Abstract
Probenecid is a highly lipid soluble benzoic acid derivative originally used to increase serum antibiotic concentrations. It was later discovered to have uricosuric effects and was FDA approved for gout therapy. It has recently been found to be a potent agonist of Transient Receptor Potential Vanilloid 2 (TRPV2). We have shown that this receptor is in the cardiomyocyte and report a positive inotropic effect of the drug. Using echocardiography, Langendorff and isolated myocytes, we measured the change in contractility and, using TRPV2−/− mice, proved that the effect was mediated by TRPV2 channels in the cardiomyocytes. Analysis of the expression of Ca2+ handling and β-adrenergic signaling pathway proteins showed that the contractility was not increased through activation of the β-ADR. We propose that the response to probenecid is due to activation of TRPV2 channels secondary to SR release of Ca2+.
Keywords: TRPV2, probenecid, echocardiography, inotropic agent
1. Introduction
Probenecid is a highly lipid soluble benzoic acid derivative developed by Sharp and Dohme [1] to decrease the renal tubular excretion of penicillin [1, 2, 3, 4, 5, 6, 7] and since then has been used to increase the serum concentration of several antibiotics and antivirals [8]. It was also found to be a competitive inhibitor of active transport processes in the brain [9], liver [10] and eye [11] and was studied in these fields; however a clinical use was not established outside of its renal effects.
Similar to other drugs which were developed to increase serum levels of antibiotics, probenecid was initially administered via slow intravenous infusion, which caused local irritation. Subsequently, probenecid was found to be rapidly absorbed following oral administration with peak serum concentrations occurring in 1 to 5 hours [3].
During the initial studies using probenecid (referred to as benemid), it became clear that probenecid had a strong uricosuric effect; this was similar to, but greater than its predecessor carinamide, and it quickly became the standard treatment for gout. Roch-Ramel et al. discovered that probenecid decreased uric acid levels in the serum via inhibition of organic acid reabsorption in the renal proximal tube by acting as a competitive inhibitor of the organic anion transporter (OAT) and thus preventing OAT-mediated reuptake of uric acid from the urine to the serum [12]. Due to its capacity as an OAT inhibitor and its minimal adverse effect profile [5, 6, 13, 14, 15] it was studied for other indications including depression and glaucoma, though it was never approved for such uses. Therefore, its clinical use has declined significantly as other therapies for gout have shown better efficacy [16].
Research interest in probenecid has recently increased with the observation that it is a potent and selective agonist of Transient Receptor Potential Vanilloid 2 (TRPV 2) channels [17]. The Transient Receptor Potential (TRP) family of channels has been studied for many years in the nephrology and neurology literature. Several TRPs have also been shown to be important mediators of vascular tone (TRPC1, TRPC6 and TRPM4), cerebral blood flow (TRPM4), neointimal hyperplasia (TRPC1) and pulmonary hypertension (TRPC6) [18]. Until recently, only a few of the channels in this family have been found to have direct cardiac effects; (e.g. TRPC3/6/7 in the development of cardiac hypertrophy in response to pressure overload [19]). With regards to the TRPV family, some members have been identified to carry a direct cardiac effect. The first study was reported by Iwata and colleagues who found that cardiac specific overexpression of TRPV2 (published as the Ca2+-permeable growth factor-regulated channel) resulted in chamber dilation of all cavities of the murine heart [20]. This study, however, did not address whether endogenous TRPV2 plays any role in the heart. Subsequently, Huang et al. [21] discovered that TRPV1−/− mice have an increased infarct size and a decreased survival rate after ligation of the left anterior descending artery in comparison to their WT littermates. Interestingly, several groups have found that TRPV1 activation with specific agonists results in protection against ischemia/reperfusion (I/R) injury [22, 23].
We performed a survey of murine and human myocardial tissue for expression of TRPV channels and established that TRPV2 was the highest of these expressed in whole heart samples and specifically in the left ventricle. This finding led us to study the cardiac effects of probenecid on the whole animal, isolated whole heart and the isolated ventricular myocyte with the hypothesis that it can modulate myocardial function.
2. Methods
2.1 Animals
All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati and in accordance with the Guide for the Care and Use of Laboratory Animals (NIH, revised 1996). All wild type (WT) mice (B6129SF2/J F2 and C57BL6J, Jackson laboratories) and TRPV2−/− mice (breeding pairs provided by Dr. M. Caterina, John’s Hopkins, Baltimore, MD) were males at 12-16 weeks of age [24].
2.2 In vivo studies
2.2.1 Studies of contractility with intravenous administration of probenecid
Echocardiographic evaluation
In order to obtain a dose response curve, male C57 WT (n= 39) mice 12-16 weeks of age were anesthetized with isoflurane while intravenous jugular access (IV) was obtained under a microscope as previously described [34]. Subsequently, an echocardiographic study with both M-mode and B-mode was obtained in parasternal long axis (PSLAX) as described below. Either saline or different doses of probenecid (increasing from 2 to 200mg/kg) were injected (bolus IV) for the initial contractility studies in WT mice.
Invasive evaluation
Once a dose range was established, a separate group of WT mice were anesthetized with an intraperitoneal injection of ketamine (50 μg/g) and inactin (thiobutabarbital, 100 μg/g, Sigma, MA). A tracheotomy was performed (PE-90), and body temperature was monitored and maintained with a feedback-controlled heating table. The right femoral artery was cannulated with fluid-filled polyethylene tubing for measurement of blood pressure and connected to a low compliance pressure transducer (COBE Cardiovascular, Arvada, CO). The right femoral vein was cannulated for delivery of drugs. A high fidelity, 1.2-French SciSence pressure catheter (SciSence, London, ON, Canada) was inserted into the right carotid artery and advanced into the left ventricle to monitor cardiac performance. ECG leads were placed on the right and left arms, and left leg and connected to a BIOAmp (AD Instruments, Colorado Springs, CO). For carotid blood flow measurements, the left carotid artery was isolated and fitted with a 0.5-PSB perivascular flow probe connected to a TS420 flowmeter (Transonic Systems, Ithaca, NY). Experimental solutions of 100μg/μl probenecid were delivered as a bolus via the femoral vein catheter at 30 and 100 mg/kg with 5 minutes between each dose. Hemodynamic variables were collected and analyzed using a MacLab 4/S system (AD Instruments, Colorado Springs, CO) and Chart software.
2.2.2 Contractility studies with WT, TRPV2+/− and TRPV2−/− mice
Based on the results of the above experiments, we determined that the dose of 100mg/kg of probenecid gave a maximum contractility response. We injected the probenecid intraperitoneal (IP) to decrease the possible stress effects of surgery. WT, TRPV2+/−, and TRPV2−/− mice were monitored by echo for 30 minutes after injection as described below.
