Abstract
This review is focused on aging-related changes in cells and extracellular matrix of the articular cartilage. Major extracellular matrix changes are a reduced thickness of cartilage, proteolysis, advanced glycation and calcification. The cellular changes include reduced cell density, cellular senescence with abnormal secretory profiles, and impaired cellular defense mechanisms and anabolic responses. The extracellular and cellular changes compound each other, leading to biomechanical dysfunction and tissue destruction. The consequences of aging-related changes for joint homeostasis and risk for osteoarthritis are discussed.
Keywords: Cartilage, Osteoarthritis, Aging, Senescence, Oxygen radicals, Autophagy
Introduction
OA is the most prevalent joint disease and aging is among its main risk factors [1, 2]. OA is rare among individuals younger than 45 years, even in those with OA risk factors, such as obesity, joint trauma, joint malalignment or abnormal shape and leg length inequality [3, 4]. Such individuals are at a higher risk of developing OA at an earlier age and with increased severity. The majority of cases develop OA after long time intervals of exposure to the effects of risk factors and this is reflected by a close correlation between increasing age and OA prevalence. While aging-related changes occur in joint tissues of all individuals, most notably in articular cartilage, symptomatic, radiographic, macroscopic or microscopic OA does not manifest in all individuals, even at advanced age [5, 6] (Figures 1, 2). This pattern of joint aging and OA suggests that aging does not necessarily cause OA but that aging-related changes provide a basis upon which OA can be initiated. Individuals with specific OA risk factors may undergo an accelerated rate of changes that are similar to those associated with aging. This notion is consistent with the general concept that aging is the consequence of an imbalance between stressors that cause damage and mechanisms that repair or protect against damage [7]. The aging process is systemic and affects all organs and tissues. However, specific changes at the organ, tissue and cellular level may exceed the extent of normal aging and trigger the development of aging-related disease in a particular organ.
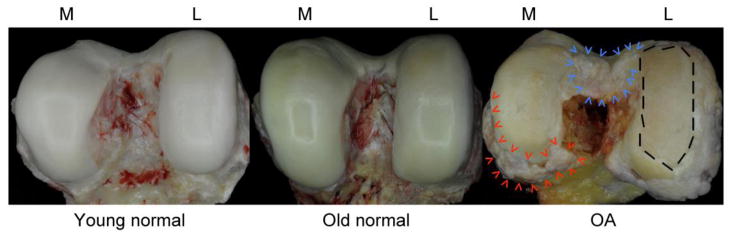
Fig. 1. Macroscopic images of human femoral condyles.
Images represent normal young (left, age 40), normal aging (center, age 76) OA (right, age 88) tissue. The old normal condyle shows intact cartilage with yellow discoloration, which is in part due to the formation of advanced glycation end products. The OA sample features large areas with complete loss of articular cartilage (dashed line on the left femoral condyle), osteophytes at the joint margins (red arrowheads) and the intercondylar notch (blue arrowheads). M=medial; L=lateral
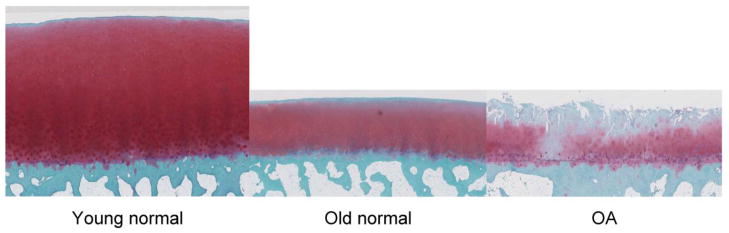
Fig. 2. Safranin O stained sections of human femoral condyles.
Images show young normal (left, age 40), old normal (center, age 76) and OA donors (right, age 88). The old normal sample has reduced tissue height, reduced safranin O staining in the superficial zone but no structural defects at the surface. The OA sample shows loss the superficial and part of the mid zone, fibrillations extending into the deep zone, areas with low cell density and cell clusters and duplication of the tidemark. All images are shown at 2.5x magnification.
OA is a disease of the joint as the functional unit or organ and affects all joint tissues. In a similar fashion, joint aging affects all tissues. Post-mitotic cells are thought to be more vulnerable to accumulation of aberrant proteins and metabolic waste [8]. This may explain in part why articular cartilage, which is composed of cells with a very low rate of replication during adulthood, is the joint tissue most affected by the aging process. Aging-related changes have been characterized in detail in extracellular matrix (ECM) and cells of articular cartilage (Table 1) and progress has been made in linking this to dysregulation of mechanisms and signaling pathways that control cellular homeostasis, activation, differentiation and survival. The influence of aging on other joint tissues, such as ligaments [9] and menisci [10] has been examined at some level of detail but not much information is available on synovial membrane and fluid. Aging-related changes in subchondral bone have been described [11] but their relationship to cartilage aging and OA development remains to be resolved [12]. Based on the more detailed information on aging-related changes in the articular cartilage and their role in initiating OA, this review summarizes manifestations of cartilage aging and discusses how these may set the stage for the development of OA.
TABLE 1.
Aging-related changes in chondrocytes
| Growth factor response and production | |
| TGFβ | ↓proliferation [79]; ↓GAG synthesis [134]; ↑pyrophosphate [44]; ↓IL-1 inhibition [135]; ↑MMP-13 [136] altered Alk1/5ratio [136] |
| IGF1 | ↓GAG synthesis [80, 81]; ↓collagen II synthesis [82]; ↑IGF-BP [80] |
| BMP7 | ↓synthesis [86]; ↑promoter methylation [87] |
| Mitochondria | ↑mtDNA damage[109] ↓activity of respiratory complexes I, II and III [110] ↓mitochondrial superoxide dismutase [113] |
| Cytoprotective Mechanisms | ↓antioxidant defense[137] ↓autophagy [122] ↓HMGB2 [62] |
| Senescence | ↑SAβ gal, telomere erosion [95] ↑GADD45β, ↑C/EBPβ, ↑p21 [138] ↓SIRT1 [139] ↑Caveolin [103] Senescence-associated secretory phenotype [48, 98] |
Articular cartilage extracellular matrix changes in aging
The principal function of the articular cartilage is to adjust to the biomechanical forces it experiences during joint movement, to absorb and distribute compressive load and to withstand shear stress [13]. As these functions are mediated by the ECM, cartilage homeostasis can functionally be defined as the condition where a normal cartilage ECM composition deals with mechanical stress without structural or cellular damage.
