Abstract
Background
Although one of the main characteristics of psychopaths is a deficit in emotion, it is unknown whether they show a fundamental impairment in appropriately recognizing their own body sensations during an emotion-inducing task.
Method
Skin conductance and heart rate were recorded in 138 males during a social stressor together with subjective reports of body sensations. Psychopathic traits were assessed using the Psychopathy Checklist – Revised (PCL-R) 2nd edition (Hare, 2003).
Results
Nonpsychopathic controls who reported higher body sensations showed higher heart rate reactivity, but this verbal-autonomic consistency was not found in psychopathic individuals. This mind-body disconnection is particularly associated with the interpersonal-affective factor of psychopathy.
Conclusions
Findings are the first to document this body sensations– autonomic mismatch in psychopaths, and suggest that somatic aphasia the inaccurate identification and recognition of one‘s own somatic states may partly underlie the interpersonal-affective features of psychopaths.
Keywords: somatic aphasia, body sensation, cardiovascular, electrodermal, psychopathy, somatic marker hypothesis
Psychopathic Individuals are characterized by a constellation of traits, including interpersonal-affective (e.g., superficial charm, manipulativeness, lack of affect and emotion) and antisocial features (e.g., impulsivity and aggression, (Hare, 2003). One prominent theoretical perspective of the underlying neurobiological basis of psychopathy is the somatic marker hypothesis (Antonio R. Damasio, 1994), which proposes that appropriate autonomic functioning is critical to experiencing emotional states that guide prosocial behavior and good decision making. It is assumed that emotions associated with previous experiences provide dispositional or visceral markers that functions as an alarm signal, alerting the individual to the potential negative outcome of a certain action. This somatic marker has been termed an “an unpleasant gut feeling” (Damasio, 1994, p 173) which includes both visceral and nonvisceral sensations in response to external stimuli, and which are associated with both positive and negative emotional states. Damage to the ventromedial prefrontal cortex and other structures involved in the representation and regulation of body states (including amygdala, insula, somatosensory cortex, cingulate, basal ganglia and brain-stem nuclei) has been argued to result in an inability to experience this “gut feeling” which in turn may predispose to psychopathic and antisocial behavior (Bechara, Damasio, Damasio, & Lee, 1999).
There has been some support for the somatic marker hypothesis. For example, using the Iowa Gambling Task, several studies have found that while nonpsychopathic individuals learned to avoid “risky” decks, psychopaths made more risky decisions over time, indicating their inability to guide behavior based on somatic markers (Blair, Colledge, & Mitchell, 2001; Mitchell, Colledge, Leonard, & Blair, 2002; van Honk, Hermans, Putman, Montagne, & Schutter, 2002). However, some have failed to find this relationship (Blair & Cipolotti, 2000), or have found that the relation is moderated by attention, anxiety, or social status (Blair & Cipolotti, 2000; Gao, Baker, Raine, Wu, & Bezdjian, 2009; Lösel & Schmucker, 2004; Schmitt, Brinkley, & Newman, 1999). Using a different paradigm, Raine and colleagues (Ishikawa, Raine, Lencz, Bihrle, & LaCasse, 2001; Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000) have provided additional supporting evidence. Individuals with high antisocial personality traits and psychopathy displayed significantly reduced autonomic reactivity when giving a speech about their personal faults compared to those with low traits, and also showed reduced gray matter in the ventromedial cortex, orbitofrontal cortex, and amygdala (Yang, Raine, Colletti, Toga, & Narr, 2010; Yang et al., 2005). Damasio suggested that this social stressor is particularly useful for eliciting secondary emotions such as guilt and embarrassment (A.R. Damasio, 2000), and that a lack of somatic markers resulted in socially abnormal behavior in psychopaths. Impairments in this cortical-subcortical network may underlie an individual‘s inability to interpret or label body sensation, which in turn may give rise to risky decision-making in psychopathic individuals (Bechara et al., 1999; Birbaumer et al., 2005; Blair, 2007; Yang et al., 2010).
