Abstract
Objectives
To determine secular trends by birth decade in body mass index (BMI), waist circumference/height (W/Ht), percent body fat (PBF), and fat free mass adjusted for height squared (FFM/Ht2) in children and adolescents aged 8–18 years.
Methods
Serial data were analyzed from 628 boys and 591girls aged 8 to 18 years who participated in the Fels Longitudinal Study. Subjects were stratified by birth decade from 1960 through 1999. Means and standard deviations were computed for all measurements by birth decade, age and sex. A repeated-measures analysis of variance was used data to ascertain secular trends separately for boys and girls.
Results
Boys and girls born in the 1990s had significantly higher mean BMI, W/Ht and PBF than did children born in previous decades. Mean FFM/Ht2 was significantly smaller in boys born in the 1990s than boys of the same age born in earlier decades. No secular trend was noted in FFM/ Ht2 in girls by decade of birth.
Conclusion
Our analysis of serial data collected over four decades confirms the secular trend in childhood BMI previously observed in successive cross-sectional studies. Our analysis discloses significant positive secular trends in W/Ht and PBF in both boys and girls and a significant negative secular trend in FFM/Ht2 in boys over the last four decades of the 20th century. The secular changes presage increases in the prevalence of conditions associated with childhood and adolescent obesity – such as hypertension, glucose intolerance, and dyslipidemia – that may appear as early as the second decade of life.
Keywords: Body Composition, Obesity, Secular Trends, Body Mass Index
INTRODUCTION
Overweight or obesity affects two of three American adults and one in three of their children. Nearly twenty years ago Kuczmarski et al., (1994) reported that obesity had become the most common and costly nutritional problem in the United States. Finkelstein et al., (2009) addressed the issue of cost and found that the annual medical expenditures in the US for obesity and its associated conditions amounted to $148 million. In an effort to elucidate the epidemic of obesity in children and adolescents Ogden et al., (2008) analyzed cross-sectional data from the National Health and Nutrition Examination Surveys (NHANES) and found that the prevalence of obesity [defined as a body mass index (BMI) > 95th percentile for age and sex] among US children and adolescents had more than doubled between 1980 and 2004, and in 2006 had reached a mean of 17%. Obesity frustrates health care providers because, once established, it is difficult to reverse, and it threatens the metabolic and cardiovascular health of both adults and children.
It is an interesting question to ascertain when the epidemic of childhood obesity began in the US and if secular trends in the prevalence of obesity can be determined by analyzing longitudinal or serial cross-sectional data sets. A secular trend is defined as differences between individuals or groups of the same age and sex that are associated with year or decade of birth (Roche 1979). Various criteria have been used for determining secular trends (Bock 1978; Kuczmarski et al., 1994; Ogden et al., 2010; Troiano et al., 1995). Bock described secular trends in height based upon inter-generational differences between same-sex parents and children at corresponding ages (Bock 1978). Cross-sectional data from repeated NHANES from 1971–2006 disclose an increased prevalence of BMI > 95th percentile for all ages in boys and girls across ethnic groups (Ogden et al., 2010).
To date, studies of secular trends toward greater prevalence of overweight and obesity have been based on BMI data in repeated cross-sectional studies. However, there are limitations regarding the use of BMI as an index of obesity. Although BMI is accepted as an index of adiposity largely independent of stature in adults, this attribute of BMI does not necessarily apply to children (Garn et al., 1986). In children, the relationships between BMI and the fat and fat-free mass of the body are complicated by varying rates of growth and levels of maturity (Daniels et al., 1997). It has not been determined whether the secular increase observed in the prevalence of BMI in children is due to an increase in fat mass, fat-free mass, or both.
Visceral adiposity is the most metabolically active compartment of adipose tissue in the body (Ibrahim, 2010), and waist circumference serves as a proxy for estimating the size of this regional fat deposit (Romaguera et al., 2010). Savva et al., (2000) reported that waist circumference and waist-for-height ratios in children function better as predictors of risk factors for cardiovascular disease than BMI; and Hurt et al., (2010) noted that while BMI alone is of limited utility for predicting adverse cardiovascular outcomes, its predictive power is strengthened when combined with waist circumference.
