Abstract
GABAA receptor modulating drugs such as benzodiazepines (BZs) have been used to treat anxiety disorders for over five decades. In order to determine whether the same or different GABAA receptor subtypes are necessary for the anxiolytic-like action of BZs in unconditioned anxiety and conditioned fear models, we investigated the role of different GABAA receptor subtypes by challenging wild type, α1(H101R), α2(H101R) and α3(H126R) mice bred on the C57BL/6J background with diazepam or chlordiazepoxide in the elevated plus maze and the fear-potentiated startle paradigms. Both drugs significantly increased open arm exploration in the elevated plus maze in wild type, α1(H101R) and α3(H126R), but this effect was abolished in α2(H101R) mice; these were expected results based on previous published results. In contrast, while administration of diazepam and chlordiazepoxide significantly attenuated fear-potentiated startle (FPS) in wild type mice and α3(H126R) mice, the fear-reducing effects of these drugs were absent in both α1(H101R) and α2(H101R) point mutants, indicating that both α1- and α2-containing GABAA receptors are necessary for BZs to exert their effects on conditioned fear responses.. Our findings illustrate both an overlap and a divergence between the GABAA receptor subtype requirements for the impact of BZs, specifically that both α1- and α2-containing GABAA receptors are necessary for BZs to reduce conditioned fear whereas only α2-containing GABAA receptors are needed for BZ-induced anxiolysis in unconditioned tests of anxiety. This raises the possibility that GABAergic pharmacological interventions for specific anxiety disorders can be differentially tailored.
Keywords: GABAA receptor, α subunit, anxiety, conditioned fear, benzodiazepine, fear-potentiated startle
1. INTRODUCTION
Anxiety disorders are the most common psychiatric disorders with a lifetime prevalence of approximately 25% (Kessler et al., 1994). While these disorders affect millions of people, the molecular basis for the development of these disorders remains largely unknown. However, both experimental and clinical data support a role of the GABAergic system and specifically of GABAA receptors in anxiety- and depression-related pathologies (Crestani et al., 1999; Kalueff and Nutt, 2007; Luscher et al., 2011; Smith and Rudolph, 2011).
GABAA receptors represent a highly diverse group of receptors with 19 known GABAA receptor subunits (α1–6, β1–3, γ1–3, δ, ε, π, ρ1–3, θ; see (Mohler et al., 2002; see Olsen and Sieghart, 2009 for a review). Benzodiazepines (BZs) such as diazepam bind to a specific site (BZ site) between the α and γ subunits and allosterically modulate GABAA receptors containing the α1, α2, α3 and/or α5 subunits. These receptors modulate processes as diverse as sleep, anxiety, learning and memory, seizures, depressive symptomatology, and reward (Rudolph and Knoflach, 2011). GABAA receptor modulating drugs such as benzodiazepines have been used to treat anxiety disorders for five decades (Shader and Greenblatt, 1993).
BZ-sensitive GABAA receptors can be rendered insensitive to this class of drugs through the replacement (i.e., point mutation) of a conserved histidine residue by arginine in the drug-binding site (Benson et al., 1998; Wieland et al., 1992). Using point mutated mice carrying diazepam-insensitive α1, α2, α3, or α5 subunits, the anxiolytic-like and sedative actions of diazepam were shown to be pharmacologically separable. Specifically, the sedative action of diazepam was blocked in α1(H101R) mice demonstrating that this property is mediated by α1-containing GABAA receptors (McKernan et al., 2000; Rudolph et al., 1999). In contrast, the anxiolytic-like actions of diazepam were absent in α2(H101R) mice when tested in unconditioned anxiety paradigms such as the elevated plus maze or the light/dark box; thus, α2-containing GABAA receptors are required for diazepam-induced anxiolysis (Low et al., 2000). Furthermore, the anxiolytic-like effects of diazepam in these unconditioned paradigms remained intact in α1-, α3- and α5-point mutant [α1(H101R), α3(H126R) and α5(H105R), respectively] mice signifying that these subunits are not necessary for the anxiolytic effect of diazepam in mice (Crestani et al., 2002; Low et al., 2000; Rudolph et al., 1999).
While the involvement of the α2-containing GABAA receptors in the modulation of anxiety by benzodiazepines is now undisputed, α3-containing GABAA receptors have also been postulated to mediate anxiolytic-like actions. In rats, TP003, a highly α3-selective agonist in vitro, displays an anxiolytic-like action in the elevated plus maze and in the stress-induced hyperthermia test. This compound also disinhibited responding in a conditioned emotional response paradigm in squirrel monkeys, suggesting that TP003 is also active in conditioned fear models (Dias et al., 2005). However, selectivity of TP003 for α3-containing GABAA receptors has not been demonstrated in vivo, and it is important to note that TP003-induced anxiolysis was observed only at high receptor occupancy levels (75%), whereas the BZ chlordiazepoxide already exerts its anxiolytic-like action at a receptor occupancy of 25% (Dias et al., 2005). Combined with the aforementioned evidence from studies with point-mutant mice, this suggests that at commonly-used therapeutic doses non-selective BZs exert their anxiolytic-like effects via α2-containing GABAA receptors. The precise role of α3-containing GABAA receptors in anxiolysis remains unclear.
It also remains unknown whether the effects of BZs on unconditioned anxiety and conditioned fear1 are mediated by the same GABAA receptor subtypes when these compounds are administered at therapeutically-relevant doses, since e.g. not only the α2 subunit, but also the α1 and α3 subunits are expressed in the lateral and basolateral amygdala (Marowsky et al., 2004), regions which have been implicated in both conditioned fear and unconditioned anxiety. The α1 subunit has also been shown to play a critical role in amygdalar plasticity and fear learning (Wiltgen et al., 2009). While previous research suggests that this subunit is not involved in the anxiolytic effects of benzodiazepines in models of unconditioned anxiety (McKernan et al., 2000; Rudolph et al., 1999), the possible contribution of this subunit to the fear reducing effects of benzodiazepines in conditioned fear paradigms has not been examined.