2.2.3 Echocardiography
All echocardiographic studies were performed with a Vevo 2100 Ultrasound system (Visualsonics, Toronto CA) with an MS400 probe (30 MHz centerline frequency) and were post-processed at a separate workstation with Vevostrain software (Vevo 2100, v1.1.1 B1455, Visualsonic, Toronto, Canada). Images were obtained from PSLAX and short axis (SAX) views at depths between 2 and 10 mm in both M-mode and B-mode. All studies on mice exposed to I/R injury included M-mode, B-mode in PSLAX and strain imaging in SAX. While for the contractility studies, only M-mode measurements were obtained from the PSLAX. Strain imaging was performed from the B-mode images and regional radial strain and circumferential displacement were measured by regional wall and summed average from the SAX images. From the M-mode images, left ventricular cavity size and wall thickness was measured and the ejection fraction (EF) and fractional shortening (FS) calculations were obtained using the Vevo software.
The change in the EF, FS, as well as strain derived parameters was obtained by subtracting the baseline value from each individual subject against subsequent time-points. In addition, the average change from baseline for each time point between 5 and 30 minutes was determined for each mouse, with measurements being taken every 5 minutes. These averages were compared between the different groups.
2.2.4 In vivo electrophysiology
Electrocardiographic data was obtained during all echocardiographic studies. These studies were subsequently analyzed by an independent, blinded reader at all time points to evaluate for electrocardiographic changes and drug induced arrhythmias. The following parameters were measured: PR interval, RR interval, QRS width and were reported as peak change while the presence of supraventricular or ventricular arrhythmias was measured as a total observed over all images obtained in 30 minutes.
2.3 Ex vivo (Langendorff) studies
Isolated heart experiments were performed as previously described [26, 27] on WT mice. After hearts achieved steady-state with pacing at 400bpm, probenecid (10−6M) was perfused into the heart using a syringe pump and continuous perfusion for up to 5 minutes. Measurements were taken every second. After the 5 minute perfusion of probenecid, the hearts were removed from the cannula and flash frozen in N2 for western blot analysis.
2.4 Molecular Studies
2.4.1 Quantitative RT-PCR
Hearts (LV) obtained for RNA isolation and qRT-PCR from WT, TRPV2 +/− and TRPV2−/− mice were flash frozen and stored at −80°C. For assessment of TRPV2 transcript levels, total RNA was isolated (RNeasy kit; Qiagen, Valencia, CA) and cDNA synthesized (high capacity RNA-to-cDNA kit; Applied Biosystems, Carlsbad, CA) per manufacturer’s instructions, using the C-terminal located primers 5′-CTACTGCTCAACATGCTC-3′ (sense) and 5′-CTCATCAGGTATACCATCC-3′ (antisense) which generate a 198 base pair product. All samples were performed in triplicate with a minimum of 3 independent experimental replicates with expression differences calculated using the delta-delta Ct approximation method with 18S mRNA as a loading control [28]. Corrections for primer efficiency were made where appropriate using the Pfaffl Method [29].
2.4.2 Western Blot Analysis
WT hearts used for protein expression were isolated from control mice (C57, 12-16 weeks old) from the Langendorff experiments. Total protein for calcium handling proteins was isolated by homogenizing whole hearts in an ice-cold buffer containing (in mM): 80 Imidazole, 300 sucrose, 1 DTT, 10 sodium metabisulfite, the protease inhibitor cocktail P8340 (Sigma, St. Louis, MO) and the phosphatase inhibitor cocktail set II (EMD, Merck, Darmstadt, Germany). Protein concentrations were determined using the BCA Protein Assay kit (Pierce, Thermo Scientific, Rockford, IL).
Aliquots of protein were separated on SDS-PAGE gels (Novex gels, Invitrogen, Eugene, OR) and then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and blocked with 5% BSA. The membranes were analyzed with primary antibodies. When appropriate, the membranes were cut in half to allow two different primary antibodies to be used. The top half would be used for one of the Ca2+ handling proteins and the bottom half (less than 60kD) would be used for the normalization protein, actin. In circumstances where it wasn’t appropriate to cut the membrane, as the two proteins were too close in size, the membranes were stripped after the first protein. Membranes were also stripped between primary antibodies for phosphorylated proteins and corresponding total protein. Table 1 shows the amount of each protein loaded, size of the protein, dilution and the manufacturer.
Table 1.
Primary antibodies for western blot analysis
| Primary antibody |
Molecular Weight (kD) |
Protein loaded/ well (mg) |
Dilution | Manufacturer |
|---|---|---|---|---|
| NCX | 112-116 | 50 | 1:500 | Swant, Bellinzona, Switzerland |
| SERCA2a | 110 | 50 | 1:1000 | Millipore, Billerica, MA |
| PLN | 25 (pentamer) 5 (monomer) |
20 | 1:5000 | Thermo Scientific, Rockford, IL |
| p-PLN (S-16) | 25 (pentamer) 5 (monomer) |
20 | 1:1000 | Millipore, Billerica, MA |
| RyR2 | 565 | 50 | 1:500 | Thermo Scientific, Rockford IL |
| p-RyR2 | 565 | 50 | 1:5000 | Badrilla, Leeds, UK |
| actin | 42 | N/A | 1:10,000 | Sigma, Waltham, MA |
NCX = Sodium calcium exchanger; SERCA2a = Sarco/endoplasmic reticulum Ca2+-ATPase 2a; PLN = Phospholamban; p-PLN = phosphorylated Phospholamban, at Serine 16; RyR2 = Ryanodine receptor 2; p-RyR = phosphorylated Ryanodine receptor 2, at Serine 2808.
2.5 Isolated myocytes, calcium uptake and handling
2.5.1 Isolation of ventricular myocytes
Hearts from WT mice (12-week old) were perfused on the Langendorff system with a modified Krebs-Henseleit buffer (KHB) composed of (mM) NaCl 118, KCl 5.4, HEPES 10, NaH2PO4 0.33, MgCl2 2, glucose 10, taurine 30, butanedione monoxime 10 (pH=7.4). Then an enzyme solution (KHB containing 0.7mg/ml type II collagenase, 0.1% BSA and 25μM CaCl2) was used to digest the hearts for 10min. Finally, ventricles of the hearts were excised, minced and filtered to obtain isolated cells. After sufficient sedimentation, the supernatant was removed and the cells were resuspended.
2.5.2 Myocyte contractility measurement
Myocytes were put on a plexiglass chamber containing a Tyrode’s solution composed of (mM) NaCl 140, KCl, 5.4, MgCl2 1, CaCl2 1.8, HEPES 5, glucose 10 (pH=7.4). Myocytes were excited under 0.5Hz field stimulation. After reaching the steady state, myocyte shortening and the rates of shortening (+dL/dt) and relengthening (−dL/dt) [30] were imaged with a CCD camera and monitored by a video-edge detector. Drugs were dissolved in Tyrode’s solution and perfused into the chamber. Data were continuously collected through the PCLAMP 9 software.
