Summary
Background
There are currently no reliable biomarkers for malignant pheochromocytomas and paragangliomas (PPGLs). This study examined whether measurements of catecholamines and their metabolites might offer utility for this purpose.
Methods
Subjects included 365 patients with PPGLs, including 105 with metastases, and a reference population of 846 without the tumor. Eighteen catecholamine-related analytes were examined in relation to tumor location, size and mutations of succinate dehydrogenase subunit B (SDHB).
Results
Receiver-operating characteristic curves indicated that plasma methoxytyramine, the O-methylated metabolite of dopamine, provided the most accurate biomarker for discriminating patients with and without metastases. Plasma methoxytyramine was 4.7-fold higher in patients with than without metastases, a difference independent of tumor burden and the associated 1.6- to 1.8-fold higher concentrations of norepinephrine and normetanephrine. Increased plasma methoxytyramine was associated with SDHB mutations and extra-adrenal disease, but was also present in patients without SDHB mutations and metastases or those with metastases secondary to adrenal tumors. High risk of malignancy associated with SDHB mutations reflected large size and extra-adrenal locations of tumors, both independent predictors of metastatic disease. A plasma methoxytyramine above 0.2 nmol/L or a tumor diameter above 5 cm indicated increased likelihood of metastatic spread, particularly when associated with an extra-adrenal location.
Interpretation
Plasma methoxytyramine is a novel biomarker for metastatic PPGLs that together with SDHB mutation status, tumor size and location provide useful information to assess the likelihood of malignancy and manage affected patients.
Keywords: pheochromocytoma, paraganglioma, metastases, methoxytyramine, dopamine, metanephrines, catecholamines, succinate dehydrogenase type B
Introduction
Pheochromocytomas and paragangliomas (PPGLs) are catecholamine-producing tumors that arise from adrenal or extra-adrenal chromaffin tissue, and which may present with metastatic involvement at diagnosis or develop metastatic spread later.1,2 Since there are no reliable histopathological methods to distinguish benign from malignant disease, diagnosis continues to rely on evidence of metastases. At that stage the prognosis is poor. There are no curative therapies. Disease is complicated by clinical manifestations of catecholamine excess, and invariably fatal.
Recognition of the shortcomings in the diagnosis and management of patients with metastatic PPGLs has stimulated considerable effort directed at identification of new biomarkers and targets for treatment. As part of this effort, transcriptomic and proteomic studies from numerous groups now indicate multitudes of differentially expressed genes or proteins and numerous pathways that might offer potential biomarkers for diagnosis or targets for treatment.3-9 There is, however, general lack of concordance of results, presumably reflecting the highly heterogeneous nature of the tumors.4 Higher malignant risk associated with tumors due to mutations of the succinate dehydrogenase subunit B (SDHB) gene or arising from extra-adrenal locations is well established,10-12 but these factors rarely receive consideration as covariates in studies directed at the discovery of new biomarkers.
As endocrine tumors, PPGLs expend considerable energy in maintaining a secretory phenotype, with cellular contents of catecholamines in some tumors approaching contents of total protein. These low molecular weight secretory molecules and their metabolites not only have well-established diagnostic utility, but also represent potential biomarkers for malignancy. Measurements of dopamine in particular have been suggested to be useful,10,13-16 with preliminary evidence indicating even better utility of its O-methylated metabolite, methoxytyramine.17
The present analysis comprehensively characterized the catecholamine metabolomic profiles of a large population of patients with PPGLs according to the presence or absence of metastases. The aim was to establish whether information from diagnostic testing of catecholamine excess might be useful for predicting the presence of metastases and how this might relate to other predictive information, including presence of SDHB mutations and differences in sizes and locations of tumors.
Methods
Subjects
The study involved retrospective analysis of data from 1211 subjects, including 365 patients with PPGLs and 846 subjects without the tumors, all of whom had biochemical testing carried out in the 15-year period between 1994 and 2009. Two hundred and seventy five patients with PPGLs were investigated at the National Institutes of Health (NIH) and the remainder at European Centers including the University Hospital of Dresden (Germany), Radboud University Medical Center at Nijmegen (The Netherlands), the University of Florence at Florence (Italy) and Sahlgrenska Academy and University Hospital at Gothenburg (Sweden). Written informed consent was obtained from subjects enrolled into Intramural Review Board approved studies at the NIH and Dresden, which allowed for collection of patient samples and data at offsite centers.
Patients with PPGLs had a mean age of 40 years (range 6 to 83 years) at initial diagnosis of tumors and included 190 males and 175 females. Adrenal and extra-adrenal locations of tumors were determined using results of imaging studies and surgical and pathological records. For patients presenting with recurrent or malignant PPGLs, careful attention was made to assess the adrenal or extra-adrenal locations of primary tumors, the presence of multi-focal disease and ages when initial tumors were first diagnosed. Patients with disease restricted to non-functional head and neck paragangliomas were not included. Patients were followed up as far as possible to assess for the presence of recurrent disease, including evidence of metastases, recurrent tumors at sites of previous resections or occurrence of new tumors at different sites as part of a multifocal presentation distinct from metastatic involvement.
Malignancy was defined by the presence of metastases at locations where chromaffin cells are normally absent (i.e., bones, lungs, liver and lymph nodes). Diagnosis of metastases to bones, lungs and liver was based on the results of computed tomography and/or magnetic resonance imaging combined with one or more functional imaging studies, these including 123I- or 131I-metaiodobenzylguanidine scintigraphy, technetium bone scans and PET scanning with 18F-fluorodeoxyglucose, 18F-fluorodopamine or 18F-fluorodihydroxyphenylanaline. Use of these scanning procedures to diagnose metastatic PPGLs in members of this patient cohort has been described in detail elsewhere.18-20 Diagnosis of lymph node metastases required pathological examination of resected nodes. Using the above criterion and diagnostic procedures, 105 of the 365 patients with PPGLs were identified with metastatic disease, a relatively high proportion (29%) reflecting disproportionate referral of patients with metastases to the NIH. Thirty-five patients were diagnosed with metastases at initial presentation of PPGLs and 70 after follow-up. Among the latter, the interval between diagnosis of primary PPGLs and metastatic disease varied between 5 months and 44 years (mean 8 years); most of these were enrolled with established metastases or suspected recurrent disease.