Despite this body of research, no study has examined whether psychopaths are unaware of their body sensations at the somatic level. It has long been theorized that emotion experiences arises directly from the perception of body changes (e.g., James 1884). Given that a fundamental emotion deficit is viewed by many as the core feature of psychopathy (Cleckley, 1976; Patrick, 1994), it could be hypothesized that psychopaths may be relatively less sensitive to their body changes during emotional events, and thus are unable to accurately perceive their somatic reactions, which may then contribute to their affective deficiency. What also appears entirely unexplored is the mismatch or decoupling between objective measures of body responses (autonomic changes), and subjective verbal reports of body changes in psychopaths. Psychopaths describe emotional experiences, albeit in a fashion that is stereotyped and at times wooden (Cleckley, 1976). For example, studies have shown that although compared to nonpsychopathic controls, psychopathic individuals show autonomic hyporesponsivity including reduced fear-potentiated startle and smaller skin conductance magnitudes, the two groups‘ subjective experience to emotional stimuli are rated similar (Benning, Patrick, & Iacono, 2005; Flor, Birbaumer, Hermann, Ziegler, & Patrick, 2002; Patrick, Bradley, & Lang, 1993). Furthermore, these psychophysiological deficits are often attributed to the interpersonal-affective factor of psychopathy (Patrick, 2007). Given that psychopaths are characterized by emotional deficits, it is possible that they are describing emotions that they think they should feel based on exteroceptive cues, but do not consciously experience. The expectation is that normal individuals may be relatively accurate in perceiving and interpreting their body changes; if they show an objective autonomic increase to a social stressor, they should be able to verbally report corresponding body sensations. In contrast, if there is a disruption in the appropriate sensation of body reactions to a stressor in psychopaths, one would expect somatic aphasia – a mismatch between the subjective report and objective autonomic measures of their body sensations.
This study attempts to address this issue by measuring skin conductance and heart rate reactivity during a social stressor in a community sample. All participants were recruited from temporary employment agencies and psychopathic traits were assessed using the PCL-R (Hare, 2003). It is hypothesized that in a social stressor: 1) psychopathic and nonpsychopathic controls will show similar verbal reports of body sensations experienced, and 2) nonpsychopathic controls who report high body sensation will exhibit high autonomic responses, whereas this consistency would not be observed in psychopaths. Finally, we aim to explore which of the two factors of psychopathy, i.e. interpersonal-affective and antisocial behavior, will be particularly associated with the mismatch between autonomic measure and verbal reports of body sensations.
Method
Participants
Participants (138 men, mean age = 35.72, SD = 8.61, range = 23 to 56 years) were recruited from temporary employment agencies in the greater Los Angeles area, and represents a different sample to that used in our prior work (Ishikawa et al., 2001; Raine et al., 2004; Yang et al., 2005). After giving written informed consent, participants were individually assessed for two days. The study and all its procedures were approved by the university‘s Institutional Review Board. An estimate of IQ scores was created by prorating four subscales of the WAIS-III (Similarities, Arithmetic, Digit Symbol and Picture Completion). Anyone who was interested in a research study was recruited and all participants were paid $100 for completing the study.
Psychopathy Assessment
Psychopathy was assessed using the Psychopathy Checklist – Revised (PCL-R): 2nd Edition (Hare, 2003), and supplemented by 10 sources of collateral data. The PCL-R: 2nd Edition consists of 20 items and reflects two factors: interpersonal-affective characteristics (Factor 1) and antisocial behavior (Factor 2). Ratings were made by the third author (RS) who received systematic training on the administration and scoring of the PCL-R by Robert D. Hare and Adelle Forth – including the completion of a series of PCL-R assessments on standardized videotaped case histories of adult male offenders (Pearson r correlations between rater‘s and standardized criterion scores: Total PCL-R = .92, Factor 1 = .93, and Factor 2 = .91). Assessments were supervised by the second author who has extensive experience in the assessment of psychopathy (AR).
