Abstract
Background
Current literature examining associations between vitamin D and chronic disease generally use a single assessment of 25-hydroxyvitamin D (25(OH)D), assuming an individual’s 25(OH)D concentration is consistent over time.
Methods
We investigated the intraindividual variability between two measures of plasma 25(OH)D concentrations collected ~5 years apart (1997-2000 to 2002-2005) in 672 postmenopausal women participating in the Women’s Health Initiative. Plasma 25(OH)D was assessed using the DiaSorin LIAISON® chemiluminescence immunoassay. The within-pair coefficient of variation (CV) was 4.9% using blinded quality control samples. Mean and standard deviations (SD) of 25(OH)D at the two time points were compared using a paired t-test. An intraindividual CV and intra-class correlation coefficient (ICC) were used to assess intraindividual variability. A Spearman correlation coefficient (r) assessed the strength of the association between the two measures and concordance in vitamin D status at two time points
Results
Mean 25(OH)D concentrations (nmol/L) significantly increased over time from 60.0 (SD=22.2) to 67.8 (SD=22.2) (p<0.05). The CV was 24.6%, the ICC (95% Confidence Interval (CI)) was 0.59 (0.54-0.64), and the Spearman r was 0.61 (95% CI=0.56-0.66). Greater concordance over 5 years was observed in participants with sufficient compared to deficient or inadequate baseline 25(OH)D concentrations (weighted kappa=0.39). Reliability measures were moderately influenced by season of blood draw and vitamin D supplement use.
Conclusion
There is moderate intraindividual variation in 25(OH)D concentrations over approximately 5 years.
Impact
These data support the use of a one-time measure of blood 25(OH)D in prospective studies with ≤ 5 years of follow-up.
Keywords: 25-hydroxyvitamin D, intraindividual variation, vitamin D, biomarkers, postmenopausal women
INTRODUCTION
Vitamin D may play a role in the pathophysiology of multiple chronic diseases states. The recent Institute of Medicine’s (IOM) report on Dietary Reference Intakes (DRIs) for calcium and vitamin D concluded that the current data are inconclusive regarding a causal role of vitamin D in disease states other than bone health (e.g., cancer, cardiovascular disease, auto-immune disease) (1). In the coming years, additional research will add to the literature on the role of vitamin D status in relation to various health outcomes. Thus, understanding variability of vitamin D status over time for use in clinical and population research is needed.
Individual vitamin D status is best assessed with blood concentrations of 25-hydroxyvitamin D (25(OH)D) which reflects exposure from all vitamin D sources (diet, supplements and sunlight) and reflects vitamin D status over an approximate preceding 3 week period (2, 3). Of the current literature assessing associations between blood measures of vitamin D and disease, most studies have used a single measure of 25(OH)D and have adjusted for date of blood draw during data analysis. These studies assume that individual vitamin D measurements are consistent over time. If this assumption is not true, then a single blood measure of 25(OH)D may have minimal relation to health beyond its short-term physiologic effects. More research is needed to better understand the intraindividual variation in blood concentrations of 25(OH)D over periods of years. This information will aid in interpreting the scientific literature and will inform on the reliability of using one measure of 25(OH)D to reflect a longer time period of exposure in studies of vitamin D status and chronic disease risk.
To date, five studies (4-8), have investigated the intraindividual variation in blood 25(OH)D concentrations over a period of 3-14 years, but 4 of 5 had small sample sizes (n<187). In the largest study, Jorde et al (7), examined the intra-individual variation in a sample of 2,688 men and women, of which, only 759 participants had blood samples drawn during the same time of year. Together these studies have found that 25(OH)D levels vary moderately within individuals over time with coefficients of variation (CV) ranging from 14.9-18.4%. Additional studies with larger sample sizes are needed to confirm these findings and explore subgroups for which variation may be lowest.
Data from the Buffalo Osteoporosis and Periodontal Disease Study, an ancillary study of the Women Health Initiative (WHI) Observation Study, provides a unique opportunity to quantify intraindividual variation in plasma 25(OH)D concentrations among a well-characterized, sample of 672 postmenopausal women with blood measures taken at two time points, approximately 5 years apart (1997-2000 to 2002-2005). The same vitamin D assay was used to measure 25(OH)D concentrations in all plasma samples from both time points over a consecutive four month period. We hypothesized that there would be minimal to moderate intraindividual variability of 25(OH)D concentrations between the two measurements. The current study will aid in better understanding how measures of vitamin D status vary over time among post-menopausal women. Specifically, this study will help interpret current and future WHI studies showing associations between vitamin D status and disease outcomes.
METHODS
Study sample
The WHI is a large multi-center study of postmenopausal women, including over 161,000 women ages 50-79 at the time of enrollment recruited from 1993 to 1998 (9, 10). The WHI includes a set of clinical trials and a prospective observational study. The participants included in the current analysis are women from the WHI Observational Study who participated in the ancillary Osteoporosis and Periodontal Disease (OsteoPerio) Study, conducted at the University at Buffalo clinical center (previously described (11)), and had available data and stored samples for studying relationships of 25(OH)D concentrations and periodontal disease. All WHI participants in the Observational Study are mutually exclusive from WHI participants in the WHI Clinical Trials (e.g., the Calcium and Vitamin D Clinical Trial).
