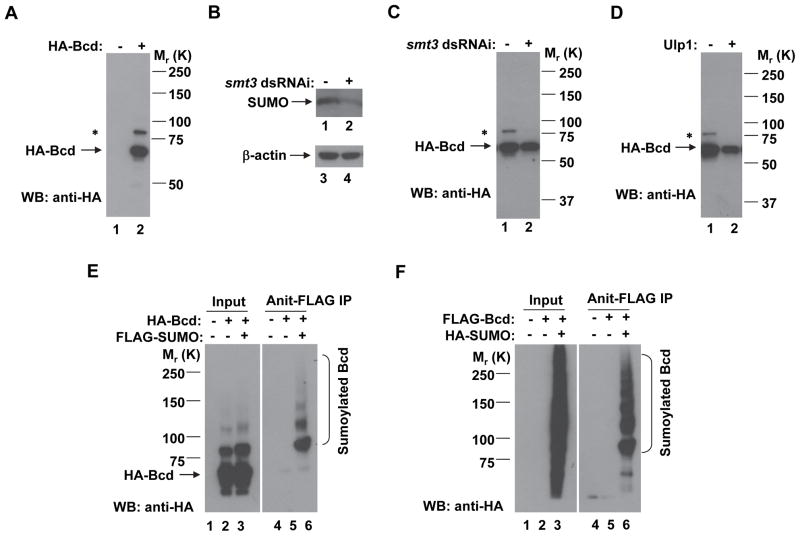
Fig. 1. Bcd is sumoylated in Drosophila S2 cells.
(A) A Western blotting (WB) detecting a minor protein species of HA-Bcd in S2 cells. Transfected cells were directly boiled in 1× SDS–PAGE loading buffer and subject to SDS-PAGE and Western blotting using an antibody against HA. HA-Bcd and the minor protein bands are marked (* for the minor band). The molecular weight standards are shown on the right.
(B) The amount of SUMO protein is reduced by smt3 dsRNAi. The loading control is represented by β-actin.
(C and D) The amount of the minor protein (relative to the full-length Bcd) is reduced by smt3 dsRNAi (C) or overexpression of Ulp1 (D).
(E) S2 cells were co-transfected with plasmids expressing FLAG-tagged SUMO and HA-tagged Bcd. Cell extracts were subject to immunoprecipitation (IP) using the anti-FLAG antibody and Western blotting using the anti-HA antibody. Sumoylated Bcd species are marked. A ladder of sumoylated Bcd bands was detected (with the lowest molecular weight (MW) band corresponding to the minor band shown in A and having the highest intensity), suggesting that, similar to ubiquitination, Bcd may be subject to poly-sumoylation.
(F) Same as panel E, except under a reciprocal experimental setting where Bcd is FLAG-tagged and SUMO is HA-tagged.
In vivo sumoylation assay
To detect sumoylated Bcd products in S2 cells, plasmids expressing both Bcd (wt or mutants) and SUMO proteins with suitable tags were co-transfected. 40 hours after transfection, cells were lysed in lysis buffer (50 mM Tris-HCl, pH7.5 and 1% SDS) for 10 min at 100°C. Cell extracts were then diluted by 1:10 in immunoprecipitation (IP) buffer (20 mM Tris-HCl, pH7.5, 150 mM NaCl, 10% glycerol, 0.1% NP-40, 1 mM DTT, complete protease inhibitor cocktail (Roche)). Immunoprecipitation (IP) and Western blotting were then performed as described previously [18].
DNA transfection and CAT reporter assay in Drosophila S2 cells
Transfection in S2 cells was carried out using the FuGENE® HD transfection reagent (Roche) according to the manufacturer’s instructions. An optimal transfection mixture contained 2 μg DNA and 6 μl FuGENE® HD transfection reagent in 100 μl serum-free medium. CAT reporter assays were carried out as described previously [17].