In this study we aim to ascertain if there are secular trends in waist circumference/height (W/Ht), percent body fat (PBF), height-adjusted fat free mass (FFM/Ht2), and BMI by using anthropometric and direct measurements of body composition collected annually from the 1960s to the present. These measurements are obtained from the Fels Longitudinal Study (FLS), in which secular trends in height have been previously documented (Roche 1992). These long-term serial data can be used to determine the temporal origins of the increase in prevalence of overweight and obesity in children over the past four decades.
SAMPLE AND METHODS
Study Sample
Data for the present study were collected from 628 white boys and 591 white girls between 8 to 18 years of age, all of whom are participants in the FLS, an ongoing study that began in 1929 and that has been described in detail by Roche (1992). Participants were enrolled at birth and were not selected with regard to factors known to be associated with body composition. Participants are drawn from a range of socioeconomic groups that reflect national SES prevalences, except that the low end of the SES scale is under-represented among those born after 1950. The Institutional Review Boards at Wright State University and Virginia Commonwealth University approved all procedures.
Measurements
Anthropometric measurements are taken following recommendations in the Anthropometric Standardization Reference Manual (Lohman et al., 1988). Stature is measured to 0.1 cm on a Holtain stadiometer (Seritex, Carlstadt, NJ), weight is measured to 0.1 kg on a beam balance scale, and waist circumference (cm) is measured at the suprailiac crest. All measurements are made twice, and a third measurement is made if the difference between the first two exceeds established tolerance (0.3 kg for weight, 0.5 cm for height, and 0.1 cm for waist circumference). BMI was expressed as kg/m2, while W/Ht was expressed as cm/m.
Body composition comprises bone mass and soft tissue mass. Soft tissue mass includes fat mass and lean mass. PBF is calculated by dividing the fat mass by the total body mass. Measures of PBF and FFM were obtained from a multicomponent model of body composition derived from data generated by hydrodensitometry and dual energy x-ray absorptiometry (DXA). Body density was determined from hydrostatic weighing, and residual volume was measured on a Gould 2100 computerized spirometer (Guo et al., 1989). In the multicomponent model of body composition, the density of the fat-free component varied due to changes in body water and bone mineral content (Guo et al., 1989; Sun et al., 2003). Therefore, we smoothed the density of FFM by using a fixed-knot cubic spline technique and calculated FFM from body density using these smoothed values (Guo et al., 1989). Measurements were also made of total body fat (TBF) and FFM by a DXA Hologic™ QDR 4500 Elite densitometer (Hologic, Waltham, MA) and a Lunar LPX machine. The coefficient of variation (CV) was 3.5% for soft tissue. Our DXA procedures have been compared and calibrated with those of underwater weighing (uww) (Guo et al., 1997). Conversion equations for each component are: TBFuww = −2.1582 + (1.1533 × TBFdxa); FFMuww = 1.8449 + (0.9329 × FFMdxa); PBFuww = −2.0337 + (1.1285 × PBFdxa). In regard to cross-calibrating the Hologic and Lunar DXA machines, DXA data were collected from 78 FLS subjects who were scanned on the same day with both the Hologic 4500 and Lunar LPX machines. The calibration equations are: HologicPBF = 5.6397 + (0.7908 × LunarPBF); R2=0.98 and SE=3.68; HologicTBF = 3.0529 + (0.8439 × LunarTBF); R2= 0.98 and SE=1.47; HologicFFM = 0.7917 + (1.0349 × LunarFFM); R2 = 0.94 and SE=4.85. FFM/Ht2 was calculated from the consolidated measure of FFM and height as FFM/stature2 in kg/m2.
Statistical Methods
Measurements of BMI, W/H, PBF and FFM/Ht2 collected from 8 to 18 years were included in the analysis. We divided subjects into four birth decades from 1960 through 1999: 1/1/1960-12/31/1969, 1/1/1970-12/31/1979, 1/1/1980-12/31/1989, and 1/1/1990-12/31/1999. In 1974, direct measurements of body composition (PBF and FFM/Ht2) were initiated among participants 8 years of age and older, so that children whose body composition was measured directly were born after 1965. Therefore, there were fewer boys and girls with complete sets of body composition data than the total sample size. The actual sample sizes available for summarization and analysis are reported in Tables 1 through 4. The missing body composition data did not affect the analysis of secular trends in BMI or W/Ht over four decades, but did limit the analyses of secular trends in PBF and FFM/Ht2 to 3.5 decades instead of 4.