Normal cartilage extracellular matrix
The normal cartilage ECM is composed of molecules that endow it with compressive stiffness, elastic properties and lubrication at its surface. The cartilage ECM is produced and maintained by the one cell type present in the tissue, the articular chondrocyte. Although it does not directly absorb much of the load placed on the joint, the cartilage deforms during joint loading and transmits the force to the underlying bone in a manner dependent on joint shape and congruity. The resiliency of cartilage and the ability to withstand compressive loads is primarily due to the large proteoglycan aggrecan. As the load is removed from the joint, water that was squeezed out during loading is attracted back in by the highly negatively charged, hydrophilic, proteoglycans. Collagen fibers, consisting primarily of type II collagen, provide the tensile strength knee with normal activities [14]. The normal articular surface is smooth with a very low coefficient of friction allowing for an efficient gliding motion during joint use. A boundary layer of lubricants is present on the cartilage surface consisting of lubricin and hyaluronic acid [15]. Lubricin was previously called “superficial zone protein” because it was noted to be produced by the superficial zone chondrocytes although it is also produced by synovial cells [16].
Aging-related changes in ECM and implications for biomechanical properties
With aging and OA, articular cartilage ECM changes in total amount, composition and undergoes proteolysis and other posttranslational modifications [17]. The superficial zone is where the earliest changes occur in human articular cartilage aging with superficial defects and fibrillation being noted [5, 18, 19]. The superficial zone is also most susceptible to mechanical injury [23, 24]. In the human knee these changes are most notable in the patella, then medial femoral condyle, followed by lateral femoral condyle [19]. These structural changes correlate with biomechanical dysfunction in cartilage [20].
In OA, increased proteolytic activity in cartilage and synovial fluid cause cartilage matrix changes [25], with an increase in degraded collagen molecules [26, 27]. This degradation is accompanied by the loss of intrinsic cartilage fluorescence [28], especially around cells in the superficial zone. In OA, there is also a decrease in fixed charge density (FCD), due to degradation and loss of aggrecan [29]. The contents of total collagen (hydroxyproline/wet weight) and pyridinoline do not change markedly with aging [30].
With normal aging, there is a marked increase in the formation of advanced glycation end-products (AGEs), including pentosidine cross-links. AGEs are produced by the spontaneous nonenzymatic glycation of proteins [31]. Although AGE formation is promoted by elevated glucose levels in diabetes, the accumulation of AGEs in articular cartilage with aging can be seen independent of diabetes and may be due in large part to the very slow turnover of cartilage matrix components noted above [32–34]. The increased cross-linking of collagen molecules that occurs as a result of AGE-formation can alter the biomechanical properties of cartilage resulting in increased stiffness and an increased susceptibility to fatigue failure [35, 36].
In addition to altering the biomechanical properties of cartilage, AGEs may interact with cell surface receptors including the Receptor for Advanced Glycation Endproducts (RAGE). RAGE has been detected on articular chondrocytes and there is evidence for an increase in RAGE levels in aging and OA [37]. Activation of chondrocyte RAGE by various ligands including S100A4 and AGE-modified bovine serum albumin has been shown to stimulate catabolic signaling pathways that result in upregulation of MMP expression and chondrocyte hypertrophy [38–40]. Although a reduction in OA severity was not seen in RAGE knock-out mice [41], the study used very young animals that were 8 weeks of age at the time OA was induced through anterior cruciate ligament disruption. It is possible that RAGE activation may play a more important role in OA as it develops in older adults where, unlike in the young animals, AGE-formation in the cartilage would have had time to accumulate.
A highly prevalent change in aging cartilage is deposition of calcium containing crystals, mainly calcium pyrophosphate (CPP) and basic calcium phosphate (BCP) [42]. In the human knee this cartilage calcification is primarily an effect of aging rather than OA and represents a precursor to increased fibrillation and OA rather than a result of OA [42]. In cartilage from patients with end-stage OA, calcification correlated with increased disease severity [43, 44]. CPP deposition is due to increased pyrophosphate production by chondrocytes from aged cartilage [44]. The presence of calcium crystals produced by chondrocytes or released into the joint space from other tissues such as the meniscus and synovium may stimulate chondrocyte production of inflammatory mediators and ECM-degrading enzymes and thus contribute to onset and progression of OA [45].
Aging-related cellular changes
Maintaining normal ECM depends on the presence of adequate numbers of functionally competent cells that are capable of synthesizing appropriate amounts of ECM components and lubricants. Cells perform these synthetic functions during normal molecular turnover, and have to adapt during periods of increased joint use or following damage.
In regard to its cellular composition, articular cartilage is less complex as compared to other tissues as it is not vascularized or innervated and does not contain tissue macrophages [13]. Although cartilage contains only cells of mesenchymal lineage, cells in adult cartilage are heterogeneous. Cell subpopulations can be distinguished in the various cartilage zones, the superficial, mid and deep zone [13]. The cells differ in morphology and gene expression [46]. The superficial zone cells are most unique and also include a large percentage of cells with features of immature or progenitor cells [47]. This cellular heterogeneity has not been systematically examined in the context of aging. In particular, most in vitro studies used mixed cell populations isolated from full thickness cartilage and it is likely that changes that are unique to any of these cell subsets have not yet been discovered.
Aging-associated cellular changes in articular cartilage include cell depletion due to different forms of cell death, impaired responses to extracellular stimuli, resulting in abnormal gene expression and cell differentiation. Molecular mechanisms accounting for these phenotype alterations are cellular senescence due to alterations in chromatin and mitochondria [48]. These are discussed in detail in the following sections.
Cell depletion: mature and progenitor cells
Chondrocytes are required for cartilage tissue homeostasis and cell loss or dysfunction could be a primary factor leading to cartilage failure. Cartilage cellularity varies among species and changes during skeletal development and aging [49–54]. The level of cartilage cellularity determines the tissue volume that is being maintained by a single chondrocyte and appears to have implications for cartilage repair. Only 19 cells per mm3 are present in the cartilage of young adults [52, 55]. In full-thickness cartilage from a variety of human joints, cell density is decreased with aging [50, 56–58]. In the articular cartilage of macroscopically normal cartilage from human femoral condyles the density of chondrocytes decreases most profoundly in the superficial zone by ~50% between age 20 and 90 [51, 53].