2.5.3 Myocyte Ca2+ transient and cytosolic Ca2+ measurement
Myocytes were loaded with Fluo-4 acetoxymethyl ester (7 μM) for 20 min at room temperature, followed by a 10 min wash with Tyrode’s solution, and then placed onto a plexiglass chamber for recording. For Ca2+ transient measurements, myocytes were excited under 0.5Hz and 3 Hz field stimulation, and the fluorescence signals were obtained using a Nikon TE 2000 microscope and collected through an InCyt Standard photometry system. For cytosolic Ca2+ analysis in quiescent myocytes, a Zeiss LSM 510 confocal microscope was used to record fluorescent images from resting myocytes. Line-scan mode was applied at 3.07ms intervals with 512 pixels spaced at 0.056μm. Drugs were dissolved in Tyrode’s solution and perfused into the chamber. Images of myocytes were recorded every 30s after drug application. Data analysis was performed using Clampfit 9.2 and Image J 1.44 software.
Water soluble probenecid (Molecular Probes, Life Technologies, Eugene, OR) was used for all of the myocyte experiments.
2.5.4 Electrophysiological recordings
Isolated myocytes were perfused with a Na+- and K+-free solution containing (mM): tetraethylammonium chloride (TEA-Cl) 137, CsCl 5.4, CaCl2 2, MgCl2 1, HEPES 5, glucose 10 and 4-aminopyridine 3 (pH=7.4). Whole-cell patch clamp recordings were performed with an Axopatch-1B amplifier. For Ca2+ current recordings, glass pipettes were filled with solution containing (mM): aspartic acid 115, CsOH 115, CsCl 20, EGTA 11, HEPES 10, MgCl2 2.5, Na-GTP 0.1, Mg-ATP 2 (pH adjusted to 7.2 with CsOH). Probenecid was dissolved in a Na+- and K+-free solution which was used to perfuse the myocytes. Data were collected using pCLAMP9 software through an Axon Digidata 1322A data acquisition system. All experiments were performed at room temperature (24°C).
2.6 Statistical Analysis
All data are expressed as means ± standard error of the mean (SEM). Results were analyzed with a paired and unpaired Student’s t-test and one-way ANOVA as needed. P values ≤0.05 were considered significant. Power analysis was employed to determine the group size necessary to determine whether significant differences exist between endpoint measures in control and experimental groups as previously described [25]. SigmaPlot was used to produce a best fit line and calculate an EC50 for the dose response curves.
3. Results
3.1 Probenecid increases in vivo contractility in WT but not TRPV2−/− mice
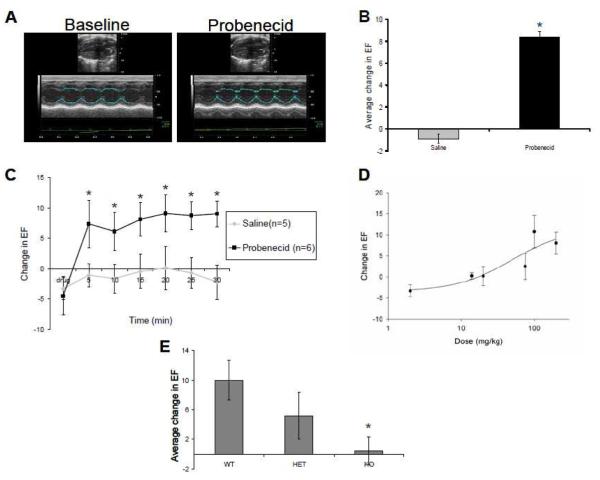
Administration of probenecid to WT mice resulted in increased contractility as measured via EF relative to EF in control mice given saline (Figure 1A). The increased contractility was noted within 5 minutes of the bolus injection with all doses at or above 75 mg/kg (peak change of 5.26±3.35, 8.40±2.80, 7.32±2.52 for 75mg/kg, 100mg/kg and 200mg/kg, respectively) (Figure 1B and 1C, data not shown for 75 and 100 mg/kg). The measured change in contractility as measured at 5 minute intervals (for 30 minutes total) revealed a dose dependent increase in contractility with an estimated EC50 of 49.33 mg/kg (Figure 1D). The EF remained at an elevated state for at least 1 hour on subjects (n=5, dose of 200 mg/kg IV) that were evaluated for a longer period of time (average increase in EF over baseline of 8.9±2.57, data not shown).
Figure 1.
Echocardiographic data. A. Representative B-mode and M-mode from long axis views for baseline and after administration of probenecid 200mg/kg IV. B. Average change (between 5 and 30 minutes) in EF from baseline after IV administration of saline and 200mg/kg probenecid, *P<0.05. C. Time course of the change in EF following IV administration of 200mg/kg probenecid, *P<0.05. D. Dose-dependent changes in EF after injection of various different concentrations of probenecid, measurements were taken every 5 minutes and the change from baseline was average form 5 to 30 minutes. E. Average change in EF following IP administration of 100mg/kg probenecid for wild type (WT), TRPV2+/− (HET) and TRPV2−/− (KO), *P<0.05.
Probenecid administered to the TRPV2+/− mice increased contractility, though the peak change in contractility was only 49% of that observed with the same dose in the WT mice (Figure 1E). Furthermore, when probenecid was administered to the TRPV2−/−, there was no discernable cardiac response noted during the 30 minutes of echocardiogram measurements.
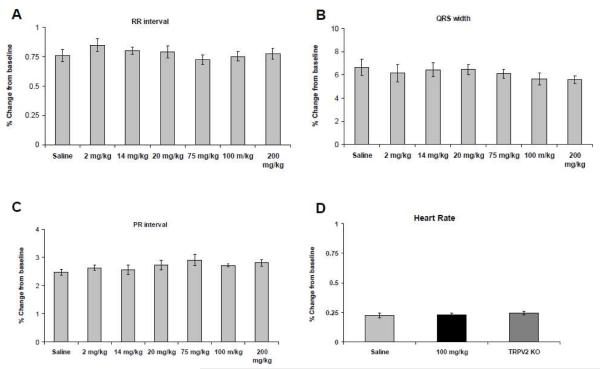
3.2 Probenecid does not cause any measurable changes in electrical conduction in vivo mice
Commonly used inotropes result in varied arrhythmias [31, 32]. We did not detect any supraventricular or ventricular arrhythmias or premature atrial or ventricular beats seen after any dose of probenecid administered in WT mice. Furthermore, at all doses there were no measured changes in any conduction intervals (Figure 2A, B and C), and there were no significant differences noted between probenecid (100 mg/kg) IP in WT and TRPV2−/− mice (Figure 2D).
Figure 2.
Electrocardiographic variables measured after various doses of probenecid were administered, A. RR interval, B. PR interval, C. QRS width and D. Heart rate. There were no statistically significant differences between any of the doses or groups.