Expanding on our prior work on community assessment with the PCL-R (Ishikawa et al., 2001), we met the challenge of using the PCL-R in a community sample by further developing a systematic and comprehensive protocol for the collation of 10 sources of objective collateral data, including professional web-based background check services. These data not only provided new additional background information for item evaluation (e.g., irresponsibility, proneness to boredom, criminal versatility), but also allowed for assessment of inconsistencies and conflicts between the participant‘s oral report and objective data reports that aid assessment of pathological lying and deception. The ten collateral data sources were as follows: (a) self-reported theft, drug offenses, and violent crime as assessed by an adult extension (Raine et al., 2000) of the National Youth Survey self-report delinquency measure (Elliott, Ageton, Huizinga, Knowles, & Canter, 1983); (b) official state-level Department of Justice criminal records for California; (c) nationwide state-level criminal and court record database searches; (d) federal criminal records database search; (e) involvement in civil action, liens, and other financial judgments; (f) personal history judgments including marriage and divorce, prior residences and relocations, relatives, and significant others; (g) data derived from, and behavioral observations made during, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1997); (h) the SCID Axis II Personality Disorders (SCID-II; First, Gibbon, Spitzer, Williams, & Benjamin, 1997). The SCID-I and II diagnoses were made by the same research assistant trained on the SCID (Ventura, Liberman, Green, Shaner, & Mintz, 1998); (i) the Interpersonal Measure of Psychopathy (IM-P; Kosson, Steuwerald, Forth, & Kirkhart, 1997), which provides an interviewer‘s ratings of an individual‘s psychopathic interpersonal behaviors, has demonstrated construct validity with the PCL-R in a prison sample, and has been validated for use with nonincarcerated samples (i.e., college students; Kosson et al., 1997); and (j) independent IM-P ratings made by two different laboratory assistants during separate phases of testing throughout the two days. A tertile split on the PCL-R score was performed, and those falling in the top and bottom third were retained. This tertile split resulted in a cut-off point of 23, i.e., subjects with total PCL-R scores equal to or larger than 23 were grouped as psychopaths (n = 44, mean PCL-R score = 28.5, range 23-39). Those scored 14 or below were grouped as nonpsychopathic controls (n = 45, mean PCL-R score = 8.0, range 0–14). This is consistent with the cut-off point used in our prior work among a completely different community sample (Ishikawa et al., 2001; Raine et al., 2004; Raine et al., 2003; Yang et al., 2005). Although it is lower than the commonly used cut-off point of 30 in incarcerated population (Hare, 2003), we used this cut-off point both to be consistent with our prior research, and also because the PCL-R (which was developed on prison samples) underestimates psychopathy scores in community samples which do not have access to the rich sources of collateral information obtained in institutionalized samples. Psychopaths had significantly higher rates of self-reported violent or nonviolent crime than the nonpsychopathic controls (all p < .001). The two groups did not differ on age, t (98) = 0.63, or IQ, t (98) = 0.52. There were significantly more American-Americans in the psychopathy group, chi;2 (1) = 5.97, p < .05. Therefore the potential confounding effect of ethnicity was examined. See Table 1 for means and standard deviations. The total PCL-R and two factor scores, e.g. interpersonal-affective and antisocial behavior, were also used in the correlational analyses.
Table 1.
Descriptive information for the total sample and psychopath/control groups
| Total sample (n = 138) | Psychopaths (n = 44) | Controls (n = 45) | |
|---|---|---|---|
| Age (in years) | 35.72(8.61) | 34.94(10.18) | 36.08(7.78) |
| IQ | 98.22(15.31) | 97.51(12.90) | 99.04(15.86) |
| Ethnicity | |||
| African-American | 61 | 25 | 14 |
| Non-African-American | 77 | 19 | 31 |
| Total PCL-R | 18.77 (9.01) | 28.03(3.91) | 7.81(3.52) |
| Factor 1a | 8.39(4.18) | 12.29(1.93) | 3.80(2.24) |
| Factor 2b | 9.06(4.60) | 13.45(2.54) | 3.49(2.10) |
| Body sensation | 22.84(7.58) | 22.64(7.37) | 23.05(8.10) |
Note. Data includes means (standard deviations), except for ethnicity where cell counts are reported.
Factor 1 = interpersonal-affective factor;
Factor 2 = antisocial behavior factor.
Psychophysiological Measures
Heart rate and skin conductance measures were recorded continuously during the psychophysiological testing session. The psychophysiological protocol lasted approximately 2 hours and included a total of 10 tasks. Each task lasted 3–8 min and the inter-task interval was about 2–5 min. In the current study, data from the social stressor task were used.
Social Stressor Task
Heart rate and skin conductance were measured during a social stressor designed to elicit secondary emotions such as embarrassment and guilt (Damasio, 2000). In this task, subjects were given two minutes to prepare a speech about their worst personal faults and weakness, followed by a two-minute period in which they gave their speech to the experimenter while being videotaped. If the participant had difficulty speaking continuously, the research assistant requested him to give specific examples of the reported fault(s) to enhance the stressful nature of the task (Raine et al., 2000; Ishikawa et al., 2001).
Apparatus and recording procedures
All psychophysiological data were collected with equipment and software from the James Long Company (1999; Caroga Lake, New York). An Isolated Bioelectric Amplifier was used and physiological data were recorded online directly into a data acquisition computer. To measure heart rate, the channel of the grounded electrocardiograph was recorded through disposable electrodes that were attached to either side of the participant‘s lowest ribs to reduce movement artifacts. Heart rate data were analyzed using the Interbeat Interval Analysis Software program (James Long Company). R-waves were sampled every 0.1 secs.