In order to determine whether the same or different GABAA receptor subtypes are necessary for the anxiolytic-like action of classical benzodiazepines in unconditioned anxiety and conditioned fear models, we investigated the role of different GABAA receptor subtypes by challenging wild type, α1(H101R), α2(H101R) and α3(H126R) mice bred on the C57BL/6J background with non-sedating doses of either diazepam or chlordiazepoxide in the elevated plus maze and the fear-potentiated startle paradigms. Activity responses in a novel open field test were also measured after drug administration to determine whether non-specific locomotor effects could impact behavior in the other two tests, particularly the elevated plus maze which is a locomotor-dependent task (Reynolds et al., 2001; but see Crestani et al., 2001).
2. MATERIALS AND METHODS
2.1. Animals
Adult male wild type, α1(H101R), α2(H101R), and α3(H126R) point mutant mice were used in our experiments. These mice were generated by the introduction of a histidine to arginine point mutation in the normally benzodiazepine-sensitive α1, α2, and α3 subunits of the GABAA receptor as previously described (Low et al., 2000; Rudolph et al., 1999) and were bred as homozygous pairings. All subjects were bred on the C57BL/6J background (original stock from Jackson Laboratory, Bar Harbor, ME) at McLean Hospital (Belmont, MA).
Subjects were group-housed (2–4 mice per cage) in Super Mouse 750™ cages containing a LifeSpan Rodent Enrichment insert (LabProducts, Seaford, DE) and covered by micro-isolator non-wire grids. Cages were maintained on individual ventilation until two weeks before the start of the behavioral experiments at which time they were moved from the colony into separate housing rooms within our behavioral suite. During this two week acclimation period, and all subsequent experimentation time, subjects were maintained off ventilation. Food (Purina Lab Diet 5P76, PMI Nutrition International, Brentwood, MO) and water were available ad libitum.
One group of subjects (elevated plus maze and open field; n=8–12/drug group/dose) were housed on a 12:12-h reverse light-dark cycle (lights on at 2000 h) and tested during the dark phase of the daily cycle beginning 1 h after lights off. A second group of subjects2 (fear-potentiated startle) were housed on a regular 12:12-h light-dark cycle (lights on at 0600 h) and experiments were conducted during the light phase of the daily cycle (between 0900 and 1700 h) to avoid ceiling effects that normally arise from the nocturnal elevation of startle responding (Chabot and Taylor, 1992). Subjects were hand carried in their home cages from their respective housing rooms to the testing rooms containing the experimental equipment. All animal procedures were approved by the Institutional Animal Care and Use Committee at McLean Hospital and were in accordance with the NIH Guide for Care and Use of Laboratory Animals (National Research Council, 1996).
2.2. Drugs
Diazepam (BIOMOL International, Plymouth Meeting, PA) was dissolved in 10% 2-Hydroxypropyl-beta-cyclodextrin (Sigma-Aldrich, St. Louis, MO) and administered at doses of 1 or 2 mg/kg. Chlordiazepoxide hydrochloride (Sigma-Aldrich) was prepared in a vehicle of 0.9% sterile saline and administered at doses of 5 or 10 mg/kg. All drugs were administered in a volume of 10 ml/kg via intraperitoneal injection 30 min before behavioral testing.
2.3. Behavioral Tests
2.3.1. Elevated Plus Maze
The apparatus consisted of two open (35 cm long × 6 cm wide) and two closed (35 cm long × 6 cm wide × 20 cm high) arms separated by a center area (5 × 5 cm); the maze was elevated 1 m above the floor. All testing was conducted in a quiet, dimly illuminated room within our behavioral suite; the illumination on the open arms was 30 lux. Subjects were placed in the center facing an open arm and allowed to freely explore the maze for 5 min. Subject activity was measured using the EthoVision XT video tracking system (Noldus Information Technology, Wageningen, Netherlands). Percentage of open arm time ((Open arm time/5min) × 100) and percentage of open arm entries ((Open arm entries/(Open arm entries + Closed arm entries)) × 100) were used as measures of anxiety where an increase in either measure is operationally defined as anxiolysis. Total distance traveled (cm) on the maze served as a within-test control of locomotor activity. The maze was wiped clean with 70% ethanol and allowed to air dry completely between subjects.
2.3.2. Novel Open Field
Subjects were tested in the novel open field 48 hours after testing in the elevated plus maze. The apparatus was an evenly illuminated (100 lux) clear Plexiglas box (42 cm high × 42 cm wide × 31 cm high). Subjects were placed in one corner of the box and allowed to freely explore it for 30 minutes. Locomotor activity, measured as the total distance traveled (cm), was analyzed using the EthoVision XT system.
2.3.3. Fear-Potentiated Startle
Fear potentiated startle (FPS) was measured using the Med-Associates Inc. (St. Albans, VT, USA) Startle Reflex System and Advance Startle software program, using a six-day protocol previously optimized for C57BL/6J mice (Smith et al., 2011). Briefly, the protocol started with three Acclimation days (days 1–3) during which animals received 50 semi-random presentations of white noise startle stimuli (20 ms; ten each of 70, 80, 85, 90 and 100 dB) with a 30-sec intertrial interval (ITI). On day 4 (PreTest), animals were first habituated to a baseline startle level through the presentation of 10 Leader white noise startles (20 ms, 85 dB). This was followed by a semi-random presentation of 20 Startle Only (20 ms, 85 dB startle) and 20 Tone + Startle (30 s, 12 kHz, 70 dB tone followed by a 20 ms, 85 dB startle) trials. On day 5 (Conditioning), animals were trained to associate the 30 s, 12 kHz, 70 dB tone (i.e., the conditioned stimulus, CS) with a 0.25 s, 0.4 mA foot shock (i.e., the unconditioned stimulus, US) through the presentation of 10 Tone + Shock pairings with a random ITI (120, 180, or 240 s). On day 6 (Test), the same experimental session presented on PreTest was repeated to examine FPS.
