Abstract
Gene therapy using angiogenic genes has emerged as a potentially viable alternative treatment strategy for myocardial ischemia. Non-specific expression of angiogenic genes, however, may result in side effects, including the growth of occult tumors. Regulation of gene expression may help to avoid the occurrence of these side effects. In this study, a plasmid expressing vascular endothelial growth factor (VEGF) was constructed with an oxygen dependent degradation (ODD) domain and a secretion signal peptide (SP) in order to stabilize the VEGF protein and facilitate the secretion of VEGF protein, specifically under hypoxic conditions. We found that this plasmid, pβ-SP-ODD-VEGF, expresses the SP-ODD-VEGF protein at increased levels under hypoxic conditions compared to normoxic conditions. Since the size of the ODD domain is almost the same as that of VEGF, the ODD-VEGF fusion protein may have lower secretion efficiency. To address this issue, a furin recognition site was located between the ODD domain and the VEGF site to facilitate elimination of the SP-ODD domain from the fusion protein before its secretion. This optimizes the likelihood that the VEGF secreted from the target cells will be wild-type VEGF. Treatment with a furin inhibitor reduced the secretion efficiency of the VEGF, indicating that furin digestion increases the secretion of VEGF. The secreted wild-type VEGF facilitated the growth of endothelial cells more efficiently under hypoxic conditions than normoxic conditions. These results suggest that this plasmid, pβ-SP-ODD-VEGF, warrants further study as a more efficient form of hypoxia-inducible gene therapy for the treatment of myocardial ischemia.
Keywords: Gene therapy, Hypoxia, VEGF, Myocardial ischemia
1. Introduction
Ischemia, a pathological condition in which organs, tissues or cells suffer from an inadequate supply of oxygen, is associated with a wide variety of disease states, including myocardial infarction, stroke and spinal cord injury [1, 2]. Myocardial ischemia, one of the leading causes of death in the world, typically occurs as a result of decreased blood flow due to stenosis in the coronary arteries. This decrease in blood flow results in a decrease in the delivery of the minimum levels of nutrients and oxygen required for normal heart function [3, 4]. The consequences of myocardial ischemia and infarction are cardiomyocyte hypertrophy, apoptotic myocyte loss, progressive collagen replacement, and enlargement of the left ventricle [5]. Unfortunately, the current pharmacological treatments for myocardial ischemia and infarction are often not sufficient to prevent remodeling of the left ventricle and the progression to heart failure. Considerable attention has focused on gene therapy as an alternative to current pharmacological therapies. Gene therapy offers the potential to directly protect cardiomyocytes against necrosis or apoptosis and thereby prevent the development of myocardial fibrosis and cardiac dysfunction [6].
In the ischemic heart, an inadequate oxygen supply prompts over-expression of hypoxia-inducible genes such as vascular endothelial growth factor (VEGF), an angiogenic protein, which helps to protect cardiomyocytes and preserve myocardial function in addition to promoting angiogenesis [7]. The level of VEGF produced endogenously in response to an ischemic insult, however, is insufficient to completely restore myocardial function [8]. Gene therapy to promote angiogenesis therefore requires the exogenous introduction of genes. Previous attempts at inducing angiogenesis have focused on enhancing the transfection of angiogenic genes in a constitutive manner. Non-specific, unregulated gene expression may lead to severe side effects, however, including tumor growth, accelerated diabetic proliferative retinopathy, and rupture of pre-existing atherosclerotic plaques. VEGF gene expression in response to ischemia, therefore, needs to be regulated [9, 10].
To the present time, only a few studies have demonstrated the ability to regulate the expression of VEGF in response to hypoxia or ischemia. At the same time, there have been significant advances in the development of novel gene carriers [11–13]. Different strategies have been used to control the transgene expression of VEGF. Controlling gene transcription is the most commonly employed strategy to allow for ischemia-inducible gene expression. At the stage of translation, hypoxia-specific stabilization improves protein stability [14]. Further, in the case of secretory proteins such as VEGF, it is necessary to proceed into the secretion pathway rapidly and to exit cells easily. While the use of a signal peptide (SP) and a protein stabilization domain is known to improve protein secretion and activity, their presence may also sometimes interfere with protein secretion and bioactivity. Separation of the therapeutic protein from these other functional domains would be achieved by the enzyme-recognition site between the protein-coding region and the other domains, allowing enzyme-mediated cleavage to result in the secretion of a wild type protein.
In the present study, we developed a new plasmid DNA (pDNA) that: 1) accelerates VEGF production; 2) stabilizes VEGF throughout the secretory pathway; and 3) results in the production of wild type VEGF under hypoxic conditions. The oxygen-dependent degradation (ODD) domain and SP derived from the Exendin-4 leader sequence were placed upstream of the VEGF-coding region to maintain VEGF stability and to facilitate the secretion of VEGF, respectively. In addition, the cleavage site recognized by furin, an enzyme present in the Golgi network, was placed in between the ODD domain and the coding region for VEGF, to promote the release of wild type VEGF. To determine the efficacy of this plasmid, the secretion and activity of VEGF were compared under hypoxic and normoxic conditions.