Among the 846 subjects without PPGLs who were included for comparative purposes, there were 379 males and 467 females. Subjects had a mean age of 41 years (range 6 to 84 years) and included 175 normotensive volunteers, 94 hypertensive volunteers and 577 patients in whom testing for PPGLs was carried out and tumors were excluded by previously described criteria.21 The use of medications known to cause false-positive elevations of plasma or urinary catecholamines and metanephrines (e.g., tricyclic antidepressants and phenoxybenzamine) constituted additional exclusion criteria.
Collections of blood, urine and surgical specimens
Blood samples from all 1211 study participants were obtained with subjects supine for at least 20 min before blood collection. Subjects were instructed to fast and abstain from caffeinated and decaffeinated beverages overnight and avoid taking acetaminophen for 5 days before blood sampling. Samples of blood were transferred into tubes containing heparin as anticoagulant and immediately placed on ice until centrifuged (4°C) to separate the plasma. Plasma samples were stored at -80°C until assayed.
Twenty-four hour urine samples were collected from 338 of the 365 patients with PPGLs and 513 of the 847 subjects without tumors. Samples were collected with hydrochloric or another acid as a preservative, total urine volume was determined and aliquots kept at 4°C until assayed.
Samples of tumor tissue were procured from 156 patients with PPGLs, generally within 1 hour of surgical resection of tumors. The x-y-z dimensions of tumors, excluding adherent tissue, were recorded. Small samples of each tumor (10 to 50 mg) were then dissected from the mass, frozen on dry ice and stored at -80°C. As part of further processing, tissue samples were weighed frozen and then homogenized in at least 5 volumes of 0.4 M perchloric acid containing 0.5 mM EDTA. Homogenized samples were centrifuged (1500 × g for 15 min at 4°C) and supernatants collected and stored at -80°C until assayed for catecholamines.
Laboratory analyses
Plasma, urinary and tissue catecholamines (norepinephrine, epinephrine and dopamine) and plasma and urinary fractionated metanephrines (normetanephrine and metanephrine) were quantified by liquid chromatography with electrochemical detection. Concentrations of catecholamines were determined after extraction from plasma or perchloric acid tissue supernatants using alumina adsorption as described previously.22 The assays of catecholamines in plasma also included measurements of three other catechols: 3,4-dihydroxyphenylalanine (DOPA), the precursor of dopamine and product of the rate limiting step in catecholamine biosynthesis; 3,4-dihydroxyphenylglycol (DHPG), a deaminated metabolite of norepinephrine and epinephrine produced principally within sympathetic nerves; and 3,4-dihydroxyphenylacetic acid, the deaminated metabolite of dopamine.
Plasma and urinary fractionated normetanephrine and metanephrine were estimated using different liquid chromatographic methods as described elsewhere.21,23 The assays in plasma also allowed simultaneous determination of methoxytyramine, the O-methylated metabolite of dopamine. Assays of plasma concentrations of metanephrine, normetanephrine and methoxytyramine were principally directed to measurements of the free metabolites (i.e., free metanephrines and methoxytyramine). However, additional measurements of the much higher concentrations of deconjugated metabolites were also carried out in 192 and 789 respective subjects with and without PPGLs. These latter measurements were carried out after incubating 200 μl aliquots of plasma with sulphatase over 30 minutes for 37°C and reflect concentrations of both free and sulphate-conjugated metabolites, similar to the measurements of urinary fractionated metanephrines.
SDHB Gene Mutation Testing
DNA for germline mutation testing of the SDHB gene was collected from 147 of the 365 patients with PPGLs. Testing, including confirmation of previous test results, was carried out at the Mayo Medical Laboratories. Eight exons of SDHB gene were amplified and sequenced by PCR based bidirectional sequencing, using an automated ABI capillary sequencer. Deletions of the SDHB gene were detected using a combination of multiple ligation-dependant probe amplification and Luminex®xTAG Technologies (Luminex Molecular Diagnostics, Inc., Toronto, Ontario, Canada).
Among the 218 patients who were not tested for SDHB gene mutations, there were 118 with already established mutations of other tumor-susceptibility genes. DNA was unavailable from the remaining 100 patients without evidence of a hereditary syndrome or germline mutation. Data from these 100 patients were excluded from analyses involving comparisons of patients with and without SDHB mutations.
Tumor size
Dimensions of primary tumors, excluding adherent tissue, were established when ever possible from records of pathological examinations. Where this was not possible (as in cases of primary tumors associated with first diagnosis of malignant disease) dimensions were established from records of imaging studies by computed tomography or magnetic resonance imaging. Volumes of tumors (V) in cubic cm were estimated using the formula for the volume of a sphere, V = 4/3 πr3, where r, the radius in cm, was derived from estimated mean diameters (the latter calculated from the cubed roots of rectangular volumes estimated from x-y-z dimensions).
Statistics
Due to the skewed distributions of plasma concentrations and urinary outputs of catecholamines and their metabolites, statistical significance of differences in neurochemical data was determined in all cases after logarithmic transformation. Differences between comparisons of two groups was determined by Students t-test and between comparisons of multiple groups by one-way or two-way ANOVA depending on respective absence or presence of more than one independent variable. Post-hoc tests associated with multiple comparisons included Dunnett's test for comparisons against a single reference group or the Tukey-Kramer test for comparisons amongst all groups. The chi-square test was used for establishing differences in distributions of non-numerical data between groups of patients with and without evidence of malignant PPGLs.
Receiver-operating characteristic (ROC) curves were established by logistic regression using the JMP statistics software package (SAS Institute, Cary, NC, USA). Estimations of optimal cut-offs for tumor diameter and plasma methoxytyramine as determinants of increased likelihood of metastatic disease were determined using values of diagnostic sensitivity (true positive rates) and 1-specificy (false positive rates) at the closest points of ROC curves to top left corners of ROC curve plots (i.e., 100% for both sensitivity and specificity). These values for diagnostic sensitivity and specificity were then used to extrapolate optimal cut-off values for tumor diameter and plasma methoxytyramine from plots of false- and true-positive rates versus tumor diameter and plasma concentrations of methoxytyramine respectively.