Results and Discussion
A modified Bcd species in Drosophila cells suggests a role of sumoylation
When wt Bcd was expressed in S2 cells (as an HA-Bcd fusion protein), a minor band of ~20 KDa larger than the full-length protein was detected in Western blotting (Fig. 1A, lane 2). The size difference suggested that it may represent the product(s) of posttranslational modifications by ubiquitin or ubiquitin-like molecules, such as SUMO. To investigate whether this minor band is a ubiquitinated product of Bcd, we treated the cells with proteasome inhibitors including MG132, lactacystin or epoxomicin. The amount of the minor band relative to that of the full-length Bcd protein was unaffected by these treatments (data not shown); nor was it affected by the overexpression of ubiquitin in cells (data not shown). These results suggest that, although Bcd is subject to ubiquitination in S2 cells [18], this minor band of Bcd is not a ubiquitinated product.
To investigate whether this minor band of Bcd is a sumoylated product, we treated S2 cells with dsRNAi against smt3, the Drosophila gene encoding SUMO. The efficiency of dsRNAi knockdown was confirmed by a reduction of the amount of SUMO protein in treated cells (Fig. 1B, lane 2). Fig. 1C shows that such a treatment dramatically reduced the relative amount of this minor band of Bcd (lane 2), suggesting it may represent a sumoylated product. Similar to ubiquitination, sumoylation is also a reversible process, and the Smt3-deconjugating enzyme Ulp1 can catalyze the reverse reaction [21]. We reasoned that, if this minor protein species is indeed a sumoylated product of Bcd, it should exhibit sensitivity to Ulp1 levels in S2 cells. Our results (Fig. 1D) show that Ulp1 overexpression in S2 cells almost completely abolished this minor band (lane 2). Together, these results suggest a role of sumoylation in the formation of the minor band of Bcd present in S2 cells.
Bcd is sumoylated in Drosophila cells
To directly visualize the sumoylated products of Bcd, we co-transfected S2 cells with plasmids expressing HA-Bcd and FLAG-SUMO. Here we used the anti-FLAG antibody to pull down the sumoylated products in cell extracts and detected Bcd by the anti-HA antibody in Western blotting. Sumoylated Bcd species were detected in the pull-down products when, and only when, both plasmids were co-transfected into the cells (Fig. 1E, lane 6; see other lanes for controls). In a reciprocal setting, we co-transfected S2 cells with plasmids expressing FLAG-Bcd and HA-SUMO. We used anti-FLAG to pull down Bcd and detected the sumoylated species by the anti-HA antibody in Western blotting. Similar results were obtained in these reciprocal experiments (Fig. 1F, lane 6, see other lanes for controls). In both experiments, we detected not only a Bcd product with a molecular weight shift indicative of mono-sumolylation, but also protein species indicative of poly-sumoylation with progressively decreasing amounts. Together, these results show that Bcd is sumoylated in Drosophila S2 cells.
Identification of K308 as the primary sumoylation site for Bcd
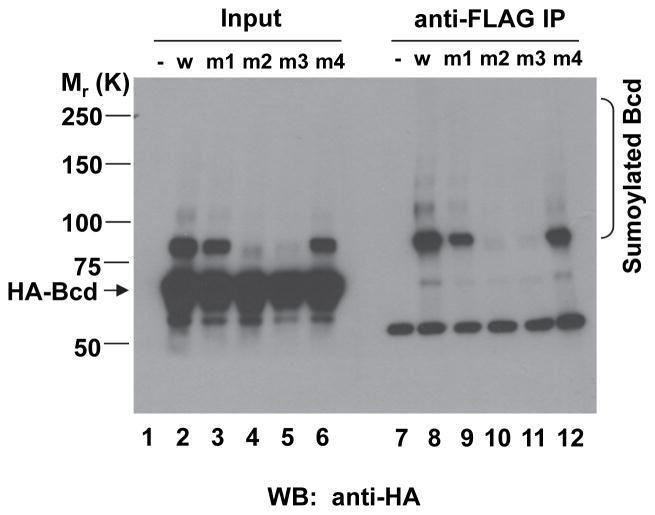
SUMO is generally conjugated to a lysine residue within a consensus motif, ψKxE/D (where ψ is a large hydrophobic residue and x is any residue) [4]. An analysis of the primary sequence of Bcd reveals three potential sumoylation motifs with lysine residues at positions 79, 308 and 355. To determine the possible roles of these lysine residues in Bcd sumoylation, we mutated them to alanines either individually or combinatorially through site-directed mutagenesis. We performed assays in S2 cells to determine the sumolyation status of these mutants. As shown in Fig. 2, sumoylated Bcd products became almost undetectable in cells expressing the Bcd mutant with K308 mutated to A (referred to as BcdK308A, lane 11). A double mutation that has both K308A and K355A alterations, also had a similar effect in preventing the formation of the sumoylated Bcd products (lane 10). In contrast, individually mutating the other two lysine residues (K79 or K355) to alanines had no detectable effects on Bcd sumoylation (lanes 9 and 12). These results suggest the lysine residue at position 308 is the primary sumoylation site for Bcd.