Cell populations in each zone of normal mature articular cartilage are characterized by unique cell organization, cell shape and gene expression or differentiation markers [13]. In contrast to cells in the other zones, SZ cells are not surrounded by an extensive pericellular matrix and they express a series of unique cell surface markers and other proteins [46]. Among these proteins, the superficial zone protein or lubricin has received much attention as an important mediator of cartilage surface lubrication and protection against damage cause by mechanical shear and compression [59]. An important and unique feature of the SZ is that it contains the highest concentration of progenitor cells that are characterized by the expression of cell surface markers such as Stro-1 or Notch-1 [47]. When isolated and cultured under appropriate conditions these cells can differentiated into adipocytes or osteoblasts, indicating multilineage differentiation potential [60, 61].
Based on the importance of the superficial zone in cartilage function and integrity, and as cell density decreases most significantly in the superficial zone, a search was performed for genes that are unique to the superficial zone and show age-related changes and this identified the High Mobility Group Protein 2 (HMGB2) [62]. Several prior observations demonstrated aging-related changes in HMGB2. In liver tissue from old rats the phosphorylation and ADP-ribosylation of HMGB2 decreased drastically [63]. In a comparison of fibroblasts from young, middle-age, and old-age humans and humans with progeria, HMGB2 was among 9 genes that were downregulated in cells from progeria patients and old individuals [64]. As HMGB2 participates in chromosomal processing and assembly, the loss of HMGB2 may cause chromosomal pathologies, which result in misregulation of genes involved in tissue homeostasis and the aging process. In articular cartilage HMGB2 expression is restricted to the superficial zone and an aging-related decrease in HMGB2 expression is linked to chondrocyte death and cartilage destruction [62]. The phenotype of Hmgb2 knock out mice includes reduced superficial zone cellularity and early onset of OA-like changes [62]. The function of HMGB2 is to support survival of superficial zone cells [65] and to maintain the immature differentiation status of mesenchymal progenitor cells [66]. These observations are only beginning to characterize aging-related changes in the superficial zone. Additional studies are required to systematically document aging-related changes in the SZ and other cartilage zones.
In OA-affected cartilage, chondrocyte proliferation in the form of “cell clusters” or “cloning” has been observed in areas of fibrillation [4, 52, 67–69]. Cells in these clusters express markers of progenitor cells and a wide spectrum of proteins associated with abnormal chondrocyte activation and differentiation [70]. These finding raise the possibility that this represents a tissue repair response of progenitor cells. However, this type of chondrocyte does not contribute significantly to repair [71] and may in fact substantially contribute to the abnormal gene expression pattern of OA cartilage [58]. The activation pattern of the cluster cells also underscores the notion that aging does not uniformly affect all cells in cartilage and that certain cell subsets in aging and OA-affected cartilage are capable of proliferation and activation.
Cell density appears to be a determinant of cartilage tissue integrity and repair capacity. Articular cartilage in the developing skeleton during childhood and adolescence appears to have an increased potential to undergo successful repair. More direct evidence is provided by observations that areas with reduced cellularity on the human femoral head were characterized by an increased frequency of cartilage fibrillation [58]. Studies on human cartilage aging indicate that individuals over 90 years of age with intact knee articular cartilage surfaces are distinguished by a significantly increased number of chondrocytes per tissue volume [51]. These reports suggest an association between viable chondrocyte cell density and maintenance of healthy ECM.
There is further evidence that chondrocyte death and proteoglycan depletion are anatomically linked and may be mechanistically related. Cartilage areas containing apoptotic cells showed proteoglycan depletion [4] and the presence of apoptotic cells also significantly correlated with nitrite production and grade of OA [72, 73].
The absence of phagocytic cells in cartilage implies that remnants of dead cells may persist in cartilage matrix and can potentially affect matrix structure and function. Chondrocyte-derived apoptotic bodies accumulate within and in the vicinity of the chondrocyte lacunae [74]. Functional analysis showed that apoptotic bodies produce pyrophosphate, contain alkaline phosphatase, and NTP pyrophosphohydrolase activities, and can precipitate calcium. These findings link apoptosis with calcification of extracellular matrix [74, 75]. Therefore, chondrocyte-derived apoptotic bodies may contribute to pathologic cartilage calcification and to the altered remodeling of the subchondral bone observed in aging and OA. This raises the possibility that apoptosis, in addition to reducing cartilage viability, may progressively contribute to its remodeling and degradation.
The type and mechanisms of cell death continue to be investigated. Several studies reported increased apoptosis in aging and OA-affected cartilage [4, 72, 75, 76]. However, there has been discussion concerning limitations of certain methods resulting in detection of a very high rate of apoptotic cells [77]. In addition, other forms of cell death have been described [78]. It remains to be determined which forms of cell death and signaling pathways are involved in the reduction in cartilage cellularity with aging. Possibilities include death receptor-mediated apoptosis, apoptosis related to endoplasmic reticulum or mitochondrial dysfunction and excessive ROS production. Chondrocyte death can also occur as part of hypertrophic or terminal differentiation. A recently described cell death by autophagy may occur in cells that have accumulated large numbers of dysfunctional organelles to a level that can no longer be restored [8].
Reduced growth factor response
With increasing age, chondrocytes become less responsive to the proliferative and anabolic effects of growth factors, which may contribute to an imbalance in anabolic and catabolic activity (Table 1). Human articular chondrocytes from older tissue donors were found to proliferate less than those from younger donors when treated with serum, TGFβ, bFGF, PDGF or IGF-1 [79]. The anabolic response to IGF-1, measured as proteoglycan or collagen synthesis, declines with increasing age [80–82] as do the responses to TGFβ [83] and BMP-6 [84]. There is also evidence for an age-related reduction in expression of TGFβ2 and TGFβ3 [83, 85] and BMP-7 (OP-1) [86]. The age-related decrease in expression of BMP-7 may be related to increased DNA methylation at the OP-1 promoter [87].
Altered cell signaling in response to growth factors may also account for the reduced anabolic response with age. Reduced IGF-1 signaling has been noted in aging rat cartilage [82] and in aged equine chondrocytes [88, 89]. IGF-1 activation of the signaling protein Akt is important for IGF-1 to promote chondrocyte survival and matrix synthesis but this activation has been found to be reduced in OA chondrocytes or in normal chondrocytes under conditions of oxidative stress [90]. Studies of TGFβ signaling have noted a change in receptors such that with age and in OA there is an increase in the ALK1 to ALK5 ratio [91]. Activation of ALK5 appears to mediate the anabolic effects of TGFβ through phosphorylation of Smad 2,3 while ALK1 promotes MMP-13 expression through Smad 1,5,8. Further evidence for promotion of catabolic signaling with aging comes from studies of chondrocytes treated with either fibronectin fragments or IL-1β. Both catabolic factors were found to stimulate more MMP-13 production in chondrocytes from older compared to younger human tissue donors [92].