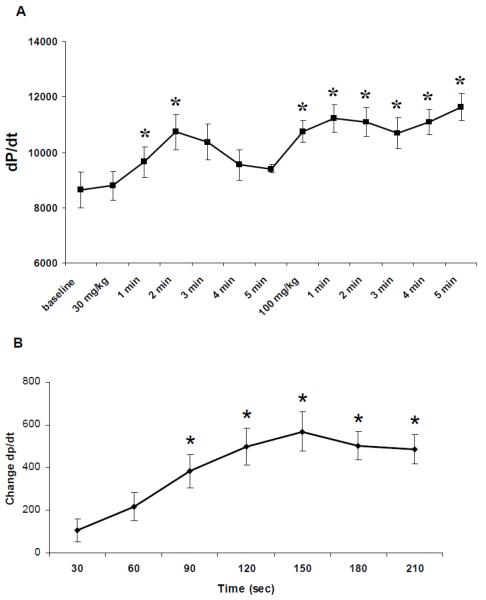
3.3 Probenecid increases contractility in in vivo hearts and in Langendorff perfused hearts
Invasive measurements of +dP/dt demonstrated an effect similar to the Langendorff perfused hearts which were used to measure the cardiac specific effects of probenecid (10−6 M) on contractility. We found that administration of probenecid in vivo at both a low (30 mg/kg) and high (100 mg/kg) dose rapidly increased the peak +dP/dt (Figure 3A). The changes in average +dP/dt over time in Langendorff perfused hearts are shown in Figure 3B. This change was found to be statistically significant from 90 to 210 seconds and reached a steady state between 120 and 180 seconds (Figure 3B). Relaxation (-dP/dt) was also affected at the same concentration of probenecid, increasing from 1812±158 to 2309±133 (P<0.01). After treatment with probenecid, the hearts were flash frozen to be used for other experiments.
Figure 3.
In vivo measurement and Langendorff perfused measurements of +dP/dt A. +dP/dt after administration of 30 mg/kg and 5 minutes following 100 mg/kg of probenecid IV, *P<0.05. B. Change in +dP/dt during perfusion of 10−6 probenecid, 90-210 sec are all significantly different from baseline, *P<0.05.
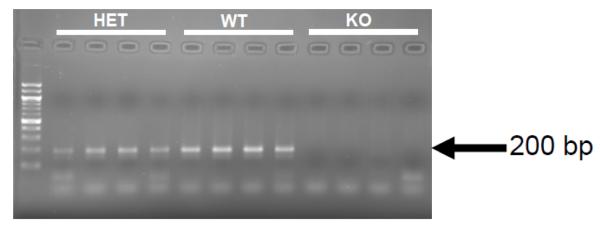
3.4 TRPV2 mRNA is found in cardiac tissue
Quantitative real time PCR from WT, TRPV2+/− and TRPV2−/− mice revealed a decrease in expression from WT to TRPV2+/− mice, with no count detected for the TRPV2−/− mice. PCR products from the qRT-PCR were run on a 2% agarose gel to verify specificity and again found expression at approximately 200 bp with no expression in the TRPV2−/− and decreased in the TRPV2+/− (Figure 4).
Figure 4.
Quantitative RT-PCR product from mRNA isolated from wild type (WT), TRPV2+/− (HET) and TRPV2−/− (KO) mouse hearts.
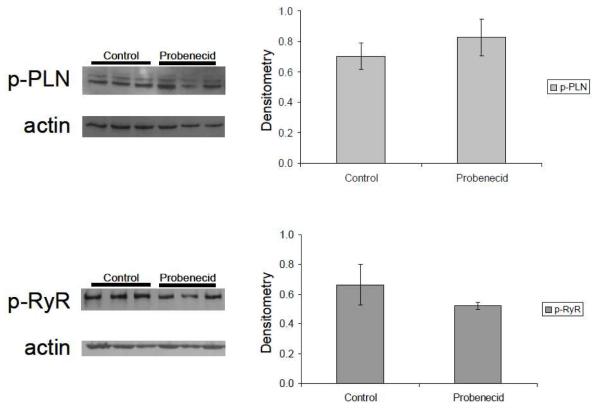
3.5 Expression levels of calcium handling proteins are unchanged after probenecid administration
Using the hearts isolated from the Langendorff procedure animals and age-matched control mouse hearts perfused with Tyrode’s solution, we assessed the expression of phospholamban (PLN) and the ryanodine receptor (RyR), phosphorylated and total protein, in the whole heart. Even though these hearts demonstrated an increase in contractility, we found no significant changes in the amount of phosphorylated or total phospholamban or ryanodine receptor in these hearts (Figure 5A and 5B) or in the ratio of p-PLN to t-PLN (Figure 5C). Furthermore, we investigated the sodium/calcium exchanger (NCX) and SERCA2a, to determine if there was another Ca2+-handling protein responsible for the increase in contractility, but we found no changes in the expression levels (data not shown).
Figure 5.
Western blot analysis of Ca2+ handling proteins. Phosphorylated Phospholamban (p-PLN) and phosphorylated Ryanodine receptor 2 (p-RyR2) did not show significant difference between saline in comparison to probenecid.
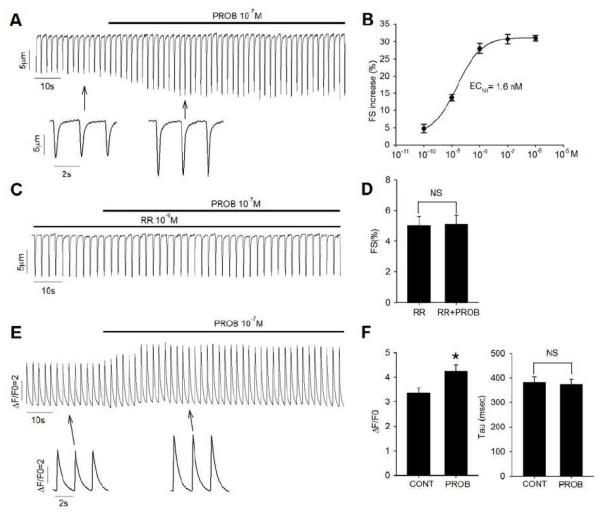
3.6 There is a dose-dependent increase of contractility by probenecid in isolated myocytes
We found that probenecid increased the contractility of isolated ventricular myocytes in a dose-dependent manner (Figure 6). Fractional shortening (FS) of myocytes was increased by 30.8 ± 1.4% (from 6.9% to 9.0%; n=6, P<0.01) when exposed to 0.1 μM probenecid at 0.5 Hz and room temp, and increased from 10.1% to 13.9% (n=4; P<0.01) at 3 Hz and 32°C; the dose-response curve for the action of probenecid had an EC50 of 1.6 nM (Figure 6A and 6B). Pretreatment of myocytes with ruthenium red, a non-selective blocker of TRPV2 channels, completely abolished probenecid’s effect on myocyte contractility (Figure 6C and 6D). In isolated myocytes, we measured the +dL/dt and −dL/dt). At room temperature, probenecid administration resulted in a change in maximum +dL/dt from 80.6±12.3 to 112.5±16.6 (n=10, P<0.01) while the −dL/dt increased from 57.7±7.4 to 77.6±8.7 μm/sec (P<0.01).
Figure 6.
Effect of probenecid on myocyte contractility. A. Representative contraction traces of ventricular myocytes upon exposure to 10−7 M probenecid (PROB). B. Dose-response curve of probenecid on myocyte contractility. Data points are averages from 4 mice and fitted to sigmoidal non-linear regression curve. C. Representative contraction traces of ventricular myocytes pretreated with 10−6 M ruthenium red (RR) and then exposed with 10−7 M probenecid. D. Average data on myocyte fractional shortening (FS) with ruthenium red pretreatment under control and 10−7 M probenecid exposure. E. Representative Ca2+ transient traces under field stimulation upon exposure 10−7 M probenecid. F. Average data on Ca2+ transient amplitude F/F0 (left) and time constant tau (right) under control and 10−7 M probenecid exposure. *P<0.001, NS P>0.2.
