Abstract
Gait and balance impairments in people with Parkinson disease (PD) may lead to falls and serious injuries. Therefore, it is critical to improve our understanding of the nature of these impairments, including how they respond to prescribed anti-Parkinson medication. This is particularly important for complex balance and gait tasks that may be associated with falls. We evaluated motor function, functional balance, and gait performance during various gait tasks in 22 people with PD off and on medication (PD OFF, PD ON) and 20 healthy older adults. Although MDS-UPDRS-III score, Berg Balance Scale, mini-Balance Evaluations Systems test, and Timed-Up-and-Go improved in PD with medication, impairments persisted in all measures on medication, compared to controls. Dual task Timed-Up-and-Go did not improve with medication, and PD ON required more time than controls. Gait velocity and stride length improved similarly with medication in PD across forward, fast, backward, dual task forward, and dual task backward gait tasks. Cadence did not change with medication, nor did it differ between PD ON and controls. Velocity and stride length were reduced in PD ON compared to controls. Velocity reductions in PD ON during fast gait were cadence-mediated, while velocity reductions in backward gait were stride length-mediated. Our results suggest functional balance improves with medication in PD and gait performance improves with medication, regardless of task complexity. Remaining impairments on medication highlight the need to examine additional therapeutic options for individuals with PD to reduce the risk of falls.
Keywords: Parkinson Disease, Medication, Gait, Balance
Introduction
Parkinson disease (PD) is a progressive neurodegenerative disease, and cardinal symptoms include tremor, rigidity, bradykinesia, and postural instability. Individuals with PD commonly experience gait and postural stability impairments which may lead to falls, mobility loss, and reduced independence [1]. PD is characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta. Motor symptoms of PD are most commonly treated pharmacologically with levodopa, a dopamine precursor. After prolonged levodopa treatment, motor response fluctuations often develop, resulting in sub-optimal medication benefits during portions of the medication cycle [2].
It is unclear how medications affect postural stability and balance in PD. Impairments in postural stability and balance have been detected in PD, regardless of medication status, using static and dynamic posturography [3–5], perturbation responses [6,7], and clinical balance assessments, including the Berg Balance Scale (BBS) [8,9]. The few studies that have evaluated balance off and on medication within a group to determine whether balance improves with medication have reported mixed results. Nova et al. [10] reported levodopa improved balance, measured by the Berg Balance Scale, in PD. In contrast, other studies suggest levodopa has minimal or perhaps even detrimental effects on postural stability and balance. Specifically, though levodopa improved limits of stability in the forward direction, it did not affect other important aspects of postural stability, including backward limits of stability, postural alignment, leaning velocity, and center of mass and center of pressure during preparation for leaning [3]. Further, early and late automatic postural responses upon backward perturbation are deficient in PD [7]. Early responses improve only slightly with medication, while later responses are not affected, implying levodopa does not substantially improve balance in PD [7]. Levodopa has also negatively impacted responses to postural displacements in PD by impairing response magnitude scaling, and reducing in torque and EMG magnitude [11].
In addition to balance deficits, reductions in preferred-pace forward gait velocity and stride length are well-documented in PD OFF and ON compared to controls [12,13], while reports regarding preferred-pace cadence are more varied. In daily life, individuals often face gait situations more complex than the forward walking at a comfortable pace, which is most commonly studied. Aspects of simple preferred-pace forward walking, including velocity and stride length, improve with dopaminergic medication [12]. However, it is unclear whether or not medication substantially improves performance of more complex gait tasks, such as walking backward or walking while completing concurrent tasks. These complex gait tasks are frequently encountered in daily activities and may increase fall risk in PD.
