Abstract
Purpose
We assessed the impact that hexaminolevulinate fluorescence cystoscopic detection of papillary, non-muscle invasive bladder cancer has on long-term recurrence rates.
Materials and Methods
Long-term follow-up was assessed in 551 participants enrolled in a prospective, randomized study of fluorescence cystoscopy for Ta or T1 urothelial bladder cancer. In the original study, 280 patients in the white light cystoscopy group and 271 in the fluorescence cystoscopy group were followed with cystoscopy for 3, 6, and 9 months following the initial resection or until recurrence. A study extension protocol obtained long-term follow-up of these patients.
Results
Follow-up information was obtained for 261 of the 280 (93%) participants in the white light group and 255 of the 271 (94%) participants in the fluorescence group. Median follow-up in the white light and fluorescence groups were 53.0 and 55.1 months, respectively. In the white light and fluorescence groups, 83 (31.8%) and 97 (38%) of the participants remained tumor free, respectively. The median recurrence free survival was 9.6 months in the white light group and 16.4 months in the fluorescence group, p = 0.04. The rates of intravesical therapy were similar in the white light (46%) and fluorescence groups (45%). Cystectomy was performed in 22/280 (7.9%) in the white light group and 13/271 (4.8%) in the fluorescence group, p = 0.16.
Conclusions
Hexaminolevulinate fluorescence cystoscopy significantly improves long-term bladder cancer recurrence free survival with a trend towards improved bladder preservation.
Keywords: urinary bladder neoplasms, 5-aminolevulinic acid hexyl ester, fluorescence, cystoscopy, recurrence
Fluorescence cystoscopy with hexaminolevulinate increases the detection of both papillary bladder cancer and carcinoma in situ.1-5 In this technique, hexaminolevulinate is administered intravesically 1 hour prior to cystoscopy and bladder tumor resection. The drug is converted locally to porphyrins that selectively accumulate in malignant cells. Through the use of blue light and selective filters, bladder cancers become more visually evident and appear red in a dark blue background. A large, multi-institutional, international, phase III study has been previously reported with primary endpoints of detection and recurrence.6 This study confirmed that fluorescence cystoscopy with hexaminolevulinate improved the detection of both papillary bladder cancer and carcinoma in situ. Furthermore, there was a significant improvement in time to recurrence in the hexaminolevulinate group at the study endpoint of 9 months.
The increased detection enabled by hexaminolevulinate fluorescence cystoscopy has been shown to result in better patient management.3 In addition, several reports document that this technique is cost effective.7,8 Nevertheless, the 9 month follow-up in this study has raised questions about the potential long-term patient benefit of this technique.9 We now provide long-term follow-up of the outcomes of the patients enrolled in the international, prospective, phase III study of hexaminolevulinate fluorescence cystoscopy vs. white light cystoscopy.6
METHODS
Patients
The complete details of this prospective, randomized trial have been previously published. Patients with suspected Ta and/or T1 urothelial bladder cancer were enrolled by 28 centers, 19 in the United States and Canada and 9 in Europe.6 By intent to treat (ITT), there were 280 patients in the white light cystoscopy group and 271 in the fluorescence cystoscopy group. Patients were followed with cystoscopy for 3, 6, and 9 months following the initial resection or until recurrence. Recurrence was to be confirmed by biopsy. Patients who had no protocol violations comprised the per protocol set (PPS) and included 200 in the white light group and 202 in the fluorescence group. A study extension protocol was written and approved by each Institutional Review Board to permit long-term follow-up of all patients in the ITT population. An attempt was made to contact all participants in the ITT population. Retrospective review captured the clinical outcome of these participants and histology was recorded when available. The study extension obtained additional follow-up data for 516 subjects (261 in the white light arm, and 255 in the fluorescence arm). Of the 551 total subjects in the study, 35 were unavailable for the assessment of recurrence (19 participants in the white light arm and 16 in the fluorescence arm). Three of the 28 centers that participated in the prospective, randomized trial did not participate in this study extension. Most of those unavailable for assessment of recurrence were from these 3 centers.
Treatment
Patients with documented Ta or T1 tumors, were randomized to have resection using conventional white light cystoscopy or fluorescence cystoscopy. The fluorescence group had 85 mg hexaminolevulinate (Photocure, Oslo, Norway) in 50 mL saline instilled into the bladder. The solution was to be retained for 1 hour. In both arms, cystoscopy was performed with a xenon light source (Karl Storz D-Light C PDD System, Karl Storz GMBH., Tuttlingen, Germany). Participants with high grade bladder cancer, T1, or CIS were to receive an induction course of BCG and 3 weekly maintenance instillations at 3 and 6 months unless contraindicated. Following completion of the original study, patients were managed according to standard clinical practice. Some participants in Europe received hexaminolevulinate fluorescence cystoscopy in follow-up. In the ITT set, the proportion of patients who subsequently had fluorescence cystoscopy was 8.9% in the white light group and 10.7% in the fluorescence group.