Skin conductance activity was recorded from bipolar leads on the distal phalanges of the index and middle fingers of the non-dominant hand using silver-silver chloride electrodes (0.8 cm in diameter). The conducting medium was K-Y lubricating jelly, surrounded by an adhesive electrode collar to delineate consistent contact area of the conducting medium with the skin. Skin conductance level was recorded using a low-pass filter set to 10 Hz. The signal was digitized at a sampling rate of 512 Hz, with 12 bits of resolution (corresponding to a step size of < .01 microsiemens).
Body Sensations Measure
After the social stressor, participant was asked to answer the question “how much did you experience the following body feelings when preparing and giving your speech?” A total of 14 items were included: lump in throat, breathing changes, stomach sensations, feel cold, feel hot, heart pounding, tense muscles, perspiration, goose pimples, facial blushing, jelly legs, hands tremble, voice tremble, and eyes well up with tears. Participants rated each of the statements on a five-point Likert scale (1= not at all, 2 = a little, 3 = sometimes, 4 = often, 5 = very often). Coefficient alpha for the scale is .86, indicating reasonable internal reliability. A total score was calculated for each participant, with higher score indicating higher body sensations. In addition, high and low sensation groups were formed using a median split.
Data Reduction and Analyses
Skin conductance and heart rate data reduction was conducted following the same procedure we had previously used in a different temporary employment agency sample (Ishikawa et al., 2001). The duration of the social stressor was 240 s, and skin conductance and heart rate recorded at baseline, T1 (time 1), T2 (time 2), and T3 (time 3) were used in the analyses. Baseline constituted a 60 s rest period immediately before the social stressor. T1 refers to the first second immediately following the directions for the task when the subject was asked to begin preparing their speech (i.e. beginning of anticipatory stress). T2 refers to 120 s into the task, when the participant received a cue to start his speech presentation (i.e. end of anticipatory stress and beginning of emotional stress). T3 refers to the end of the task at 240 s, when he was asked to stop his speech (i.e. end of emotional stress). For each of these four time points, heart rate in beats per minute was calculated by doubling the number of beats recorded during the 30 s either preceding or following each time point (i.e., baseline = 1–30 s before the beginning of the social stressor; T1 = 1–30 s of the speech preparation period, or beginning of anticipatory stress; T2 = 90–120 s of the speech preparation period, or end of anticipatory stress; T3 = 210–240 s at the end of the speech presentation, or end of emotional stress).
Repeated-measures analysis of variance (ANOVA) was conducted on the 4 time points (Baseline, T1, T2, and T3) in a 2 (psychopaths vs. controls) x 2 (high vs. low body sensation groups) design for heart rate and skin conductance measures separately. One-way ANOVAs and t-tests were then followed as needed. All tests of significance are 2-tailed with an alpha level of .05. Cohen‘s d (0.2, small; 0.5 median; 0.8 large effect, (Cohen, 1988)) and partial η2 were used to measure effect sizes.
Finally, skin conductance level change score was calculated for each participant by subtracting skin conductance level at baseline from that at T2. Heart rate change score was computed similarly. Autonomic-body sensations mismatch scores were then computed by calculating the differences between Z-standardized heart rate or skin conductance level changes and Z-standardized body sensation scores for each subject. Higher (positive) values indicate greater autonomic responding relative to subjective (verbal) reports (i.e. heart rate or skin conductance level changes > body sensations) and indicate autonomic-verbal or emotional mismatch (Bonanno, Znoj, Siddique, & Horowitz, 1999). Pearson correlation coefficients were calculated to examine the relations between skin conductance- and heart rate-derived mismatch scores, total psychopathy and its two sub-factors.
Results
Descriptive Statistics
Means and standard deviations of the main variables for the sample and each group are displayed in Table 1. The two groups did not differ on body sensations, t (98) = 0.26, all p > .05. Age and IQ were not correlated with any of the psychopathy or psychophysiological variables, nor were they related to body sensations, r < .09, p > .05.
Body Sensation and Autonomic Changes to the Social Stressor
Heart rate
Repeated measures ANOVA revealed a significant main effect of Time, F (3, 83) = 16.19, p < .001, η2 = 0.369, and a significant psychopathy by time interaction, F (3, 83) = 3.99, p < .01, η2 = 0.126. Compared to the controls, psychopaths had significantly lower heart rate at T1, t (91) = 2.24, p < .05, d = 0.465, and showed a trend of lower heart rate at other time points, p > .14, d < 0.308.