There were 2,249 women who participated in the WHI Observational Study at the Buffalo center. The details of the OsteoPerio Study eligibility, recruitment and follow-up study have been previously described (11, 12). In brief, all women were invited to participate in the OsteoPerio Study at the WHI year 3 clinic visit (1997-2000). Of these, 549 women were unable to be reached, not interested, deceased, canceled after accepting, or were temporarily ineligible and 338 women did not meet the specified inclusion criteria. Among the remaining 1,362 individuals, 5 did not complete or return study questionnaires and 16 did not have adequate oral x-rays needed for determination of alveolar crestal height. This left a sample of 1,341 women with available data on periodontal disease. There were 934 women who had plasma samples available for 25(OH)D assessment at OsteoPerio baseline (1997-2000). There were 231 women who did not return for follow-up (36 were deceased, 76 were ineligible, 112 were not interested, and 7 were lost or withdrew from WHI) and 30 women did participate in follow-up but did not provide a blood sample. Therefore, 673 women also had available plasma drawn for 25(OH)D assessment at the 5-year follow-up study (2002-2005). The Institutional Review Board at the University at Buffalo approved all protocols and consent forms and participants provided written informed consent for all study activities in the WHI and ancillary OsteoPerio Study.
Data collection
At the baseline OsteoPerio visit participants completed a standardized set of self-administered questionnaires pertaining to personal and family health history and lifestyle habits, and had an extensive clinic examination following standardized protocols (11, 12). The physical examination components included measured height and weight, and a fasting blood draw. Questionnaire information included participation in recreational physical activity (13) and intake of vitamin D from foods assessed from a food frequency questionnaires (14). Participants were asked to bring in current medications and supplements they were taking and reported their dose and frequency of use. The same clinic examinations, questionnaires, and medication and supplement use inventory were repeated at the OsteoPerio five year follow-up visit (2002-2005).
Assessment of 25-hydroxyvitamin D status
At both OsteoPerio baseline and follow-up, fasting blood samples were collected from participants by a trained phlebotomist and processed using a standardized protocol. The average time between blood sample at baseline and follow-up was 5.1 years. Effort was made in the OsteoPerio study to bring women in for the baseline and follow-up study visits at the same time of year, as near as feasible to their baseline calendar date, because previous data has shown that bone tissue mass varies by season (15).
Plasma samples were stored in 0.5 ml straws liquid nitrogen at −196 °C until the time of 25(OH)D measurements when they were removed from liquid nitrogen, put in a −80°C freezer, thawed and aloquited under standard protocol into croyvials. Samples were refrozen and shipped on dry ice to the Heartland Assays, Inc., (Ames, IA) laboratory where they were thawed and assayed. All assays were run over a consecutive 4 month period. Assays for plasma drawn at baseline were run first followed by assays for plasma drawn at follow-up. Duplicate participant samples were run in separate batches.
Plasma 25(OH)D concentrations were measured by competitive chemiluminescence immunoassay using the DiaSorin LIAISON 25(OH)D assay. The within-pair CV was 4.9% using the investigators’ blinded duplicate quality control samples that were nested in each batch. One woman was excluded from the analysis because her baseline 25(OH)D concentration was extremely high (530 nmol/L) reportedly due to taking a high levels of vitamin D supplements for calcium deficiency leaving 672 participants for the current analysis. The majority (84.2%) of participants had bloods drawn at follow-up within 90 days from their baseline month of blood draw due to the study’s efforts to bring women in at follow-up at a similar time of year.
Statistical Analysis
Basic descriptive characteristics of this study sample were described at OsteoPerio baseline including age, race, education, smoking status, hormone therapy use, body mass index (BMI), and physical activity levels. Plasma concentrations of 25(OH)D, season of blood draw (January-March, April-June, July-September, October-December), and intake of vitamin D from foods and supplements (IU/day) at baseline and follow-up were compared to describe changes in vitamin D status and intake over time. Paired t-tests or Bowker’s test of symmetry, where applicable, were conducted to examine difference between baseline and follow-up concentrations of plasma 25(OH)D and vitamin D intake.
Spearman correlation coefficients (r) were calculated to determine the strength of the relationship between baseline and follow-up 25(OH)D concentrations. Additionally, an intra-class correlation coefficient (ICC) and an intraindividual coefficient of variation (CV) were estimated to assess intraindividual variation in plasma 25(OH)D concentrations between the two time points. The ICC was estimated using ICC=Vb/Vt, where Vb=variance between individuals and Vt=total variance (16). The higher the ICC, the greater the proportion of total variation in 25(OH)D concentrations between time points owed to differences between individuals, rather than intraindividual variability or measurement errors. The intraindividual CV was computed, using the root mean square approach as described by Bland (17).
In exploratory analyses, the influence of season of blood draw and supplement use patterns over time on the reliability of two measures of 25(OH)D 5 years apart were examined. The above analyses were repeated among participants whose bloods were drawn (1) in the same season (winter [January-March], spring [April-June], summer [July-September], and fall [October-December]) at baseline and follow-up (n=444), (2) in different seasons at baseline and follow-up (using the 4 noted seasons) (n=228), and (3) opposite times of year (e.g., January-March compared to July-September) between baseline and follow-up (n=49 of the 228). Similarly, analyses were repeated for participants who reported (1) no use of vitamin D supplements at baseline and follow-up (n=54), (2) vitamin D supplement use at baseline but not follow-up (n=18), (3) no vitamin D supplement use at baseline but vitamin D supplement use at follow-up (n=133), and (4) vitamin D supplement use at both time points (n=463). Participants were further collapsed into two groups representing women whose use of vitamin D supplements did not change over time (n=517) and women whose vitamin D supplement use did change over time (n=151) and analyses were repeated in these two groups.