Increases in the likelihood of metastatic disease with increasing mean tumor diameter or plasma concentrations of methoxytyramine in patients presenting with adrenal or extra-adrenal primary tumors were calculated assuming overall frequencies of metastatic disease of 10% for adrenal tumors and 36% for extra-adrenal tumors. Cumulative frequencies of metastatic disease within the patient cohort were estimated as a function of increasing mean tumor diameter and plasma concentrations of methoxytyramine; using the overall 29% frequency of metastatic disease within the patient cohort, these frequencies were normalized to those expected for the respective 10% and 36% assumed frequencies for adrenal and extra-adrenal tumors.
Results
Clinical Characteristics
Among patients with PPGLs and no metastases, 86% had adrenal tumors, compared to 14% with extra-adrenal tumors (Table 1). Among patients with metastatic disease these proportions were reversed (P<0.001); 32% had adrenal primary tumors and 68% primary tumors with an extra-adrenal local. Primary tumors among patients with metastases were 3.6-fold larger (P<0.001) in volume than among those without metastases, reflecting mean tumor diameters of 7.2 and 4.7 cm. Forty-eight patients were identified with mutations of the SDHB gene, including 41 with and 7 without metastases.
Table 1.
Demographic Data, Clinical Characteristics and Catecholamine Metabolomic Profiles in Patients with and without Metastatic PPGLs
| Reference Population | Patient Population | ||||
|---|---|---|---|---|---|
| |
|
|
|||
| Demographic/Clinical Data | No tumor | No Metastases | Metastases | P value* | |
| N | 846 | 260 | 105 | ||
| Age (Mean±SD) | 41±14 | 41±17 | 37±17 | 0.0399 | |
| Gender (M/F) | 467 / 379 | 129 / 131 | 60 / 45 | 0.2047 | |
| Tumor location (A/E/B)† | no tumors | 223/28/9 | 34/65/6 | 0.0001 | |
| Tumor Volume (Mean±SE)‡ | no tumors | 54±6 | 195±26 | 0.0001 | |
| SDHB mutation (yes/no)§ | not tested | 7 / 253 | 41 / 64 | 0.0001 | |
| Norepinephrine & metabolites | Mean Ref. Intervals | Mean 95% CI | Mean 95% CI | P value* | AUC |
|---|---|---|---|---|---|
| Plasma NE (nmol/L) | 1.46 [0.51-4.18] | 5.57 (4.91-6.35) | 10.02 (7.75-12.94) | 0.0001 | 0.630 |
| Plasma f-NMN (nmol/L) | 0.29 [0.11-0.74] | 3.52 (3.05-4.07) | 5.51 (4.10-7.40) | 0.0031 | 0.603 |
| Plasma d-NMN (nmol/L) | 10.54 [3.85-28.12] | 78.3 (65.3-93.98) | 145.8 (99.4-213.9) | 0.0037 | 0.656 |
| Plasma DHPG (nmol/L) | 4.88 [2.69-8.88] | 7.86 (7.35-8.41) | 9.20 (8.16-10.36) | 0.0174 | 0.582 |
| Urine NE (μmol/day) | 0.22 [0.08-0.61] | 0.99 (0.84-1.16) | 1.48 (1.13-1.93) | 0.0094 | 0.587 |
| Urine d-NMN (umol/day) | 1.37 [0.45-4.14] | 9.02 (7.63-10.68) | 14.92 (11.01-20.21) | 0.0023 | 0.613 |
| Urine VMA (μmol/day) | 17.61 [7.06-43.39] | 48.95 (43.08-55.62) | 69.68 (56.15-86.47) | 0.0054 | 0.616 |
| Epinephrine & metabolites | Mean Ref. Intervals | Mean 95% CI | Mean 95% CI | P value* | AUC |
|---|---|---|---|---|---|
| Plasma EPI (nmol/L) | 0.09 [0.01-0.67] | 0.30 (0.25-0.36) | 0.17 (0.13-0.22) | 0.0012 | 0.626 |
| Plasma f-MN (nmol/L) | 0.13 [0.04-0.45] | 0.66 (0.54-0.81) | 0.24 (0.18-0.33) | 0.0001 | 0.683 |
| Plasma d-MN (nmol/L) | 3.91 [1.00-14.90] | 21.05 (16.20-27.36) | 10.8 (6.70-17.31) | 0.0232 | 0.624 |
| Urine EPI (μmol/day) | 0.02 [0.01-0.09] | 0.07 (0.06-0.9) | 0.03 (0.02-0.04) | 0.0001 | 0.663 |
| Urine d-MN (μmol/day) | 0.39 [0.09-1.56] | 2.19 (1.69-2.85) | 0.92 (0.66-1.28) | 0.0001 | 0.661 |
| Dopamine, DOPA & metabolites | Mean Ref. Intervals | Mean 95% CI | Mean 95% CI | P value* | AUC |
|---|---|---|---|---|---|
| Plasma DA (nmol/L) | 0.05 [0.01-0.41] | 0.13 (0.11-0.15) | 0.40 (0.25-0.62) | 0.0001 | 0.630 |
| Plasma f-MTY (nmol/L) | 0.02 [0.00-0.11] | 0.07 (0.06-0.08) | 0.33 (0.22-0.50) | 0.0001 | 0.739 |
| Plasma d-MTY (nmol/L) | 1.22 [0.20-7.41] | 3.79 (3.10-4.63) | 7.91 (3.70-16.92) | 0.0097 | 0.591 |
| Plasma DOPAC (nmol/L) | 7.92 [3.78-16.59] | 8.95 (8.35-9.59) | 10.59 (8.96-12.52) | 0.0313 | 0.564 |
| Plasma DOPA (nmol/L) | 7.94 [4.98-12.63] | 9.51 (8.94-10.11) | 11.69 (10.16-13.46) | 0.0023 | 0.549 |
| Urine DA (μmol/day) | 1.19 [0.39-3.66] | 1.70 (1.57-1.84) | 2.41 (1.97-2.95) | 0.0006 | 0.592 |
Denotes significance of difference between groups with and without metastases
Tumor location indicates adrenal (A), extra-adrenal (E) or both adrenal and extra-adrenal (B) primary tumors
Tumor size indicates the volume (cubic cm) of the primary tumor or sets of multifocal primary tumors first diagnosed
Numbers of patients with no SDHB mutations detected include 100 who were not tested and in who SDHB mutations remain possible (80 without metastases and 20 with metastases who were excluded from subsequent comparisons of patients with and with SDHB mutations).