Evidence regarding complex gait tasks is relatively sparse. During fast as possible walking, velocity [1,14,15], stride length [1,14,15] and cadence [14] are reduced in PD OFF [15] and ON [1,14,15], compared to controls. Levodopa improves fast gait velocity and stride length in PD, while cadence remains unchanged [15]. During backward walking, PD ON and controls reduce velocity and stride length, while maintaining a similar cadence, compared to forward walking [16]. Backward gait velocity and stride length are also reduced in PD ON, compared to controls [16]. The effects of medication on backward walking are unknown. When people with PD on medication engage in a secondary task while walking forward, velocity and stride length decrease [17]. Similar to forward gait, backward gait also degrades in controls and PD ON with the addition of a secondary task, but the added task more severely affects forward and backward gait in PD[18]. Nearly all dual task studies have been performed in PD on medication. As a result, it is unclear how medication affects dual task forward or backward gait or if potential changes with medication are similar to those in single task walking.
It is important to better understand balance and gait impairments in PD because these deficits may increase the likelihood of falls and serious injuries. Further, individuals with PD likely respond to challenging balance and gait situations in daily life during both optimal and sub-optimal medication response [19], and relatively little is known about performance of these tasks in the off-medication state. The present study aimed to address several deficits in our current understanding of medication effects on balance and complex gait tasks in individuals with PD. We hypothesized improvements would occur in clinical balance measures and preferred-pace gait in PD with medication, while other more complex gait tasks would not improve or would improve to a lesser extent. Secondarily, we aimed to determine if balance deficits were present and if certain gait tasks were more impaired in PD ON, compared to healthy older adults (controls). We expected that even on medication, those with PD would exhibit balance deficits and impairments across all gait tasks, compared to controls.
Methods
Participants
We recruited 29 participants from the Movement Disorders Center at Washington University who had been diagnosed with idiopathic Parkinson disease. Inclusion criteria for PD participants were: currently taking levodopa for PD with good drug response, ambulatory, no prior brain surgery, MMSE score ≥ 24, and no recent surgeries or injuries affecting movement. Of the 29 people with PD recruited, data were excluded for 5 who did not experience substantial motor benefit with medications (Movement Disorders Society Unified Parkinson Disease Rating Scale-III (MDS-UPDRS-III) [20] score improvement < 20% from off to on), and 2 with MMSE scores less than 24. We also recruited 21 healthy older adults (controls) without PD. Inclusion criteria for controls were: ambulatory, MMSE score ≥ 24, no recent surgeries or injuries affecting movement, and no history or symptoms of neurological diseases. One control was excluded due to presence of tremor possibly indicative of a neurological disease. A total of 22 participants with PD and 20 controls were included in analyses. All individuals with PD were taking a carbidopa/levodopa medication (LEDD: 968.4±603.7). Additionally, 2 were taking amantadine, 2 rasagiline, 4 ropinirole, and 1 entacapone. Participants provided written informed consent prior to participation, and this study was approved by the Washington University Human Research Protection Office.
Data Collection and Analysis
Participants were tested on one day. All tasks were completed once in controls and twice in PD, first off (after overnight withdrawal from PD medications) and then on medication (about 50 minutes after taking medication). The MDS-UPDRS-III motor scale was given prior to initial testing to assess overall motor impairment.
Participants completed the Activities-Specific Balance Confidence (ABC) Scale [21] once during testing. Postural stability was assessed by the same physical therapist for all participants using two different clinical balance tests: the Berg Balance Scale (BBS) [22] and the Mini-Balance Evaluations Systems Test of dynamic balance (mini-BESTest) [23]. Timed-Up-and-Go (TUG) and dual task TUG (DT-TUG) times were extracted from the mini-BESTest to measure functional mobility with and without a dual task. The dual task was random number listing. BBS scores, mini-BESTest scores, TUG, and DT-TUG were compared in PD OFF and ON with paired T-tests. Independent T-tests were used to compare BBS scores, mini-BESTest scores, TUG, and DT-TUG between PD ON and controls. Bonferroni corrections were used for multiple comparisons across balance and functional measures. If data were not normally distributed, values were log-transformed or equivalent non-parametric statistics were used. We ran Pearson correlations to assess relationships between balance confidence (total ABC score) and mini-BESTest and BBS scores.