In this paradigm, the fear-potentiation of the startle reflex is defined as a significantly increased startle response in trials when the CS is presented (Tone + Startle trials) after conditioning (i.e., during Test) compared to PreTest. Percent FPS was calculated using mean startle amplitude values from each trial type during Test with the following formula: [((Tone + Startle) − (Startle Only) / (Startle Only)] × 100. Subjects were matched into different treatment groups using startle amplitude and percent change in startle amplitude on Tone + Startle trials versus Startle Only trials during PreTest (same formula used to calculate percent FPS) such that each group had equivalent levels of baseline startle and unconditioned effects of the tone on startle. The testing chambers were wiped with distilled water between animals and cleaned with 70% ethanol at the end of each testing day.
2.4. Statistical Analyses
Data were expressed as means and standard errors of the mean (SEM) and analyzed using the SAS statistical software version 9.1 (SAS Institute, Inc., Cary, NC) and SigmaPlot software version 11.0 (Systat Software, Inc., Chicago, IL). Elevated plus maze and open field data were analyzed with a two-way analysis of variance (ANOVA) using genotype and drug-dose as the factors, followed by post hoc Dunnett’s tests (Control levels set as “wild-type” for genotype and “vehicle” for drug-dose) to compare the effect of individual drug doses to the corresponding vehicle control condition in each genotype.
Due to the more complex nature of the FPS data, the analyses were performed using factorial ANOVAs or repeated individual ANOVAs for each genotype group depending on the variable tested. A Bonferroni correction was used when the nature of the data required repeated ANOVAs; more stringent Tukey’s post hoc tests were used instead of Dunnett’s where significant effects were found in the initial ANOVAs.
FPS startle amplitude data were analyzed within genotype with two-way ANOVAs (drug dose x trial type); a Bonferroni correction was applied to account for the multiple comparisons. Significant main effects and interactions were followed up with ANOVAs and Tukey’s post hoc tests. Percent FPS data were first analyzed with a two-way ANOVA (genotype x drug dose); significant main effects and interactions were followed up with ANOVAs and Tukey’s post hoc tests. Effects of drug within genotype were next examined using one-way ANOVAs and Tukey’s post hoc tests; a Bonferroni correction was used to account for multiple comparisons due to the repeated analyses through individual one-way ANOVAs in each genotype. The significance level for all tests was set at p<0.05.
3. RESULTS
3.1. Elevated Plus Maze
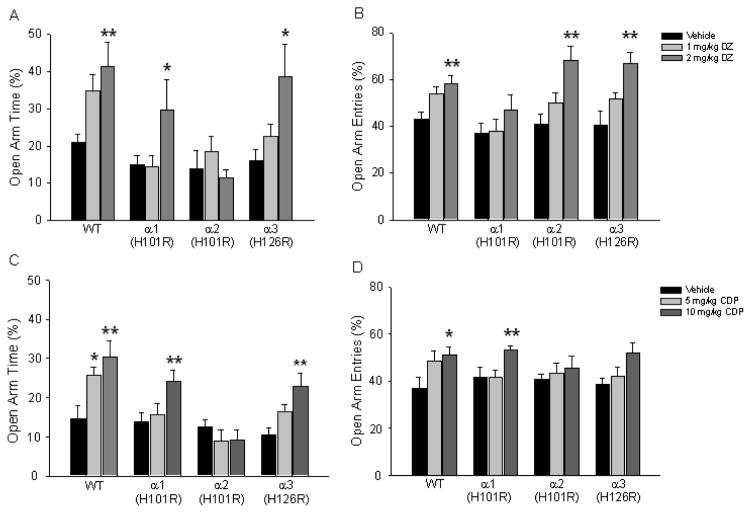
The effects of diazepam dose and genotype on the percentage of time spent in the open arms and percentage of open arm entries were examined by 4×3 two-way (Genotype x Drug dose) ANOVAs. Percent open arm time was significantly affected by genotype (F(3,145)=5.55, p<0.01), drug dose (F(2,145)=9.61, p<0.01) and the genotype x drug dose interaction (F(6, 145)=2.13, p<0.05) (Fig. 1A). Post hoc Dunnett tests revealed that the 2 mg/kg dose of diazepam increased percent open arm time in wild type (p<0.01), α1(H101R) (p<0.05) and α3(H126R) (p<0.05) mice, while diazepam administration had no effect in α2(H101R) animals. A significant drug dose (F(2,145)=18.80, p<0.01) main effect was observed on percentage open arm entries (Fig. 1B). Post hoc tests showed that diazepam (2 mg/kg) increased percentage entries into the open arms in wild type (p<0.01), α2(H101R) (p<0.01) and α3(H126R) (p<0.01) animals. Analyses of the total distance traveled during the 5 min test session revealed no significant genotype x drug interactions. These results are similar to studies previously reported using 129X1/SvJ mice (Low et al., 2000; Rudolph et al., 1999) with respect to open arm time and demonstrate that the anxiolytic-like effect of diazepam is also observed in C57BL/6J mice.
Figure 1.
Open arm exploration in the elevated plus maze. Percent open arm time and entries were examined when wild type, α1(H101R), α2(H101R) and α3(H126R) point mutant mice were administered diazepam (A, B) or chlordiazepoxide (C, D) or their corresponding vehicle. DZ=diazepam. CDP=chlordiazepoxide. *p<.0.05. **p<0.01.