Catecholamine metabolomic data are shown as geometric means with reference intervals (Ref.Intervals) determined from the 2.5 and 97.5 percentiles of the reference population and the 95% confidence intervals of the mean (95% CI) shown for the two PPGL patient groups. All 18 analytes were higher (P<0.007) in both groups of PPGL patients compared to the reference group.
Abbreviations: AUC, area under ROC curves; NE; norepinephrine; f-NMN, free normetanephrine; d-NMN, deconjugated normetanephrine; DHPG, 3,4-dihydroxyphenylglycol; VMA, vanillylmandelic acid; EPI, epinephrine; f-MN, free metanephrine; d-MN, deconjugated metanephrine; DA, dopamine; f-MTY, free methoxytyramine; d-MTY, deconjugated methoxytyramine; DOPAC; 3-4-dihydroxyphenylacetic acid; DOPA, 3,4-dihydroxyphenylalanine.
Catecholamine Metabolomic Profiles
All 18 catecholamine-related analytes were increased (P<0.007) to variable extents in patients with and without metastases compared to reference subjects (Table 1). There were also variable differences in patterns of increases among the 3 groups of catecholamines and their metabolites. Differences were particularly striking for plasma dopamine and its metabolites, which were up to 370% higher (P<0.001) in patients with than without metastases. In contrast, norepinephrine and its metabolites were up to 60% higher (P<0.02) in patients with than without metastases, whereas plasma epinephrine and its metabolites were as much as 63% lower (P<0.03).
Areas under ROC curves indicated that among all 18 catecholamine-related analytes plasma free methoxytyramine provided the most accurate biomarker for discriminating patients with and without metastases (Table 1). Among patients with metastases, 66% had increases of plasma methoxytyramine, a higher (P<0.001) proportion than the 32% of patients without metastases (Fig.1A). Similarly, plasma and urinary dopamine were respectively increased above the upper reference intervals in 35% and 26% of patients with metastases, higher (P<0.001) proportions than the 8% and 5% in patients without metastases (Fig.1B&C). Measurements of dopamine were, however, less sensitive (P<0.001) than plasma methoxytyramine for indicating tumoral dopamine production.
Figure 1.
Dot plots showing normalized logarithmic distributions of plasma concentrations of methoxytyramine (panel A) and dopamine (panel B) and urinary outputs of dopamine (panel C) in patients with PPGLs according to presence or absence of metastases. Values for disease secondary to tumors with an exclusive adrenal location are illustrated by empty circles (○). Values for disease secondary to tumors with extra-adrenal locations are illustrated by solid circles (●). The dashed horizontal lines show the upper reference limits determined from 97.5 percentiles of the reference population, as indicated by the displayed values to the upper left of dashed lines in nmol/L (see also Table 1).
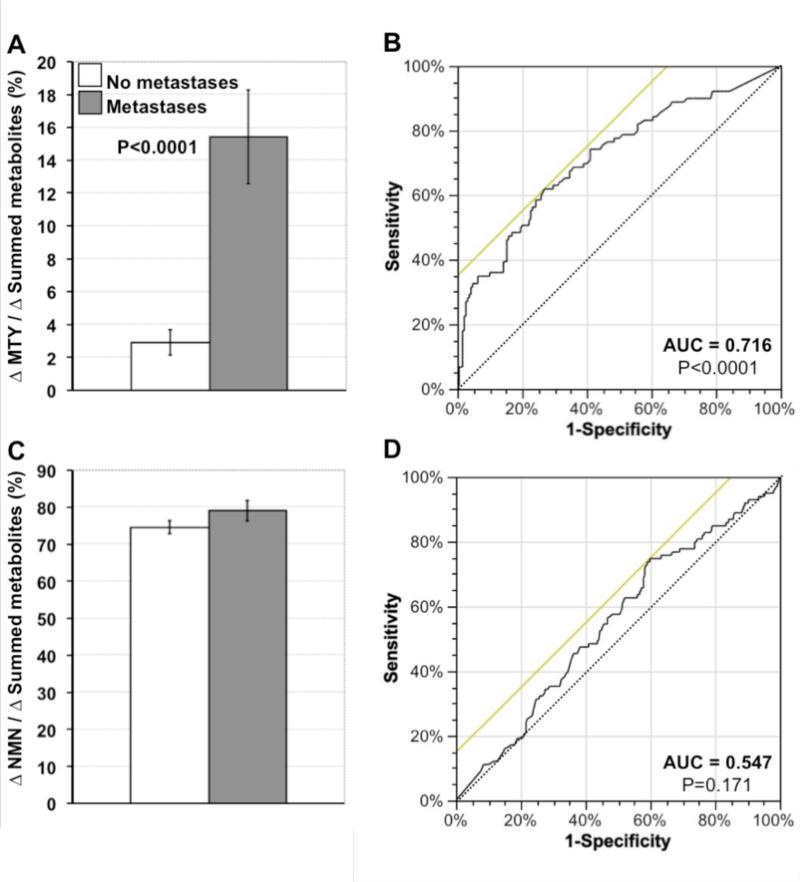
Increases above reference levels of plasma methoxytyramine relative to summed increases of all O-methylated metabolites, which are independent of tumor burden, were 5.3-fold larger (P<0.0001) in patients with than without metastases (Fig.2A). In contrast, relative increases of plasma normetanephrine did not differ (Fig. 2C). ROC curves indicated that the discriminating power of methoxytyramine as a biomarker of malignancy was retained (AUC=0.716, P<0.0001), whereas that of normetanephrine was lost (AUC=0.547, P=0.171), after conversion into variables independent of tumor burden (Fig.2B&D).
Figure 2.
Comparative assessment of relative increases of plasma free methoxytyramine (MTY, A & B) and normetanephrine (NMN, C & D) as biomarkers for metastatic PPGLs. For this analysis mean±SE increases of methoxytyramine and normetanephrine above mean reference levels (from Table 1) are shown as percents of summed increases above reference for all three O-methylated metabolites (A & C). These relative increases, which are independent of tumor burden, indicate that increases in methoxytyramine represent only 2.9±0.8% of combined increases of all metabolites in patients without metastases, but 15.4±2.9% of the total in patients with metastases (A). In contrast, relative increases of plasma normetanephrine are similar in both groups (C). ROC curves, relating rates of true-positive (sensitivity) versus false-positive results (1-specificity) for relative increases of methoxytyramine (B) and normetanephrine (D) as biomarkers of metastatic disease, indicate significant diagnostic discriminatory power for methoxytyramine (P<0.0001), but lack of discriminatory power for normetanephrine (P=0.171).