Gait was evaluated using a 4.8m GAITRite instrumented walkway (CIR Systems, Havertown, PA). Participants performed three trials each of preferred-pace forward (Fwd), fast as possible forward (Fast), preferred-pace backward (Bkd), and preferred-pace dual task forward (DT-Fwd) and backward (DT-Bkd) gait. The order of gait tasks was randomized for each participant to avoid crossover effects. During dual task trials, participants walked forward or backward at preferred pace while completing a phonemic listing task (list words starting with a certain letter). A different letter was given for each trial, and correct words and errors were quantified. Letters were grouped with two consonants and one vowel (JOF, PAS, NID, GYC, REB, MUW). Letter groups were balanced based on first letter frequency in words within the English language to form groups of equivalent difficulty, and letter groups were randomly assigned for dual tasks for each participant. Seated cognitive task performance was tested in three 10 second trials. For each gait task, three trials were averaged for each participant. Velocity was the primary gait variable, and stride length and cadence were also examined. Repeated measures ANOVAs were used to evaluate gait variables across five gait tasks in PD OFF and ON. Mixed model ANOVAs were used to assess gait variables across the five gait tasks in PD ON and controls. Greenhouse-Geisser corrections were used for sphericity violations.
Results
Functional Measures and Balance
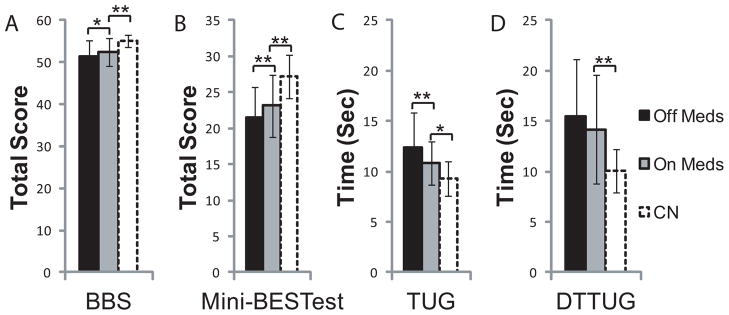
Participant characteristics are shown in Table 1, and all results are summarized in Table 2. MDS-UPDRS-III improved in PD with medication (t(21)=7.83, p<0.001). Further, BBS (Z= −2.25, p=0.05) and mini-BESTest scores (t(21)= −2.96, p=0.01) improved with medication (Fig 1A,B). TUG time (Fig 1C) decreased with medication (t(21)=3.46, p=0.004), but DT-TUG time (Fig 1D) did not improve (t(21)= −1.17, p=0.52).
Table 1.
Participant Characteristics
| PD OFF | PD ON | CN | |
|---|---|---|---|
| Total n | 22 | 20 | |
| Age (yrs) | 71.3 ± 7.6 | 72.1 ± 6.1 | |
| Males/Females | 13/9 | 10/10 | |
| MMSE | 28.0 ± 1.8 | 28.7 ± 1.2 | |
| ABC Scale | 74.3 ± 18.1 | 92.9 ± 11.2 | |
| Disease Duration (yrs) | 7.0 ± 4.2 | NA | |
| MDS-UPDRS-III | 38.1 ± 9.2 | 25.3 ± 6.9 | NA |
| H&Y Stage | 2.4 ± 0.3 | 2.2 ± 0.3 | NA |
Values are M ± SD. OFF medication H&Y scores were 2 for 6 participants, 2.5 for 13 participants, and 3 for 3 participants. ON medication H&Y scores were 2 for 15 participants, 2.5 for 6 participants, and 3 for 1 participant.
Table 2.