The analysis of chlordiazepoxide effect on the percentage of time spent in the open arms revealed a significant main effect of genotype (F(3,128)=11.82, p<0.01, a significant main effect of drug-dose (F(2,128)= 10.17, p<0.01) and a significant genotype x drug-dose interaction (F(6,128)=2.59, p<0.05; Fig. 1C). Post hoc Dunnett’s tests revealed that the increase was observed following both 5 mg/kg (p<0.01) and 10 mg/kg (p<0.01) doses in wild type mice, whereas only the 10 mg/kg dose caused a significant increase in open arm time for α1(H101R) (p<0.01) and α3(H126R) (p<0.01) mice. The percentage of open arm entries was affected significantly only by drug-dose (F(2,128)=8.67, p<0.01) with the 10 mg/kg dose of chlordiazepoxide causing an increase in open-arm entries regardless of genotype (p<0.05; Fig. 1D). However, individual post-hoc Dunnett comparisons within each genotype revealed that the 10 mg/kg dose of chlordiazepoxide caused an increase in percentage open arm entries in wild-type (p<0.01), α1H(101R) (P<0.05) and α3(H126R) (p<0.01) mice while this effect was abolished in α2(H101R) animals. The 5 mg/kg dose of chlordiazepoxide was also effective in increasing percentage open arm entries in wild type mice only (P<0.05). Analyses of the total distance traveled revealed no significant genotype x drug interactions demonstrating that exploration of elevated plus maze was not attributed to either baseline differences in locomotor activity between the different genotypes or drug-induced changes.
3.2. Open Field Test
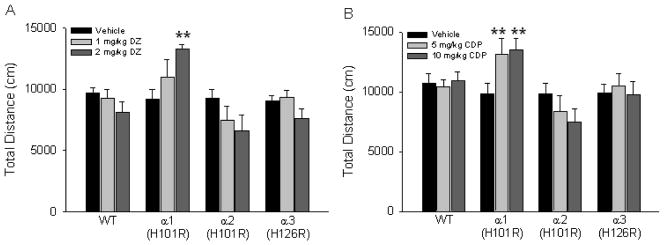
To further exclude the possibility that a potential sedative effect of diazepam or chlordiazepoxide could have confounded the results in the elevated plus maze, we also assessed locomotor activity in the novel open field. Figure 2 shows how each drug affected locomotor activity in a 30 min test session. The analyses of the data collected with neither diazepam nor chlordiazepoxide revealed a significant main effect of drug-dose, genotype or an interaction (Fig. 2B). However, Dunnett’s tests comparing individual drug groups to the corresponding vehicle group within each genotype showed that the α1(H101R) point mutant mice were significantly more active after administration of 2 mg/kg diazepam compared to vehicle-treated mice (p<0.05; Fig. 2A). Similarly, both doses of chlordiazepoxide significantly increased the total distance traveled in the open field by α1(H101R) point mutants compared to vehicle-treated controls (Dunnett’s post hoc test p<0.05 for both 5mg/kg and 10mg/kg doses; Fig. 2B). These results are consistent with previous studies that reported a locomotor-stimulating effect of diazepam in α1(H101R) mice bred on a mixed and a 129x1/SvJ background (Crestani et al., 2000; McKernan et al., 2000). As the duration of the elevated plus maze test was only 5 min, we also examined the first 5 min of the open field test in order to assure that possible effects during this time period were not masked over the 30 min testing period of the open field test. Interestingly, this analysis revealed no significant effects of drug, genotype or interaction on the total distance moved with either drug, suggesting that the locomotor-stimulating effect of diazepam in α1(H101R) animals in our experiments was observed at further time points during the 30 min open field test.
Figure 2.
Locomotor activity in a novel open field. Mean total distance traveled in a novel open field was examined when mice from the different genotypes were administered (A) diazepam (DZ) or (B) chlordiazepoxide (CDP). **p<0.01
3.3. Fear-Potentiated Startle
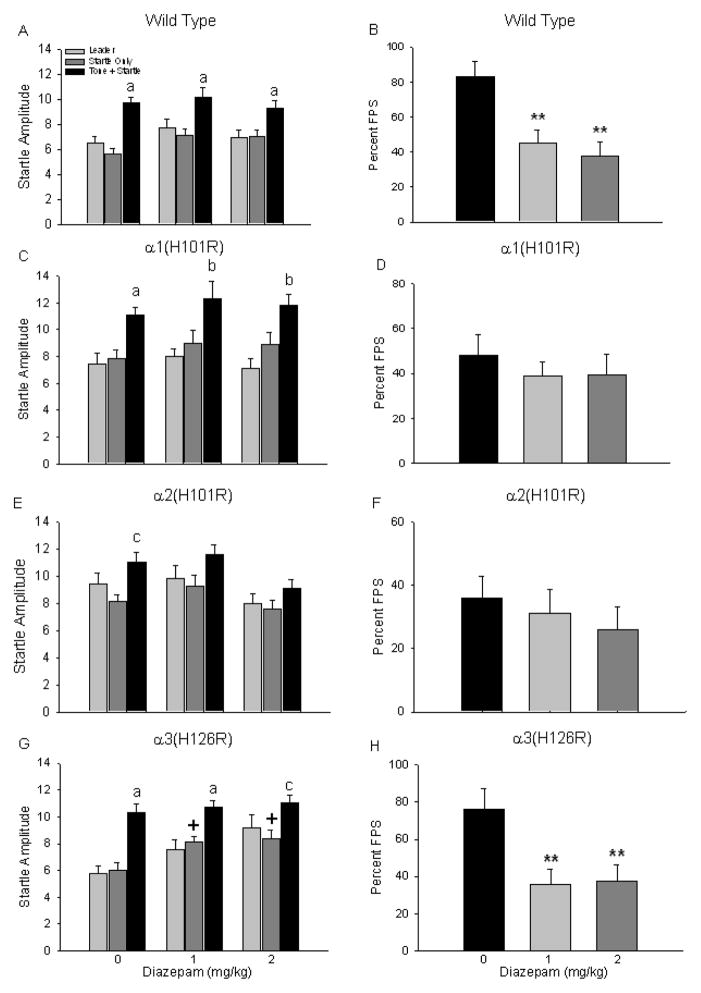
The effects of pretreatment with diazepam on FPS in wild type, α1(H101R), α2(H101R), and α3(H126R) point mutant mice are depicted in Figure 3. A two-way (genotype x drug dose) ANOVA examining percent FPS data revealed a significant main effect of genotype F(3,142)=5.17, p<0.01) where, overall, WT percent FPS responses were significantly greater than percent FPS responses of α2(H101R) point mutants (one-way ANOVA and Tukey’s post hoc test, p<0.05). However, this was likely driven by the fact that vehicle-treated wild type mice had greater percent FPS values than α2(H101R). The overall two-way ANOVA also revealed a significant main effect of drug F(2,142)=1147, p<0.01 where vehicle-treated mice had greater startle responses than mice treated with either dose of diazepam (Tukey’s post hoc, p<0.05), but this was attributed to the fact that both wild type and α3(H126R) vehicle-treated mice had greater percent FPS values than α1(H101R) and α2(H101R) point mutants.