Increases in plasma methoxytyramine represented more than 50% of the combined total of increases of all three O-methylated metabolites in 14 patients with metastases. One of these patients with high levels of methoxytyramine (14.2 nmol/L) and dopamine (17.5 nmol/L) was operated for a solitary 10×5×5 cm retroperitoneal tumor after which there was no evidence of residual disease. Five years later the patient again showed elevations of plasma methoxytyramine (2.2 nmol/L) and dopamine (4.6 nmol/L) with normal levels of other amines and metastases in liver, bones and lungs.
Methoxytyramine and Metanephrine in Relation to Tumor Location
Patients with extra-adrenal primary tumors had plasma concentrations of methoxytyramine that were 274% higher (P<0.001) and plasma concentrations of metanephrine 78% lower (P<0.001) than in patients with adrenal tumors. Higher concentrations of methoxytyramine in patients with than without metastases were retained after separation of patients into subgroups according to locations of primary tumors (Fig.2A). In contrast, the lower plasma concentrations of metanephrine in patients with than without metastases were not retained after similar subgroup separation of patients (Fig. B).
Among the 34 patients with metastases secondary to adrenal tumors, 14 (41%) had increased plasma concentrations of metanephrine, a proportion that did not differ significantly from the 56% of patients with epinephrine-producing adrenal tumors and no metastases. Among the 14 patients with metastases secondary to epinephrine-producing adrenal primary tumors, 11 had their adrenal tumors removed an average of 9 years earlier. Despite absence of adrenal primary tumors, all had clear evidence of epinephrine production originating from distant metastases, with increases in plasma metanephrine averaging 41% of combined increases of all O-methylated metabolites.
Tumor Characteristics according SDHB Mutation Status
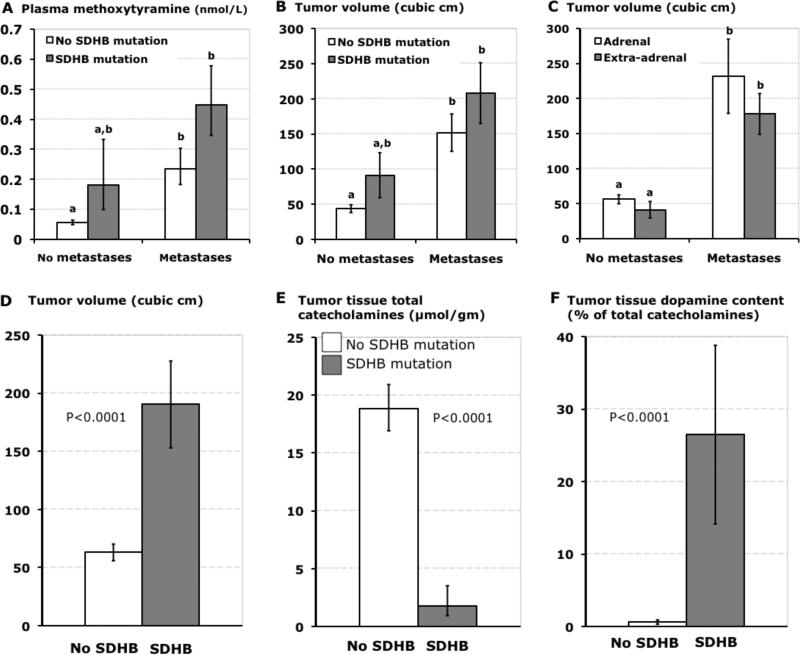
Plasma concentrations of methoxytyramine were 400% higher (P<0.001) in patients with than without SDHB mutations. However, after separating patients into subgroups according to presence or absence of metastases, the significance of differences between groups with and without SDHB mutations was not retained (Fig.4A). Plasma concentrations of methoxytyramine remained higher (P<0.05) in patients with than without metastases in the absence of SDHB mutations
Figure 4.
Bar graphs (means±SE) illustrating distinguishing characteristics of tumors due to SDHB mutations. Plasma concentrations of methoxytyramine and volumes of PPGLs in patients with and without SDHB mutations are shown sub-grouped according to presence of absence of metastases in panels A and B, whereas volumes of primary tumors according to adrenal and extra-adrenal locations are shown sub-grouped according to presence of absence of metastases in panel C. Tumor volumes, tissue concentrations of total catecholamines and percent contents of dopamine in patients with and without SDHB mutations are shown respectively in panels D, E and F. Data are restricted to patients with established mutations or in whom SDHB mutations were excluded by gene testing. Tumor volumes represent those for primary tumors. Data for tumor tissue catecholamines are from primary tumors resected from 11 patients with SDHB mutations (7 with distant metastases) and 145 without SDHB mutations. a,bDifferent alphabetic characters in upper panels indicate significant differences (P<0.05) between groups, whereas presence of identical characters indicates lack of a significant difference.
A 3.5-fold larger (P<0.001) volume of primary tumors in patients with than without metastases was also retained after exclusion of patients with SDHB mutations (Fig.4B). Furthermore, differences (P<0.001) in sizes of primary tumors in patients with and without metastases were retained after the two groups were separated into subgroups according to adrenal and extra-adrenal locations of primary tumors (Fig.4C); the two groups of patients who developed metastases secondary to adrenal or extra-adrenal tumors both had similar 4.1- to 4.3-fold larger volumes of primary tumors than respective groups without metastases. Among the 48 patients with PPGLs due to SDHB mutations, 43 had tumors with exclusive extra-adrenal locations and 5 that included an adrenal location. Furthermore, among the patients with extra-adrenal tumors, 43% had SDHB mutations. After excluding patients with SDHB mutations the proportion of patients with metastatic disease secondary to extra-adrenal tumors remained higher (P<0.001) than that due to adrenal tumors (48% versus 14%), indicating a 3.4-fold higher prevalence of malignancy associated with extra-adrenal than adrenal tumors, independent of any increased risk associated with SDHB mutations. Nevertheless, comparisons of patients with and without SDHB mutations who had malignant disease secondary to extra-adrenal tumors (87% versus 48%) indicated that an SDHB mutation imparted an additional 1.8-fold higher (P<0.001) risk for malignant disease.