Linear regression was conducted to understand the general magnitude of the relationship between 25(OH)D status at the two time points. Effect modification of the linear regression analysis of follow-up 25(OH)D concentrations on baseline 25(OH)D concentrations by season of blood draw (same season or different season of blood draw at both time points), as well as by supplement use (consistent use over time or change in use over time), were conducted. A p-value of <0.05 was considered statistically significant.
Lastly, deciles were created for baseline and follow-up 25(182 OH)D concentrations independently. Participants were also categorized at baseline and follow-up according to clinical cutpoints of vitamin D status defined by the recent IOM report: persons at risk for deficiency (<30 nmol/L), persons at risk for inadequacy (≥30 to <50 nmol/L) (18). Two categories were investigated as being adequate, ≥50 nmol/L to <75 nmol/L and ≥75 nmol/L. The cross tabulation of deciles and clinically defined cutpoints assessed at baseline and follow-up was examined to determine how an individuals’ placement in a category changed over time. Weighted kappa statistics using the Cicchetti-Allison methods for weights were calculated using both decile and clinical categories (18).
Data analysis and figures were completed using SAS® version 9.2.
RESULTS
Table 1 shows descriptive characteristics of our study sample at baseline. The mean age at baseline was 65.7 years and most of the participants were Caucasian and highly educated. The majority of participants were never smokers (55.1%) and current users of hormone therapy (51.2%). The mean BMI at baseline was 26.6 kilograms/meter2 (kg/m2) and mean physical activity levels at baseline were 14.5 metabolic equivalents task (MET) hours/week.
Table 1.
Descriptive characteristics of participants with plasma 25-hydroxyvitamin D (25(OH)D) concentrations at Osteoporosis and Periodontal Disease (OsteoPerio) Study baseline (1997-2000) and follow-up (2002-2005) (n=672).
| Age at visit (years), (mean ± standard deviation (SD)) | 65.7 ± 6.7 |
|---|---|
| Race/Ethnicity, n (%) | |
| Caucasian | 663 (98.7%) |
| African American, | 4 (0.6%) |
| American Indian/Alaskan Native | 4 (0.6%) |
| Asian/Pacific Islander | 1 (0.2%) |
|
| |
| Education Level, n (%) | |
| Some high school | 11 (1.6%) |
| High school diploma/GEDa | 121 (18.0%) |
| School after high school | 211 (31.4%) |
| College degree or higher | 321 (47.8%) |
| N Missing | 8 (1.2%) |
|
| |
| Smoking status, n (%) | |
| Current | 14 (2.1%) |
| Former | 288 (42.9%) |
| Never | 370 (55.1%) |
|
| |
| Hormone Therapy Use, n (% any use)b | 344 (51.2%) |
|
| |
| Body Mass Index (kg/m2) c, (mean ± SD) | 26.6 ± 4.9 |
|
| |
| Physical Activity (METd - hours/week), (mean ± SD) | 14.5 ± 14.4 |
General Equivalency Diploma
Current use of estrogen and/or progestin.
Kilograms/meters2
metabolic equivalents task
Table 2 shows the mean 25(OH)D concentrations, the percentage of individuals classified into clinical vitamin D levels, and the mean vitamin D intake from food and supplements at baseline and follow-up. The mean 25(OH)D concentration significantly increased between baseline (60.0 nmol/L) and follow-up (67.8 nmol/L). At follow-up as compared to baseline there were fewer participants classified as at risk for deficiency or inadequacy (19.8% versus 32%). The mean vitamin D intake from foods, the percentage of any vitamin D supplement use, and the percentage of high dose vitamin D supplements use increased from baseline to follow-up. As a result, the mean intake of vitamin 205 D from foods and supplements combined also increased from 536.9 to 775.7 IU/day.
Table 2.
Plasma 25-hydroxyvitamin D (25(OH)D) concentrations, season of blood draw, and vitamin D intake from foods and supplements at Osteoporosis and Periodontal Disease (OsteoPerio) Study baseline (1997-2000) and follow-up (2002-2005) among women with plasma 25(OH)D concentrations at both time points (n=672).
| Baseline | Follow up | |
|---|---|---|
| Plasma 25(OH)D concentrations (nmol/L), (mean ± SD) | 60.0 ± 22.2 | 67.8 ± 22.2* |
|
| ||
| Plasma 25(OH)D concentrations (nmol/L), (range) | 5.9 - 147.2 | 13.0 - 185.8 |
|
| ||
| Clinical 25(OH)D Levels, n (%) | ||
| At risk for deficiency (<30 nmol/L) | 58 (8.6%) | 22 (3.7%) * |
| At risk for inadequacy (≥30 to <50 nmol/L) | 157 (23.4%) | 108 (16.1%) |
| Adequate (≥50 to <75 nmol/L) | 305 (45.4%) | 317 (47.2%) |
| Adequate (≥75 nmol/l) | 152 (22.6%) | 225 (33.5%) |
|
| ||
| Season of blood draw, n (%) | ||
| January-March | 143 (21.3%) | 160 (23.8%) * |
| April-June | 163 (24.3%) | 180 (26.8%) |
| July-September | 182 (27.1%) | 168 (25.0%) |
| October-December | 184 (27.4%) | 164 (24.4%) |
|
| ||
| Vitamin D intake from foods (IU/day), (mean ± SD) | 200.5 ± 130.3 | 246.4 ± 245.2* |
|
| ||
| Vitamin D supplement usage, n (%) | ||
| None | 189 (28.1%) | 72 (10.7%)* |
| ≤ 400 IU/day | 324 (48.2%) | 237 (35.3%) |
| > 400 IU/day | 159 (23.7%) | 359 (53.4%) |
| N Missing | 0 | 4 (0.6%) |
|
| ||
| Total Vitamin D intake from foods and supplements | 536.9 ± 319.7 | 775.7 ± 439.1* |
| (IU/day), (mean ± SD) | ||
p-value <0.05 for paired t-test when comparing means of continuous variables or Bowker’s test of symmetry when comparing proportions of categorical variables at baseline vs. follow-up.