Figure 3.
Impact of diazepam on fear-potentiated startle responses. Mean startle amplitude and percent fear-potentiated startle were examined after wild type (A, B), α1(H101R) (C, D), α2(H101R0 (E, F) and α3(H126R) (G, H) mice were administered diazepam (0, 1, 2 mg/kg) on Test. a=startle amplitude on Tone+Startle trials significantly different from both Leader and Startle Only trials. b=startle amplitude on Tone+Startle trials significantly different from Leader trials. c= startle amplitude on Tone+Startle trials significantly different from Startle Only trials. +=startle amplitude on Test Startle Only trials for mice that received drug significantly greater than the same trial type in mice treated with vehicle. **p<0.01.
In wild type mice, a two-way ANOVA (trial type x drug dose) revealed a significant main effect of trial type (F(2,135)=24.82, p<0.01) on startle amplitude. Subject startle responses were significantly elevated on Tone+Startle trials compared to both Leader and Startle Only trials (post hoc tests p<0.05) for each drug condition (Fig. 3A). A one-way ANOVA showed a significant effect of drug treatment on percent FPS (F(2,45)=9.14, p<0.01); diazepam reduced percent FPS by approximately 50% (Tukey’s post hoc test, p<0.05 for both each dose) when compared to vehicle-treated wild types (Fig. 3B).
While α1(H101R) point mutants also exhibited significantly increased startle responding on Tone+Startle trials compared to both Leader or Startle Only trials during Test (significant main effect of trial type: F(2,87)=20.60, p<0.01; post hoc tests p<0.05 for each drug condition), there was no impact of diazepam on percent FPS values (one-way ANOVA F(2,29)=0.38, p=0.68) (Figs. 3C and 3D). Startle responding on Tone+Startle trials was also significantly elevated when examined in the overall two-way ANOVA (significant main effect of trial type, F(2,123)=3.44, p<0.01) for the α2(H101R) point mutants, but individual post hoc ANOVAs showed that responding on this trial type was significantly different from baseline startle responses during Startle Only trials for vehicle-treated mice only (F(2,45)=4.42, p<0.05; post hoc ANOVA p<0.05) (Fig. 3E). Percent FPS was not impacted by diazepam pre-treatment during Test (F(2,41)=0.49, p=0.62) (Fig. 3F), a result similar to that observed in the α1(H101R) point mutants.
α3(H126R) point mutant mice that were administered diazepam exhibited a pattern of startle responding on Test similar to that observed in wild types. Results of a two-way ANOVA revealed a significant main effect of trial type (F(2,84)=25.33, p<0.01; post hoc ANOVAs p<0.05) on startle amplitude such that responses on Tone+Startle trials were significantly elevated compared to responses generated during Leader or Startle Only trials regardless of drug treatment condition (Fig. 3G). However, this analysis also revealed a significant main effect of drug treatment on startle responding (F(2,84)=8.84, p<0.01); further analysis showed that baseline startle levels on Startle Only trials were significantly elevated for diazepam-treated mice (both doses) when compared to mice that were administered vehicle (one-way ANOVA, F(2,28)=5.50, p<0.01, Tukey’s post hoc test, p<0.05). Therefore, while diazepam-treated mice had significantly lower percent FPS values than vehicle-treated mice (one-way ANOVA, F(2,28)=5.66, p<0.01) (Fig. 3H), this reduction was driven by elevated baseline startle responses. Analysis of baseline startle responses for all other groups revealed no changes across treatment groups.
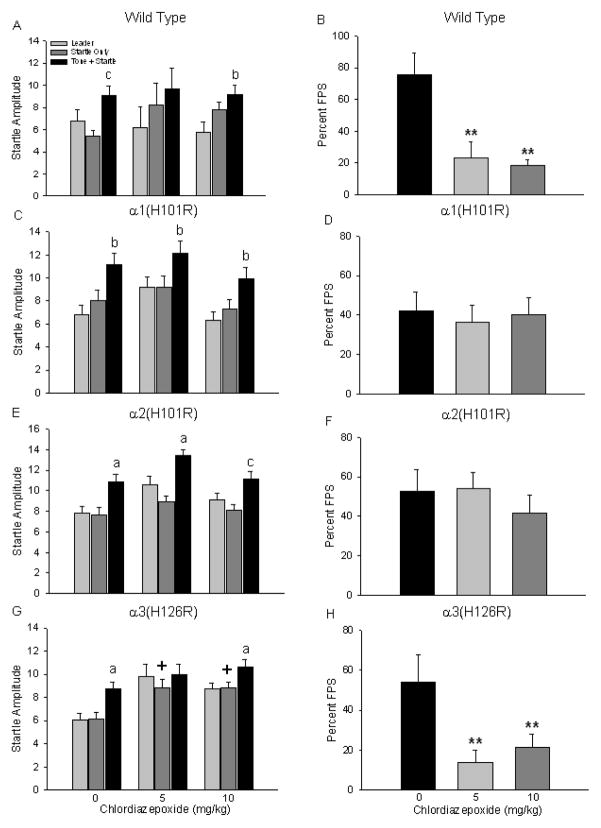
Figure 4 depicts FPS responding when subjects were pretreated with chlordiazepoxide. Results of a two-way (genotype x drug dose) ANOVA examining percent FPS data revealed a significant main effect of drug dose only F(2,115)=8.01, p<0.0001). Overall, vehicle-treated mice had significantly greater startle responses compared to mice treated with either dose of chlordiazepoxide (Tukey’s post hoc, p<0.05).