Gait and Balance in PD OFF and ON Medication and Controls
| PD OFF | PD ON | CN | |
|---|---|---|---|
| Balance Tests | n = 22 | n=20 | |
| Berg Balance Scale*+ | 52.0, 5 | 53.5, 4 | 55.0, 2 |
| Mini-BESTest*+ | 21.6 ± 4.1 | 23.1 ± 4.3 | 27.3 ± 3.0 |
| TUG*+ | 12.4 ± 3.4 | 10.9 ± 2.1 | 9.3 ± 1.7 |
| DT-TUG+ | 15.4 ± 5.8 | 14.2 ± 5.4 | 10.0 ± 2.1 |
| Gait Tasks | n=21 | n=20 | |
| Velocity (cm/sec)*+• | |||
| Fwd▲ | 107.1 ± 19.7 | 112.6 ± 15.7 | 122.2 ± 19.0 |
| Fast▲ | 153.6 ± 33.2 | 157.7 ± 28.9 | 182.4 ± 14.1 |
| Bkd▲ | 63.0 ± 24.9 | 68.6 ± 20.4 | 85.2 ± 29.4 |
| DT-Fwd | 83.4 ± 25.4 | 94.6 ± 23.6 | 91.8 ± 22.9 |
| DT-Bkd | 47.9 ± 26.3 | 54.5 ± 21.9 | 64.2 ± 25.6 |
| Stride Length (cm)*+• | |||
| Fwd | 121.6 ± 17.1 | 126.7 ± 12.5 | 131.2 ± 15.3 |
| Fast | 145.3 ± 22.4 | 148.2 ± 18.5 | 153.0 ± 25.0 |
| Bkd▲ | 69.4 ± 24.7 | 77.4 ± 19.1 | 95.3 ± 22.7 |
| DT-Fwd | 105.3 ± 23.6 | 114.5 ± 18.8 | 117.7 ± 18.6 |
| DT-Bkd▲ | 59.6 ± 29.8 | 68.2 ± 21.1 | 85.5 ± 24.1 |
| Cadence (steps/min)• | |||
| Fwd | 107.6 ± 12.8 | 107.0 ± 9.5 | 112.0 ± 10.4 |
| Fast▲ | 126.2 ± 14.1 | 127.9 ± 15.8 | 142.4 ± 15.5 |
| Bkd | 109.6 ± 21.2 | 108.5 ± 18.0 | 111.8 ± 29.4 |
| DT-Fwd | 95.8 ± 13.5 | 98.6 ± 13.0 | 93.5 ±16.5 |
| DT-Bkd | 106.5 ± 23.1 | 104.8 ± 33.9 | 96.6 ± 20.0 |
Values are means ± standard deviations for all values except Berg Balance Scale, where medians and interquartile ranges are listed.
Significant medication effect for PD OFF vs. PD ON
Significant group effect for PD ON vs. CN
Significant gait task effect for PD OFF vs. PD ON, as well as PD ON vs. CN
Significant group × gait task interaction for PD ON vs. CN
Figure 1.
Balance and Functional measures including BBS (A), mini-BESTest (B), TUG (C), and DT-TUG (D) are compared between PD OFF and ON medication, as well as between PD ON and Controls. BBS, mini-BESTest, and TUG improved in PD with medication. Impairments were detected in all measures in PD ON compared to Controls. Error Bars are SD. *p≤0.05, **p≤0.01, ***p≤0.001.
All functional and balance measures, including mini-BESTest scores (t(40)=3.54, p=0.002), BBS (U=96.6, p=0.002), TUG time (t(40)= −2.60, p=0.03), and DT-TUG time (t(40)= −3.42, p=0.002), were impaired in PD ON compared to controls.