Volumes of tumors were 3-fold larger (P<0.001) in patients with than without SDHB mutations (Fig.4D). Tissue concentrations of catecholamines in tumors from patients with SDHB mutations were less than 10% those of tumors patients without SDHB mutations (Fig.4E). In primary tumors from patients with SDHB mutations, most of whom had metastatic disease, contents of dopamine averaged 26% of total catecholamine contents, a 40-fold larger (P<0.001) proportion than in other tumors, all without metastatic involvement (Fig.4F).
Likelihood of Malignancy as a Function of Tumor Diameter and Plasma Methoxytyramine
Analysis of ROC curves relating rates of true- and false-positive results for mean tumor diameter (Fig.5A) and methoxytyramine (Fig.5B) as determinants of malignancy revealed optimal cut-offs of 5 cm for tumor diameter (Fig.5C) and 0.2 nmol/L for plasma methoxytyramine (Fig.5D). At these cutoffs, diagnostic sensitivities and specificities were respectively 65% and 80% for tumor diameter and 57% and 85% for plasma methoxytyramine. Above these cut-offs the likelihood of malignancy increased with increasing tumor diameter (Fig.5E) and plasma methoxytyramine (Fig.5F).
Figure 5.
Analyses of tumor diameter (A,C,E) and plasma methoxytyramine (B,D,F) as predictors of metastatic disease. ROC curves relating changes in true-positive results (sensitivity) and false-positive results (1-specificity) for predicting the presence of malignancy as a function of changing cut-offs for mean tumor diameter are shown in panel A and for plasma methoxytyramine in panel B. Points of ROC curves closest to top left corners indicate values for true- and false-positive results used to estimate optimal cut-offs for tumor diameter in panel C and plasma methoxytyramine in panel D. Likelihoods of metastatic disease as a function of increasing tumor diameter in panel E and of increasing plasma methoxytyramine in panel F are shown separately for tumors with adrenal and extra-adrenal locations, assuming an overall frequency of malignancy of 10% for adrenal tumors and 36% for extra-adrenal tumors. Likelihoods of malignancy were then estimated based on differences in cumulative frequencies of tumors with and without metastases as a function of increasing mean diameter or plasma concentration of methoxytyramine.
For adrenal tumors the likelihood of metastases increased from less than 6% for tumors smaller than 5 cm to over 50% for tumors larger than 10 cm; for extra-adrenal tumors the likelihood of malignancy increased to over 80% for tumors 9 cm and larger (Fig.5E). Similarly, the likelihood of metastases in patients with adrenal tumors increased from less than 10% for patients with normal plasma concentrations of methoxytyramine to over 33% in patients with levels higher than 3 nmol/L (Fig.5F). For extra-adrenal tumors the likelihood of malignancy increased to over 70% for patients with concentrations of methoxytyramine above 3 nmol/L.
Discussion
This study involving a large cohort of well-characterized patients with PPGLs establishes plasma free methoxytyramine as a novel biomarker of metastatic disease that together with presence of SDHB mutations, large size and extra-adrenal locations of primary tumors should prove useful for indicating the likelihood of malignancy. Among the 18 biomarkers examined, this metabolite of dopamine showed the largest diagnostic signal, with close to 5-fold higher levels in patients with than without metastases. Several previous studies suggested that increased dopamine could have prognostic significance for metastatic PPGLs, but almost all relied on urinary measurements of dopamine.10, 13-16 Ours is the first study to examine plasma methoxytyramine, which we show to be a more sensitive biomarker of tumoral dopamine production and metastatic spread than either plasma or urinary dopamine.
The larger increases of plasma and urinary norepinephrine and its metabolites, but smaller increases of epinephrine and metanephrine in patients with than without metastases are consistent with previous reports.16,24,25 As suggested in those reports, larger increases of norepinephrine in malignant than benign disease most likely reflect increased tumor burden associated with metastatic spread.24,25 As shown by the relative increments of metabolites, which are unrelated to tumor size, this explanation cannot account for the larger increases in plasma methoxytyramine in patients with than without metastases.
Although tumoral production of methoxytyramine correlates with presence of SDHB mutations and extra-adrenal location of primary tumors, our data also indicate that excess dopamine production is not confined to metastatic tumors with these characteristics. This is in contrast to the lower levels of metanephrine in patients with than without metastases, which reflects the high risk of malignancy associated with extra-adrenal tumors that rarely produce epinephrine.
Production of metanephrine by metastases arising from some adrenal primary tumors establishes that epinephrine synthesis can be maintained without proximity to sources of adrenal steroids. This and findings of similar dopamine-producing characteristics in both primary tumors and their associated metastases indicate that loss of catecholamine phenotypic features is not a characteristic of malignant transformation. Thus, in addition to pointing to the presence of metastases, it is likely that an elevated methoxytyramine should prove useful for predicting metastatic spread. This possibility is also supported by findings in one patient of predominant increases in plasma methoxytyramine that preceded by 5 years appearance of metastases.
As in the study by Ayala-Ramirez et al.,26 referral of patients with malignant PPGLs was likely responsible for the higher proportion of patients with metastatic disease in the present patient cohort than in nine other studies.10,27-34 Analysis of these earlier studies indicates a 14% overall prevalence of malignant PPGLs and a 3.6-fold higher risk of malignancy associated with extra-adrenal than adrenal tumors (Table 2). This is consistent with the present findings of a 3.4-fold higher risk of malignancy due to extra-adrenal than adrenal tumors, even after exclusion of patients with SDHB mutations, which in turn indicates that high malignant risk associated with SDHB mutations largely reflects the predominant extra-adrenal locations of associated tumors.
Table 2.