Statistical Methods
To integrate the data collected from the original study with that of the extension study, the following data convention rules were established. If any of the 551 ITT subjects were not enrolled in the extension study, the last available visit date from the pivotal study was used as a censoring timepoint. The number of intravesical (BCG and chemotherapy) treatments were calculated as the number observed in the pivotal study, plus the number in the extension. Demographic information, including age, gender, and ethnicity, was obtained from the pivotal study database. In the pivotal study, tumor stage was collected per lesion, whereas the extension study collected an overall assessment for tumor stage at each recurrence. The databases were integrated by determining the maximum stage across all lesions in the pivotal study.
For missing date information, imputation methods were employed in order to determine time-to-event analyses. If only year was present, the date was imputed to be 01-Jan of that year. If only month/year was present, the day was imputed as the 1st of that month. All time-to-event models were analyzed using Kaplan-Meier methods. The 25th, 50th, and 75th percentiles for survival times and the 95% confidence interval of the median were calculated for each treatment group using Kaplan-Meier product-limit estimation. The time to events were compared between procedure groups using the log-rank test. The Wilcoxon test was added for recurrence time-to-event analyses. Month and day imputations were similar in the two groups and varied by analysis. Month imputations by recurrence were 0 in the white light group and 1 in the fluorescence group. Day imputations for recurrence were 5 in the white light group and 6 in the fluorescence group. Month imputations for death were 4 in the white light group and 5 in the fluorescence group. Day imputations for death were 3 in the white light group and 2 in the fluorescence group.
Results
As previously reported, the white light and fluorescence cystoscopy groups were similar in age, gender, race, bladder cancer history, and prior Intravesical therapy.6 The study revision provided additional data for 94% of the patients. Follow-up information was obtained for 261 of the 280 (93%) participants in the white light group and 255 of the 271 (94%) participants in the fluorescence group. Mean and median follow-up in the white light group were 53.6 and 53.0 months, respectively. Mean and median follow-up in the fluorescence group were 54.6 and 55.1 months, respectively. Following the initial resection, 83 (31.8%) and 97 (38%) of the participants remained tumor free in the white light and fluorescence groups respectively, p = 0.14.
In the original study, BCG was administered to 55 (20%) of the participants in the white light group and 50 (19%) of those in the fluorescence group. Including the study extension, a total of 253 participants (130 [46%] in the white light and 123 [45%] in the fluorescence group) were reported as receiving any type of intravesical therapy. Of the participants that received BCG or intravesical chemotherapy, 127 were in the white light group, and 123 were in the fluorescence group. Participants that received both BCG and intravesical chemotherapy totaled 16 in the white light group and 14 in the fluorescence group.
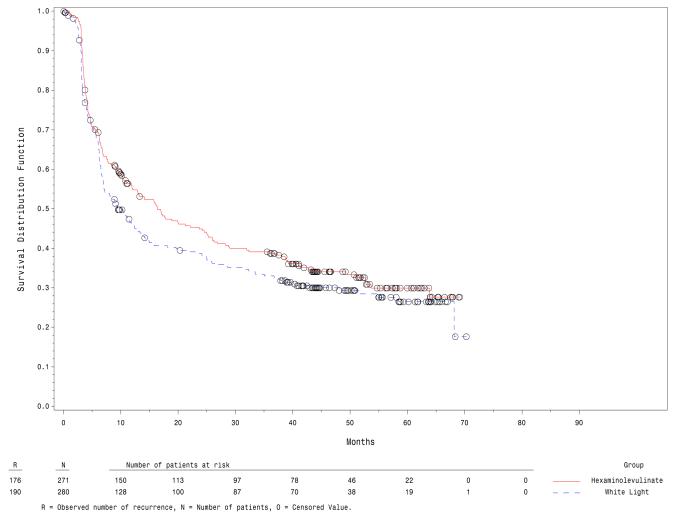
The median recurrence free survival was 9.6 months in the white light group and 16.4 months in the fluorescence group, figure 1. Recurrence free survival was significantly better in the fluorescence group, p = 0.04. At first recurrence, 9 (3.5%) in the white light group and 6 (2.4%) in the fluorescence group had T2-4 bladder cancer. The overall development of T2-4 bladder cancer was reported in 16 (6.1%) in the white light group and 8 (3.1%) in the fluorescence group (p = 0.066). The rate of cystectomy was 7.9% (22/280) in the white light group and 4.8% (13/271) in the fluorescence cystoscopy group (p = 0.16). Radiation therapy was given to 4 patients in the white light group, but none in the fluorescence group received radiation. Twelve participants had radiation or systemic chemotherapy and cystectomy (7 in the white light group and 5 in the fluorescence group).