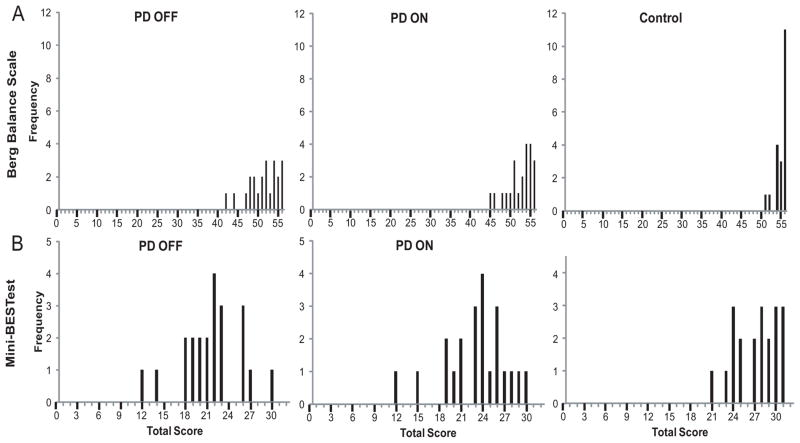
Overall, performance on the mini-BESTest (r=0.66, n= 64, p<0.001) and BBS (r=0.58, n=64, p<0.001) were correlated with total ABC score, indicating better performance on each balance measure was related to higher balance confidence. Further, mini-BESTest scores were significantly correlated with BBS scores (r=0.83, n=64, p<0.001). Though results were similar using either balance test, scores for the BBS tended to cluster toward the top of the range for all groups, suggesting a ceiling effect, compared to mini-BESTest scores (Fig 2).
Figure 2.
Frequency distribution of scores for the BBS (A) and mini-BESTest (B) are shown for PD OFF, PD ON, and Controls. Scores on the BBS tended to cluster at the upper end of the range for all groups, compared to the more normal distribution in mini-BESTest scores, suggesting the possibility of ceiling effects with the BBS.
Gait
One participant was unable to walk backward independently off medication, and was excluded from gait analyses. Seated and DT-Fwd phonemic listing performance (correct word rate and error rates) did not differ between PD ON and OFF or between PD ON and controls (p>0.05). Correct word rate during DT-Bkd gait improved in PD with medication (p=0.003), but correct word rate did not differ between PD ON and controls, and error rates did not differ in either comparison (p>0.05).
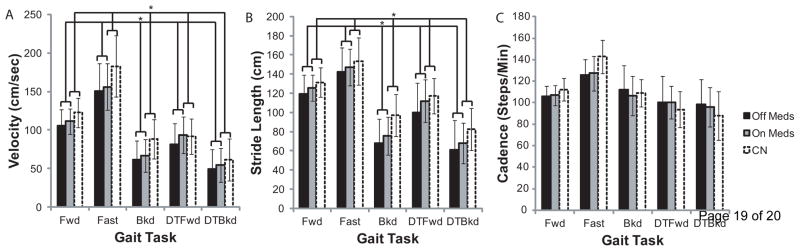
When comparing gait tasks between PD OFF and PD ON, medication was associated with increased velocity (F(1,20)=6.73, p=0.017) and increased stride length (F(1,20)=5.88, p=0.025). Cadence (F(1,20)=0.015, p=0.90) was not affected by medication. There were significant effects of gait task on velocity (F(2.20,43.98)=198.72, p<0.001), stride length (F(2.17,43.42)=210.16, p<0.001), and cadence (F(2.20,43.89)=28.34, p<0.001). For velocity and stride length, differences were detected in all pairwise comparisons (p<0.05), and values were highest during Fast gait, followed by Fwd, DT-Fwd, Bkd, and DT-Bkd. Cadence was higher in Fast gait, compared to other gait tasks (p≤0.001). Cadence was also higher in Fwd compared to DT-Fwd (p<0.001), and Bkd compared to DT-Bkd (p=0.01). There were no significant interactions.