Meta-analysis of Nine Studies of Metastatic PPGLs
| Percent Malignant |
Percent |
||||
|---|---|---|---|---|---|
| Study | N | Adrenal | Extra-adrenal | Total | Extra-adrenal |
| Remine et al. 1974 | 138 | 9.7% (12/124) | 42.9% (6/14) | 13.0% | 10.1% |
| Proye et al., 1994 | 310 | 11.6% (31/268) | 40.5% (17/42) | 15.5% | 13.5% |
| Goldstein et al., 1999 | 99 | 11.0% (8/73) | 23.1% (6/26) | 14.1% | 26.3% |
| Mannelli et al., 1999 | 281 | 6.7% (17/253) | 32.1% (9/28) | 9.2% | 9.9% |
| John et al., 1999 | 95 | 6.5% (5/77) | 27.8% (5/18) | 10.5% | 18.9% |
| Edstrom Elder et al., 2003 | 85 | 10.0% (7/70) | 46.7% (7/15) | 16.5% | 17.6% |
| Amar et al., 2005 | 192 | 11.4% (19/167) | 40.0% (10/25) | 15.1% | 13.0% |
| Feng et al., 2011 | 136 | 16.6% (17/102) | 41.2% (14/34) | 22.7% | 25.0% |
| Park et al., 2011 | 152 | 9.5% (13/137) | 26.7% (4/15) | 11.2% | 9.9% |
| Total | 1488 | 10.1% (129/1271) | 35.9% (78/217) | 13.9% | 14.6% |
The association of large tumor size with increased risk of malignancy is in agreement with several previous studies.10,26,27,33,34 However, we also show here that the higher risk of malignancy with increasing tumor size is independent of tumor location. Thus, increased tumor size and extra-adrenal location both contribute additively to increased risk of metastatic disease, fully accounting for the high malignancy rate associated with SDHB mutations.
As suggested elsewhere,11 large size of PPGLs in patients with SDHB mutations may reflect delayed diagnosis, a possibility supported by the present findings of lower tumor tissue concentrations of catecholamines in SDHB-associated PPGLs than in other tumors. As shown elsewhere,35 immature catecholamine biosynthetic phenotypes of PPGLs in patients with SDHB mutations can result in an asymptomatic presentation and a delay in detection until tumors attain a large enough size to cause clinical complications. Possibly also, energy not utilized for maintaining the catecholamine secretory phenotype is available for more aggressive growth.
The above considerations underscore the importance of genetic testing in patients with PPGLs and surveillance programs in all patients with identified mutations. Detection and resection of tumors at an earlier stage and smaller size can be expected to be particularly important for reducing malignant risk and minimizing subsequent morbidity and mortality in patients with SDHB mutations.
The overlap in distributions in plasma methoxytyramine in patients with and without metastases indicates that these measurements cannot be used to accurately predict or exclude the presence of malignancy. Nevertheless, as the currently only identified circulating biomarker of metastatic PPGLs and in the absence of more reliable circulating biomarkers, these measurements may serve to assess the relative likelihood of metastatic disease, supplementing predictive information derived from considerations of tumor size, location and SDHB mutation status.
Since identification of metastatic spread continues to rely heavily on extensive and expensive use of functional imaging studies,18 predictive information about the likelihood of metastases may be used to justify such studies. This in turn provides promise for identifying metastatic spread at an earlier stage when currently available or emerging therapies might offer an improved outcome. Due to the substantial amounts of methoxytyramine produced by a significant proportion of metastatic PPGLs these measurements should also offer utility in patient management as surrogate biomarkers to assess tumor burden, disease progression and response to treatment.
As in other studies, a limitation of this study is that without satisfactory methods to exclude future development of metastases there is likelihood that some patients were misclassified. Consequently, the utility of various indices examined for prediction of malignancy is likely underestimated. Accurate assessment and validation of such utility requires long-term patient follow-up. As indicated by the considerable time period of up to 44 years between initial presentation of tumors and development of metastases, follow-up should be life-long, beyond the time frame of most studies.
In summary, this study establishes plasma free methoxytyramine as a novel biomarker for metastatic PPGLs, clarifies tumor location and size as malignant risk factors and the contributions of these to the high risk of malignancy associated with SDHB mutations. These data provide useful information for improving management of patients with PPGLs and for recognizing risk factors of metastatic disease.
Supplementary Material
Figure 3.
Bar graphs showing plasma concentrations of methoxytyramine (A) and metanephrine (B) in subgroups of patients with and without metastases according to adrenal versus extra-adrenal locations of primary tumors. Results are shown as geometric means±SE. a,b,cPresence of different alphabetic characters indicate significant differences (P<0.05) between groups, whereas presence of identical characters indicates lack of significant differences.
Acknowledgments
Thanks are extended to Thanh-Truc Huynh, Nan Qin, Stephanie Fliedner, Kathryn King and Tamara Prodanov for technical help or assistance with collections of patient materials and data.
Funding: European Union 7th Framework Program (ENS@T-CANCER; HEALTH-F2-2010-259735), National Institutes of Health and the Deutsche Forschungsgesellschaft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: This manuscript is the last of a series utilizing a comprehensive dataset from a cohort of 1211 subjects. Originally, all manuscripts in the series were compiled as part of a single article totaling over 12,500 words. That original article was split into four separate manuscripts, each focusing on a separate topic. Since the presented data are inter-related, the ideal presentation would have been as a single article. Journal requirements, however, do not allow this. Nevertheless, all effort has been made to avoid presentation of duplicate or redundant material in each of the four manuscripts. The data in the present manuscript represent unique data on malignant pheochromocytoma and paraganglioma that are not are presented in the other three manuscripts in the series.