The distribution of changes in 25(OH)D (follow-up minus baseline) concentrations was examined. There were 42.4%, 69.9% and 86.6% participants whose 25(OH)D concentrations remained within a 10, 20, and 30 nmol/L range, respectively, between the two time points. Among participants whose blood was drawn in the same season at both time points, there were 44.4%, 71.9%, and 87.8% participants whose 25(OH)D concentrations remained within a 10, 20, and 30 nmol/L range, respectively.
In the overall sample, the Spearman r and 95% confidence interval (CI) was r=0.61 (95% CI: 0.56-0.66) and the intraindividual CV was 24.6% (Table 3). The ICC was modest at 0.59 (95% CI: 0.54-0.64). Individuals who had their blood draw in the same compared to different seasons at baseline and follow-up had slightly stronger reliability measures, as expected (Table 3). Among participants whose bloods were drawn in the same season, reliability measures were strongest among women whose bloods were drawn in summer and fall at both time points compared to participants whose bloods were drawn in winter and spring. Spearman correlations and ICCs were larger for participants who reported no vitamin D supplement use at both time points and for participants who reported use only at baseline. The largest CV, indicating the greatest intraindividual variation, was 33.7% among participants who reported use at follow-up but not baseline. When the data was combined to compare participants who did and did not change their vitamin D supplement use over time, the reliability measures were stronger in those with stable supplementation use over time, as expected.
Table 3.
Spearman correlation coefficients (r) and 95% confidence intervals (CIs), intraindividual coefficients of variation (CV), intra-class correlation coefficients (ICC) and 95% CIs are presented for repeat measures of 25-hydroxyvitamin D (25(OH)D) at baseline (1997-2000) and follow-up (2002-2005) among postmenopausal women from the Osteoporosis and Periodontal Disease (OsteoPerio) Study (n=672) with and without stratification by season of blood draw and supplement use patterns.
| Sample | N | Spearman r (95% CI) |
CV | ICC (95% CI) |
|---|---|---|---|---|
| All participants | 672 | 0.61 (0.56-0.66) | 24.6% | 0.59 (0.54-0.64) |
| Same seasona at baseline and follow-up | 444 | 0.68 (0.63-0.73) | 23.8% | 0.64 (0.58-0.69) |
| Same season: Winter at baseline and follow-up | 98 | 0.59 (0.44-0.71) | 27.7% | 0.60 (0.45-0.71) |
| Same season: Spring at baseline and follow-up | 116 | 0.66 (0.54-0.75) | 26.5% | 0.62 (0.50-0.72) |
| Same season: Summer at baseline and follow-up | 122 | 0.75 (0.66-0.82) | 19.3% | 0.63 (0.51-0.72) |
| Same season: Fall at baseline and follow-up | 108 | 0.68 (0.56-0.77) | 21.2% | 0.67 (0.56-0.77) |
| Different seasons at baseline and follow-up | 228 | 0.49 (0.38-0.58) | 26.0% | 0.48 (0.37-0.57) |
| Six months apart at baseline and follow-up | 49 | 0.58 (0.35-0.74) | 30.1% | 0.59 (0.38-0.74) |
| No vitamin D supplement useb at baseline or follow-up | 54 | 0.72 (0.56-0.73) | 25.5% | 0.65 (0.47-0.78) |
| Vitamin D supplement use at baseline but not follow-up | 18 | 0.72 (0.36-0.88) | 21.8% | 0.78 (0.52-0.91) |
| Vitamin D supplement use at follow-up but not baseline | 133 | 0.57 (0.45-0.68) | 33.7% | 0.48 (0.34-0.60) |
| Vitamin D supplement use at baseline and follow-up | 463 | 0.59 (0.52-0.64) | 21.3% | 0.55 (0.49-0.61) |
| No changec in vitamin D supplement use between baseline and follow-up |
517 | 0.62 (0.56-0.67) | 21.8% | 0.60 (0.55-0.66) |
| Vitamin D supplement use changed between baseline and follow-up |
151 | 0.57 (0.45-0.67) | 32.5% | 0.51 (0.39-0.62) |
Seasons are defined by the following four seasons: winter (January-March), spring (April-June), summer (July-September), and fall (October-December).
Four participants (2 users, 2 were non users) who reported vitamin D supplements use at baseline and are missing follow-up data and are excluded. Use of vitamin D supplements is defined as any use of a vitamin D supplement.