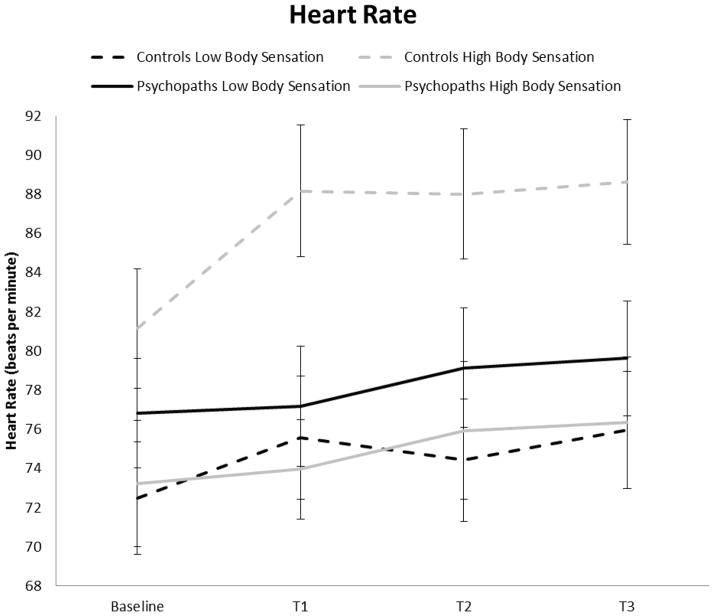
More importantly, the psychopathy by body sensation group interaction was significant, F (1, 85) = 6.15, p < .05, η2 = 0.067. For controls, high body sensation group showed significantly higher heart rate than the low sensation group, F (1, 43) = 7.74, p < .01, η2 = 0.153. In contrast, for psychopaths, high and low body sensation groups did not differ on heart rate, F (1, 42) < 1, p = .45, η2 = 0.013. No other main or interaction effects were significant, all p > .15. Figure 1 shows heart rate (and standard errors) during the rest, social stressor, and recovery periods for high and low body sensation groups in control and psychopaths separately.1
Figure 1.
Heart rate (± SE) during the rest, social stressor, and recovery periods for high and low body sensation groups in controls and psychopaths.
Skin conductance levels
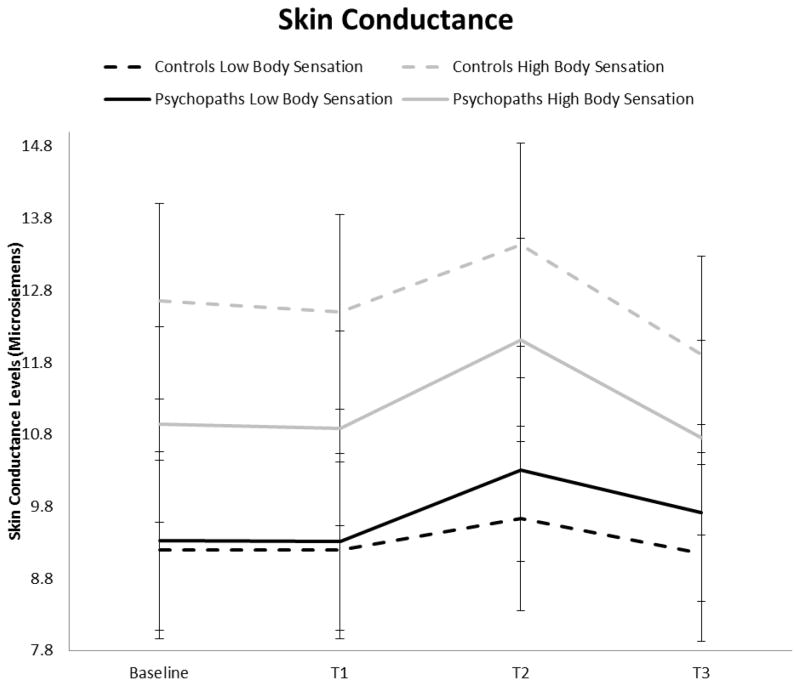
A significant main effect of Time was found, F (3, 82) = 23.46, p < .001, η2 = 0.460. The high body sensation group had significantly higher skin conductance levels than the low sensation group, F (3, 82) = 4.86, p <.01, η2 = 0.151. No other main or interaction effects were significant. Specifically, contrary to our prediction, the psychopathy by body sensation group interaction was not significant, F (1, 84) < 1, p > .48. However, skin conductance levels for high and low body sensation groups in controls and psychopaths are displayed in Figure 2 for illustration purposes.
Figure 2.
Skin conductance levels (± SE) during the rest, social stressor, and recovery periods for high and low body sensation groups in controls and psychopaths.