Table 1.
Body Mass Index (BMI), kg/m2) by Age from 8 to 18 Years and Decades of Birth
| Age | Birth Decade (BD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | 1960≤BD<1970 | 1970≤BD<1980 | 1980≤BD<1990 | 1990≤BD<2000 | ||||||||
| Boys | n | mean | std | n | mean | std | n | mean | std | n | mean | std |
| 8≤age<9 | 68 | 16.18 * | 1.99 | 57 | 15.94 * | 1.83 | 43 | 16.06 | 2.51 | 35 | 16.90 | 3.01 |
| 9≤age<10 | 65 | 16.73 * | 2.56 | 46 | 16.87 * | 2.56 | 43 | 17.00 | 3.24 | 30 | 17.61 | 4.31 |
| 10≤age<11 | 73 | 17.02 * | 2.44 | 62 | 16.94 * | 2.89 | 46 | 17.71 | 3.80 | 37 | 18.77 | 5.05 |
| 11≤age<12 | 73 | 17.53 * | 2.61 | 59 | 17.77 * | 3.28 | 48 | 18.06 | 3.71 | 36 | 18.41 | 3.92 |
| 12≤age<13 | 70 | 18.15 * | 3.04 | 60 | 18.22 * | 3.27 | 41 | 19.15 | 4.41 | 41 | 20.30 | 4.80 |
| 13≤age<14 | 64 | 18.76 * | 3.47 | 58 | 19.01 * | 3.46 | 44 | 19.73 | 4.53 | 30 | 20.65 | 4.66 |
| 14≤age<15 | 64 | 19.64 * | 3.56 | 59 | 20.06 * | 3.73 | 51 | 20.97 | 4.77 | 29 | 22.12 | 5.77 |
| 15≤age<16 | 61 | 20.19 * | 3.36 | 58 | 20.76 * | 4.10 | 41 | 21.05 | 4.38 | 29 | 22.72 | 5.22 |
| 16≤age<17 | 52 | 21.38 * | 4.26 | 43 | 20.95 * | 4.20 | 40 | 22.20 | 5.01 | 20 | 22.70 | 4.75 |
| 17≤age<18 | 48 | 21.73 * | 4.40 | 43 | 21.40 * | 4.29 | 44 | 22.26 | 4.45 | 15 | 22.18 | 3.79 |
| Girls | ||||||||||||
| 8≤age<9 | 72 | 16.39 * | 2.08 | 51 | 16.78 * | 2.14 | 57 | 16.52 * | 2.48 | 34 | 18.35 | 6.65 |
| 9≤age<10 | 81 | 16.93 * | 2.27 | 51 | 17.11 * | 2.49 | 54 | 16.83 * | 2.67 | 27 | 18.10 | 3.70 |
| 10≤age<11 | 73 | 17.54 * | 2.46 | 53 | 17.94 * | 2.59 | 55 | 17.84 * | 3.00 | 24 | 18.70 | 3.67 |
| 11≤age<12 | 75 | 17.96 * | 2.45 | 55 | 18.38 * | 2.83 | 57 | 18.66 * | 3.42 | 19 | 18.81 | 3.14 |
| 12≤age<13 | 74 | 19.06 * | 2.84 | 52 | 19.59 * | 3.19 | 57 | 19.83 * | 3.64 | 18 | 19.83 | 3.44 |
| 13≤age<14 | 73 | 20.13 * | 2.97 | 51 | 20.10 * | 2.90 | 46 | 20.69 * | 3.83 | 20 | 20.63 | 2.88 |
| 14≤age<15 | 55 | 21.05 * | 3.05 | 38 | 20.39 * | 3.35 | 46 | 21.88 * | 3.98 | 15 | 22.57 | 3.26 |
| 15≤age<16 | 45 | 21.32 * | 3.31 | 45 | 21.09 * | 3.03 | 28 | 22.30 * | 3.82 | 19 | 22.81 | 3.69 |
| 16≤age<17 | 53 | 21.90 * | 2.99 | 42 | 21.37 * | 3.27 | 40 | 22.29 * | 3.77 | 11 | 22.54 | 4.51 |
| 17≤age<18 | 55 | 21.63 * | 3.38 | 38 | 21.65 * | 2.67 | 48 | 23.87 * | 5.32 | 11 | 23.37 | 5.29 |
Mean BMI significantly less than mean BMI for 1990–2000 (p-value < 0.05).