Change in vitamin D supplement use is defined as a person who reported using any amount of vitamin D supplements at baseline and reported using no vitamin D supplements at follow-up and vice versa.
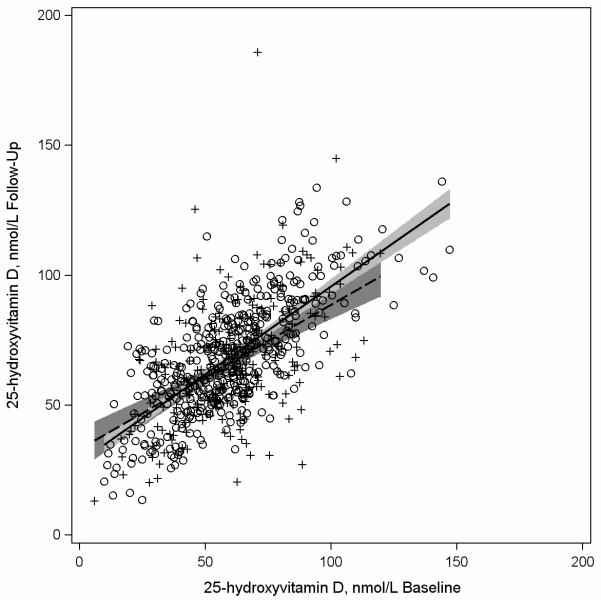
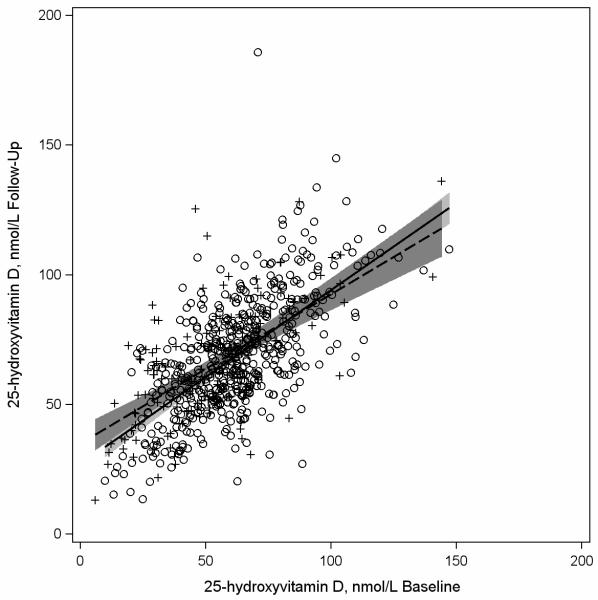
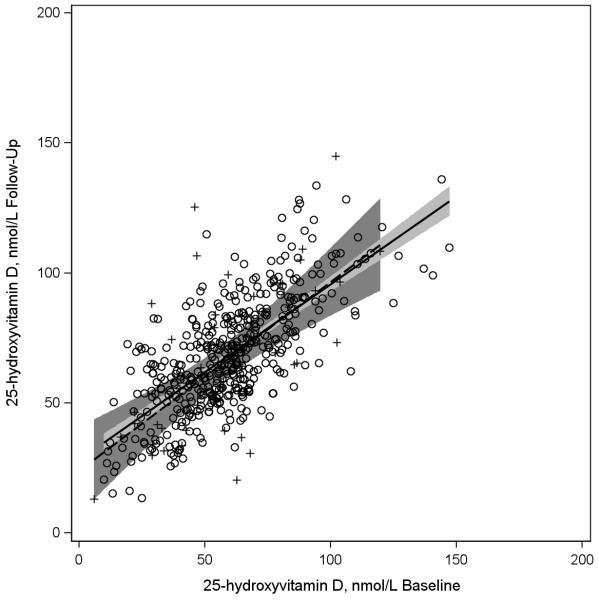
Figures 1-3 are scatter plots comparing baseline and follow-up concentrations of 25(OH)D. The depicted regression lines of 25(OH)D concentrations at follow-up regressed on 25(OH)D concentrations at baseline describe the magnitude of the 228 relationship among participants whose bloods were drawn in similar (n=444) compared to different (n=228) seasons at both time points (Figure 1), among participants whose bloods were drawn in similar seasons (n=444) compared to opposite times of year at baseline and follow-up (n=49) (Figure 2), and among participants who had similar (n=517) and different (n=151) vitamin D supplement use patterns at baseline and follow up (Figure 3). In Figure 1 the regression lines are slightly different and the interaction between baseline 25(OH)D concentrations and season of blood draw (similar or different at both time points) was borderline statistically significant at p=0.059. In Figure 2, the regression lines are more similar to each other than depicted in Figure 1 and the interaction between baseline 25(OH)D concentrations and season of blood draw was p=0.60. In Figure 3 the regression lines for participants with consistent and inconsistent supplement use over time were also similar to each other and the interaction between baseline 25(OH)D concentrations and consistent supplement use over time (yes, no) was p=0.16.
Figure 1.
Scatterplot of plasma 25-hydroxyvitamin D (25(OH)D) concentrations assessed at follow-up (2002-2005) versus baseline (1997-2000) among participants in the Osteoporosis and Periodontal Disease (OsteoPerio) Study (n=672) with least squares regression linesa representing participants with bloods drawn in the same (n=444) and different (n=228) seasonsb at baseline and follow-up.
a Solid regression line and circles (O) represents women with bloods drawn at baseline and follow-up in the same season. Dashed regression line and plus signs (+) represents women with bloods drawn in different seasons at baseline and follow-up.
b Seasons are defined by the following four seasons: winter (January-March), spring (April-June), summer (July-September), and fall (October-December).