Fig. 2. Identification of K308 as the primary sumoylation site for Bcd.
Experimental procedures are described in Fig. 1E. Here, both wt Bcd (w; lane 8) and Bcd mutants (lanes 9–12) were tested, with the mutants having the following alterations: K79A (m1; lane 9), K308A and K355A (m2; lane 10), K308A (m3; lane 11) or K355A (m4; lane 12). Results from a control experiment with the expression vector alone (−) are shown in lane 7. The non-sumoylated and sumoylated Bcd protein species are marked, with molecular weight standards shown on the left. The detected heavy chain of IgG is marked with *. Input lanes 1–6 show Western blotting results from cell extracts prior to IP.
Bcd activity is subject to inhibition by sumoylation
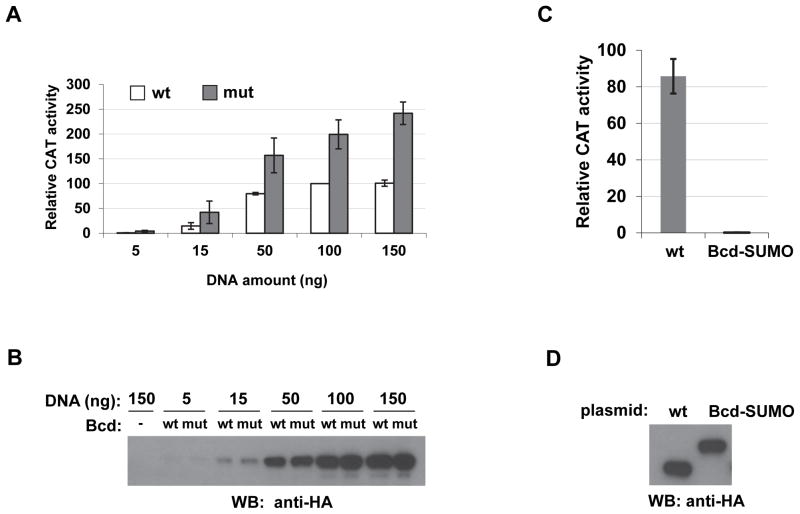
Sumoylation has been implicated to have important regulatory roles in modulating the function of transcriptional activators [22,23]. To determine specifically whether the function of Bcd as an activator is subject to regulation by the sumoylation status of itself, we performed reporter assays in S2 cells. Here we used a Bcd-responsive reporter gene expressing chloramphenicol acetyltransferase (CAT). The expression of this reporter, hb-CAT, is controlled by the ~250bp hb enhancer element [17]. We co-transfected cells with effector plasmids expressing either the wt or mutant forms of Bcd. Fig. 3A shows that both wt Bcd and BcdK308A activated the hb-CAT reporter in a dose-dependent manner. However, BcdK308A achieved higher levels of reporter activity than wt Bcd at all concentrations tested. The difference between the plateau levels of the reporter activity is >2 fold (p = 4.5 × 10−3 at 150 ng effecter plasmids, n = 3). The activity difference between BcdK308A and wt Bcd is not due to differences in their accumulated protein levels in cells (Fig. 3B). These results suggest that sumoylation of Bcd is inhibitory to its ability to activate transcription, and removing the primary sumoylation site of Bcd renders the mutant protein insensitive to this inhibitory mechanism.
Fig. 3. Sumoylation of Bcd negatively affects its activator function in Drosophila S2 cells.