Associations between Autonomic-Body Sensation Mismatch and Psychopathy
Skin conductance- or heart rate- derived mismatch scores were not significantly correlated with PCL-R total scores. However, the skin conductance-derived mismatch score was positively correlated with the Factor 1 of psychopathy, r (137) = 0.19, p < .05, indicating that individuals with a higher autonomic-body sensation mismatch had a higher degree of interpersonal-affective psychopathy features. Heart rate-derived mismatch score was not significantly correlated with either of the two factor scores, r < .10.2
Potential Confounds
The significant mismatch between body sensations and heart rate reactivity could be a function of the fact that many psychopaths are characterized by pathological lying. To test this alternative, we repeated the above MANOVA analyses using PCL-R Item 4 (pathological lying) as a covariate for heart rate measures. The psychopathy group by body sensation group interaction effect remained significant, F (1, 84) = 6.39, p < .05, η2 = .07, suggesting that psychopaths genuinely are less capable of matching their personal experience of body sensations to a stressor to objective autonomic reality. Similarly, after controlling for ethnicity (African-American vs. non-African-American), the psychopathy group by body sensation group interaction effect remained significant, F (1, 84) = 5.86, p = .018, η2 = .071.
Discussion
Main Findings
Consistent with our hypothesis, psychopathic individuals are not accurately aware of their body states as objectively measured by heart rate changes during the social stressor. Specifically, although nonpsychopathic controls who show increased heart rate verbally reported corresponding higher body sensations, this consistency was not observed in psychopaths. Furthermore, this autonomic-body sensation mismatch was associated with the interpersonal-affective factor of psychopathy in particular. To our knowledge, findings are the first to document this mind-body disconnection in psychopathy, and suggest that somatic aphasia – the inaccurate identification and recognizing of one‘s somatic states – may partly underlie the emotional/affective impairment in psychopaths.
Mechanisms
Findings are broadly consistent with Damasio‘s somatic marker model (Antonio R. Damasio, 1994). They also potentially bear a relation to Cleckley‘s “semantic aphasia” theory of psychopathy (Cleckley, 1976). Cleckley in discussing a core feature of psychopaths commented “… it is unknown if they truly understand the meaning of their own words, a condition called semantic aphasia‘” (Cleckley, 1976, p 378). According to Cleckley, psychopaths are fundamentally deficient in the capacity for normal emotional experiences and exhibit a global awareness issue, which includes their genuine unawareness of the negative consequences of their own behavior on their victims. Cleckley‘s notion that psychopaths have a general failure in the ability to understand their own words bears partial similarity with the current findings that show that psychopaths inappropriately self-report their body sensations during a stressful task. In this context, our findings suggest that psychopaths may at least in part be characterized by a lack of fundamental somatic awareness - “somatic aphasia” - in response to the social stressor.
Although psychopaths had lower heart rate responses than the controls, as can be seen in Figures 1 and 2, the two groups showed comparable variance in their autonomic measures at each time point. Therefore, it is unlikely that the somatic aphasia in psychopaths was mainly due to their lack of reactivity to social stressor. An important question concerns how this somatic aphasia predisposes to psychopathy. According to Damasio, the disconnection between the mind and body is part of the causal chain that leads to psychopathy (Antonio R. Damasio, 1994). The neural circuit believed to underlie somatic markers, including the insula, amygdala, and the medial prefrontal cortex, has been found to be critically involved in the detection of emotional significance and the generation of negative affective states in response to emotional faces, pictures, and recall of personal life events (Phan et al., 2005), which in turn have been found to be disturbed in offenders with psychopathic traits (Kiehl et al., 2001). Consequently, this disruption in appropriate sensing of body reactions may indicate dysfunction of this cortical-subcortical network, which partly contributes to the inaccurate perception and interpretation of emotion in psychopathic individuals. One caveat is that Damasio‘s model is tagging emotional valence in general, whereas our study only focuses on negative emotion in a socially stressful condition. Future studies may examine if this somatic aphasia in psychopathy is specific to negative affect in particular, or generalizes to broader emotional events, including those with a positive valence.
As predicted, psychopaths‘ verbal reports of body sensations were not significantly different from those of nonpsychopathic controls. Psychopaths may describe emotions that they think that they should feel based on exteroceptive cues, although they do not consciously experience such cues. Essentially, psychopaths are as aware as others of the social demand characteristics of a given situation – in this case a laboratory “stressor” which the average person would expect to result in emotional stress. While psychopaths may subjectively express emotions such as remorse for their acts in verbal terms when expected to do so, they are viewed as lacking true experience of such emotions and hence sincerity (Hare, 2003).