The effect of probenecid on the myocyte Ca2+ transient was also examined. Probenecid caused a significant increase in Ca2+ transient amplitude (F/F0) (Figure 6E and 6F), which was consistent with the fractional shortening increase found in myocytes. The concentration of 0.1 μM probenecid increased the Ca2+ transient amplitude (F/F0) from 3.35 ± 0.20 to 4.25 ± 0.25 (P<0.001), while the decay rate (Tau) of Ca2+ transient was not affected by probenecid treatment (Figure 6F).
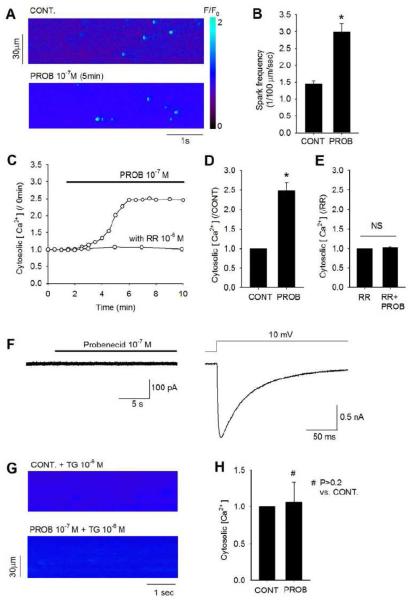
3.7 Cytosolic Ca2+ increase by probenecid in isolated myocytes
The effects of probenecid on cytosolic Ca2+ and SR Ca2+ release were further examined using confocal imaging. Probenecid (0.1 μM) caused a marked increase in SR release (as measured by Ca2+ spark frequency) as well as a gradual increase in cytosolic Ca2+ concentration (Figure 7A and 7B). On average, the spark frequency was increased two fold, from 1.45 ± 0.09 to 2.99 ± 0.24. The cytosolic Ca2+ began to increase after 1 minute and typically reached a peak at approximately 5 min after probenecid treatment (Figure 7C). Once steady state was achieved, cytosolic Ca2+ was increased 2.5 ± 0.2 fold under probenecid treatment compared to control (Figure 7D). When ruthenium red (1 μM) was applied to the myocytes, the increase of cytosolic Ca2+ by probenecid was fully blocked (Figure 7E). Patch clamp experiments indicate that probenecid does not trigger any significant transmembrane Ca2+ influx. No measurable inward Ca2+ current was detected when myocytes were held at −70 mV and exposed to 0.1 μM probenecid (Figure 7F, left). However, depolarization of the cell membrane from −70mV to +10 mV elicited a robust L-type Ca2+ current (Figure 7F, right). Increased SR Ca2+ release appeared to play a major role in generating the increased cytosolic Ca2+ level. Emptying of SR content with thapsigargin, a SERCA blocker, abolished the change in cytosolic Ca2+ by probenecid (Figure 7G and 7H).
Figure 7.
Effect of probenecid on myocyte cytosolic Ca2+. A. Images of confocal microscopic line-scan of cytosolic Ca2+ from a myocyte under control and upon exposure to 10−7 M probenecid for 5 minutes. A heat map is shown to indicate the F/F0 intensity (valued from 0 to 2). B. Average data on Ca2+ spark frequency under control and upon exposure to 10−7 M probenecid for 5 minutes. *P<0.001. C. Time courses of cytosolic Ca2+ in myocytes with and without 10−6 M ruthenium red (RR) pretreatment upon exposure to 10−7 M probenecid. All the data points are normalized to time 0 and probenecid was applied at 1 minute. D. Average data on myocyte cytosolic Ca2+ under control and 10−7 M probenecid exposure (for 5 minutes) *P<0.001. E. Average data on myocyte cytosolic Ca2+ pretreated with 10−6 M ruthenium red under control and 10−7 M probenecid exposure (for 5 minutes). NS: P>0.9. F. Patch clamp data from the same myocyte showing no inward Ca2+ current with 10−7 M probenecid treatment (left) and L-type Ca2+ current elicited by depolarization voltage step to +10 mV. The myocyte is held at −70 mV. G. Images of confocal microscopic line-scan of cytosolic Ca2+ from a myocyte pretreated for 15 minutes with 10−6 M thapsigargin (TG), before and after treatment with 10−7 M probenecid for 5 minutes. H. Average data on myocyte cytosolic Ca2+ under control and after 10−7 M probenecid treatment, in myocytes pretreated with 10−6 M thapsigargin.
4. Discussion
We have shown that probenecid, a well-tolerated FDA approved drug has positive inotropic properties which were previously not recognized, and present evidence that the effect is mediated by TRPV2 channels. This is a significant finding, and has immediate implications for patients with heart disease that are currently receiving probenecid in the therapy of gout. Potential use of probenecid in cardiac disease patients requires a thorough analysis regarding its mechanism(s) of action, dosing, target and off-target effects.
Our studies indicated that probenecid increased contractility in the mouse heart as determined by echocardiography and invasive measurements in a dose-dependent manner with the initial doses based on previously published work [33, 34]. However, these studies required the introduction of a jugular catheter which was traumatic to the animals and likely stimulated a sympathetic response. We then studied IP injection at a lower dose (100 mg/kg) and found it to have similar effects with a simpler method of administration (and therefore this method and dose was used for the remainder of the studies).
Probenecid has been described to cause transient increases of certain monoamines in the brain, CSF and plasma [35]. These studies suggested that probenecid therapy could raise the concentrations of 5-HIAA and HVA (acid metabolites of serotonin and dopamine, respectively) in dogs [36] and the levels of norepinephrine [37] or DOPAC (also an acid metabolite of dopamine) in humans [38, 39]. We performed a series of experiments to determine if the β-adrenergic (β-ADR) pathway was being stimulated by the administration of probenecid. We concluded that the increased contractility observed in the in vivo experiments occurred through a calcium dependent mechanism and not through β-ADR mediated phosphorylation of RyR or PLN. This is significant because β-ADR stimulation has been extensively described as having cardiotoxic effects resulting in increased infarct size [40] through increased oxygen demand [41], induction of cardiocyte apoptosis [42] and hypertrophy [43], while β-antagonism is known to be cardioprotective and safe in the treatment of heart failure [44, 45]. Hence, β-ADR stimulation of the heart (directly or indirectly) results in increased contractility but with increased ischemia and cell death and is considered injurious in patients with ischemic heart disease. We have also shown through ex vivo and in vitro experiments that the mechanism of action of probenecid (i.e. causing increased contractility) is not dependent on these pathways and can be potentially useful as a positive inotrope in patients with ischemic heart disease. Furthermore, a very thorough analysis of all electrocardiographic traces before, during and after administration of probenecid IP or IV on WT and TRPV2−/− mice demonstrated no arrhythmias and no significant changes in any of the measured electrocardiographic variables. This is in stark contrast with commonly used inotropes which result in significant arrythmias, including atrial tachycardia and AV conduction deficits (digoxin) and ventricular and supraventricular arrhythmias (dopamine, dobutamine, isoprotenelol) [31, 32].