Figure 3.
Scatterplot of plasma 25-hydroxvitamin D (25(OH)D) concentrations assessed at follow-up (2002-2005) versus baseline (1997-2000) among participants in the Osteoporosis and Periodontal Disease (OsteoPerio) Study (n=672) with least squares regression linesa representing participants with similar (n=517) and different (n=151) self-reported vitamin D supplement useb at baseline and follow-up.
a Solid regression line and circles (O) represents women with similar self-reported vitamin D supplement use at baseline and follow-up. Dashed regression line and plus signs (+) represents women with different self-reported vitamin D supplement use at baseline and follow-up.
b Similar self-reported vitamin D supplement use included women who reported use at both baseline and follow-up or no use at both time points. Different self-reported vitamin D supplement use included women who reported use at baseline but no use at follow-up or no use at baseline and use at follow-up.
Figure 2.
Scatterplot of plasma 25-hydroxvitamin D (25(OH)D) concentrations assessed at follow-up (2002-2005) versus baseline (1997-2000) among participants in the Osteoporosis and Periodontal Disease (OsteoPerio) Study (n=672) with least squares regression linesa representing participants with bloods drawn in the same (n=444) seasonb and at opposite times of yearb (n=49) at baseline and follow-up.
a Solid regression line and circles (O) represents women with bloods drawn at baseline and follow-up in the same season. Dashed regression line and plus signs (+) represents women with bloods drawn at opposite times of year at baseline and follow-up.
b Seasons are defined by the following four seasons: winter (January-March), spring (April-June), summer (July-September), and fall (October-December). There were 444 women with bloods drawn at baseline and follow-up in the same season and 49 women with bloods drawn at baseline and follow-up at opposite times of year (January-March compared to July-September or April-June compared to October-December).
Weighted kappas were calculated comparing agreement between deciles and clinical vitamin D status at each time point. The weighted kappa was 0.42 (95% CI: 0.38-0.47) and 0.39 (95% CI: 0.34-0.44) using deciles and clinical vitamin D status, respectively. Among women with bloods drawn in the same season of blood draw (fall, winter, summer or spring), the weighted kappas were 0.48 (95% CI: 0.43-0.54) for deciles and 0.42 (95% CI: 0.36-0.49) for clinical vitamin D status. Table 4 shows the concordance of classification using clinical categories for plasma 25(OH)D concentrations at baseline and at follow-up. There was greater concordance among those who were adequate at baseline and less concordance among those at risk for deficiency or insufficiency.
Table 4.
Concordance of classification of clinical categories of plasma 25-hydroxvitmain D (25(OH)D) concentrations at follow-up (2002-2005) based on clinical categories at baseline (1997-2000) among 672 participants in the Osteoporosis and Periodontal Disease (OsteoPerio) Study. N and row percent presented.
| Follow-up: At Risk for Deficiency (<30 nmol/L) |
Follow-up: At Risk for Inadequacy (≥30 to <50 nmol/L) |
Follow-up: Adequate (≥50 to <75 nmol/L) |
Follow-up: Adequate (≥75 nmol/L) |
|
|---|---|---|---|---|
| Baseline: At Risk for Deficiency (<30 nmol/L) |
14 (24.1%) |
22 (37.9%) |
20 (34.5%) |
2 (3.5%) |
| Baseline: At Risk for Inadequacy (≥30 to <50 nmol/L) |
6 (3.8%) |
52 (33.1%) |
80 (51.0%) |
19 (12.1%) |
| Baseline: Adequate (≥50 to <75 nmol/L) |
1 (0.3%) |
30 (9.8%) |
176 (57.7%) |
98 (32.1%) |
| Baseline: Adequate (≥75 nmol/L) |
1 (0.7%) |
5 (3.3%) |
41 (27.0%) |
106 (69.7%) |
DISCUSSION
In this study we evaluated intraindividual variability of plasma 25(251 OH)D concentrations measured approximately 5 years apart in a group of 672 postmenopausal women. The mean 25(OH)D concentration increased (60.0 to 67.8 nmol/L) and the percent of women classified as either at risk for deficiency or inadequacy (<50 nmol/L) decreased (32% to 19.8%) over time. Comparing 25(OH)D concentrations at baseline and follow-up, we observed a moderate ICC of 0.59 (95% CI: 0.54-0.64), an intraindividual CV of 24.6%, and a moderate Spearman r of 0.61 (95% CI=0.56-0.66). The weighted kappa comparing the same clinical status cutpoints for vitamin D status at baseline and follow-up was fair at 0.39 (95% CI: 0.34-0.44). Together, these data suggest there is moderate intraindividual variability of 25(OH)D concentrations over a five year period. Overall, these data support that a one-time assessment of plasma 25(OH)D adequately reflects vitamin D status over a five year period in postmenopausal women.