Figure 1.
Kaplan-Meier plot of recurrence free survival (ITT).
There were 44 (15.7%) deaths in the white light group and 39 (14.4%) in the fluorescence group. Known bladder cancer related deaths were 8 (2.9%) and 6 (2.2%) in the white light and fluorescence groups, respectively. Assuming the deaths were bladder cancer related when there was incomplete information, the number of bladder cancer related deaths was 24 (8.6%) and 19 (7.0%) in the white light and fluorescence groups, respectively.
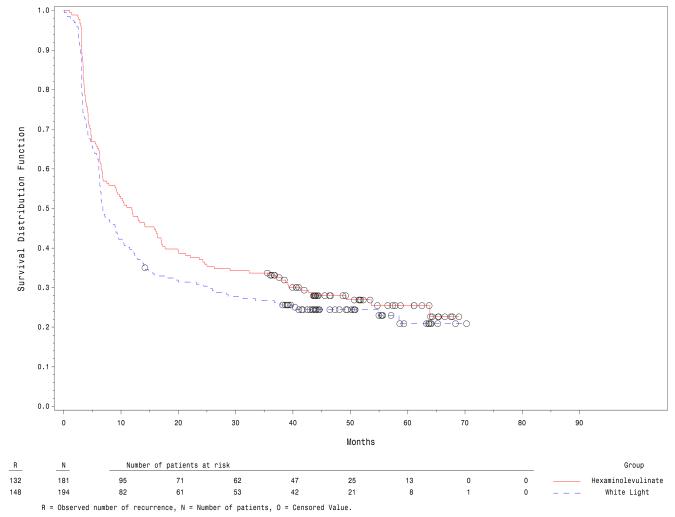
The PPS comprised study participants that met all protocol requirements. Similar results were reported for the PPS and the ITT populations. The median time to recurrence in the PPS is shown in figure 2 and was 6.8 months in the white light group and 11.8 months in the fluorescence group, p = 0.04.
Figure 2.
Kaplan-Meier plot of recurrence free survival in the per protocol set.
Discussion
Fluorescence cystoscopy became clinically possible with the report of increased detection of bladder cancer using 5-aminolevulinic acid.10 Hexaminolevulinate, a second generation drug, made fluorescence cystoscopy practical by requiring a much shorter exposure time (1 hour) to achieve good quality fluorescence.1 Multi-institutional studies of fluorescence cystoscopy with either 5-aminolevulinic acid or hexaminolevulinate have demonstrated increased detection of both carcinoma in situ and papillary bladder cancer.6,11 While it is reasonable to hypothesize that better visualization of bladder cancer results in more complete resection and improved disease free interval, conflicting data from different studies have made this conclusion controversial.
Randomized small studies by experienced investigators using 5-aminolevulinc acid demonstrated that fluorescence cystoscopy results in improved disease free survival that is durable for 8 years or more.12-14 However, a recently reported multi-institutional study of 5-aminolevulinic acid did not confirm this finding.11 While the cause for the differences in the outcomes of these studies cannot be known with certainty, the negative multi-institutional study had investigators with limited experience with fluorescence and the mean exposure to 5-aminolevulinic acid was only 2.1 hours, less than optimal to achieve fluorescence with this drug.
The large multi-institutional study of hexaminolevulinate in patients with primary or recurrent Ta and/or T1 bladder cancer also had investigators inexperienced with fluorescence but demonstrated an improved disease free survival at 9 months.6 Continued surveillance of the participants in this study now with a median follow-up over 4 years demonstrates that the improved outcome is durable and clinically meaningful. Disease free survival is significantly better in the group that was randomized to fluorescence cystoscopy, 16.4 vs. 9.4 months. Furthermore, the fluorescence group had an absolute improvement in tumor free survival of more than 6%. This improved benefit was accompanied by a trend toward improved bladder preservation. It is important to note that the continued surveillance was not powered to show statistical significance for any clinical parameters since power calculations were only performed on the initial study. Statistical proof of improved survival and bladder preservation would require a significantly larger study with longer follow-up.
Most studies of fluorescence cystoscopy have used little or no adjuvant intravesical therapy. Our study restricted BCG to individuals with T1 or CIS. An important question is whether adjuvant intravesical therapy would decrease the beneficial results of fluorescence. A recent randomized prospective study compared hexaminolevulinate enabled fluorescence cystoscopy to white light cystoscopy with perioperative mitomycin in both arms.15 Furthermore, patients with intermediate-risk tumors received adjuvant mitomycin and those with high-risk tumors received BCG. This study found fluorescence cystoscopy was beneficial with improvements in disease recurrence at 3 months (7.2% vs. 15.8%), 1 year (21.6% vs. 31.2%) and 2 years (32.5% vs. 45.6%).