Cellular senescence
Cellular senescence classically refers to the limited proliferative capacity of normal differentiated cells resulting in growth arrest which, as described by Hayflick, generally occurs after 30–40 population doublings [93]. This is often referred to as replicative senescence, which has been associated with features of a senescent phenotype including enlarged flattened cells in culture, expression of the senescence-associated β-galactosidase (SA-βgal), and the presence of markers of DNA damage [94]. Another feature of senescence is shortening of the ends of chromosomes, called telomeres, which occurs with each cycle of cell division in normal somatic cells that lack the enzyme telomerase. Although chondrocytes have been shown to exhibit telomere shortening with age [95], it would seem unlikely that this is due to multiple rounds of cell division since chondrocytes in normal adult articular cartilage appear to be well differentiated post-mitotic cells. It is rare to find evidence of cell division in normal articular cartilage [96, 97]. However, chondrocyte proliferation is a feature seen in OA tissue and both telomere shortening and the presence of SA-βgal have been observed in chondrocytes in OA lesions [98].
Besides replicative senescence, telomere shortening can be seen as a result of extrinsic or “stress-induced” senescence [99, 100]. This form of cell senescence can be promoted by the chronic effects of oxidative damage and inflammation that result in DNA damage that includes damage to the telomeres. In a pig model of spontaneous cartilage degeneration, there was increased DNA damage in the OA-affected tissue and similar damage was caused by IL-1 induced nitric oxide production [101].
In vitro experiments using peroxide to induce oxidative stress [102] and IL-1 as a form of chronic inflammation have been shown to induce markers of senescence in chondrocytes [103]. In the study by Dai et al, the induction of senescence required expression of the membrane protein caveolin that has been associated with senescence in other cell types [103]. Injurious mechanical loading may be a stimulus for excessive ROS production in cartilage [104, 105] and could thus contribute to stress-induced chondrocyte senescence and OA. Stress induced chondrocyte senescence has also been observed when chondrocytes were treated with oxidized low-density lipoprotein (LDL) that could form when LDL present in synovial fluid reacts with ROS [106].
Senescent chondrocytes could contribute to the pro-inflammatory state and excessive production of MMPs observed in OA cartilage by assuming the senescence-associated secretory phenotype (SASP), which has been observed in other types of senescent cells [107]. Many of the “SASP factors” such as IL-1, IL-6, IL-7, IL-8, GROα, MCP-2, and MMP-3 are also produced by OA chondrocytes. In a study of human OA cartilage, RNA levels of MMP-1, -8, -13, and TIMP-1 were altered in cells isolated from lesion sites, where telomere shortening and SA-βgal were observed, but also in sites distal to the lesions where a lower number of cells exhibited the senescent changes [98]. Although a direct link between the SASP and chondrocyte senescence was not made, the tissues studied were from end-stage disease and only RNA levels of a limited number of factors were studied, suggesting future studies should examine earlier stages of disease and measure levels of the secreted proteins.
Mitochondria
The mitochondrial theory of aging is the most extensively studied and implies that accumulation of mitochondrial DNA (mtDNA) mutations, lead to reduced ATP production and increased ROS production [7]. It has also been proposed that aging can be caused by an alteration of the redox homeostasis, since mitochondria regulate the relative levels of NADH/NAD+, NADPH/NADP+, and GSH/GSSG [108]. Despite the critical role of mitochondria in various aspects of cellular aging, there is very limited information on specific changes in aging cartilage. Chondrocytes from endstage OA cartilage showed more mtDNA damage than cells from normal tissue and this was accompanied by reduced mtDNA repair capacity, and increased apoptosis in OA chondrocytes following exposure to oxygen radicals [109]. In OA chondrocytes, the activity of respiratory complexes I, II and III is reduced, resulting in a decreased mitochondrial bioenergetic reserve [110]. Protection of human chondrocytes from mtDNA damage by the mitochondria-targeted DNA repair enzyme hOGG1 rescued mtDNA integrity, preserved ATP levels, reestablished mitochondrial transcription, and significantly diminished apoptosis following IL-1β and TNF-β exposure [111].
Anti-oxidant defense
Given that excessive levels of ROS can contribute to aging in many tissues including cartilage, it is important to determine if reduced levels of anti-oxidants occur with aging in cartilage. A study of rat articular cartilage noted an age-related decrease in catalase but not superoxide dismutase or glutathione peroxidase [112] while a proteomic analysis of mitochondrial proteins in human chondrocytes revealed an age-related decrease in the mitochondrial superoxide dismutase (SOD2) [113]. SOD2 has also been noted to be decreased in OA chondrocytes and this was associated with an increase in promoter methylation [114]. Extracellular superoxide dismutase has also been shown to be decreased in human OA cartilage [115] and a gene microarray study revealed decreased expression of SOD2 and SOD3 as well as glutathione peroxidase 3 in human OA cartilage [116]. Glutathione is an important intracellular anti-oxidant and a study of normal human ankle cartilage from tissue donors found an age-related decrease in the amount of reduced relative to oxidized glutathione, which compromised the anti-oxidant defense system demonstrated by increased susceptibility to oxidant-mediated chondrocyte death [117].
Mechanisms for removal of altered proteins and organelles
Mechanisms that are responsible for removal of misfolded proteins and dysfunctional organelles are essential to maintain normal cell function and survival. Aging-related deficiencies in these mechanisms have been observed in model organisms and several human tissues and can contribute to the development of disease [118, 119].
Autophagy is a central homeostasis mechanism to eliminate damaged organelles, long-lived or aberrant proteins and superfluous portions of the cytoplasm [120]. Substrates are enclosed in a double membrane, the autophagosome, which fuses with lysosomes, allowing enzymatic substrate degradation. Cleavage products are recycled for use in biosynthesis or as energy sources [121].
Cartilage aging in humans or mice is associated with reduced expression of autophagy regulators, which is most profound in the superficial zone in the weightbearing areas of cartilage. Cartilage that is deficient in autophagy has reduced cellularity and extracellular matrix damage [122]. Mechanical injury to cartilage explants also suppresses autophagy regulators [123].