Reliability measures were also examined in participants with similar and different seasons of blood draw at baseline and follow-up. The reliability of repeat 25(OH)D measures in different seasons at baseline and follow-up were comparable, although slightly weaker, to the reliability of repeat measures of 25(OH)D when blood was drawn in the same season at both time points. The correlation coefficients observed in our study between repeat 25(OH)D measures in different seasons (r=0.49) and opposite seasons (r=0.58) were slightly higher than the correlation coefficient observed in a previous study (r=0.462) (19) that measured 25(OH)D concentrations in 121 Japanese women (aged 45-81 years) in both summer and winter months approximately 1.5 years apart. Other previously noted studies (4-8) on the variation in blood measures of 25(OH)D over time did not present data comparing the variation in blood measures drawn in different seasons.
The reliability of repeat 25(OH)D measures among participants with different supplementation practices over time was also compared. Overall, reliability measures were greater among participants with stable supplementation patterns over time compared to those whose supplementation patterns changed over time. We did observed strong reliability measures among the 18 participants who reported using vitamin D supplements at baseline but not follow-up, but this subset had a relatively small sample size. Although we did not observe tremendous difference in 5-year variation in serum vitamin D concentrations within strata of seasonal and supplemental vitamin D use, there appears to be some. Future studies should include collection of information on seasonal and supplemental vitamin D use to further examine the potential modifying effect these factors could have on etiologic associations between serum vitamin D exposures assessed at a single time point and future disease risk.
This is the second largest study to our knowledge that has assessed intraindividual variability in 25(OH)D concentrations. Our results support findings from previous work (4-6, 8), which concluded that vitamin D concentrations are reliable over extended periods of time (e.g., approximately 5 years). We observed a Spearman r of 0.68 between 25(OH)D concentrations taken 5 year apart in the same season among 444 participants. This is higher than the Spearman correlation (r=0.53) between two same season measures of plasma 25(OH)D separated by five years reported by Hoffman et al. (6) in 29 men and women (55-70 years). Jorde et al. (7) also reported a similar correlation (Pearson r=0.52) between two measures of plasma 25(OH)D concentrations in 759 Norwegian men and women (50-74 years) with measures assessed at the same time of the year 14 years apart.
We observed an intra-individual CV of 24.6% that was slightly higher than what has been presented previously in the literature. Renjmark et al. (5) reported an intra-individual CV of 16% for repeat measures over 4 years in 187 peri-and postmenopausal women. 296 Kotsopoulos et al. (8) reported an intra-individual CV of 18.4% with bloods drawn 3 times over a 3 year period. Similarly, a CV of 14.9% was reported in the earlier mentioned study by Hoffman et al. (6). We also observed a slightly lower ICC (0.59) than that reported by Hoffman et al. (6) of 0.71, and Kotsopoulos et al. (8) of 0.72, but the ICC in our study increased to 0.64 when our sample was limited to women with blood draws in the same season.
In our study, we found that 42.4% of the sample had a change in 25(OH)D concentrations of less than 10 nmol/L over 5 years. Similarly, Jorde et. al. (7) observed that 45.8% of their participants also had a change in 25(OH)D plasma measurements of less than 10 nmol/L over 14 years. Therefore, over a 5 to 14 year period it appears that ~40% of individuals will have minimal (<10 nmol/L) changes in their 25(OH)D concentrations.
We observed an increase in mean 25(OH)D concentrations from baseline to follow-up from 60.0 to 67.8 nmol/L even though one might expect a decrease in concentrations with increasing age. This suggests that, in some women, true change in 25(OH)D concentrations is occurring over time. However, the agreement measures still suggest that this change moderately influences ranking of women in the cohort with respect to 25(OH)D concentrations. The observed mean increase in 25(OH)D concentrations over time could be explained in part by the observed increases in vitamin D intake from foods and supplements from baseline to follow-up. This change could also be explained by an interaction between increased vitamin D intake and baseline 25(OH)D concentrations. Additionally, changes in other factors over time such as sun exposure practices or percent body fat may also influence 25(OH)D status. To what extent certain participant characteristics and behaviors can explain change in 25(OH)D between baseline and follow-up was not the primary focus of this study but remains to be better understood. Previous work in WHI participants (20) showed that only 21% of the variation in 25(OH)D concentrations could be explained by the factors of season, vitamin D intake, waist circumference, recreational physical activity, race-ethnicity, regional solar irradiance and age, with age explaining the least amount of variation among all factors. It seems unlikely that assay drift could explain our results as blind duplicates run consistently throughout the 4 months of 25(OH)D assessment had a CV of 4.9%. Explication of factors explaining change in vitamin D status over time and potential approaches to limit or correct their influence on longitudinal vitamin D status deserves further study.
This study has a number of strengths and limitations. One strength is our study’s large sample size. This is the largest study (n=672) to date assessing intraindividual 25(OH)D variability in postmenopausal women. The current study helps to interpret and analyze past and future epidemiologic studies of 25(OH)D and disease, particularly studies in postmenopausal women. Intraindividual variability in 25(OH)D, when used as an exposure for disease occurrence, likely would result in an attenuated estimates of association (e.g., relative risk) (21). However, it is beyond the scope of this study to determine the nature and magnitude of the potential bias.
Second, the same lab and assay, with high quality control measures, was used to determine both baseline and follow-up plasma 25(OH)D concentrations over the same 4 month period. Measuring samples over a continuous 4 month period may minimize lab drifts which may occur if samples were measured 5 years apart. Third, bloods were drawn in the majority (66%) of participants in the same season at both time points. Additionally, all women in our study were from the same latitude, which helped control, in part, for confounding from sun exposure.