2. Materials and Methods
2.1 Materials
Branched poly(ethylenimine) (bPEI, Mw: 25 kDa), cobalt chloride (CoCl2), and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were purchased from SigmaAldrich (St. Louis, MO). All cell culture products including fetal bovine serum (FBS), Dulbecco’s phosphate buffered saline (DPBS), and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Invitrogen (GibcoBRL, Carlsbad, CA). Furin inhibitor II was obtained from EMD chemicals Inc. (Gibbstown, NJ). Human VEGF ELISA kit and carboxy-DCFDA were purchased from Invitrogen (Camarillo, CA). The LDH cytotoxicity assay kit II was obtained from Abcam Inc. (Cambridge, MA). Transwell plates were purchased from Corning Inc. (Corning, NY).
2.2 Construction of pβ-SP-ODD-VEGF
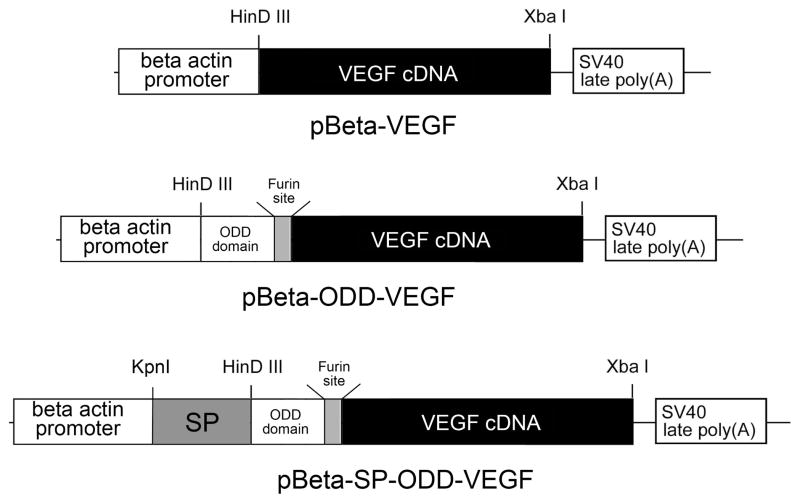
The VEGF cDNA was amplified by PCR using pSV-VEGF as a template. The primers for the PCR were as follows: forward, 5’-GCTCTAGACGTCAACGTCGTATGAA-CTTTCTGCTGTCTTG-3’, backward, 5’-TCCCCCCGGGTTACCGCCTCGGCTTGTCAC-3’. The XbaI and XmaI recognition sites were introduced to the primers, respectively, for cloning convenience. In addition, the furin recognition site was inserted at the upstream of the start codon of the VEGF cDNA. The amplified VEGF cDNA was inserted into the pβ, resulting in construction of pβ-VEGF. The ODD cDNA was amplified by PCR using pSV-Luc-ODD as a template. The primers for the PCR were as follows: forward, 5’-GGGGTACCATGGCCGCTGGAGACACAATCAT-3’; backward, 5’-GCTCT-AGACTGCTGGAATACTGTAACTG-3’. The KpnI and XbaI sites were underlined. The ODD cDNA was inserted upstream of the furin-VEGF cDNA at the sites of KpnI and XbaI in pβ-VEGF, resulting in construction of pβ-ODD-VEGF. Finally, the secretion SP was amplified by PCR using pUAS-SP-GLP-1 as a template. The primers were as follows: forward, 5’-CCCAAGCTTATGAAGATCATCCTGTGGCT-3’; backward, 5’-GGGGTAC-CGGCGAAGCTCTCGGAGCTGG -3’. The HindIII and KpnI sites were underlined. The SP cDNA was inserted into pβ-ODD-VEGF at the sites of HinDIII and KpnI, producing pβ-SP-ODD-VEGF. The plasmid constructions were confirmed by direct sequencing and restriction enzyme reactions (Figure 1). The RTP801-VEGF plasmid (pRTP-VEGF), a hypoxia-inducible VEGF plasmid developed previously by our group, was used as a positive control for comparison of relative VEGF production and secretion [15].
Figure 1.
Schematic representation of the construction of the plasmids.