(A) The BcdK308A mutant activates reporter expression to higher levels in S2 cells. A hb-CAT reporter was co-transfected into S2 cells with increasing amounts of effector plasmids expressing either HA-tagged wt Bcd or BcdK308A (shown as mut, which corresponds to m3 in Fig. 2.). CAT activities shown are relative to those obtained from cells transfected with 100 ng effector plasmid expressing wt Bcd (set to 100). Error bars show standard deviations from three independent experiments.
(B) Western blotting results showing comparable levels of wt Bcd and BcdK308A mutant. Loading volume was adjusted by transfection efficiency determined by β-galactosidase activities expressed from a lacZ control plasmid.
(C) CAT reporter assays comparing the activities of HA-tagged wt Bcd and a fusion protein Bcd-SUMO. The mean and standard deviation of relative CAT activities (from three independent experiments) are: 85.79 ± 9.45 and 0.22 ± 0.06 for wt Bcd and Bcd-SUMO, respectively. To specifically evaluate the activation function of Bcd-SUMO, the CAT assays were performed under conditions where similar protein levels were expressed for Bcd and Bcd-SUMO (see panel D).
(D) A Western blotting showing HA-tagged Bcd and Bcd-SUMO levels in the same cells used for CAT reporter assays in C. The effector plasmid was: 50ng and 300ng for Bcd and Bcd-SUMO, respectively, to achieve comparable protein levels in cells. The loading amount for each lane was adjusted by β-galactosidase activities expressed from a lacZ control plasmid.
The SUMO moiety covalently linked to Bcd is sufficient to inhibit its activity
To directly and specifically document the inhibitory role of SUMO on Bcd activity, we covalently linked SUMO to the C terminus of HA-Bcd as a fusion protein. To prevent this fusion protein itself from becoming conjugated to other proteins (as sumoylation substrates), we mutated the GG residues (which are needed for its conjugation with protein substrates [24]) at the C terminus of SUMO to AA. Compared with wt Bcd, this fusion protein, referred to as Bcd-SUMO, almost completely lost its ability to activate hb-CAT (Fig. 3C) when the two proteins were expressed at comparable levels in cells (Fig. 3D). Together, these results suggest that the SUMO moiety has an intrinsic inhibitory activity for the activator function of Bcd.
Concluding remarks
Our experiments document that Bcd is a substrate of sumoylation in S2 cells. The use of a specific Bcd mutant that has a single amino acid change and is defective of being sumoylated shows that the function of Bcd as an activator is subject to inhibition by its own sumoylation status. A single SUMO moiety covalently linked to Bcd is sufficient to confer such an inhibitory effect, suggesting an intrinsic inhibitory activity of this moiety for the activator function of Bcd. Our reporter assay results (Fig. 3A and 3B) show the Bcd level in cells where half of maximal reporter activities were detected was not significantly different between wt and mutant proteins, suggesting that sumoylation primarily inhibits the activation function of Bcd. A recent study has shown that Bcd is also subject to sumoylation in early Drosophila embryos [25]. The BcdK308A mutant generated in this study has enabled us to investigate how the activity of Bcd, a direct and specific substrate of sumoylation, is regulated by this post-translational modification during development. As detailed elsewhere, our results show that this Bcd mutant also has an increased ability (of a comparable magnitude) to activate hb transcription in embryos. Unlike cultured cells, cells (nuclei) in the early Drosophila embryo undergo synchronous mitotic divisions, allowing us to follow Bcd activity as a function of time. Our studies in embryos suggest a role of Bcd sumoylation in modulating the length of the action time of Bcd during a mitotic interphase in early embryos.
Our results described here identify a specific regulatory role of sumoylation on the activator function of Bcd. They suggest that, although an impaired sumoylation pathway (caused by lwr mutations in embryos) has been shown to affect the nuclear import of Bcd [19], the primary regulatory role of sumoylation on Bcd as a direct substrate is the inhibition of its ability to activate transcription. As shown both our own studies in embryos (detailed elsewhere) and those in [26], a Bcd mutant lacking its primary sumoylation site forms a normal gradient and remains localized to the nucleus. Thus, the nuclear import defect of Bcd in lwr mutant embryos must be reflective of the dependence of other molecules, such as those in the nuclear import machinery [19], on sumoylation as substrates. Our studies thus illustrate the importance of delineating the specificity of the regulatory roles of post-translational modifications such as sumoylation.