Table 4.
Fat-free Mass/Height2 (kg/m2) by Annual Age from 8 to 18 Years and Decades of Birth.
| Age | Birth Decade (BD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | 1960≤BD<1970 | 1970≤BD<1980 | 1980≤BD<1990 | 1990≤BD<2000 | ||||||||
| Boys | n | mean | std | n | mean | std | n | mean | std | n | mean | std |
| 8≤age<9 | 6 | 14.09 | 1.05 | 6 | 14.53 | 2.00 | 35 | 13.46 | 1.33 | 35 | 14.01 | 1.58 |
| 9≤age<10 | 10 | 14.76 | 1.33 | 21 | 13.64 | 1.08 | 41 | 13.92 | 1.71 | 28 | 14.18 | 1.95 |
| 10≤age<11 | 9 | 14.93 | 0.88 | 32 | 13.67 | 1.40 | 34 | 14.28 | 1.36 | 35 | 14.18 | 1.87 |
| 11≤age<12 | 20 | 14.37 | 0.76 | 41 | 13.99 | 1.33 | 45 | 14.23 | 1.58 | 32 | 14.36 | 1.68 |
| 12≤age<13 | 28 | 15.28 | 1.40 | 39 | 14.66 | 1.82 | 37 | 15.03 | 1.74 | 37 | 15.04 | 1.94 |
| 13≤age<14 | 24 | 15.59 | 1.94 | 42 | 15.20 | 2.04 | 34 | 15.49 | 1.97 | 28 | 15.65 | 1.98 |
| 14≤age<15 | 25 | 16.69* | 1.91 | 37 | 16.36 | 2.03 | 47 | 16.72 | 2.26 | 28 | 16.35 | 2.47 |
| 15≤age<16 | 27 | 17.90* | 2.07 | 44 | 17.56 | 2.18 | 38 | 17.28* | 2.41 | 23 | 16.62 | 1.85 |
| 16≤age<17 | 38 | 19.01* | 2.10 | 40 | 17.76 | 2.51 | 36 | 18.41* | 2.37 | 19 | 17.61 | 2.24 |
| 17≤age<18 | 26 | 18.95* | 1.62 | 43 | 18.49 | 2.21 | 43 | 18.72* | 2.11 | 14 | 17.71 | 2.29 |
| Girls | ||||||||||||
| 8≤age<9 | 5 | 12.59 | 1.48 | 8 | 11.97 | 1.97 | 38 | 13.63 | 1.50 | 30 | 13.21 | 1.54 |
| 9≤age<10 | 4 | 13.72 | 1.27 | 20 | 13.44 | 1.50 | 38 | 13.36 | 1.74 | 25 | 13.31 | 1.22 |
| 10≤age<11 | 6 | 14.36 | 1.00 | 30 | 13.69 | 1.46 | 49 | 13.62 | 1.85 | 21 | 13.67 | 1.38 |
| 11≤age<12 | 15 | 14.02 | 1.42 | 33 | 13.84 | 1.56 | 48 | 14.13 | 2.16 | 15 | 13.76 | 1.61 |
| 12≤age<13 | 19 | 14.72 | 1.50 | 34 | 14.40 | 1.71 | 47 | 14.57 | 1.86 | 17 | 14.16 | 1.66 |
| 13≤age<14 | 26 | 15.13 | 1.49 | 34 | 15.14 | 1.75 | 41 | 15.49 | 2.18 | 17 | 15.18 | 1.26 |
| 14≤age<15 | 30 | 15.86 | 1.22 | 32 | 15.48 | 1.69 | 41 | 16.07 | 1.96 | 15 | 15.52 | 1.23 |
| 15≤age<16 | 22 | 16.25 | 1.67 | 42 | 16.04 | 1.46 | 28 | 16.29 | 1.92 | 17 | 15.71 | 1.74 |
| 16≤age<17 | 27 | 16.34 | 1.41 | 39 | 15.89 | 1.5 | 40 | 15.95 | 2.07 | 10 | 15.43 | 2 |
| 17≤age<18 | 30 | 16.11 | 1.51 | 36 | 16.12 | 1.64 | 47 | 16.39 | 2.11 | 10 | 16.04 | 2.08 |
Mean BMI significantly less than mean BMI for 1990–2000 (p-value < 0.05).