2.3 Transfection and VEGF Quantification
H9C2 cells were cultured in DMEM containing 10% FBS and 1% antibiotics at 37ºC under 5% CO2. When cell confluence reached 80%, cells were seeded on 24-well plates at a density of 2.0 × 104 cells/well. After 24 hrs of incubation, the culture media was replaced with plain media containing pDNA/PEI polyplexes prepared by mixing 1 μg pDNA and 1 μg PEI. After 4 hrs, the cells were washed with PBS and cultured with DMEM. For the creation of hypoxic conditions, concentrations of CoCl2 were added to the cells and the culture plates were placed in a hypoxia chamber. The cell culture media was collected 24 hrs or 48 hrs after transfection and the cells were lysed with 150 μl of reporter lysis buffer for 20 min. The cells were harvested and centrifuged for 30s at 13,000 rpm. The amount of VEGF production and secretion were quantified using a VEGF ELISA kit according to the manufacturer’s protocol.
2.4 Reactive Oxygen Species (ROS)
Cells were prepared as described above. Phenol red-free DMEM containing 5μmol carboxy-DCFDA replaced the culture media following exposure to hypoxic conditions. Cells were incubated for 30 min, washed with PBS, and cultured in DMEM without phenol red. After 2 hrs of incubation, fluorescence intensities were recorded at Ex=495 nm and Em=520 nm using a fluorescence reader. ROS values were calculated as the percent ROS present relative to normoxic conditions.
2.5 Cell Viability and Cytotoxicity
Cell viability under hypoxic conditions was determined by MTT assay. MTT was added to the cells at the end of cell incubation and the cell viability was calculated as a relative value. Cytotoxicity was also measured concurrently with a VEGF ELISA using an LDH cytotoxicity assay kit according to the manufacturer’s instruction.
2.6 Human Umbilical Vein Endothelial Cells (HUVEC) Proliferation
HUVECs were cultured using F-12K medium containing endothelial cell growth supplement (ECGS) and 10% FBS. The HUVECs were seeded on the inner wells of transwell plates at a density of 5 × 103 cells/well. H9C2 cells were seeded on 24-well plates and transfected with pDNA/PEI polyplexes. The inner wells were relocated in the 24-well plates, where H9C2 cells were seeded 4 hrs following transfection. HUVEC proliferation was assessed by CyQUANT® NF Cell Proliferation Assay Kit (Molecular Probes; Eugene, OR) and HUVEC viability was determined by MTT assay at 48 hrs post-transfection.
2.7 Furin Inhibitor
H9C2 cells were seeded on 24-well plates as described above. The furin inhibitor was added to the cells at final concentrations of 0, 1, and 10 μmol 8 hrs following seeding. The cells were transfected with polyplexes of pDNA/PEI in the absence of the furin inhibitor after 16 hrs of cell incubation. The culture media was replaced with complete media containing the furin inhibitor at 4 hrs post-transfection. After 44 hrs of incubation, the culture media was collected, the cells were lysed and the presence of VEGF determined by ELISA.
3. Results
3.1 VEGF Secretion Patterns under CoCl2-Induced Hypoxia
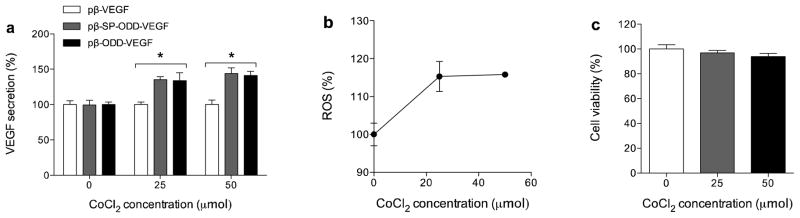
Since transfection of the pDNA has to be maximized to see differences in performance of the pDNA constructs, polyethylenimine (PEI), a cationic polymer for gene delivery, was used to evaluate which pDNA construct produced the highest level of VEGF expression or secretion under hypoxic condition. Despite its high transfection efficiency, PEI is toxic to cells. Therefore, the weight ratio of PEI to DNA was fixed at 1:1, which is typically associated with less than 10% cytotoxicity. This serves to minimize the effects of PEI on cell viability. H9C2 cells were incubated in the presence of various concentrations of CoCl2 to mimic hypoxic conditions. To establish a benchmark secretion for VEGF, a non-hypoxia-responsible plasmid, pβ-VEGF, was referenced to 100%. Since the three plasmids share a common promoter, the total amount of VEGF expression might be expected to be similar. The secretion of VEGF, however, was significantly increased by the presence of the SP and ODD domains as shown in figure 2a. The relative amount of VEGF secretion was increased by the pβ-SP-ODD-VEGF plasmid and the pβ-ODD-VEGF plasmid in the presence of CoCl2. At a concentration of 50 μmol CoCl2, both plasmids increased the production of VEGF by approximately 50% compared to that in the absence of CoCl2. As it is difficult to directly determine the degree of hypoxia in cell culture, measurement of reactive oxygen species (ROS) is a widely accepted indirect method to quantify the degree of hypoxia. We measured the level of production of ROS to confirm that the cells were cultured under hypoxic conditions. The generation of-ROS was dependent on the concentration of CoCl2 up to 25 μmol, as shown in figure 2b. These results indicate that both plasmids enhance VEGF secretion in response to CoCl2-induced hypoxia and there is no artifact caused by CoCl2 toxicity (figure 2c). Since there may be a difference in the intracellular environments between CoCl2-induced hypoxia and atmosphere-induced hypoxia, we also chose to study the production of VEGF under conditions of atmosphere-induced hypoxia.