An important question concerns whether the mind-body disconnection in psychopaths as indicated by the failure of their subjective reports of body sensations to match heart rate change to the stressor is a simple function of the fact that many psychopaths are characterized by pathological lying. Such pathological lying would be expected to result in a null association between reported body sensations and objectively-measured autonomic changes. If this were the case, controlling for this pathological lying feature of psychopathy should nullify the effect. However, findings remained significant after controlling for pathological lying. In addition, the finding that psychopaths scored significantly higher than the nonpsychopathic controls on the self-reported crime provides further evidence that the subjective report of body sensations are trustworthy. This suggests that a genuine mind-body disconnection is present in psychopaths, rather than some artifact of pathological lying. Psychopaths seem genuinely unable to appropriately appreciate their body sensations in response to emotional events.
Our findings suggest that the autonomic-body sensation mismatch is particularly associated with the interpersonal-affective factor of psychopathy. Interpretation of this link must proceed cautiously, however, because this significant association was only observed when the skin conductance-derived mismatch score was used. It is worth noting that it is not uncommon to observe discrepant findings when using different autonomic measures such as heart rate and skin conductance (Ishikawa et al., 2001; Wang, Baker, Gao, Raine, & Lozano, in press). This may be due to the distinctive neuroanatomical mechanisms involved in the autonomic measure in question. For example, skin conductance is solely controlled by the sympathetic nervous system, whereas heart rate reflects the complex interactions between sympathetic and parasympathetic nervous system activity (Hugdahl, 2001). Nevertheless, the finding of a mismatch between skin conductance levels and verbal report of body sensation is consistent with prior studies showing reduced skin conductance responses both when anticipating and reacting to aversive stimuli in psychopathic individuals (Birbaumer et al., 2005; Flor et al., 2002; Wang et al., in press). Future studies with larger sample size are needed to replicate and extend our findings in other autonomic domains.
Limitations
Limitations of this study include the fact that the sample consisted only of men, and findings may not generalize to psychopathic women. Group sizes were modest, although correlational analyses on the larger sample suggested a similar pattern of findings. This study‘s PCL-R cutoff of 23 is somewhat lower than that recommended for incarcerated psychopaths (Hare, 1991). However, when we reconducted the main analyses after using the recommended cutoff of 30, none of the findings changed significantly.3 Given that there is a paucity of information available to assess psychopathic traits in community sample, additional information including records of institution disciplinary actions, medical records, psychiatric reports, and police reports with both detailed description of offenses as well as victim statements shall be considered in future studies to obtain a full picture of their psychological symptomatology and behavior patterns. We also caution that the psychopathy by body sensations group interaction was observed for heart rate but not for skin conductance, although the skin conductance-derived mismatch score was positively and significantly correlated with Factor 1 psychopathy scores. Nevertheless, the psychopathy by body sensation interaction effect for heart rate changes seems in parallel with the vast findings of lower heart rate activity/reactivity in antisocial populations in general (Ortiz & Raine, 2004). Finally, we focused on negative affect in a social stressor, so it is unknown whether the same autonomic-body sensation mismatch generalizes to emotional experiences with a positive valence. It has been found that psychopathic individuals do not show emotional deficits in response to neutral or positive stimuli (Blair, Budhani, Colledge, & Scott, 2005; Dadds et al., 2006; Kimonis, Frick, Fazekas, & Loney, 2006), we therefore hypothesize that this autonomic-body sensation mismatch does not generalize to these conditions. Further research is needed to test this hypothesis.
In summary, the present study examined self-report and psychophysiological assessment of emotional experiences in a community sample at high risk for psychopathy. In contrast to nonpsychopathic controls, psychopathic individuals showed a verbal-autonomic inconsistency, and this mind-body disconnection is particularly associated with the interpersonal-affective factor of psychopathy. Although this deficit has never been overly discussed in psychopathy, our findings, together with some preliminary evidence from prior studies, suggest that somatic aphasia the inaccurate identification and recognition of one‘s own somatic states – may indicate a deficient somatic marker in these individuals and partly contribute to their affective deficiency.
Highlights.
In contrast to nonpsychopathic controls, psychopathic individuals are not accurately aware of their body states as objectively measured by heart rate changes during the social stressor.
The mind-body disconnection is particularly associated with the interpersonal-affective factor of psychopathy.
Findings suggest that somatic – aphasia the inaccurate identification and recognizing of one’s somatic states – may partly underlie the emotional/affective impairment in psychopaths.
Acknowledgments
This study was supported by a grant to the second author from the National Institute of Mental Health (K02 MH01114).