References
- 1.Eisenhofer G, Bornstein SR, Brouwers FM, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004 Sep;11(3):423–436. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 2.Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007 Sep;14(3):569–585. doi: 10.1677/ERC-07-0074. [DOI] [PubMed] [Google Scholar]
- 3.Brouwers FM, Petricoin EF, 3rd, Ksinantova L, et al. Low molecular weight proteomic information distinguishes metastatic from benign pheochromocytoma. Endocr Relat Cancer. 2005 Jun;12(2):263–272. doi: 10.1677/erc.1.00913. [DOI] [PubMed] [Google Scholar]
- 4.Brouwers FM, Elkahloun AG, Munson PJ, et al. Gene expression profiling of benign and malignant pheochromocytoma. Ann N Y Acad Sci. 2006 Aug;1073:541–556. doi: 10.1196/annals.1353.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong VE, Kennedy T, Al-Ahmadie H, et al. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery. 2008 Jun;143(6):759–768. doi: 10.1016/j.surg.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Suh I, Shibru D, Eisenhofer G, et al. Candidate genes associated with malignant pheochromocytomas by genome-wide expression profiling. Ann Surg. 2009 Dec;250(6):983–990. doi: 10.1097/SLA.0b013e3181b248bb. [DOI] [PubMed] [Google Scholar]
- 7.Thouennon E, Pierre A, Tanguy Y, et al. Expression of trophic amidated peptides and their receptors in benign and malignant pheochromocytomas: high expression of adrenomedullin RDC1 receptor and implication in tumoral cell survival. Endocr Relat Cancer. 2010;17(3):637–651. doi: 10.1677/ERC-10-0109. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann J, Fendrich V, Holler JP, et al. Microarray analysis reveals differential expression of benign and malignant pheochromocytoma. Endocr Relat Cancer. 2010 Jun 18;17:743–756. doi: 10.1677/ERC-09-0118. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Rochow GY, Jackson NE, Conaglen JV, et al. MicroRNA profiling of benign and malignant pheochromocytoma identifies novel diagnostic and therapeutic targets. Endocr Relat Cancer. 2010 Jul 12;17(835-846) doi: 10.1677/ERC-10-0142. [DOI] [PubMed] [Google Scholar]
- 10.John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999 Apr;53(4):679–683. doi: 10.1016/s0090-4295(98)00612-8. [DOI] [PubMed] [Google Scholar]
- 11.Amar L, Bertherat J, Baudin E, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005 Dec 1;23(34):8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 12.Brouwers FM, Eisenhofer G, Tao JJ, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab. 2006 Nov;91(11):4505–4509. doi: 10.1210/jc.2006-0423. [DOI] [PubMed] [Google Scholar]
- 13.Tippett PA, McEwan AJ, Ackery DM. A re-evaluation of dopamine excretion in phaeochromocytoma. Clin Endocrinol (Oxf) 1986 Oct;25(4):401–410. doi: 10.1111/j.1365-2265.1986.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 14.Proye C, Vix M, Goropoulos A, Kerlo P, Lecomte-Houcke M. High incidence of malignant pheochromocytoma in a surgical unit. 26 cases out of 100 patients operated from 1971 to 1991. J Endocrinol Invest. 1992 Oct;15(9):651–663. doi: 10.1007/BF03345810. [DOI] [PubMed] [Google Scholar]
- 15.Januszewicz W, Wocial B, Januszewicz A, Gryglas P, Prejbisz A. Dopamine and dopa urinary excretion in patients with pheochromocytoma--diagnostic implications. Blood Press. 2001;10(4):212–216. doi: 10.1080/08037050152669729. [DOI] [PubMed] [Google Scholar]
- 16.van der Harst E, de Herder WW, de Krijger RR, et al. The value of plasma markers for the clinical behaviour of phaeochromocytomas. Eur J Endocrinol. 2002 Jul;147(1):85–94. doi: 10.1530/eje.0.1470085. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhofer G, Goldstein DS, Sullivan P, et al. Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005 Jan 11;90:2068–2075. doi: 10.1210/jc.2004-2025. [DOI] [PubMed] [Google Scholar]
- 18.Timmers HJ, Kozupa A, Chen CC, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007 Jun 1;25(16):2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 19.Timmers HJ, Eisenhofer G, Carrasquillo JA, et al. Use of 6-[18F]-fluorodopamine positron emission tomography (PET) as first-line investigation for the diagnosis and localization of nonmetastatic and metastatic phaeochromocytoma (PHEO). Clin Endocrinol (Oxf) 2009 Jul;71(1):11–17. doi: 10.1111/j.1365-2265.2008.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluorodeoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009 Dec;94(12):4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? Jama. 2002 Mar 20;287(11):1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhofer G, Goldstein DS, Stull R, et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986 Nov;32(11):2030–2033. [PubMed] [Google Scholar]
- 23.Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem. 1993 Jan;39(1):97–103. [PubMed] [Google Scholar]
- 24.Rao F, Keiser HR, O'Connor DT. Malignant pheochromocytoma. Chromaffin granule transmitters and response to treatment. Hypertension. 2000 Dec;36(6):1045–1052. doi: 10.1161/01.hyp.36.6.1045. [DOI] [PubMed] [Google Scholar]
- 25.Amar L, Peyrard S, Rossignol P, Zinzindohoue F, Gimenez-Roqueplo AP, Plouin PF. Changes in urinary total metanephrine excretion in recurrent and malignant pheochromocytomas and secreting paragangliomas. Ann N Y Acad Sci. 2006 Aug;1073:383–391. doi: 10.1196/annals.1353.042. [DOI] [PubMed] [Google Scholar]
- 26.Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011 Mar;96(3):717–725. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 27.Remine W, Chong G, van Heerden J, Sheps S, Harrison EJ. Current management of pheochromocytoma. Ann Surg. 1974;179:740–748. doi: 10.1097/00000658-197405000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proye CA, Vix M, Jansson S, Tisell LE, Dralle H, Hiller W. “The” pheochromocytoma: a benign, intra-adrenal, hypertensive, sporadic unilateral tumor. Does it exist? World J Surg. 1994 Jul-Aug;18(4):467–472. doi: 10.1007/BF00353738. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein RE, O'Neill JA, Jr., Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999 Jun;229(6):755–764. doi: 10.1097/00000658-199906000-00001. discussion 764-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannelli M, Ianni L, Cilotti A, Conti A. Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol. 1999 Dec;141(6):619–624. doi: 10.1530/eje.0.1410619. [DOI] [PubMed] [Google Scholar]
- 31.Edstrom Elder E, Hjelm Skog AL, Hoog A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003 Apr;29(3):278–283. doi: 10.1053/ejso.2002.1413. [DOI] [PubMed] [Google Scholar]
- 32.Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005 Apr;90(4):2110–2116. doi: 10.1210/jc.2004-1398. [DOI] [PubMed] [Google Scholar]
- 33.Feng F, Zhu Y, Wang X, et al. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol. 2011 May;185(5):1583–1590. doi: 10.1016/j.juro.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 34.Park J, Song C, Park M, et al. Predictive characteristics of malignant pheochromocytoma. Korean J Urol. 2011 Apr;52(4):241–246. doi: 10.4111/kju.2011.52.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmers HJ, Pacak K, Huynh TT, et al. Biochemically Silent Abdominal Paragangliomas in Patients with Mutations in the Sdhb Gene. J Clin Endocrinol Metab. 2008 Oct 7;93:4826–4832. doi: 10.1210/jc.2008-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.