Figure 2.
VEGF secretion under conditions of CoCl2-induced hypoxia. (a) VEGF secretion, (b) ROS, and (c) cell viability in the presence of CoCl2. Secreted VEGF was measured in the culture media and ROS levels were measured to determine the degree of hypoxia. All results are expressed as relative values to pβ-VEGF (data presented as mean ± SD; *p<0.001, one-way ANOVA).
3.2 VEGF Secretion Patterns Under Hypoxic Conditions
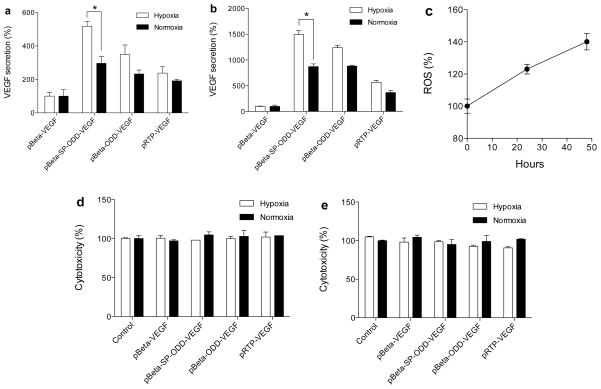
Cells were cultured under the same transfection conditions described above except for the source of hypoxia. A gas mixture composed of 94% N2, 5% CO2, and 1% O2 was used to create hypoxic conditions in the cell culture chamber. The cells were grown under these hypoxic conditions for 24 hrs and 48 hrs following transfection. Figure 3a shows the secretion of VEGF after 24 hrs of incubation and figure 3b shows the secretion of VEGF after 48 hrs of incubation. At 48 hrs post-transfection, the secretion of VEGF under hypoxic conditions increased 1.7-fold following transfection with the pβ-SP-ODD-VEGF plasmid and increased 1.5-fold following transfection with the pβ-ODD-VEGF plasmid, compared to normoxic conditions. At the same time, the level of ROS was increased as shown in figure 3c. Transfection with either plasmid was not associated with cytotoxicity (figure 3d, e). Regardless of the etiology of the hypoxic conditions present, the pβ-SP-ODD-VEGF plasmid produced the highest level of VEGF secretion in response to hypoxia. VEGF secretion increased more than 200% at 24 hrs and 300% at 48 hrs following transfection with the pβ-SP-ODD-VEGF plasmid compared to transfection with the pRTP-VEGF plasmid. These results indicate that the pβ-SP-ODD-VEGF plasmid is the most effective plasmid construct for promoting the secretion of VEGF under hypoxic conditions.
Figure 3.
VEGF secretion under the conditions of atmosphere-induced hypoxia. (a) At 24 hrs and (b) at 48 hrs of hypoxia, (c) ROS, and cytotoxicity at (d) 24 hrs and (e) 48 hrs. VEGF levels were quantified in the culture media; the state of hypoxia was confirmed by measuring ROS. Data are presented as relative VEGF secretion compared to pβ-VEGF (data presented as the mean ± SD; *p<0.01).
3.3 Bioactivity of the Secreted VEGF
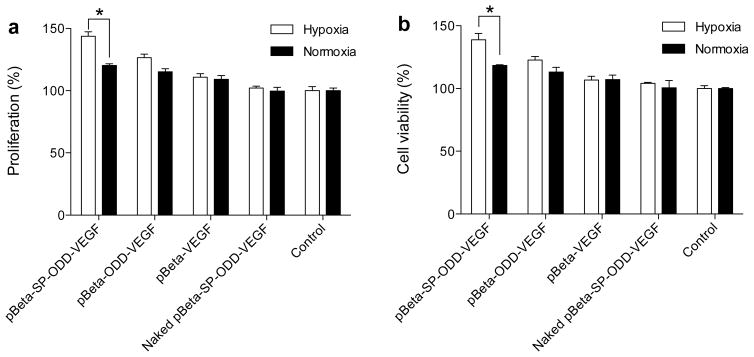
Bioactivity of the secreted VEGF was evaluated to determine its therapeutic potential. H9C2 cells were cultured in the outer wells of transwell plates while HUVEC were seeded in the inner wells of the plates. The transwell plates were incubated in the absence of any growth supplements. The HUVEC proliferated significantly when exposed to the culture media containing VEGF that was secreted from either the pβ-SP-ODD-VEGF- or the pβ-ODD-VEGF-transfected H9C2 cells. As shown in figure 4, transfection with the pβ-SP-ODD-VEGF resulted in relative HUVEC proliferation of ~140% under hypoxic conditions and ~115% under normoxic conditions while transfection with pβ-ODD-VEGF resulted in relative HUVEC proliferation of ~120% under hypoxic conditions and ~110% under normoxic conditions.