Footnotes
To examine the degree to which the single item of “heart pounding” contributes to the effects, further analyses were conducted. After removing this item from the body sensation composite, the results remained the same.
Similar associations were observed when the “perspiration” item was removed from the body sensation composite.
Because of space limitations, full statistics are not presented here but are available on request.
Financial Disclosures
Drs. Yu Gao, Adrian Raine, and Robert A. Schug report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopaths. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. Journal of Child Psychology and Psychiatry. 2005;46:327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal. A case of 'acquired sociopathy'. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Colledge E, Mitchell DGV. Somatic markers and response reversal: Is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? Journal of Abnormal Child Psychology. 2001;29:499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Znoj H, Siddique HI, Horowitz MJ. Verbal-autonomic dissociation and adaptation to midlife conjugal loss: a follow-up at 25 months. Cognitive Therapy and Research. 1999;23:605–624. [Google Scholar]
- Cleckley HC. The mask of sanity. St. Louis: Mosby; 1976. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, Abeygunawardane AI. Attention to the eyes and fear-recognition deficits in child psychopathy. British Journal of Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error: Emotion, reason, and the human brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Damasio AR. A neural basis for sociopathy. Archives of General Psychiatry. 2000;57:128–129. [Google Scholar]
- Elliott DS, Ageton SS, Huizinga D, Knowles BA, Canter RJ. Prevalence and incidence of delinquent behavior: 1976–1980 (National Youth Survey, Report No. 26) Boulder, CO: Behavior Research Institute; 1983. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. User's guide for the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User's guide for the Structured Clinical Interview for DSM-IV Axis I Disorders - Clinical Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: Peripheral and central correlates. Psychophysiology. 2002;39:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Gao Y, Baker L, Raine A, Wu H, Bezdjian S. Brief report: Interaction between social class and risky decision-making in children with psychopathic tendencies. Journal of Adolescence. 2009;32:409–414. doi: 10.1016/j.adolescence.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised (PCL-R) 2. Toronto, Canada: Multi-Health Systems; 2003. [Google Scholar]
- Hugdahl K. Psychophysiology The mind-body perspective. Cambridge, Massachusetts: Harvard University Press; 2001. [Google Scholar]
- Ishikawa SS, Raine A, Lencz T, Bihrle S, LaCasse L. Autonomic stress reactivity and executive functions in successful and unsuccessful criminal psychopaths from the community. Journal of Abnormal Psychology. 2001;110:423–432. doi: 10.1037//0021-843x.110.3.423. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50(9):677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Fazekas H, Loney BR. Psychopathy, aggression, and the processing of emotional stimuli in non-referred girls and boys. Behavioral Sciences and the Law. 2006;24:21–37. doi: 10.1002/bsl.668. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Steuwerald BL, Forth AE, Kirkhart KJ. A new method for assessing the interpersonal behavior of psychopathic individuals: Preliminary validation studies. Psychological Assessment. 1997;9:89–101. [Google Scholar]
- Lösel F, Schmucker M. Psychopathy, risk taking, and attention: A differentiated test of the somatic maker hypothesis. Journal of Abnormal Psychology. 2004;113:522–529. doi: 10.1037/0021-843X.113.4.522. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Colledge E, Leonard A, Blair RJR. Risky decisions and response, reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;40:2013–2022. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of American Academy of Child and Adolescent Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Getting to the heart of psychopathy. In: Herve H, Yuille JC, editors. Psychopathy: Theory, research, and social implications. Hillsdale, NJ: Lawrence Erlbaum Associates; 2007. pp. 207–252. [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural sustrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, Colletti P. Hippocampal structural asymmetry in unsuccessful psychopaths. Biological Psychiatry. 2004;55:185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Taylor K, Hellige HB, Bihrle S, LaCasse L, et al. Corpus callosum abnormalities in psychopathic antisocial individuals. Archives of General Psychiatry. 2003;60:1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- Schmitt WA, Brinkley CA, Newman JP. Somatic markers and response reversal: Is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? Journal of Abnormal Psychology. 1999;108:538–543. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- van Honk J, Hermans EJ, Putman P, Montagne B, Schutter DJLG. Defective somatic markers in sub-clinical psychopathy. NeuroReport. 2002;13:1025–1027. doi: 10.1097/00001756-200206120-00009. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Wang P, Baker LA, Gao Y, Raine A, Lozano DI. Psychopathy and physiological responses to aversive stimuli in children aged 9–10 years. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-011-9606-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. Journal of Abnormal Psychology. 2010;119(3):546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry. 2005;57:1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]