We used a mixed-effect repeated measure analysis of variance (ANOVA) model to test for secular trends in BMI, W/Ht, PBF, FFM/Ht2 separately in boys and girls. Childhood age, birth decade classification and the interaction between childhood age and birth decade classification were included as fixed effects, while a subject-specific random effect was used to account for within-subject dependence, which was modeled using an auto-regressive correlation pattern (Laird and Ware, 1982). Childhood age was included as a third-degree polynomial for BMI, W/Ht and FFM/Ht2, since the growth curves for those measures were non-linear (Guo et al., 2000), while childhood age was included as a two-degree polynomial for boys’ PBF and as a one-degree polynomial for girls’ PBF. The polynomial degree to which childhood age was modeled was chosen by minimizing Akaike’s Information Criterion (Akaike 1974), which measures the adequacy of the model fit to the data while penalizing for model complexity. We used a 5% significance level for all comparisons. The values of BMI, W/Ht, PBF and FFM/Ht2 were summarized with means and standard deviations for each childhood age, birth decade and sex. All analyses were performed using the MIXED procedure in the SAS statistical software (v. 9.2, Cary, NC, U.S.A.). The computation was performed using the mixed procedure in SAS (Statistical Analysis Software Institute Inc. 2008).
RESULTS
We uncovered significant secular trends among children and adolescents in the FLS who were born from 1960 through 1999. Cohorts born in the 1960s and 1970s were leaner during late childhood and adolescence than cohorts born in the 1980s and 1990s. This secular trend was significant over a period of four decades in both boys and girls.
In our longitudinal model, we evaluated age-related linear and curvilinear trends and their interactions with decades of birth. BMI increased curvilinearly from 8 to 18 years of age in both boys and girls. In girls, a secular trend of increasing values for weight was detected from 1960 through 1999, and the secular trend varied by age in that the most pronounced differences were detected at older ages. In both sexes mean W/H differed significantly between cohorts born in the 1960s and the 1990s and between cohorts born in the 1970s and the 1990s. Our observations show that boys born from 1970 through 1999 exhibited a large increase in mean body fat. We also found a significant secular increase in PBF by decade in girls. Girls born in the 1970s had greater PBF than those born in the 1960s, and they were also heavier and taller.