While the initial in vivo experiments only describe an increase in contractility, and not a mechanism, we subsequently performed a series of Langendorff experiments in an attempt to separate the systemic from the cardiac specific effects of probenecid. These experiments showed an increase in contractility similar to that which was observed in vivo. We used 10−6M concentration as a series of preliminary experiments showed this dose to result in the most reproducible response. Furthermore, the fact that these experiments resulted in corresponding changes in contractility within a similar time frame (5 minutes in all cases) argues convincingly against a significant systemic increase in adrenergic drive as the main cause for the positive inotropism. We also found that the increase in contractility was associated with an increased rate of relaxation in isolated myocytes when we measured the rate of shortening (+dL/dt) and the rate of relengthening (−dL/dt), and even though they are not identical, they are comparable to rate of contractility (+dP/dt) and relaxation (−dP/dt) found in ex vivo studies.
Probenecid was identified as a TRPV2 agonist by Bang et al. in 2007 using TRPV2-expressing HEK293 cells. They performed fluo-3 AM calcium imaging experiments and discovered that probenecid elicited a significant increase in cytosolic Ca2+ in TRPV2-expressing cells but not in cells expressing other thermo-TRP channels, including TRPV1, TRPV3, TRPV4, TRPM8 and TRPA1 [17]. As a member of the thermoTRP channel family, TRPV2 is a Ca2+ selective channel activated by noxious heat (> 52°C) and activation of TRPV2 has been shown to lead to Ca2+ influx in neuron cells localized to dorsal root ganglia [46]. Based upon our early results and this literature, we hypothesized that, in cardiac myocytes, probenecid’s stimulatory effects on cytosolic Ca2+ and myocyte contractility involves activation of the TRPV2 channel. This hypothesis is supported by the ability of ruthenium red (RR), a blocker of TRPV2 channels, to fully abolish probenecid’s effects on both contractility and cytosolic Ca2+ level. RR has been established as a general antagonist for TRPV channels, acting by blocking their aqueous pores [47], however, it has also been shown to affect mitochondrial calcium uptake [48] and ryanodine receptor calcium release [49].
Having obtained TRPV2−/− mice [24], we performed functional experiments in vivo which showed that TRPV2+/− mice had an approximately 50% decrease in contractility, while TRPV2−/− mice had absolutely no change in function after exposure to probenecid (100 mg/kg). These findings argue for the necessity of TRPV2 receptors for increased contractility, but do not prove (as it is a whole body and not a cardiac specific knockout), that the effect is directly dependent on these receptors in the cardiomyocyte.
At the cellular level, we have shown that probenecid increased contractility in mouse ventricular myocytes, though we did not investigate the cardiac fibroblasts separately. TRPV2 has been found to be present and active in various types of fibroblasts [50], which may potentially play a role in the observed effects. We also found that probenecid increased the amplitude of the Ca2+ transients, and in quiescent myocytes we observed a gradual increase of the cytosolic Ca2+ concentration. Intriguingly, we did not detect any measurable inward Ca2+ current upon exposure to probenecid, suggesting that Ca2+ influx into the myocyte was not the direct source of the probenecid-triggered increase in cytosolic Ca2+ levels. Rather, enhanced SR Ca2+ release appeared to play a major role in the cytosolic Ca2+ increase. We found that probenecid markedly increased Ca2+ spark frequency and emptying the SR with a SERCA blocker abolished the effect of probenecid on cytosolic Ca2+.
The presence of TRPV2 channels in cardiac myocytes has been described by Iwata and colleagues [20]. They report that TRPV2 channels are present specifically in the intercalated discs (though less so in the peripheral sarcolemma and the cell interior) and that cell stretch increases the translocation to the sarcolemma. Muraki et al. described the channel in arterial myocytes of mice and, in a separate set of over-expression experiments, reported stretch induced activation [51]. Activation of TRPV2 channels may involve translocation from intracellular compartments to the cell membrane [50]. Researchers have found TRPV2 expressed in the intracellular stores of neuroendocrine cells [52] and in the endoplasmic reticulum of macrophages [53] and pancreatic β-cells [54]; all of whom reported potential translocation to the cell membrane under assorted conditions [55]. Our future planned studies will evaluate the possibility of TRPV2 translocation and activation with isolated myocytes at baseline and after various stimuli. We do not fully understand the mechanism underlying the effect of probenecid on SR Ca2+ release. One possible mechanism is that probenecid, via TRPV2 channels, results in a local increase in Ca2+ concentration and triggers the activation of nearby ryanodine receptors. Given that probenecid does not affect phosphorylation of either PLN or ryanodine receptors, our results are consistent with a direct action of probenecid on cellular Ca2+ content, which increased the Ca2+ release beat-to-beat, resulting in enhanced contractility. Further, the effect of probenecid that we describe is upon physiologic conditions and not in an overexpression mouse model that was previously described (and found to result in cavity dilation) [20].
As probenecid has been used for decades and in a variety of clinical scenarios, it would be fair to question why this effect hasn’t been described previously. As we have noted with the in vivo dose response experiments, the EC50 of 49.3 mg/kg is roughly equivalent to the higher limits of the currently accepted human dose of 1 to 3 grams of probenecid orally as it is highly bioavailable after an oral dose [56]. Interestingly, studies that describe an effect of probenecid on cardiac function were published in 1954 and 1955 and appear to have not been recognized at the time [13, 14]. In these studies, probenecid, still being referred to as benemid, was administered orally to patients with “uncomplicated congestive heart failure” and observed a strong diuretic effect (average diuresis of 2.7 liters per day). This study was based on a previous report of carinamide (a precursor to probenecid) that mentioned (but never proved) a potential diuretic effect along with a uricosuric effect [57]. The authors attributed the observed diuresis only to the renal aspect of probenecid and not to a potential cardiac effect. We scoured the literature, including the original FDA submissions for probenecid approval and found no other references or comments reporting an increase in diuresis specifically relating to probenecid (though several did report on its interactions with other diuretics) [58]. The occasional studies that refer to cardiac issues and probenecid are limited to its use in inflammation and infection but not direct effects on cardiac function [12].
5. Conclusion
We have identified a novel property of probenecid in enhancing calcium handling and myocardial contractility in the heart. The fact that we have done this with an FDA approved drug which has been used in thousands of patients and is known to have a limited adverse profile argues strongly for the potential clinical applicability of our finding. Furthermore, the fact that there is at least one previous study which documented improved diuresis in congestive heart failure implies a high likelihood that this effect is clinically relevant. We have also demonstrated a novel target for this action, TRPV2. TRP channels are ubiquitous in the mammalian species and have recently been studied in the immune and nervous system of humans, where probenecid has been found to be a strong agonist of TRPV2. There are only a few studies that have described members of the TRP family in the heart (specifically TRPC and TRPM) and their association with calcium handling and hypertrophy. To the best of our knowledge, we are the first to describe TRPV2 channels in the heart and believe that they (and related TRP channels) may constitute an important group of proteins for further study in animals and humans.