Figure 4.
Activity of the secreted VEGF as determined by proliferation of HUVECs. HUVEC (a) proliferation and (b) cell viability. HUVECs were cultured in the inner wells of transwell plates while pDNA-transfected H9C2 cells were grown in the outer wells of the transwell plate. The degree of proliferation is presented as a percentage relative to the control group of pβ-VEGF transfected cells (data presented as the mean ± SD, *p<0.01).
3.4 Effect of the SP-Domain and the Furin-Recognition Site on VEGF Secretion
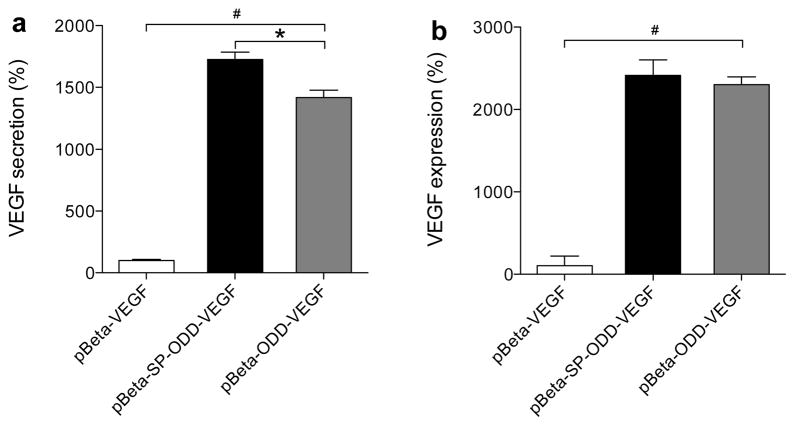
We hypothesized that the SP-domain would facilitate VEGF secretion and that the furin-recognition site would increase VEGF secretion under hypoxic conditions. The amount of VEGF secreted and the amount of VEGF remaining in cells were compared to determine the effect of the SP-domain on the secretion of VEGF. VEGF secretion in the pβ-SP-ODD-VEGF transfected cells was ~1700% and in the pβ-ODD-VEGF transfected cells was ~1300% of the levels in the pβ-VEGF transfected cells (Figure 5a). The VEGF remaining in the pβ-SP-ODD-VEGF transfected cells was ~2300% and in the pβ-ODD-VEGF transfected cells ~2200% of the levels in the pβ-VEGF transfected cells (Figure 5b). The difference in VEGF secretion between the pβ-SP-ODD-VEGF transfected cells and the pβ-ODD-VEGF transfected cells was greater than the amount of VEGF remaining in the cells.
Figure 5.
Effect of the SP domain on VEGF secretion. (a) VEGF secreted into the media and (b) VEGF remaining in the cells. VEGF secretion was determined by quantifying the amount of VEGF in the culture media and the amount of VEGF remaining in the cells as measured in the cell lysates. VEGF secretion is expressed as a value relative to the secretion of VEGF following transfection with pβ-VEGF (data presented as the mean ± SD; #p<0.0001 one-way ANOVA, *p<0.05).
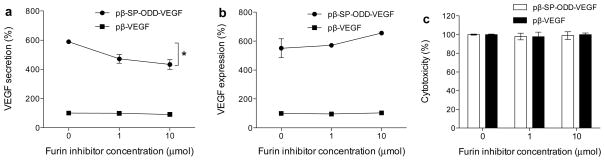
Figure 6 illustrates that furin plays a crucial role in facilitating VEGF secretion. H9C2 cells were cultured in the presence or absence of the furin inhibitor. Relative amounts of the VEGF secreted and the VEGF remaining intra-cellularly are shown in figures 6. The pβ-VEGF plasmid, which contains no furin-recognition site, was used as a positive control. The amounts of VEGF measured in all test groups were calculated relative to the positive control of the pβ-VEGF plasmid, which did not contain the furin inhibitor. VEGF secretion decreased with increasing concentration of the furin inhibitor (figure 6a). The relative VEGF secretion was ~600% in the absence of the furin inhibitor and decreased to ~400% in the presence of 10 μmol of the furin inhibitor. The levels of the VEGF remaining in the cells were ~550% without the furin inhibitor and ~650% with 10 μmol of the furin inhibitor. There was a negligible difference in the amount of VEGF measured in all of the pβ-VEGF treatment groups. Cytotoxicity as a consequence of the furin inhibitor was studied to determine its influence on cell viability (Figure 6c). The relative cell viability was ~100% under all test conditions, indicating that the furin inhibitor does not increase cytotoxicity.