Body Mass Index
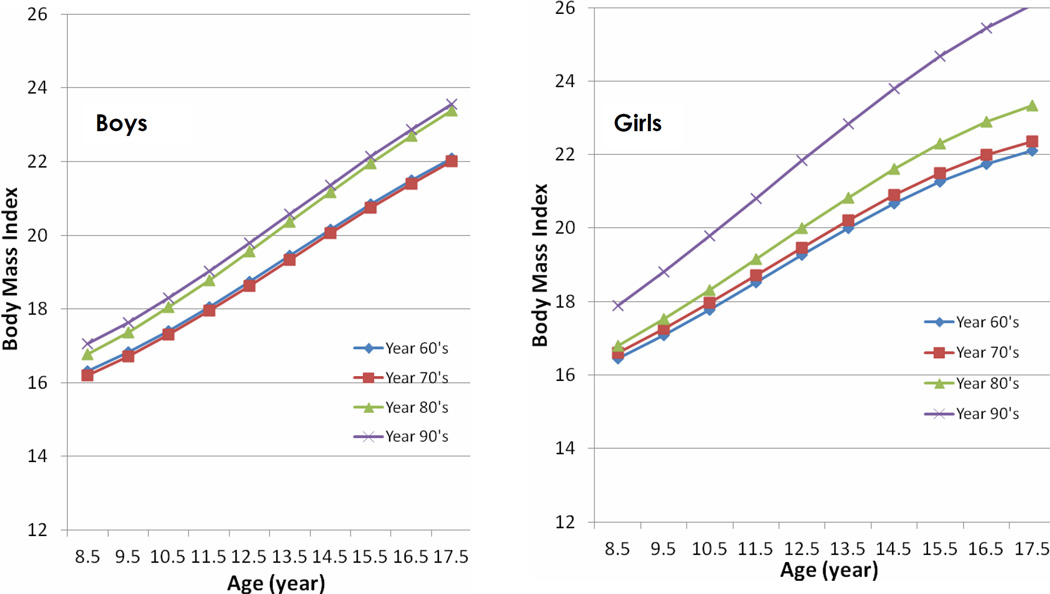
The mean BMI and standard deviation for each birth decade from the 1960s to the 1990s and age are presented in Table 1 for boys and girls separately, and the estimated BMI trajectories for each birth decade and gender are shown in Figure 1. Girls born in the 1990s had significantly greater mean BMI than girls born in the three prior decades for ages 8 through 18 (p<0.05). We found a sex difference in the secular trends. Boys born in the 1990s had significantly greater mean BMI than boys born in the 1960s and the 1970s at all ages. However, the mean BMIs did not differ significantly between the cohort born in the 1980s and the cohort born in the 1990s. It thus appears that a secular trend of increased BMI began in boys in the 1980s, one decade earlier than in girls.
Figure 1.
Estimated mean Body Mass Index (BMI, kg/m2) by childhood age and birth decade for boys and girls. Childhood age measured in years (8 to 18), while birth decade categorized as 1960 through 1969, 1970 through 1979, 1980 through 1989, and 1990 through 1999.
Waist Circumference/Height
The means and standard deviations for W/Ht are presented in Table 2 for each birth decade in both genders, while the estimated W/Ht trajectories are provided in Figure 2. The mixed model results indicated a significant increase in mean W/Ht in boys aged 8–18 born in the 1980s and 1990s compared to boys born in the 1960s and 1970s. Girls aged 8–18 born in the 1990s had significantly greater mean W/Ht than girls born in the three prior decades. We found a sex-associated difference in the mean W/Ht growth trajectories, in that mean W/Ht in girls increased with increasing age in all birth decades, whereas boys showed no significant increase in slope with increasing age in the W/Ht trajectories in any birth decade. This observation indicates that boys add abdominal girth in constant proportion to their increasing height from ages 8 through 18, while girls add abdominal girth at an increased rate with increasing age as their growth in stature slows.
Table 2.
Waist Circumference/Height (cm/m) by Age from 8 to 18 Years and Decades of Birth.