Comparisons between PD ON and Controls indicated significant group effects for velocity (F(1,40)=4.30, p=0.045) and stride length (F(1,40)=4.19, p=0.047), where each was higher in controls. Cadence (F(1,40)=0.10. p=0.75) did not differ between groups. There were significant effects of gait task on velocity (F(2.62,104.68)=281.37, p<0.001), stride length (F(2.24,89.74)=311.86, p<0.001), and cadence (F(2.38,95.13)=68.51, p<0.001). For velocity and stride length, differences were detected in all pairwise comparisons (p<0.001), and values followed the same pattern described previously. Cadence followed a similar pattern (p<0.01), except there were no differences between Fwd and Bkd gait (p=1.0), nor between DT-Fwd and DT-Bkd gait (p=1.0). There was a significant interaction for velocity (F(4,37)=4.68, p=0.001), where controls were faster than PD ON during Fwd (p=0.05), Fast (p=0.018), and Bkd (p=0.005) gait. Further, there was an interaction for stride length (F(4,37)=4.38, p=0.002), where controls had longer strides than PD ON during Bkd (p=0.002) and DT-Bkd (p=0.022) gait. There was also a significant interaction for cadence (F(4,37)=3.68, p<0.001), where controls used a higher cadence during Fast gait (p=0.003).
Discussion
Balance and complex gait performance were evaluated in people with mild to moderate PD OFF and ON PD medication. Additionally, performance was compared between PD ON and controls to determine if impairments remained even when on medication. MDS-UPDRS-III, TUG, and balance test scores improved in PD with medication. Deficits remained in all functional and balance measures in PD ON compared to controls. Gait velocity and stride length improved similarly in PD ON across gait tasks, but impairments in these gait parameters persisted on medication compared to controls. Cadence did not change with medication, nor did it differ between controls and PD ON.
Functional Measures and Balance
As previously documented, MDS-UPDRS-III scores [24] and TUG [8,25] improved with medication. Though TUG and DT-Fwd gait improved with medication, DT-TUG time did not improve significantly. However, on average, DT-TUG time was reduced on medication. Also, different concurrent tasks were used for the DT-TUG (random number listing) and DT-Fwd (phonemic listing) tasks. It is possible that differential effects of medication may relate to differences in the dual tasks used.
As expected, and in agreement with a previous study [8], balance improved with medication according to both the BBS and mini-BESTest. This suggests functional balance does improve with prescribed dopaminergic medication in PD. However, these results conflict with prior studies reporting levodopa has little effect [3,7] or negative effects on postural stability and balance [11], predominantly measured with posturography. Balance is a complex motor behavior involving various components, some of which may be more levodopa-responsive. For example, the five mini-BESTest items assessing balance during gait tasks accounted for 46% of the average mini-BESTest score improvement with medication. Additionally, the two BBS items involving turning accounted for 52% of the average BBS score improvement from off to on. Aspects of gait [12,15] and turning [26] are known to improve with medication. Alternatively, aspects of postural stability, particularly those measured more precisely via posturography, may be more impacted by levodopa-induced dyskinesias, which may trigger instability or falls [27]. Only two of our 22 participants exhibited dyskinesia so this likely did not compromise balance in this study, but may factor into previous studies.
Gait
Our study was novel in evaluating medication effects across various gait tasks, including backward, dual task forward, and dual task backward gait. We expected robust improvements in forward and fast gait with medication, and less pronounced improvements in other, more complex gait tasks that may be controlled by neural systems distinct from those for simple forward walking [28,29]. Contrary to our hypothesis, similar improvements in velocity and stride length occurred across all gait tasks, regardless of complexity, while cadence was unchanged [12,13]. Individuals with PD are thought to exhibit reduced gait velocity due to difficulty with internal, automatic control of stride length [14]. Internal stride length control may be impaired because of deficits in motor cue production at the level of the basal ganglia or deficits in motor planning and preparation involving basal ganglia-supplementary motor area connections [14]. Dopaminergic medications may improve general gait performance in PD by facilitating stride length control.