Figure 6.
Effects of the furin-cleavage site on VEGF secretion. (a) VEGF secretion into the culture media, (b) VEGF remaining in the cells, and (c) cytotoxicity in the presence of the furin inhibitor. The cells were incubated in the presence of the furin inhibitor and transfected with pDNA. The changes in the VEGF secretion pattern were determined by measuring VEGF in the culture media and in the cell lysates. VEGF expression was calculated as a percentage relative to VEGF expression following transfection with the pβ-VEGF plasmid at the furin inhibitor concentration of 0 μmol (data presented as the mean ± SD; *p<0.001 one-way ANOVA).
4. Discussion
The VEGF family includes five glycoproteins referred to as VEGF-A, -B, -C, -D, and placental growth factor (PGF). VEGF, conventionally indicating VEGF-A, is a well-known angiogenic protein that exerts its activity on surrounding endothelial cells via paracrine, endocrine, and autocrine mechanisms [16]. Binding of VEGF to its receptors activates a variety of signal pathways for angiogenesis, vasculogenesis, and lymphangiogenesis. The key steps in VEGF-mediated angiogenesis are VEGF secretion and receptor binding; therefore, enhanced secretion of wild-type VEGF is necessary for successful VEGF-based gene therapy. All cells have a constitutive secretory pathway (CSP) and dedicated secretory cells contain a regulated secretory pathway (RSP) [17]. These mechanisms, however, are not available to enhance VEGF secretion when the VEGF gene is introduced exogenously. Fusion of VEGF with a bioactive signal peptide that targets the endoplasmic reticulum (ER) can accelerate the secretion of VEGF through the Golgi/trans-Golgi network (TGN).
The signal peptide located upstream in Exendin-4 (position 1 – 23) is a highly conserved sequence between Exendin-4, -3, and -2 and functions to direct its downstream protein to the secretory pathway [18, 19]. To utilize this mechanism in the present study, the signal peptide was cloned into the plasmid upstream of the VEGF-coding region to facilitate VEGF secretion and post-translational processing of VEGF. In order to demonstrate the effect of the SP domain on VEGF secretion, the amount of VEGF produced by the plasmid constructs with and without the signal peptide was compared. Increases in VEGF secretion in the presence of the SP domain compared to that in the absence of the SP domain under both types of hypoxic conditions are due to the acceleration in VEGF secretion by the SP domain (Figure 2 and 3). Concurrent observations confirm that the SP domain significantly increases VEGF secretion but not VEGF expression (Figure 5). Since the signal peptide is not responsive to hypoxia, it also has an influence on VEGF secretion under normoxic conditions. VEGF is capable of inducing proliferation of endothelial cells because it is one of the cytokines that act on endothelial cell proliferation during angiogenesis [20]. Thus, endothelial cells proliferate in the presence of secreted VEGF, with the proliferation rate depending on the amount of VEGF secreted. HUVEC proliferation occurred in direct proportion to the secretion of VEGF, suggesting that the SP domain is responsible for the increase in the secretion of VEGF. These results confirm that the plasmid containing the SP domain accelerates the secretion of VEGF compared to the plasmid construct without the SP domain.
Hypoxia-responsible gene expression can be primarily regulated in three ways: 1) transcriptional regulation; 2) post-transcriptional regulation, and 3) post-translational regulation [1]. Hypoxia-inducible factor-1 is accepted as the most important element in transcriptional regulation. In post-transcriptional regulation, VEGF mRNA is stabilized under hypoxic conditions by the cooperation of the 5'- and 3'-untranslated regions (UTRs) and the coding region. The best-studied strategy to control gene expression under hypoxic conditions in post-translational regulation is the fusion of the ODD domain and the therapeutic gene in order to stabilize the protein produced [21, 22]. The use of the ODD domain constitutes a powerful tool to increase the resident time of proteins, which typically have a short half-life. VEGF in particular needs to be stabilized following expression because its half-life is very short [23–25]. The increase in VEGF secretion seen with the pβ-SP-ODD-VEGF and the pβ-ODD-VEGF plasmids relative to pβ-VEGF is due to the stabilizing effect of the ODD domain during post-translational regulation. The ODD domain, therefore, plays an important role in stabilizing VEGF and thereby increasing half-life of VEGF under hypoxic conditions.