Both a strength and a limitation of the current study is the time between 25(OH)D measurements. While five years is a significant amount of time that allows for 25(OH)D concentrations to fluctuate, it is only a small portion of an individual’s life. Vitamin D status may fluctuate more over longer periods of time. Therefore, studies with repeat measures of vitamin D over longer time periods are warranted.
Another limitation is that our study results may only be applicable to Caucasian women living in Northern latitudes who use vitamin D supplements. Our study participants were all from the Buffalo, NY area and the majority were consistent supplement users of vitamin D over time (71.9% at baseline and 88.7% at follow-up). Population data from the National Health and Nutrition Examination Survey (NHANES) (22) showed that 49.7% of women 60 years and older used vitamin D supplements from 1999-2002 and this number increased to 56.3% from 2003-2006. Although the women in the present study have a higher prevalence of supplement use, both NHANES and the OsteoPerio study showed that older women were increasing their vitamin D supplement use over comparable time periods. The higher level of supplement use, in addition to the primarily Caucasian sample limits generalizability of study results. Inferences about intraindividual variability over time in other racial/ethnic groups should be made with caution.
Based on the present study findings, we conclude that using a one-time assessment of 25(OH)D concentrations moderately reflects vitamin D status of individuals over an approximate 5 year period among individuals living at a similar latitude. Reliability of repeat measures appears to be moderately influenced by vitamin D supplement use and season of blood draw. Additional studies are warranted to 1) capture 25(OH)D trends using more frequent serial measurements of blood 25(OH)D concentrations and over longer periods of time, 2) to better understand the ranking of individuals when 25(OH)D measures are taken in extreme seasons, and 3) to better understand the reliability of measures in racially/ethnically diverse groups.
ACKNOWLEDGEMENTS
We thank the women who generously contributed their time to participate in the OsteoPerio Study.
Amy E. Millen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
GRANT SUPPORT
This research is supported by NIH grants 1R01DE13505 (awarded to J Wactawski-Wende), 1R21DE020918 (awarded to AE Millen) from the National Institute of Dental and Craniofacial Research (NIDCR) and a grant awarded to J Wactawski-Wende from the Department of Defense (DAMD179616319).
The WHI Program is supported by Contracts from the National Heart, Lung and Blood Institute, NIH (N01WH32122). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Footnotes
DISCLOSURES/CONFLICTS OF INTEREST
A.E. Millen is a Co-investigator on a vitamin D related grant funded by the Mushroom Council (#10008). R.L. Horst is the Owner/Director of Heartland Assay, LLC.
All other authors have no disclosures or conflicts of interest.
REFERENCES
- 1.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. The National Academy Press; Washington DC: 2011. [Google Scholar]
- 2.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–91S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 3.Barragry JM, France MW, Corless D, Gupta SP, Switala S, Boucher BJ, et al. Intestinal cholecalciferol absorption in the elderly and in younger adults. Clin Sci Mol Med. 1978;55:213–20. doi: 10.1042/cs0550213. [DOI] [PubMed] [Google Scholar]
- 4.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–65. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 5.Rejnmark L, Lauridsen AL, Brot C, Vestergaard P, Heickendorff L, Nexo E, et al. Vitamin D and its binding protein Gc: long-term variability in peri- and postmenopausal women with and without hormone replacement therapy. Scand J Clin Lab Invest. 2006;66:227–38. doi: 10.1080/00365510600570623. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19:927–31. doi: 10.1158/1055-9965.EPI-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–8. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 8.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–46. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 10.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 11.Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76:2116–24. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]
- 12.Bole C, Wactawski-Wende J, Hovey KM, Genco RJ, Hausmann E. Clinical and community risk models of incident tooth loss in postmenopausal women from the Buffalo Osteo Perio Study. Community Dent Oral Epidemiol. 2010;38:487–97. doi: 10.1111/j.1600-0528.2010.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–8. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 15.Dawson-Hughes B, Harris S. Regional changes in body composition by time of year in healthy postmenopausal women. Am J Clin Nutr. 1992;56:307–13. doi: 10.1093/ajcn/56.2.307. [DOI] [PubMed] [Google Scholar]
- 16.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2nd ed. Jones and Bartlett Publishers, LLC; Sudbury, MA: 2007. Quality Assurance and Control; pp. 297–347. [Google Scholar]
- 17. [accessed 14 February 2012];How should I calculate a within-subject coefficient of variation? Internet: http://www-users.york.ac.uk/~mb55/meas/cv.htm.
- 18.Armstrong BK, White E, Saracci R. Principles of exposure measurement in epidemiology. Oxford University Press; Oxford, United Kingdom: 1994. [Google Scholar]
- 19.Nakamura K, Nashimoto M, Yamamoto M. Summer/winter differences in the serum 25-hydroxyvitamin D3 and parathyroid hormone levels of Japanese women. Int J Biometeorol. 2000;44:186–9. doi: 10.1007/s004840000067. [DOI] [PubMed] [Google Scholar]
- 20.Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, Liu S, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women’s Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–35. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol. 1992;136:1400–13. doi: 10.1093/oxfordjournals.aje.a116453. [DOI] [PubMed] [Google Scholar]
- 22.Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, et al. NCHS data brief, no 61. National Center for Health Statistics; Hyattsville, MD: 2011. Dietary supplement use among U.S. adults has increased since NHANES III (1988-1994) [PubMed] [Google Scholar]