As anticipated, based on evidence that backward movements are particularly impaired in PD, performance was worse in backward than in forward gait tasks. Deficits in Bkd walking velocity have been reported in a group of people with PD whose forward walking velocity was similar to controls [16]. Backward walking deficits may be particularly important, as many falls in those with PD occur following backward perturbations or during backward movements [6]. Like backward walking, dual task walking was also impaired in the PD group and improved with medication. This is consistent with previous literature [17,30]. Secondary task performance was similar off and on medication except in DT-Bkd, where both gait and cognitive tasks improved on medication. The cognitive dual task performance was similar between PD ON and controls in seated, DT-Fwd, and DT-Bkd trials. Individuals were equally able to complete the task, and dual task performance was not sacrificed in favor of locomotor tasks in any group. Cadence was also similar between groups for all gait tasks, but gait velocity and stride length remained impaired in PD ON [1,13]. Impairments in spatial gait parameters in PD ON were consistently present across various gait tasks, compared to controls. Interaction effects indicated reductions in gait velocity in PD ON during fast gait were cadence-mediated whereas reductions in velocity in PD ON during backward gait were stride length-mediated.
Conclusions
Our results suggest that anti-Parkinson medications enhance overall functional balance and improve performance of various gait tasks similarly regardless of task complexity. Though performance improved with medication, substantial impairments in balance and gait remained in PD ON compared to controls. These remaining impairments may contribute to balance loss and falls in this population, and future research should continue investigating novel treatment options and strategies to improve gait and balance in PD.
Figure 3.
Gait analyses compared gait velocity (A), stride length (B), and cadence (C) between PD OFF and ON medication, as well as between PD ON and Controls. Velocity and stride length improved with medication in PD, while cadence did not change. Velocity and stride length were impaired in PD ON vs. Controls. Impairments in Bkd and DT-Bkd velocity in PD ON vs. Controls were likely mediated by reduced stride length, while deficits in Fast velocity were likely mediated by reduced cadence. Error Bars are SD, * indicates p<0.05.
Highlights.
Medication effects on balance and complex gait were examined in Parkinson’s disease.
Clinical balance measures improved with medication.
Complex gait tasks improved similarly with medication, regardless of task complexity.
Deficits remained in all gait and balance measures in PD on medicine versus controls.
Acknowledgments
Research support was provided by the National Institute of Health/National Institute of Neurological Disease and Stroke Award Number F31 NS071639, the National Institute of Health/National Center for Medical Rehabilitation Research Award Number R01 HD056015, the Parkinson’s Disease Foundation, the American Parkinson Disease Association (APDA) Advanced Center for PD Research at Washington University School of Medicine, and the Greater Saint Louis Chapter of the APDA. We would like to thank Samantha Herriott and Laura Pilgrim for assisting with data processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris ME, Iansek R, Matyas TA, Summers JJ. Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994;57(12):1532–4. doi: 10.1136/jnnp.57.12.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hametner E, Seppi K, Poewe W. The clinical spectrum of levodopa-induced motor complications. J Neurol. 2010;257(Suppl 2):S268–75. doi: 10.1007/s00415-010-5719-9. [DOI] [PubMed] [Google Scholar]