While the presence of the ODD domain can play a positive role in post-translational regulation, its large molecular weight may cause an abnormal folding or a decrease in secretion of the therapeutic protein, thereby diminishing the efficacy of the secreted therapeutic protein [1, 26]. These potential shortcomings may be overcome by the use of a short ODD domain composed of 18 core amino acids [26]. Even this shorter ODD domain, however, may interfere with the interaction between VEGF and its receptors. Enzymatic degradation between the ODD domain and VEGF helps to ensure that the wild-type form of VEGF is secreted following processing in the Golgi network [27]. Furin, a member of the subtilisin-like proprotein convertase family that exists in the TGN, processes latent precursor proteins into their biologically active products in the secretory pathway [28]. Furin recognizes a conserved polybasic R-X-R/K-R site and cleaves that site downstream of the target sequence [29, 30]. In these experiments, R-G-R-R, a furin-recognition site, was inserted between the ODD domain and the VEGF-coding region to enhance the secretion of wild type VEGF [19]. The decrease in VEGF secretion seen with the increase in the concentration of the furin inhibitor suggests that VEGF secretion is accelerated by the cleavage of the SP and the ODD domains. The finding that the furin inhibitor hinders VEGF secretion and increases the intra-cellular concentration of VEGF also confirms that the furin-recognition site is a powerful tool to enhance the secretion of wild-type VEGF.
5. Conclusion
A new plasmid containing the SP domain, the ODD domain, and the furin-cleavage site is a promising construct for enhancing VEGF secretion and the activity of VEGF under hypoxic conditions. The SP domain directs VEGF into the secretory pathway while the ODD domain stabilizes the expression of VEGF. The furin-recognition site provides a specific region to separate VEGF from the SP and ODD domains, resulting in more efficient secretion of wild-type VEGF. The combination of these three functional domains in one plasmid increases the secretion of VEGF under hypoxic conditions. This pβ-SP-ODD-VEGF plasmid results in more efficient production of wild-type VEGF than the pRPT-VEGF plasmid, demonstrating that this new plasmid construct is superior to other hypoxia-inducible VEGF plasmids. The findings of this study suggest that pβ-SP-ODD-VEGF may be a promising gene construct for the treatment of a variety of clinically important ischemic disease states.
Acknowledgments
This work was supported by NIH grants HL065477 (SW Kim) and HL071541 (DA Bull). Minhyung Lee was supported by the International Exchange Program for University Researchers (013-2011-1-E00060) funded by the National Research Foundation of Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim HA, Mahato RI, Lee M. Hypoxia-specific gene expression for ischemic disease gene therapy. Adv Drug Deliv Rev. 2009;61:614–622. doi: 10.1016/j.addr.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Nam HY, McGinn A, Kim PH, Kim SW, Bull DA. Primary cardiomyocyte-targeted bioreducible polymer for efficient gene delivery to the myocardium. Biomaterials. 2010;31:8081–8087. doi: 10.1016/j.biomaterials.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGinn AN, Nam HY, Ou M, Hu N, Straub CM, Yockman JW, Bull DA, Kim SW. Bioreducible polymer-transfected skeletal myoblasts for VEGF delivery to acutely ischemic myocardium. Biomaterials. 2011;32:942–949. doi: 10.1016/j.biomaterials.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Won YW, Kim JK, Cha MJ, Hwang KC, Choi D, Kim YH. Prolongation and enhancement of the anti-apoptotic effects of PTD-Hsp27 fusion proteins using an injectable thermo-reversible gel in a rat myocardial infarction model. J Control Release. 2010;144:181–189. doi: 10.1016/j.jconrel.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Choi D, Hwang KC, Lee KY, Kim YH. Ischemic heart diseases: current treatments and future. J Control Release. 2009;140:194–202. doi: 10.1016/j.jconrel.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Nam HY, Kim J, Kim S, Yockman JW, Kim SW, Bull DA. Cell penetrating peptide conjugated bioreducible polymer for siRNA delivery. Biomaterials. 2011;32:5213–5222. doi: 10.1016/j.biomaterials.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haider H, Ye L, Jiang S, Ge R, Law PK, Chua T, Wong P, Sim EK. Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. J Mol Med (Berl) 2004;82:539–549. doi: 10.1007/s00109-004-0546-z. [DOI] [PubMed] [Google Scholar]