Hexaminolevulinate induced fluorescence cystoscopy has little toxicity.6 While fluorescence cystoscopy does require some training, the learning curve is short. This multi-institutional study included many sites that had no prior experience with this technique. Investigators naïve to fluorescence underwent training with an experienced evaluator on five procedures. Although it is likely that additional experience will increase the skill with which this technique is applied, experienced urologists can quickly become competent in fluorescence cystoscopy. Adding fluorescence to conventional cystoscopy requires little training, can be cost effective, and provides meaningful improvement in disease free survival.
Conclusions
Hexaminolevulinate fluorescence cystoscopy significantly improves long-term bladder cancer recurrence free survival with a trend towards improved bladder preservation.
Abbreviations and Acronyms
- ITT
intent to treat
- PPS
per protocol set
- CIS
Carcinoma in situ
Contributor Information
H. Barton Grossman, Department of Urology), The University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Yves Fradet, Department of Urology, University Hospital, Tübingen (AS), CHUQ Hotel-Dieu de Quebec, Quebec, Canada.
Lance A. Mynderse, Mayo Clinic, Rochester, Minnesota
Martin Kriegmair, Urology Clinic, München-Planegg, Munich, Germany.
J. Alfred Witjes, Radboud University, Nijmegen Medical Centre, Nijmegen, Netherlands.
Mark S. Soloway, University of Miami School of Medicine, Miami, Florida
Alexander Karl, Department of Urology, Ludwig-Maximilians-University, Campus Grosshadern,), Munich, Germany.
Maximilian Burger, Caritas St Josef Medical Center, University of Regensburg, Regensburg.
References
- 1.Jichlinski P, Guillou L, Karlsen SJ, et al. Hexyl aminolevulinate fluorescence cystoscopy: A new diagnostic tool for the photodiagnosis of superficial bladder cancer - A multicenter study. J Urol. 2003;170:226. doi: 10.1097/01.ju.0000060782.52358.04. [DOI] [PubMed] [Google Scholar]
- 2.Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004;171:135. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- 3.Jocham D, Witjes F, Wagner S, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2005;174:862. doi: 10.1097/01.ju.0000169257.19841.2a. [DOI] [PubMed] [Google Scholar]
- 4.Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: A phase III, multicenter study. J Urol. 2007;178:68. doi: 10.1016/j.juro.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Grossman HB, Gomella L, Fradet Y, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol. 2007;178:62. doi: 10.1016/j.juro.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010;184:1907. doi: 10.1016/j.juro.2010.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaak D, Wieland WF, Stief CG, et al. Routine use of photodynamic diagnosis of bladder cancer: practical and economic issues. European Urology Supplements. 2008;7:536. [Google Scholar]
- 8.Malmstrom PU, Hedelin H, Thomas YK, et al. Fluorescence-guided transurethral resection of bladder cancer using hexaminolevulinate: analysis of health economic impact in Sweden. Scand J Urol Nephrol. 2009;43:192. doi: 10.1080/00365590902808541. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg GD. Editorial comment. J Urol. 2010;184:1913. doi: 10.1016/j.juro.2010.06.163. [DOI] [PubMed] [Google Scholar]
- 10.Kriegmair M, Baumgartner R, Knuechel R, et al. Fluorescence photodetection of neoplastic urothelial lesions following intravesical instillation of 5-aminolevulinic acid. Urology. 1994;44:836. doi: 10.1016/s0090-4295(94)80167-3. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher MC, Holmang S, Davidsson T, et al. Transurethral resection of non-muscle-invasive bladder transitional cell cancers with or without 5-aminolevulinic Acid under visible and fluorescent light: results of a prospective, randomised, multicentre study. Eur Urol. 2010;57:293. doi: 10.1016/j.eururo.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Babjuk M, Soukup V, Petrik R, et al. 5-aminolaevulinic acid-induced fluorescence cystoscopy during transurethral resection reduces the risk of recurrence in stage Ta/T1 bladder cancer. BJU Int. 2005;96:798. doi: 10.1111/j.1464-410X.2004.05715.x. [DOI] [PubMed] [Google Scholar]
- 13.Daniltchenko DI, Riedl CR, Sachs MD, et al. Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. J Urol. 2005;174:2129. doi: 10.1097/01.ju.0000181814.73466.14. [DOI] [PubMed] [Google Scholar]
- 14.Denzinger S, Burger M, Walter B, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology. 2007;69:675. doi: 10.1016/j.urology.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Geavlete B, Multescu R, Georgescu D, et al. Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int. 2011 Jun 28; doi: 10.1111/j.1464-410X.2011.10374.x. doi: 10.1111/j.1464-410X.2011.10374.x. [DOI] [PubMed] [Google Scholar]