The protein kinase mammalian target of rapamycin (mTOR), as part of the complex mTORC1 is a key regulator and suppressor of autophagy. Excess mTOR activation has been linked to aging on the basis of results from genetic and pharmacological studies [118]. Major effects of mTOR are the inhibition of autophagy and the stimulation of protein synthesis. Chronic mTOR activation thus can potentially lead to increased accumulation of aggregation-prone proteins [124]. Senescent cells are enlarged or hypertrophic and this as well as the abnormal gene expression can be reversed by the mTOR inhibitor rapamycin [125],
Recently, abnormal mTOR signaling has been associated with autophagy to promote the secretory phenotype of senescent cells and the release of factors known to contribute to defective renewal and dysfunction of aging tissues [126]. In a model of cellular senescence a new cell compartment was discovered, the TORC autophagy secretory colocalization compartment, (TASCC) where TORC dependent synthesis of senescence-associated proteins, including proinflammatory mediators occurs.
Pharmacologic inhibition of mTORC-1 by rapamycin activates autophagy in chondrocytes and has protective effects against mechanical injury induced ECM damage and cell death in cartilage explants [123]. In experimental OA, rapamycin also reduced the severity of cartilage degradation and this was associated with a preservation of cartilage cellularity and reduction in inflammatory mediators [127].
The unfolded protein response (UPR) is a related homeostasis mechanism that is activated in response to disturbances in the protein-folding capacity of the endoplasmic reticulum or ER stress [128]. The UPR can restore ER function, while failure to adapt to ER stress or irreversible ER stress can lead to cell death and elimination of damaged cells. Defective UPR is linked with aging-related diseases and mediated by oxidative stress and accumulation of misfolded proteins [129]. ER stress can be triggered in chondrocytes by mutated type X collagen and contributes to the pathology of chondrodysplasia [130]. Site-1 protease regulates components of UPR pathways. Cartilage specific deletions results in abnormal growth plate development due to intracellular accumulation of type II collagen and chondrocyte apoptosis[131].
In advanced OA there is evidence for upregulation of ER stress markers [132] but aging-related changes in the ER stress response that precede the manifestation of overt OA remain to be investigated.
Conclusions
A growing body of evidence documents aging-associated changes in cartilage ECM and cells. Changes in these two compartments presumably aggravate each other since altered ECM may lead to increased mechanical stress to the cells while cell loss or dysfunction leads to deficient ECM synthesis and increased degradation.
Survival and normal function of postmitotic cells like mature articular chondrocytes depends on their ability to cope with stress [8]. Conceptually, age-related pathologies originate from limitations in the maintenance and repair mechanisms of DNA, by anomalies in the antioxidant mechanisms that contribute to the detoxification of reactive oxygen species or by abnormalities in mechanisms for removal of abnormal proteins and organelles. As summarized above, these mechanisms have been at least partially investigated in cartilage and there is evidence for deficits in some of these cellular homeostasis mechanisms.
Although useful information has been generated, there are limitations in previous studies of cartilage or chondrocyte aging. A clear distinction between aging and OA has not always been provided so that it can be difficult to differentiate primary aging-related changes from those that are part of the OA process. Future studies require more detailed consideration of cartilage heterogeneity within a joint such as areas exposed to different levels of load bearing. It will also be important to address how aging affects cell subpopulations in the different zones of cartilage. A need and opportunity exists to study basic mechanisms of aging that have been discovered in model organisms or other tissues for their role in cartilage aging. Furthermore, it is necessary to integrate existing and future information on individual mechanisms of aging into networks that allow understanding of interactions and hierarchies of changes.
The most important cellular changes that have been documented in aging cartilage are reduced cellularity and abnormal cell activation and differentiation. These changes provide a basis upon which the cartilage remodeling and destruction process is triggered that ultimately manifests as OA. The potential clinical significance of studies of cartilage aging is in the discovery of approaches that slow or reverse aging-associated changes and thus prevent the onset of OA. Given the limited success in developing effective treatments for established OA [133] maintaining joint health to delay tissue aging is an even more important objective.
Acknowledgments
The research by M.L. is supported by grants from the National Institute on Aging (AG007996), the National Institute of Arthritis, Musculoskeletal and Skin Diseases (AR056026) and the Sam and Rose Stein Endowment Fund.
The research by R.F.L. is supported by grants from the National Institute on Aging (P30 AG021332), the National Institute of Arthritis, Musculoskeletal and Skin Diseases (AR49003) and the Arthritis Foundation and the Dorothy Rhyne and Willard Duke Kimbrell Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Martin Lotz, Email: mlotz@scripps.edu.
Richard F. Loeser, Email: rloeser@wakehealth.edu.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis and Rheumatism. 1998;41:1632–8. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Temple-Wong MM, Bae WC, Chen MQ, Bugbee WD, Amiel D, Coutts RD, Lotz M, Sah RL. Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2009;17:1469–76. doi: 10.1016/j.joca.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goekoop RJ, Kloppenburg M, Kroon HM, Dirkse LE, Huizinga TW, Westendorp RG, Gussekloo J. Determinants of absence of osteoarthritis in old age. Scand J Rheumatol. 2011;40:68–3. doi: 10.3109/03009742.2010.500618. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Vicencio JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo A, Tasdemir E, Morselli E, Ben Younes A, Maiuri MC, Lavandero S, Kroemer G. Senescence, apoptosis or autophagy? When a damaged cell must decide its path--a mini-review. Gerontology. 2008;54:92–9. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa A, Otsuki S, Pauli C, Steklov N, Kinoshita M, Koziol J, D’Lima DD, Lotz M. Anterior cruciate ligament changes in human joint in aging and osteoarthritis. Arthritis Rheum. doi: 10.1002/art.33417. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, Lotz MK, D’Lima DD. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–41. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada K, Healey R, Amiel D, Lotz M, Coutts R. Subchondral bone of the human knee joint in aging and osteoarthritis. Osteoarthritis Cartilage. 2002;10:360–9. doi: 10.1053/joca.2002.0525. [DOI] [PubMed] [Google Scholar]
- 12.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 13.Poole AR. Cartilage in health and disease. In: Koopman WJ, editor. Arthritis and Allied Conditions. Philadelphia: Williams and Wilkins; 2005. [Google Scholar]
- 14.Mundermann A, Dyrby CO, D’Lima DD, Colwell CW, Jr, Andriacchi TP. In vivo knee loading characteristics during activities of daily living as measured by an instrumented total knee replacement. J Orthop Res. 2008;26:1167–72. doi: 10.1002/jor.20655. [DOI] [PubMed] [Google Scholar]