Research Highlights.
We describe transient receptor potential vanilloid 2 (TRPV2) in the murine heart
We have identified a novel property of probenecid as a positive inotrope
Probenecid does not cause a positive inotropic response in TRPV2−/− mice
Probenecid increases myocyte cytosolic calcium concentration
Probenecid does not cause electrophysiologic changes in the murine heart or myocyte
Acknowledgements
The authors would like to thank Evangelia Kranias PhD and Neal Weintraub MD for their helpful suggestions, guidance and critique during the development of this paper. We would also like to thank Michael Caterina MD PhD and his staff for supplying the breeding pairs for the TRPV2 −/− mice. Lastly, we wish to acknowledge the technical support of Myc Mcguinness, Donna Gering and Jackie Belew.
Funding
This study was supported by National Institute of Health Grants HL007382 (L.H. and M.T.), HL64018 (W.C.), HL26057 (W.C.), ES017263 (H-S.W), HL091478 (W.K.J.) and University of Cincinnati CCTST grant T1 UL1RR026314 (J.R.). Additional funding for this study was supported in part by the Cardiovascular Center of Excellence at the University of Cincinnati College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None declared.
References
- 1.Beyer RH, Wiebelhaus VD, Russe HF, Peck HM, McKinney SE. Benemid: An anticatabolite; its pharmacological properties. Fed Proc. 1950;9:258. [Google Scholar]
- 2.Beyer KH, Russo HF, Tillson EK, Miller AK, Verwey WF, Gass SR. Benemid,’ p-(di-n-propylsulfamyl)-benzoic acid; its renal affinity and its elimination. Am J Physiol. 1951;166(3):625–40. doi: 10.1152/ajplegacy.1951.166.3.625. [DOI] [PubMed] [Google Scholar]
- 3.Boger WP, Beatty JO, Pitts F, Flippin HF. The influence of a new benzoic acid derivative on the metabolism of para-aminosalicylic acid (PAS) and penicillin. Ann Intern Med. 1950A;33(1):18–31. doi: 10.7326/0003-4819-33-1-18. [DOI] [PubMed] [Google Scholar]
- 4.Boger WP, Pitts FW, Gallagher ME. Benemid and Carinamide: Comparison of effect on Para-amino-salicylic acid (PAS) Plasma Concentrations. J of Lab Clin Med. 1950B;36:276–82. [PubMed] [Google Scholar]
- 5.Boger WP, Strickland SC. Probenecid (Benemid): Its uses and side effects in 2502 Patients. AMA Arch Intern Med. 1955;95:83–92. doi: 10.1001/archinte.1955.00250070099012. [DOI] [PubMed] [Google Scholar]
- 6.Burnell JM, Kirby WMM. Effectiveness of a new compound, Benemid, in elevating serum penicillin concentrations. J Clin Invest. 1951;30:697–700. doi: 10.1172/JCI102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meads M, Knight VH, Izlar HR., Jr The enhancement of serum penicillin in man by benemid. South Med J. 1951;44(4):297–302. [PubMed] [Google Scholar]
- 8.Butler D. Wartime tactics doubles power of scarce bird-flu drug. Nature. 2005;438:6. doi: 10.1038/438006a. [DOI] [PubMed] [Google Scholar]
- 9.Korf J, van Praag HM. The intravenous probenecid test: a possible aid in evaluation of the serotonin hypothesis on the pathogenesis of depressions. Psychopharmacologia. 1970;18(1):129–32. doi: 10.1007/BF00402393. [DOI] [PubMed] [Google Scholar]
- 10.Kenwright S, Levi AJ. Impairment of hepatic uptake of rifamycin antibiotics by probenecid, and its therapeutic implications. Lancet. 1973;302(7843):1401–5. doi: 10.1016/s0140-6736(73)92799-2. [DOI] [PubMed] [Google Scholar]
- 11.Forbes M, Becker B. The transport of organic anions by the rabbit eye. II. In vivo transport of iodopyracet (Diodrast) Am J Ophthalmol. 1960;50:867–75. doi: 10.1016/0002-9394(60)90339-1. [DOI] [PubMed] [Google Scholar]
- 12.Roch-Ramel F, Guisan B. Renal Transport of Urate in Humans. News Physiol Sci. 1999;14:80–4. doi: 10.1152/physiologyonline.1999.14.2.80. [DOI] [PubMed] [Google Scholar]
- 13.Bronsky D, Dubin A, Kusher DS. Diuretic Action of Benemid: Its effects upon the urinary excretion of sodium, chloride, potassium, and water in edematous subjects. Am J Med. 1954;18:259–66. doi: 10.1016/0002-9343(55)90241-2. [DOI] [PubMed] [Google Scholar]
- 14.Kushner D, Dubin A, Bronsky D. Effect of Benemid on excretion of water, sodium and chloride in congestive heart failure. Fed Proc. 1954;13:435. [Google Scholar]
- 15.Reynolds ES, Schlant RC, Gonick HC, Dammin GJ. Fatal Massive Necrosis of the liver as a manifestation of hypersensitivity to Probenecid. N Engl J Med. 1957;256:592–6. doi: 10.1056/NEJM195703282561304. [DOI] [PubMed] [Google Scholar]
- 16.Rider TG, Jordan KM. The modern management of gout. Rheumatology. 2010;49:830. doi: 10.1093/rheumatology/kep306. [DOI] [PubMed] [Google Scholar]
- 17.Bang S, Kim KY, Too S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by Probenecid. Neuroscience Letters. 2007;435:120–5. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, et al. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–31. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA. 2010;107:7000–5. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–67. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W, Rubinstein J, Prieto AR, Thang LV, Wang DH. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension. 2009;53(2):243–50. doi: 10.1161/HYPERTENSIONAHA.108.118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda K, Tsuji F, Hirata T, Takaoka M, Matsumura Y. Preventive effect of TRPV1 agonists capsaicin and reiniferatoxin on ischemia/reprofusion-induced renal injury in rats. J Cardiovasc Pharmacol. 2008;51:513–20. doi: 10.1097/FJC.0b013e31816f6884. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–23. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- 24.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP Vanilloid 2 knockout mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J of Neurosci. 2011;31(32):11425–36. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren X, Wang Y, Jones WK. TNF-α is required for late ischemic preconditioning but not for remote preconditioning of trauma. J Surg Res. 2004;121:120–9. doi: 10.1016/j.jss.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Mundiña-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem. 1996;271:33561–7. doi: 10.1074/jbc.271.52.33561. [DOI] [PubMed] [Google Scholar]