- 8.Brogi E, Schatteman G, Wu T, Kim EA, Varticovski L, Keyt B, Isner JM. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest. 1996;97:469–476. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 10.Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- 11.Ah Kim H, Lee S, Park JH, Lee BW, Ihm SH, Kim TI, Kim SW, Ko KS, Lee M. Enhanced protection of Ins-1 beta cells from apoptosis under hypoxia by delivery of DNA encoding secretion signal peptide-linked exendin-4. J Drug Target. 2009;17:242–248. doi: 10.1080/10611860902718664. [DOI] [PubMed] [Google Scholar]
- 12.Yockman JW, Kim SW, Bull DA. Women and heart disease--physiologic regulation of gene delivery and expression: bioreducible polymers and ischemia-inducible gene therapies for the treatment of ischemic heart disease. Adv Drug Deliv Rev. 2009;61:863–870. doi: 10.1016/j.addr.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JM, Lee M, Kim KH, Ha Y, Choi JK, Park SR, Park H, Park HC, Ahn CH, Kim SW, Choi BH. Gene therapy of neural cell injuries in vitro using the hypoxia-inducible GM-CSF expression plasmids and water-soluble lipopolymer (WSLP) J Control Release. 2009;133:60–67. doi: 10.1016/j.jconrel.2008.09.080. [DOI] [PubMed] [Google Scholar]
- 14.Yockman JW, Choi D, Whitten MG, Chang CW, Kastenmeier A, Erickson H, Albanil A, Lee M, Kim SW, Bull DA. Polymeric gene delivery of ischemia-inducible VEGF significantly attenuates infarct size and apoptosis following myocardial infarct. Gene Ther. 2009;16:127–135. doi: 10.1038/gt.2008.146. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Bikram M, Oh S, Bull DA, Kim SW. Sp1-dependent regulation of the RTP801 promoter and its application to hypoxia-inducible VEGF plasmid for ischemic disease. Pharm Res. 2004;21:736–741. doi: 10.1023/b:pham.0000026421.09367.b3. [DOI] [PubMed] [Google Scholar]
- 16.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voutetakis A, Cotrim AP, Rowzee A, Zheng C, Rathod T, Yanik T, Loh YP, Baum BJ, Cawley NX. Systemic delivery of bioactive glucagon-like peptide 1 after adenoviral-mediated gene transfer in the murine salivary gland. Endocrinology. 2010;151:4566–4572. doi: 10.1210/en.2010-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons GB, Souza DW, Wu H, Yu D, Wadsworth SG, Gregory RJ, Armentano D. Ectopic expression of glucagon-like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007;14:38–48. doi: 10.1038/sj.gt.3302842. [DOI] [PubMed] [Google Scholar]
- 19.Oh S, Lee M, Ko KS, Choi S, Kim SW. GLP-1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003;7:478–483. doi: 10.1016/s1525-0016(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 20.Porter AM, Klinge CM, Gobin AS. Biomimetic hydrogels with VEGF induce angiogenic processes in both hUVEC and hMEC. Biomacromolecules. 2011;12:242–246. doi: 10.1021/bm101220b. [DOI] [PubMed] [Google Scholar]
- 21.Kim HA, Lim S, Moon HH, Kim SW, Hwang KC, Lee M, Kim SH, Choi D. Hypoxia-inducible vascular endothelial growth factor gene therapy using the oxygen-dependent degradation domain in myocardial ischemia. Pharm Res. 2010;27:2075–2084. doi: 10.1007/s11095-010-0206-7. [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Liu ML, Kim HA, Lee M, An S, Oh J, Cho J, Yi S, Kim K, Yoon D, Ha Y. Role of the oxygen-dependent degradation domain in a hypoxia-inducible gene expression system in vascular endothelial growth factor gene therapy. Spine (Phila Pa 1976) 2009;34:E952–958. doi: 10.1097/BRS.0b013e3181c4af80. [DOI] [PubMed] [Google Scholar]
- 23.Stefanini MO, Wu FT, Mac Gabhann F, Popel AS. A compartment model of VEGF distribution in blood, healthy and diseased tissues. BMC Syst Biol. 2008;2:77. doi: 10.1186/1752-0509-2-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George ML, Eccles SA, Tutton MG, Abulafi AM, Swift RI. Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: clinical evidence of platelet scavenging? Clin Cancer Res. 2000;6:3147–3152. [PubMed] [Google Scholar]
- 25.Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, Papadopoulos N, Pyles EA, Torri A, Wiegand SJ, Thurston G, Stahl N, Yancopoulos GD. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A. 2007;104:18363–18370. doi: 10.1073/pnas.0708865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada H, Hiraoka M, Kizaka-Kondoh S. Antitumor effect of TAT-oxygen-dependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res. 2002;62:2013–2018. [PubMed] [Google Scholar]
- 27.Kim TI, Lee M, Kim SW. Efficient GLP-1 gene delivery using two-step transcription amplification plasmid system with a secretion signal peptide and arginine-grafted bioreducible polymer. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- 30.Walker JA, Molloy SS, Thomas G, Sakaguchi T, Yoshida T, Chambers TM, Kawaoka Y. Sequence specificity of furin, a proprotein-processing endoprotease, for the hemagglutinin of a virulent avian influenza virus. J Virol. 1994;68:1213–1218. doi: 10.1128/jvi.68.2.1213-1218.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]