Highlights.
Bicoid is sumoylated in Drosophila cells
K308 is the major sumoylation site for Bicoid
The activator function of Bicoid is subject to inhibition by its own sumoylation status
Acknowledgments
We thank members of our groups at CCHMC for discussion and assistance, in particular, David Cheung for technical support. We thank Dr. Anne Dejean for kindly providing the anti-SUMO antibodies used in our studies. This work was supported in part by grants from NIH and NSF (to J.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–82. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 2.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–80. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 4.Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11:861–71. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–56. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 6.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 7.Arias AM, Hayward P. Filtering transcriptional noise during development: concepts and mechanisms. Nat Rev Genet. 2006;7:34–44. doi: 10.1038/nrg1750. [DOI] [PubMed] [Google Scholar]
- 8.Kerszberg M, Wolpert L. Specifying positional information in the embryo: looking beyond morphogens. Cell. 2007;130:205–9. doi: 10.1016/j.cell.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007;128:245–56. doi: 10.1016/j.cell.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Wartlick O, Kicheva A, Gonzalez-Gaitan M. Morphogen gradient formation. Cold Spring Harb Perspect Biol. 2009;1:a001255. doi: 10.1101/cshperspect.a001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 12.Ephrussi A, St Johnston D. Seeing is believing: the bicoid morphogen gradient matures. Cell. 2004;116:143–52. doi: 10.1016/s0092-8674(04)00037-6. [DOI] [PubMed] [Google Scholar]
- 13.Driever W, Nusslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 14.Driever W, Thoma G, Nusslein-Volhard C. Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature. 1989;340:363–7. doi: 10.1038/340363a0. [DOI] [PubMed] [Google Scholar]
- 15.Struhl G, Struhl K, Macdonald PM. The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell. 1989;57:1259–73. doi: 10.1016/0092-8674(89)90062-7. [DOI] [PubMed] [Google Scholar]
- 16.Driever W, Nusslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989;337:138–43. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, et al. The activity of the Drosophila morphogenetic protein Bicoid is inhibited by a domain located outside its homeodomain. Development. 2002;129:1669–80. doi: 10.1242/dev.129.7.1669. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Ma J. Fates-shifted is an F-box protein that targets Bicoid for degradation and regulates developmental fate determination in Drosophila embryos. Nat Cell Biol. 2011;13:22–9. doi: 10.1038/ncb2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epps JL, Tanda S. The Drosophila semushi mutation blocks nuclear import of bicoid during embryogenesis. Curr Biol. 1998;8:1277–80. doi: 10.1016/s0960-9822(07)00538-6. [DOI] [PubMed] [Google Scholar]
- 20.Lehembre F, Badenhorst P, Muller S, Travers A, Schweisguth F, Dejean A. Covalent modification of the transcriptional repressor tramtrack by the ubiquitin-related protein Smt3 in Drosophila flies. Mol Cell Biol. 2000;20:1072–82. doi: 10.1128/mcb.20.3.1072-1082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhaskar V, Smith M, Courey AJ. Conjugation of Smt3 to dorsal may potentiate the Drosophila immune response. Mol Cell Biol. 2002;22:492–504. doi: 10.1128/MCB.22.2.492-504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–59. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang J, Gill G. SUMO engages multiple corepressors to regulate chromatin structure and transcription. Epigenetics. 2009;4:440–4. doi: 10.4161/epi.4.7.9807. [DOI] [PubMed] [Google Scholar]
- 24.Johnson ES, Schwienhorst I, Dohmen RJ, Blobel G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 1997;16:5509–19. doi: 10.1093/emboj/16.18.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie M, Xie Y, Loo JA, Courey AJ. Genetic and proteomic evidence for roles of Drosophila SUMO in cell cycle control, Ras signaling, and early pattern formation. PLoS One. 2009;4:e5905. doi: 10.1371/journal.pone.0005905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm O, Wieschaus E. The Bicoid gradient is shaped independently of nuclei. Development. 2010;137:2857–62. doi: 10.1242/dev.052589. [DOI] [PMC free article] [PubMed] [Google Scholar]