| Age | Birth Decade (BD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | 1960≤BD<1970 | 1970≤BD<1980 | 1980≤BD<1990 | 1990≤BD<2000 | ||||||||
| Boys | n | mean | std | n | mean | std | n | mean | std | n | mean | std |
| 8≤age<9 | 14 | 0.44* | 0.03 | 57 | 0.45* | 0.04 | 43 | 0.45 | 0.04 | 35 | 0.47 | 0.05 |
| 9≤age<10 | 19 | 0.44* | 0.03 | 46 | 0.45* | 0.05 | 43 | 0.46 | 0.06 | 30 | 0.47 | 0.07 |
| 10≤age<11 | 32 | 0.44* | 0.03 | 62 | 0.45* | 0.06 | 46 | 0.46 | 0.07 | 37 | 0.48 | 0.08 |
| 11≤age<12 | 38 | 0.44* | 0.03 | 59 | 0.45* | 0.06 | 48 | 0.46 | 0.06 | 36 | 0.46 | 0.06 |
| 12≤age<13 | 43 | 0.44* | 0.03 | 60 | 0.45* | 0.06 | 41 | 0.46 | 0.07 | 41 | 0.48 | 0.07 |
| 13≤age<14 | 43 | 0.43* | 0.03 | 58 | 0.44* | 0.05 | 44 | 0.46 | 0.08 | 30 | 0.47 | 0.07 |
| 14≤age<15 | 48 | 0.43* | 0.03 | 59 | 0.44* | 0.06 | 51 | 0.46 | 0.07 | 29 | 0.48 | 0.09 |
| 15≤age<16 | 53 | 0.43* | 0.03 | 58 | 0.44* | 0.06 | 41 | 0.45 | 0.07 | 29 | 0.48 | 0.08 |
| 16≤age<17 | 50 | 0.44* | 0.06 | 43 | 0.44* | 0.06 | 40 | 0.45 | 0.08 | 20 | 0.47 | 0.07 |
| 17≤age<18 | 48 | 0.44* | 0.06 | 43 | 0.44* | 0.06 | 44 | 0.45 | 0.06 | 15 | 0.46 | 0.05 |
| Girls | ||||||||||||
| 8≤age<9 | 13 | 0.46* | 0.06 | 51 | 0.46* | 0.03 | 57 | 0.46* | 0.05 | 34 | 0.48 | 0.05 |
| 9≤age<10 | 23 | 0.45* | 0.04 | 51 | 0.45* | 0.04 | 54 | 0.46* | 0.05 | 27 | 0.48 | 0.06 |
| 10≤age<11 | 22 | 0.46* | 0.05 | 53 | 0.45* | 0.04 | 55 | 0.46* | 0.05 | 24 | 0.48 | 0.06 |
| 11≤age<12 | 31 | 0.44* | 0.04 | 55 | 0.44* | 0.04 | 57 | 0.46* | 0.06 | 19 | 0.47 | 0.05 |
| 12≤age<13 | 41 | 0.45* | 0.04 | 52 | 0.45* | 0.04 | 57 | 0.47* | 0.06 | 18 | 0.46 | 0.05 |
| 13≤age<14 | 49 | 0.45* | 0.04 | 51 | 0.44* | 0.05 | 46 | 0.47* | 0.06 | 20 | 0.47 | 0.05 |
| 14≤age<15 | 46 | 0.46* | 0.05 | 38 | 0.45* | 0.05 | 46 | 0.48* | 0.06 | 15 | 0.49 | 0.05 |
| 15≤age<16 | 37 | 0.45* | 0.05 | 45 | 0.45* | 0.05 | 28 | 0.49* | 0.07 | 19 | 0.49 | 0.06 |
| 16≤age<17 | 50 | 0.46* | 0.05 | 42 | 0.46* | 0.05 | 40 | 0.49* | 0.06 | 11 | 0.49 | 0.06 |
| 17≤age<18 | 55 | 0.46* | 0.05 | 38 | 0.46* | 0.04 | 48 | 0.50* | 0.07 | 11 | 0.50 | 0.09 |
Mean BMI significantly less than mean BMI for 1990–2000 (p-value < 0.05).
Figure 2.
Estimated mean Waist Circumference / Height (cm/m) by childhood age and birth decade for boys and girls. Childhood age measured in years (8 to 18), while birth decade categorized as 1960 through 1969, 1970 through 1979, 1980 through 1989, and 1990 through 1999.