- 15.Greene GW, Banquy X, Lee DW, Lowrey DD, Yu J, Israelachvili JN. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci U S A. 2011;108:5255–9. doi: 10.1073/pnas.1101002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–20. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 17.Maroudas A. Different ways of expressing concentration of cartilage constituents with special reference to the tissue’s organization and functional properties. In: Maroudas A, Kuettner KE, editors. Methods in Cartilage Research. New York: Academic Press; 1990. pp. 211–9. [Google Scholar]
- 18.Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Annals of the Rheumatic Diseases. 1972;31:457–64. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meachim G, Emery IH. Quantitative aspects of patello-femoral cartilage fibrillation in Liverpool necropsies. Ann Rheum Dis. 1974;33:39–47. doi: 10.1136/ard.33.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. Journal of Bone and Joint Surgery. 1982;64:88–94. [PubMed] [Google Scholar]
- 21.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–69. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae WC, Temple MM, Amiel D, Coutts RD, Niederauer GG, Sah RL. Indentation testing of human cartilage: sensitivity to articular surface degeneration. Arthritis Rheum. 2003;48:3382–94. doi: 10.1002/art.11347. [DOI] [PubMed] [Google Scholar]
- 23.Otsuki S, Brinson DC, Creighton L, Kinoshita M, Sah RL, D’Lima D, Lotz M. The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 2008;58:1076–85. doi: 10.1002/art.23381. [DOI] [PubMed] [Google Scholar]
- 24.Carames B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, Lotz M. Mechanical injury suppresses autophagy regulators and its pharmacological activation results in chondroprotection. Arthritis Rheum. 2011 doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. Journal of Clinical Investigation. 1989;84:678–85. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodge GR, Poole AR. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. Journal of Clinical Investigation. 1989;83:647–61. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bank RA, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber FP, te Koppele JM. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biology. 1997;16:233–43. doi: 10.1016/s0945-053x(97)90012-3. [DOI] [PubMed] [Google Scholar]
- 28.Gibson GJ, Verner JJ, Nelson FR, Lin DL. Degradation of the cartilage collagen matrix associated with changes in chondrocytes in osteoarthrosis. Assessment by loss of background fluorescence and immunodetection of matrix components. Journal of Orthopaedic Research. 2001;19:33–42. doi: 10.1016/S0736-0266(00)00008-5. [DOI] [PubMed] [Google Scholar]
- 29.Maroudas A. Physico-chemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Tunbridge Wells: Pitman Medical; 1979. pp. 215–90. [Google Scholar]
- 30.Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochemical Journal. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verzijl N, Bank RA, TeKoppele JM, DeGroot J. AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616–22. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochemical Journal. 1998;330 ( Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pokharna HK, Monnier V, Boja B, Moskowitz RW. Lysyl oxidase and Maillard reaction-mediated crosslinks in aging and osteoarthritic rabbit cartilage. Journal of Orthopaedic Research. 1995;13:13–21. doi: 10.1002/jor.1100130105. [DOI] [PubMed] [Google Scholar]
- 34.Uchiyama A, Ohishi T, Takahashi M, Kushida K, Inoue T, Fujie M, Horiuchi K. Fluorophores from aging human articular cartilage. J Biochem. 1991;110:714–8. doi: 10.1093/oxfordjournals.jbchem.a123646. [DOI] [PubMed] [Google Scholar]
- 35.Chen AC, Temple MM, Ng DM, Verzijl N, DeGroot J, TeKoppele JM, Sah RL. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212–7. doi: 10.1002/art.10627. [DOI] [PubMed] [Google Scholar]
- 36.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330 ( Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–2911. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 39.Steenvoorden MM, Huizinga TW, Verzijl N, Bank RA, Ronday HK, Luning HA, Lafeber FP, Toes RE, DeGroot J. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–63. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 40.Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J Immunol. 2005;175:8296–302. doi: 10.4049/jimmunol.175.12.8296. [DOI] [PubMed] [Google Scholar]
- 41.Cecil DL, Appleton CT, Polewski MD, Mort JS, Schmidt AM, Bendele A, Beier F, Terkeltaub R. The pattern recognition receptor CD36 is a chondrocyte hypertrophy marker associated with suppression of catabolic responses and promotion of repair responses to inflammatory stimuli. J Immunol. 2009;182:5024–31. doi: 10.4049/jimmunol.0803603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitsuyama H, Healey RM, Terkeltaub RA, Coutts RD, Amiel D. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthritis Cartilage. 2007;15:559–65. doi: 10.1016/j.joca.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, Rutsch F, Schafer FK, Niggemeyer O, Steinhagen J, Lohmann CH, Pap T, Ruther W. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 44.Rosen F, McCabe G, Quach J, Solan J, Terkeltaub R, Seegmiller JE, Lotz M. Differential effects of aging on human chondrocyte responses to transforming growth factor beta: increased pyrophosphate production and decreased cell proliferation. Arthritis Rheum. 1997;40:1275–81. doi: 10.1002/1529-0131(199707)40:7<1275::AID-ART12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 45.Terkeltaub RA. What does cartilage calcification tell us about osteoarthritis? J Rheumatol. 2002;29:411–5. [PubMed] [Google Scholar]
- 46.Fukui N, Miyamoto Y, Nakajima M, Ikeda Y, Hikita A, Furukawa H, Mitomi H, Tanaka N, Katsuragawa Y, Yamamoto S, Sawabe M, Juji T, Mori T, Suzuki R, Ikegawa S. Zonal gene expression of chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 2008;58:3843–53. doi: 10.1002/art.24036. [DOI] [PubMed] [Google Scholar]
- 47.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–9. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilmore RS, Palfrey AJ. Chondrocyte distribution in the articular cartilage of human femoral condyles. Journal of Anatomy. 1988;157:23–31. [PMC free article] [PubMed] [Google Scholar]
- 50.Meachim G, Collins DH. Cell counts of normal and osteoarthritic articular cartilage in relation to the uptake of sulphate (35SO4) in vitro. Annals of the Rheumatic Diseases. 1962;21:45–50. doi: 10.1136/ard.21.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quintero M, Mitrovic DR, Stankovic A, de Seze S, Miravet L, Ryckewaert A. Cellular aspects of the aging of articular cartilage. I. Condylar cartilage with a normal surface sampled from normal knees. Revue du Rhumatisme et des Maladies Osteo-Articulaires. 1984;51:375–9. [PubMed] [Google Scholar]
- 52.Quintero M, Mitrovic DR, Stankovic MA, de Seze S, Miravet L, Ryckewaert A. Cellular aspects of the aging of the articular cartilage. II. Condylar cartilage with fissured surface taken from normal and arthritic kneesf. Rev Rhum Mal Osteoartic. 1984;51:445–9. [PubMed] [Google Scholar]
- 53.Stockwell RA. The cell density of human articular and costal cartilage. Journal of Anatomy. 1967;101:753–63. [PMC free article] [PubMed] [Google Scholar]
- 54.Stockwell RA. Cell density, cell size, and cartilage thickness in adult mammalian articular cartilage. J Anat. 1971;108:584–93. [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed AM, Burke DL, Yu A. In-vitro measurement of static pressure distribution in synovial joints--Part II: Retropatellar surface. J Biomech Eng. 1983;105:226–36. doi: 10.1115/1.3138410. [DOI] [PubMed] [Google Scholar]