Figure 4.
Impact of chlordiazepoxide on fear-potentiated startle responses. Mean startle amplitude and percent fear-potentiated startle were examined after wild type (A, B), α1(H101R) (C, D), α2(H101R0 (E, F) and α3(H126R) (G, H) mice were administered chlordiazepoxide (0, 5, 10 mg/kg) on Test. All symbols are the same as in Figure 3.
Examination of wild type startle responses using a two-way ANOVA (trial type x drug dose) revealed a significant main effect of trial type (F(2,57)=8.16, p<0.01); post-hoc analysis showed that subject startle responses were significantly elevated on Tone+Startle trials compared to either Leader or Startle Only trials for subjects treated with vehicle (p<0.05) or 10 mg/kg chlordiazepoxide (p<0.05) (Fig. 4A). A one-way ANOVA showed a significant effect of chlordiazepoxide pretreatment on percent FPS (F(2,19)=9.84, p<0.01) such that subjects treated with either dose of the drug had significantly attenuated startle responses during test compared to vehicle-treated wild types (Fig. 4B).
Using the same two-way ANOVA, analysis of startle responding in α1(H101R) point mutants also revealed a significant main effect of trial type (F(2,78)=13.43, p<0.01); startle responses on Tone+Startle trials were significantly elevated compared to Leader trials in all treatment groups (post hocs, p<0.05) (Fig. 4C). Percent FPS was not impacted by chlordiazepoxide since subjects pretreated with this drug responded like vehicle-treated mice during Test (F(2,26)=0.12, p=0.89) (Fig. 4D). Likewise, α2(H101R) point mutants also exhibited significantly higher startle responses on Tone+Startle trials compared to Leader and Startle Only trials (significant main effect of trial type, F(2,111)=22.79, p<0.01; post hocs, p<0.05) (Fig. 4E) but administration of chlordiazepoxide (both doses) had no impact on percent FPS when compared to vehicle treated mice (one-way ANOVA, F(2,37)=0.50, p=0.61) (Fig. 4F).
Lastly, α3(H126R) point mutant mice that received chlordiazepoxide exhibited a pattern of startle responding similar to that observed in wild types; this response pattern was also the same as that observed when mice from these same genotypes were administered diazepam. Startle responding on Tone+Startle trials was significantly elevated compared to Leader and Startle Only trials for mice that received vehicle and the highest dose of the drug (significant main effect of trial type, F(2,99)=6.04, p<0.01; post hocs, p<0.05) (Fig. 4G). However, as with diazepam-treated α3(H126R) point mutants, the two-way ANOVA showed that there was also a significant main effect of drug treatment on startle responses (F(2,99)=12.39, p<0.01). Follow up analysis showed that baseline startle levels on Startle Only trials were significantly elevated for mice treated with chlordiazepoxide (both doses) than vehicle-treated mice (one-way ANOVA, F(2,33)=6.27, p<0.01, Tukey’s post hoc test, p<0.05). Hence, even though chlordiazepoxide-treated α3(H126R) point mutants had significantly lower percent FPS values than vehicle-treated mice (one-way ANOVA, F(2,33)=5.15, p<0.05) (Fig. 4H), this reduction was also driven by elevated baseline startle responses. Examination of the baseline startle responses for all other groups on Startle Only trials revealed no changes across treatment groups. Together, the FPS data indicate that both α1- and α2-containing GABAA receptors are necessary for benzodiazepines to exert their effects on conditioned fear responses.
4. DISCUSSION
The above findings provide evidence for a differential role of GABAA receptor subtypes in mediating the effects of benzodiazepines on unconditioned anxiety and conditioned fear responses at the same drug doses. In the elevated plus maze, both diazepam and chlordiazepoxide significantly increased the amount of open arm exploration in wild type, α1(H101R) and α3(H126R) mice but this effect was abolished in α2(H101R) mice when either drug was administered. These results are similar to studies previously reported using point mutants generated on the 129X1/SvJ background (Low et al., 2000; Rudolph et al., 1999) suggesting that the specific role of α2-containing GABAA receptors in unconditioned anxiety also occurs in C57BL/6J mice. Moreover, we extend these earlier findings to another benzodiazepine, chlordiazepoxide.
The locomotor-dependent nature of the elevated plus maze, suggests that the anxiolytic effects, or the lack thereof, reported in mice of different genotypes here may be confounded by unforeseen baseline locomotor activity differences between the genotypes. However, our analyses of the total distance traveled on the elevated plus maze did not reveal such an effect. We further investigated this possibility in a novel open field and found that baseline activity levels of vehicle-treated mice also did not differ across genotypes in this test. Together, these locomotor activity data demonstrate that there were no inherent baseline activity differences between the different groups of mice tested.