- 27.Said M, Vittone L, Mundiña-Weilenmann C, Ferrero P, Kranias EG, Mattiazzi A. Role of dual-site phospholamban phosphorylation in the stunned heart from phospholamban site-specific mutants. Am J Physiol Heart Circ Physiol. 2003;285:H1198–205. doi: 10.1152/ajpheart.00209.2003. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, II, Walsh RA, et al. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest. 1996;97:533–9. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouvy ML, Heerdink ER, De Bruin ML, Herings RMC, Leufkens HGM, Hoes AW. Use of Sympathomimetic Drugs Leads to Increased Risk of Hospitalization for Arrhythmias in Patients With Congestive Heart Failure. Arch Intern Med. 2000;160:2477–80. doi: 10.1001/archinte.160.16.2477. [DOI] [PubMed] [Google Scholar]
- 32.Williamson KM, Thrasher KA, Fulton KB, LaPointe NMA, Dunham GD, Cooper AA, et al. Digoxin Toxicity: An Evaluation in Current Clinical Practice. Arch Intern Med. 1998;158:2444–9. doi: 10.1001/archinte.158.22.2444. [DOI] [PubMed] [Google Scholar]
- 33.Vamos E, Pardutz A, Fejes A, Tajti J, Toldi J, Vecsei L. Modulatory effects of probenecid on the nitroglycerin-induced changes in the rat caudal tribeminal nucleus. Eur J Pharmacol. 2009;621:33–7. doi: 10.1016/j.ejphar.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Vecsei L, Miller J, MacGarvey U, Beal MF. Kynurenine and probenecid inhibit pentylenetetrazol- and NMDLA-induced seizures and increase kynurenic acid concentrations in the brain. Brain Res Bull. 1992;28:233–8. doi: 10.1016/0361-9230(92)90184-y. [DOI] [PubMed] [Google Scholar]
- 35.Neff NH, Tozer TN, Brodie BB. Application of steady-state kinetics to studies of the transfer of 5-hydroxyindolacetic acid and from brain to plasma. J Pharmacol Exp Ther. 1967;158:214–8. [PubMed] [Google Scholar]
- 36.Guldberg HC, Ashcroft GW, Crawford TBB. Concentration of 5-hydroxyindolyacetic acid and homovanillic acid in the cerebrospinal fluid of the dog before and during treatment with Probenecid. Life Sci. 1966;5:1571–5. doi: 10.1016/0024-3205(66)91026-5. [DOI] [PubMed] [Google Scholar]
- 37.Lake CR, Wood JH, Ziegler MG, Ebert MH, Kopin IJ. Probenecid-Induced norepinephrine elevations in plasma and csf. Arch Gen Psychiatry. 1978;35:237–40. doi: 10.1001/archpsyc.1978.01770260115014. [DOI] [PubMed] [Google Scholar]
- 38.Gordon EK, Markey SP, Sherman RL, Kopin IJ. Conjugated 3,4 dihydroxyphenyl acetic acid (DOPAC) in human and monkey cerebrospinal fluid and rat brain and the effects of Probenecid. Life Sci. 1976;18:1285–92. doi: 10.1016/0024-3205(76)90206-x. [DOI] [PubMed] [Google Scholar]
- 39.Tamarkin NR, Goodwin FK, Axelrod J. Rapid elevation of biogenic amine metabolites in human csf following probenecid. Life Sci. 1970;9:1397–408. doi: 10.1016/0024-3205(70)90134-7. [DOI] [PubMed] [Google Scholar]
- 40.Maroko PR, Kjekshus JK, Sobel BE, Watanabe T, Covell JW, Ross J, Jr, et al. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971;43:67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- 41.Davidson S, Maroko PR, Braunwald E. Effects of isoproterenol on contractile function of the ischemic and anoxic heart. Am J Physiol. 1974;227(2):439–43. doi: 10.1152/ajplegacy.1974.227.2.439. [DOI] [PubMed] [Google Scholar]
- 42.Shizukuda Y, Buttrick PM, Geenen DL, Borczuk AC, Kitsis RN, Sonnenblick EH. beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol Heart Circ Physiol. 1998;275(3 Pt 2):H961–8. doi: 10.1152/ajpheart.1998.275.3.H961. [DOI] [PubMed] [Google Scholar]
- 43.Simspon P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983;72(2):732–38. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bangalore S, Messerli FH, Kostis JB, Pepine CJ. Cardiovascular Protection Using Beta-Blockers A Critical Review of the Evidence. J Am Coll of Cardiol. 2007;50:563–72. doi: 10.1016/j.jacc.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 45.Feldman D, Elton TS, Menachemi DM, Wexler RK. Heart rate control with adrenergic blockade: Clinical outcomes in cardiovascular medicine. Vasc Health Risk Manag. 2010;6:387–97. doi: 10.2147/vhrm.s10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci. 2010;30:4601–12. doi: 10.1523/JNEUROSCI.5830-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vriens J, Appendino G, Nilius B. Pharmacology of Vanilloid Transient Receptor Potential cation channels. Mol Pharmacol. 2009;75:1262–79. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- 48.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, et al. Mitochondrial calcium signaling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–60. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L, Lai FA, Cohn A, Etter E, Guerrero A, Fay FS, et al. Evidence for a Ca(2+)-gated ryanodine-sensitive Ca2+ release channel in visceral muscle. Proc Natl Acad Sci USA. 1994;91:3294–8. doi: 10.1073/pnas.91.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stokes AJ, Wakano C, del Carmen KA, Koblan-Huberson M, Turner H. Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. J Cell Biochem. 2005;94(4):669–83. doi: 10.1002/jcb.20331. [DOI] [PubMed] [Google Scholar]
- 51.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, et al. TRPV2 Is a Component of Osmotically Sensitive Cation Channels in Murine Aortic Myocytes. Circ Res. 2003;93:829–38. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 52.Boels K, Glassmeier G, Herrman D, Riedel B, Hampe W, Kojima I, et al. The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci. 2001;114(Pt. 20):3599–606. doi: 10.1242/jcs.114.20.3599. [DOI] [PubMed] [Google Scholar]
- 53.Nagasawa M, Nakagawa Y, Tanaka S, Kojima T. Chemotactic Peptide fMetLeuPhe Induces Translocation of the TRPV2 Channel in Macrophages. J Cell Physiol. 2007;210:692–702. doi: 10.1002/jcp.20883. [DOI] [PubMed] [Google Scholar]
- 54.Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. Regulation of Calcium-Permeable TRPV2 Channel by Insulin in Pancreatic β-Cells. Diabetes. 2009;58:174–84. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanzaki M, Zhang Y-Q, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1(3):165–70. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 56.Selen A, Amidon GL, Welling PG. Pharmacokinetics of probenecid following oral doses to human volunteers. J Pharm Sci. 1982;71(11):1238–42. doi: 10.1002/jps.2600711114. [DOI] [PubMed] [Google Scholar]
- 57.Gutman AB, Yu TF. Benemid (p-di-n-propylsulfamyl)-benzoic acid) as uricosuric agent in chronic gouty arthritis. Trans Assoc Am Physicians. 1951;64:279–88. [PubMed] [Google Scholar]
- 58.Smith DE, Gee WL, Brater DC, Lin ET, Benet LZ. Preliminary evaluation of furosemide-probenecid interaction in humans. J Pharmacol Sci. 1980;69(5):571–5. doi: 10.1002/jps.2600690526. [DOI] [PubMed] [Google Scholar]