- 56.Stockwell RA, Meachim G. The chondrocytes. In: Freeman MAR, editor. Adult Articular Cartilage. Tunbridge Wells: Pitman Medical; 1979. pp. 69–144. [Google Scholar]
- 57.Vignon E, Arlot M, Meunier P, Vignon G. Quantitative histological changes in osteoarthritic hip cartilage. Morphometric analysis of 29 osteoarthritic and 26 normal human femoral heads. Clin Orthop Relat Res. 1974:269–78. [PubMed] [Google Scholar]
- 58.Vignon E, Arlot M, Patricot LM, Vignon G. The cell density of human femoral head cartilage. Clin Orthop Relat Res. 1976:303–8. [PubMed] [Google Scholar]
- 59.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535–41. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 60.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–32. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 61.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–97. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, Lotz M. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–6. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thakur MK, Prasad S. Age-specific methylation of high-mobility-group proteins of the rat liver and its modulation by spermine and sodium butyrate. Mutat Res. 1989;219:107–11. doi: 10.1016/0921-8734(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 64.Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–92. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 65.Taniguchi N, Carames B, Kawakami Y, Amendt BA, Komiya S, Lotz M. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via beta-catenin pathway. Proc Natl Acad Sci U S A. 2009;106:16817–22. doi: 10.1073/pnas.0904414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taniguchi N, Carames B, Hsu E, Cherqui S, Kawakami Y, Lotz M. Expression Patterns and Function of Chromatin Protein HMGB2 during Mesenchymal Stem Cell Differentiation. J Biol Chem. 2011;286:41489–98. doi: 10.1074/jbc.M111.236984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hashimoto S, Takahashi K, Amiel D, Coutts RD, Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis and Rheumatism. 1998;41:1266–74. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 68.Mankin H. The metabolism of articular cartilage in health and disease. In: Burleigh P, Poole AR, editors. Dynamics of connective tissue macromolecules. Amsterdam: North-Holland; 1975. pp. 327–56. [Google Scholar]
- 69.Vignon E, Arlot M, Vignon G. The cellularity of fibrillated articular cartilage. A comparative study of age-related and osteoarthrotic cartilage lesions from the human femoral head. Pathologie Biologie. 1977;25:29–32. [PubMed] [Google Scholar]
- 70.Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D. Cartilage cell clusters. Arthritis Rheum. 2010;62:2206–18. doi: 10.1002/art.27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moriizumi T, Yamashita N, Okada Y. Papain-induced changes in the guinea pig knee joint with special reference to cartilage healing. Virchows Archiv B, Cell Pathology Including Molecular Pathology. 1986;51:461–74. doi: 10.1007/BF02899052. [DOI] [PubMed] [Google Scholar]
- 72.Du W, Mills I, Sumpio BE. Cyclic strain causes heterogeneous induction of transcription factors, AP-1, CRE binding protein and NF-kB, in endothelial cells: species and vascular bed diversity. Journal of Biomechanics. 1995;28:1485–91. doi: 10.1016/0021-9290(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 73.Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, Currie MG, Martel-Pelletier J. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis and Rheumatism. 2000;43:1290–9. doi: 10.1002/1529-0131(200006)43:6<1290::AID-ANR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto S, Ochs RL, Rosen F, Quach J, McCabe G, Solan J, Seegmiller JE, Terkeltaub R, Lotz M. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3094–9. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kouri JB, Aguilera JM, Reyes J, Lozoya KA, Gonzalez S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. Journal of Rheumatology. 2000;27:1005–19. [PubMed] [Google Scholar]
- 76.Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anatomical Record. 1998;250:418–25. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 77.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis and Rheumatism. 2001;44:1304–12. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 78.Roach HI, Aigner T, Kouri JB. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9:265–77. doi: 10.1023/b:appt.0000025803.17498.26. [DOI] [PubMed] [Google Scholar]
- 79.Guerne PA, Blanco F, Kaelin A, Desgeorges A, Lotz M. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum. 1995;38:960–8. doi: 10.1002/art.1780380712. [DOI] [PubMed] [Google Scholar]
- 80.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–8. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 81.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–20. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 82.Messai H, Duchossoy Y, Khatib A, Panasyuk A, Mitrovic DR. Articular chondrocytes from aging rats respond poorly to insulin-like growth factor-1: an altered signaling pathway. Mech Ageing Dev. 2000;115:21–37. doi: 10.1016/s0047-6374(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 83.Blaney Davidson EN, Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–47. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bobacz K, Gruber R, Soleiman A, Erlacher L, Smolen JS, Graninger WB. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum. 2003;48:2501–8. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- 85.Loeser RF, Olex A, McNulty MA, Carlson CS, Callahan M, Ferguson C, Chou J, Leng X, Fetrow JS. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2011 doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002;1588:126–34. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 87.Loeser RF, Im HJ, Richardson B, Lu Q, Chubinskaya S. Methylation of the OP-1 promoter: potential role in the age-related decline in OP-1 expression in cartilage. Osteoarthritis Cartilage. 2009;17:513–7. doi: 10.1016/j.joca.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortier LA, Miller BJ. Signaling through the small G-protein Cdc42 is involved in insulin-like growth factor-I resistance in aging articular chondrocytes. J Orthop Res. 2006;24:1765–72. doi: 10.1002/jor.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boehm AK, Seth M, Mayr KG, Fortier LA. Hsp90 mediates insulin-like growth factor 1 and interleukin-1beta signaling in an age-dependent manner in equine articular chondrocytes. Arthritis Rheum. 2007;56:2335–43. doi: 10.1002/art.22664. [DOI] [PubMed] [Google Scholar]
- 90.Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J Biol Chem. 2009;284:31972–81. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Kraan PM, Blaney Davidson EN, van den Berg WB. A role for age-related changes in TGFbeta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther. 2010;12:201. doi: 10.1186/ar2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forsyth CB, Cole A, Murphy G, Bienias JL, Im HJ, Loeser RF., Jr Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J Gerontol A Biol Sci Med Sci. 2005;60:1118–24. doi: 10.1093/gerona/60.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayflick L. Intracellular determinants of cell aging. Mech Ageing Dev. 1984;28:177–85. doi: 10.1016/0047-6374(84)90018-6. [DOI] [PubMed] [Google Scholar]
- 94.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–56. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B172–9. doi: 10.1093/gerona/56.4.b172. [DOI] [PubMed] [Google Scholar]