It was also important to determine whether activity in either the elevated plus maze or the open field could have been impacted by drug, even though both drugs were administered at non-sedating doses. While activity in the open field was not affected by either diazepam or chlordiazepoxide in wild type, α2(H101R) or α3(H126R) point mutant mice, both drugs elicited hyperactivity in the α1(H101R) mice in the novel open field only. This finding is in line with previous reports demonstrating a similar response following diazepam administration in α1(H101R) mice bred on different strain backgrounds (Crestani et al., 2000; McKernan et al., 2000); this hyperactivity was observed only when the animals were tested in an unfamiliar environment (Crestani et al., 2000), an effect that was interpreted as reduced neophobia and/or heightened arousal in response to stressors in α1(H101R) point mutant mice.
Although both the elevated plus maze and the open field were novel to all mice in this study, it is possible that the inherent differences of each test as well as the increased duration of the open field test (i.e., 30 min) compared to the elevated plus maze test (i.e., 5 min) revealed a benzodiazepine-induced hyperactivity in the α1(H101R) point mutants only in the open field. This may be due to the fact that in a longer test, there is more time for habituation to the novelty of the environment, which may make it possible for non-anxiety dependent locomotor effects to be unmasked. Most importantly, locomotor activity measures from both tests indicate that neither diazepam nor chlordiazepoxide led to a reduction in locomotor activity at the doses employed (i.e., sedation), a response that could have impacted exploration of anxiety-provoking locations on the elevated plus maze and startle reflex responses in the FPS experiments.
The effects of diazepam and chlordiazepoxide on conditioned fear responses were assessed with the fear-potentiated startle paradigm. We previously showed that both drugs significantly attenuated the potentiation of the startle response on Tone+Startle trials, and did not impact baseline startle on Startle Only trials in wild type C57BL/6J mice (Smith et al., 2011). Here, we hypothesized that similar effects would be observed in wild type, α1(H101R), and α3(H126R) mice but that the fear-reducing effects of benzodiazepines would be blocked in α2(H101R) mice, a pattern of responding that would be consistent with findings from unconditioned tests of anxiety.
As expected, administration of diazepam and chlordiazepoxide significantly attenuated FPS in wild type mice and α3(H126R) mice at all administered doses, while the fear-reducing effects of these drugs were blocked in α2(H101R) point mutants. Surprisingly, we also observed that the fear-reducing effects of diazepam and chlordiazepoxide were blocked in α1(H101R) mice; FPS remained unchanged in drug-treated mice compared to those that received vehicle. These data indicate that both α1- and α2-containing GABAA receptors are necessary for benzodiazepines to exert their effects on conditioned fear responses whereas only α2-containing GABAA receptors are needed for benzodiazepine-induced anxiolysis in unconditioned tests of anxiety, such as the elevated plus maze and the black white box (Low et al., 2000; Rudolph et al., 1999).
Our finding that α1-containing GABAA receptors are required for the fear-reducing effects in the fear-potentiated startle paradigm are at variance with earlier reports that examined this response in rats. L-838,417, a multispecific agent with agonistic properties at α2-, α3-, and α5-subunit-containing receptors, and an antagonist at α1-containing receptors was reported to have an anxiolytic-like profile in a fear-potentiated startle test in rats (McKernan et al., 2000). This finding suggested that α1-containing GABAA receptors were not necessary for the attenuation of the FPS response. However, these authors evaluated startle responding differently in their subjects compared to the present study. While they did not calculate percent FPS values, they looked at the mean startle amplitude under CS+ and CS− conditions separately. While under CS+ (i.e., light presentation) conditions the mean startle amplitude was reduced in drug-treated animals, it appears that the mean startle amplitude was also reduced under CS− (i.e., dark) conditions; it was not reported whether or not the latter effect was statistically significant. Our calculation of percent FPS values from Fig. 5d of this previous report suggests that L-838,417 likely does not reduce conditioned startle responses in the CS+ condition. Rather, the reduction of FPS reported in the CS+ condition is confounded by an increase in baseline startle responses in the CS− condition according to this figure.
In another FPS study with rats, the α2-/α3-selective agonist TPA023 reduced light-dark difference scores, indicating that TP023 reduces potentiation of the fear response (Atack et al., 2006). It is possible that either species differences between rats and mice, or specifics of the respective behavioral protocols (e.g., conditioning to light in rats versus conditioning to tone in mice) contributes to the apparent differences in outcomes. It should also be noted that the FPS experiments in the current work were conducted using low anxiolytic doses of the two drugs. Our data do not exclude the possibility that at higher drug doses only α1- or only α2-containing GABAA receptors may lead to fear reduction. This also translates to other positive modulators of these receptors, where the efficacy and concentration of the drug may lead to different requirements to produce behavioral effects.
The involvement of α3-containing GABAA receptors in anxiolysis is somewhat controversial. While an initial examination of the FPS responses in the α3(H126R) point mutant mice indicated that diazepam and chlordiazepoxide reduced percent FPS during test, this reduced value was in fact driven by increased baseline startle responses on Startle Only trials in drug-treated animals of this genotype. In contrast, the administration of benzodiazepines did not have nonspecific effects on baseline startle amplitude (either an increase or decrease) to the white noise stimulus in wild type, α1(H101R), and α2(H101R) mice. That the two different benzodiazepines failed to abolish the fear-potentiation of the startle reflex in the α1 and α2 point mutants when the white noise stimulus was preceded by a fear-conditioned stimulus demonstrates that this represents a specific and genuine fear-reducing effect in this paradigm in these mice.