- 3.Mancini M, Rocchi L, Horak FB, Chiari L. Effects of Parkinson’s disease and levodopa on functional limits of stability. Clin Biomech (Bristol, Avon) 2008;23(4):450–8. doi: 10.1016/j.clinbiomech.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesan M, Pal PK, Gupta A, Sathyaprabha TN. Dynamic posturography in evaluation of balance in patients of Parkinson’s disease with normal pull test: concept of a diagonal pull test. Parkinsonism Relat Disord. 2010;16(9):595–9. doi: 10.1016/j.parkreldis.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Colnat-Coulbois S, Gauchard GC, Maillard L, Barroche G, Vespignani H, Auque J, Perrin PP. Management of postural sensory conflict and dynamic balance control in late-stage Parkinson’s disease. Neuroscience. 2011;193:363–9. doi: 10.1016/j.neuroscience.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 6.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol. 2005;193(2):504–21. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson’s disease. Mov Disord. 1996;11(5):509–21. doi: 10.1002/mds.870110506. [DOI] [PubMed] [Google Scholar]
- 8.Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson’s disease. Exp Neurol. 2009;219(2):430–8. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brotherton SS, Williams HG, Gossard JL, Hussey JR, McClenaghan BA, Eleazer P. Are measures employed in the assessment of balance useful for detecting differences among groups that vary by age and disease state? J Geriatr Phys Ther. 2005;28(1):14–9. doi: 10.1519/00139143-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Nova IC, Perracini MR, Ferraz HB. Levodopa effect upon functional balance of Parkinson’s disease patients. Parkinsonism Relat Disord. 2004;10(7):411–5. doi: 10.1016/j.parkreldis.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75(6):2380–96. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 12.Bohnen NI, Cham R. Postural control, gait, and dopamine functions in parkinsonian movement disorders. Clin Geriatr Med. 2006;22(4):797–812. vi. doi: 10.1016/j.cger.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Morris ME, Huxham F, McGinley J, Dodd K, Iansek R. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech (Bristol, Avon) 2001;16(6):459–70. doi: 10.1016/s0268-0033(01)00035-3. [DOI] [PubMed] [Google Scholar]
- 14.Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 1994;117 (Pt 5):1169–81. doi: 10.1093/brain/117.5.1169. [DOI] [PubMed] [Google Scholar]
- 15.Chien SL, Lin SZ, Liang CC, Soong YS, Lin SH, Hsin YL, Lee CW, Chen SY. The efficacy of quantitative gait analysis by the GAITRite system in evaluation of parkinsonian bradykinesia. Parkinsonism Relat Disord. 2006;12(7):438–42. doi: 10.1016/j.parkreldis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Hackney ME, Earhart GM. Backward walking in Parkinson’s disease. Mov Disord. 2009;24(2):218–23. doi: 10.1002/mds.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012:918719. doi: 10.1155/2012/918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackney ME, Earhart GM. The Effects of a Secondary Task on Forward and Backward Walking in Parkinson’s Disease. Neurorehabil Neural Repair. 2009 doi: 10.1177/1545968309341061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord. 2011;17(3):166–71. doi: 10.1016/j.parkreldis.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 21.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 22.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 (Suppl 2):S7–11. [PubMed] [Google Scholar]
- 23.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42(4):323–31. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahn S. Levodopa in the treatment of Parkinson’s disease. J Neural Transm Suppl. 2006;(71):1–15. doi: 10.1007/978-3-211-33328-0_1. [DOI] [PubMed] [Google Scholar]
- 25.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther. 2001;81(2):810–8. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 26.McNeely ME, Earhart GM. The Effects of Medication on Turning in People with Parkinson Disease with and without Freezing of Gait. Journal of Parkinson’s Disease. 2011;1(3):259–270. doi: 10.3233/JPD-2011-11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloem BR. Postural instability in Parkinson’s disease. Clin Neurol Neurosurg. 1992;94 (Suppl):S41–5. doi: 10.1016/0303-8467(92)90018-x. [DOI] [PubMed] [Google Scholar]
- 28.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10(8):1055–62. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- 29.Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80(4):1868–85. doi: 10.1152/jn.1998.80.4.1868. [DOI] [PubMed] [Google Scholar]
- 30.Lord S, Baker K, Nieuwboer A, Burn D, Rochester L. Gait variability in Parkinson’s disease: an indicator of non-dopaminergic contributors to gait dysfunction? J Neurol. 2011;258(4):566–72. doi: 10.1007/s00415-010-5789-8. [DOI] [PubMed] [Google Scholar]