- 96.Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. J Bone Joint Surg. 1963;45-A:529–540. [Google Scholar]
- 97.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–12. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 98.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 99.Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5:1–10. doi: 10.1023/b:bgen.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 100.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 101.Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008;16:624–30. doi: 10.1016/j.joca.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P. Oxidative stress induces senescence in chondrocytes. J Orthop Res. 2011;29:1114–1120. doi: 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]
- 103.Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo K, Kato T, Nishioka K, Yudoh K. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–31. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- 104.Yamazaki K, Fukuda K, Matsukawa M, Hara F, Matsushita T, Yamamoto N, Yoshida K, Munakata H, Hamanishi C. Cyclic tensile stretch loaded on bovine chondrocytes causes depolymerization of hyaluronan: involvement of reactive oxygen species. Arthritis Rheum. 2003;48:3151–8. doi: 10.1002/art.11305. [DOI] [PubMed] [Google Scholar]
- 105.Green DM, Noble PC, Ahuero JS, Birdsall HH. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis Rheum. 2006;54:1509–17. doi: 10.1002/art.21812. [DOI] [PubMed] [Google Scholar]
- 106.Zushi S, Akagi M, Kishimoto H, Teramura T, Sawamura T, Hamanishi C. Induction of bovine articular chondrocyte senescence with oxidized low-density lipoprotein through lectin-like oxidized low-density lipoprotein receptor 1. Arthritis Rheum. 2009;60:3007–16. doi: 10.1002/art.24816. [DOI] [PubMed] [Google Scholar]
- 107.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–46. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leeuwenburgh C, Pamplona R, Sanz A. Mitochondria and aging. J Aging Res. 2011;2011:782946. doi: 10.4061/2011/782946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grishko VI, Ho R, Wilson GL, Pearsall AWt. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthritis Cartilage. 2009;17:107–13. doi: 10.1016/j.joca.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–9. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 111.Kim J, Xu M, Xo R, Mates A, Wilson GL, Pearsall AWt, Grishko V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage. 2010;18:424–32. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 112.Jallali N, Ridha H, Thrasivoulou C, Underwood C, Butler PE, Cowen T. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–22. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 113.Ruiz-Romero C, Lopez-Armada MJ, Blanco FJ. Mitochondrial proteomic characterization of human normal articular chondrocytes. Osteoarthritis Cartilage. 2006;14:507–18. doi: 10.1016/j.joca.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 114.Scott JL, Gabrielides C, Davidson RK, Swingler TE, Clark IM, Wallis GA, Boot-Handford RP, Kirkwood TB, Taylor RW, Young DA. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69:1502–10. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Regan E, Flannelly J, Bowler R, Tran K, Nicks M, Carbone BD, Glueck D, Heijnen H, Mason R, Crapo J. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005;52:3479–91. doi: 10.1002/art.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayr F, Zimmer R, Bartnik E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–44. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 117.Del Carlo M, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–30. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 118.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–95. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 119.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 120.Lotz MK, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7:579–87. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- 122.Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caramés B, Taniguchi N, Seino D, Blanco F, D’Lima DD, Lotz M. Mechanical injury suppresses autophagy regulators and its pharmacological activation results in chondroprotection. Arthritis Rheum. doi: 10.1002/art.33444. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–95. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 125.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–95. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 126.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, Tavare S, Inoki K, Shimizu S. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–70. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Caramés B, Hasegawa A, Taniguchi N, Blanco F, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2011-200557. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Austin RC. The unfolded protein response in health and disease. Antioxid Redox Signal. 2009;11:2279–87. doi: 10.1089/ars.2009.2686. [DOI] [PubMed] [Google Scholar]
- 129.Naidoo N. The endoplasmic reticulum stress response and aging. Rev Neurosci. 2009;20:23–37. doi: 10.1515/revneuro.2009.20.1.23. [DOI] [PubMed] [Google Scholar]
- 130.Rajpar MH, McDermott B, Kung L, Eardley R, Knowles L, Heeran M, Thornton DJ, Wilson R, Bateman JF, Poulsom R, Arvan P, Kadler KE, Briggs MD, Boot-Handford RP. Targeted induction of endoplasmic reticulum stress induces cartilage pathology. PLoS Genet. 2009;5:e1000691. doi: 10.1371/journal.pgen.1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patra D, DeLassus E, Hayashi S, Sandell LJ. Site-1 protease is essential to growth plate maintenance and is a critical regulator of chondrocyte hypertrophic differentiation in postnatal mice. J Biol Chem. 2011;286:29227–40. doi: 10.1074/jbc.M110.208686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nugent AE, Speicher DM, Gradisar I, McBurney DL, Baraga A, Doane KJ, Horton WE., Jr Advanced osteoarthritis in humans is associated with altered collagen VI expression and upregulation of ER-stress markers Grp78 and bag-1. J Histochem Cytochem. 2009;57:923–31. doi: 10.1369/jhc.2009.953893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hellio Le Graverand-Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17:1393–401. doi: 10.1016/j.joca.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 134.Iqbal J, Dudhia J, Bird JL, Bayliss MT. Age-related effects of TGF-beta on proteoglycan synthesis in equine articular cartilage. Biochem Biophys Res Commun. 2000;274:467–71. doi: 10.1006/bbrc.2000.3167. [DOI] [PubMed] [Google Scholar]
- 135.Scharstuhl A, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61:1095–8. doi: 10.1136/ard.61.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van der Kraan PM, Goumans MJ, Blaney Davidson E, Ten Dijke P. Age-dependent alteration of TGF-beta signalling in osteoarthritis. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carlo MD, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–30. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 138.Shimada H, Sakakima H, Tsuchimochi K, Matsuda F, Komiya S, Goldring MB, Ijiri K. Senescence of chondrocytes in aging articular cartilage: GADD45beta mediates p21 expression in association with C/EBPbeta in senescence-accelerated mice. Pathol Res Pract. 2011;207:225–31. doi: 10.1016/j.prp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P. Oxidative stress induces senescence in chondrocytes. J Orthop Res. 2011;29:1114–20. doi: 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]