α1- and α2-containing GABAA receptors are highly expressed in the amygdala (Fritschy and Mohler, 1995; Pirker et al., 2000), a neuroanatomical structure that is strongly implicated in mediating both anxiety and fear responses (Engin and Treit, 2008). Whereas α1-containing GABAA receptors are expressed in the lateral and basolateral nuclei of the amygdala, but are largely absent in the central nucleus, α2-containing GABAA receptors are expressed both in the lateral/basolateral and central nuclei at high levels (Marowsky et al., 2004). Using single, double, and triple point-mutated mice, it has been shown that the effects of diazepam on the peak IPSC amplitude in the lateral/basolateral amygdala are mediated by α1- and α2-containing GABAA receptors, but not by α3- and α5-containing GABAA receptors, and the effects of diazepam on peak IPSC amplitudes in the central amygdala are mediated by α2-containing GABAA receptors, but not by α1-, α3-, and α5-containing GABAA receptors (Marowsky et al., 2004). These findings suggest a functional role of α1-containing GABAA receptors in the lateral/basolateral amygdala and of α2-containing GABAA receptors in both the lateral/basolateral and central amygdala.
Previous work has shown that infusions of benzodiazepines into the amygdala significantly increases exploration of anxiogenic locations in unconditioned tests of anxiety (See Engin and Treit, 2008 for a review) demonstrating that the anxiolytic effects of benzodiazepines are critically mediated by the GABAergic system in this neural structure. There is evidence that the α1-containing GABAA receptors in the lateral amygdala underlie fear learning and plasticity (Wiltgen et al., 2009) and that the α1 subunits in this particular neural structure are important mediators of emotional responses. Our data are, to our knowledge, the first to demonstrate in any anxiety or fear paradigm that α1-containing GABAA receptors are involved in the fear-reducing effects of benzodiazepines, and that both the α1- and α2-containing GABAA receptors are necessary for this effect. Although not formally tested here, we posit that these effects are likely mediated by the α1 and α2 subunit containing GABAA receptors in the amygdala.
Unconditioned and conditioned anxiety paradigms likely model different aspects of anxiety disorders in humans. For example, the elevated plus maze has some face and predictive validity for generalized anxiety disorders, while fear-potentiated startle is frequently considered to be a model of post-traumatic stress disorder. Overall, our findings illustrate both some overlap and some divergence between the GABAA receptor subtype requirements for the impact of BZs on unconditioned anxiety and conditioned fear. Specifically, the α2-containing GABAA receptors are required for BZ-induced anxiolysis while both α1- and α2-containing GABAA receptors are necessary for benzodiazepines to exert their fear-reducing effects. This raises the possibility that GABAergic pharmacological interventions for specific anxiety disorders can be differentially tailored. While specific α2-agonists may be useful to treat generalized anxiety and panic disorder, α1/2-agonists may be more effective in the treatment of phobias and post-traumatic stress disorder. Future studies are therefore needed to elucidate the exact processes (e.g., learning, memory and/or fear expression) mediated by α1-containing GABAA receptors in conditioned fear responses.
Highlights.
We used H-R point mutated mice to study benzodiazepine effects on anxiety and fear.
α2-subunits are required for benzodiazepine-induced anxiolysis in elevated plus maze.
α1- and α2-subunits are required for benzodiazepine-induced reduction of conditioned fear.
Pharmacological interventions for specific anxiety disorders can be differentially tailored.
Acknowledgments
The project described was supported by Award Number R01MH080006 of the National Institute of Mental Health to UR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
While different definitions of anxiety and fear, as well as conventions of using the terms interchangeably, are common in the literature, for the purposes of this manuscript, we use the term “fear” only when referring to learned fear responses and the term “anxiety” only when referring to unconditioned responses.
Data from wild type FPS subjects were previously published in Smith et al, 2011. These data were collected at the same time as the drug data in this experiment.
Disclosure / Conflict of Interest
UR is a consultant for Sunovion and for Concert Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro JL, Whiting P, Dawson GR, McKernan RM. TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. Journal of Pharmacology & Experimental Therapeutics. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Benson JA, Low K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Letters. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Circadian modulation of the rat acoustic startle response. Behavioral Neuroscience. 1992;106:846–852. doi: 10.1037//0735-7044.106.5.846. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature Neuroscience. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Crestani F, Martin JR, Mohler H, Rudolph U. Resolving differences in GABAA receptor mutant mouse studies. Nature Neuroscience. 2000;3:1059–1059. doi: 10.1038/80553. [DOI] [PubMed] [Google Scholar]
- Crestani F, Mohler H, Rudolph U. Anxiolytic-like action of diazepam: mediated by GABAA receptors containing the alpha2 subunit. Trends in Pharmacological Sciences. 2001;22:403–403. [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. Journal of Neuroscience. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D. The effects of intra-cerebral drug infusions on animals’ unconditioned fear reactions: a systematic review. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:1399–1419. doi: 10.1016/j.pnpbp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. Journal of Comparative Neurology. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depression & Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Molecular Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. European Journal of Neuroscience. 2004;20:1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nature Neuroscience. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. Journal of Pharmacology & Experimental Therapeutics. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, McKernan RM, Dawson GR. Anxiolytic-like action of diazepam: which GABA(A) receptor subtype is involved? Trends in Pharmacological Sciences. 2001;22:402–403. doi: 10.1016/s0165-6147(00)01773-9. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABA(A) receptor subtypes. Nature Reviews Drug Discovery. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shader RI, Greenblatt DJ. Use of benzodiazepines in anxiety disorders. New England Journal of Medicine. 1993;328:1398–1405. doi: 10.1056/NEJM199305133281907. [DOI] [PubMed] [Google Scholar]
- Smith KS, Meloni EG, Myers KM, Van’t Veer A, Carlezon WA, Jr, Rudolph U. Reduction of fear-potentiated startle by benzodiazepines in C57BL/6J mice. Psychopharmacology. 213:697–706. doi: 10.1007/s00213-010-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Rudolph U. Anxiety and depression: Mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2011;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. Journal of Biological Chemistry. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Wiltgen BJ, Godsil BP, Peng Z, Saab F, June HL, Linn ML, Cook JM, Houser CR, O’Dell TJ, Homanics GE, Fanselow MS. The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala. Frontiers in Behavioral Neuroscience 3. 2009;37:1–12. doi: 10.3389/neuro.08.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]