Percent of Body Fat
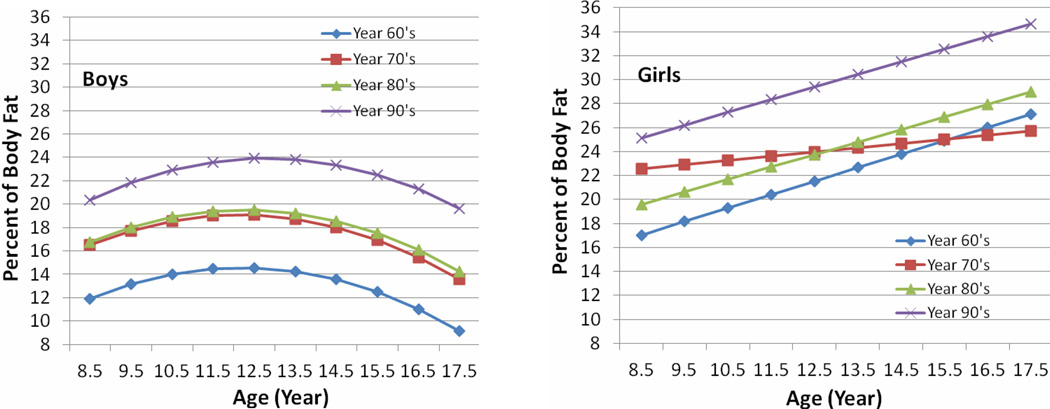
The means and standard deviations of PBF for boys and girls from ages 8 to 18 by decade of birth from 1960 through1999 are shown in Table 3. The mean PBF for both boys and girls aged 8 to 18 born in the 1990s was significantly larger than the corresponding mean PBF for boys born in the three prior decades. The estimated PBF growth trajectories seen in Figure 3 differed between boys and girls. In boys, mean PBF increased with age to reach a maximum at age 12 years and decreased thereafter, while in girls mean PBF increased linearly with age. Girls had significantly larger mean PBF than boys at all ages from 8 to 18 years in all four birth decades
Table 3.
Percent Body Fat (PBF) by Age from 8 to 18 Years and Decades of Birth.
| Age | Birth Decade (BD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | 1960≤BD<1970 | 1970≤BD<1980 | 1980≤BD<1990 | 1990≤BD<2000 | ||||||||
| Boys | N | mean | std | n | mean | std | n | mean | std | n | mean | std |
| 8≤age<9 | 6 | 12.96* | 10.34 | 5 | 17.57* | 12.46 | 34 | 15.20* | 9.89 | 33 | 18.23* | 11.21 |
| 9≤age<10 | 9 | 12.65* | 7.38 | 21 | 16.97* | 9.26 | 40 | 17.25* | 11.83 | 26 | 22.97* | 10.12 |
| 10≤age<11 | 9 | 15.86* | 7.64 | 31 | 18.22* | 9.79 | 34 | 17.71* | 12.17 | 35 | 23.05* | 11.70 |
| 11≤age<12 | 20 | 14.96* | 5.16 | 41 | 18.95* | 9.21 | 45 | 19.45* | 9.65 | 32 | 21.56* | 9.54 |
| 12≤age<13 | 28 | 16.67* | 6.48 | 39 | 20.18* | 7.86 | 36 | 20.00* | 10.14 | 37 | 24.64* | 10.39 |
| 13≤age<14 | 24 | 14.15* | 6.51 | 42 | 19.58* | 7.77 | 34 | 18.82* | 9.25 | 28 | 23.06* | 9.57 |
| 14≤age<15 | 25 | 12.43* | 5.35 | 37 | 18.42* | 11.32 | 47 | 18.30* | 9.41 | 28 | 23.49* | 11.49 |
| 15≤age<16 | 27 | 8.54* | 4.69 | 44 | 15.54* | 8.09 | 38 | 16.66* | 10.31 | 23 | 18.77* | 8.01 |
| 16≤age<17 | 38 | 10.70* | 7.77 | 40 | 14.52* | 7.58 | 35 | 15.14* | 7.95 | 19 | 19.48* | 8.27 |
| 17≤age<18 | 26 | 10.11* | 4.93 | 43 | 12.39* | 7.75 | 42 | 13.19* | 7.34 | 14 | 18.56* | 5.87 |
| Girls | ||||||||||||
| 8≤age<9 | 5 | 16.45* | 7.45 | 8 | 28.61* | 4.78 | 36 | 18.91* | 10.33 | 30 | 26.09* | 12.21 |
| 9≤age<10 | 4 | 22.32* | 11.73 | 20 | 22.35* | 9.69 | 38 | 21.22* | 9.63 | 25 | 24.88* | 10.20 |
| 10≤age<11 | 7 | 18.58* | 11.58 | 30 | 23.55* | 8.26 | 49 | 23.20* | 8.54 | 21 | 26.36* | 10.27 |
| 11≤age<12 | 16 | 22.93* | 7.27 | 33 | 23.80* | 7.77 | 48 | 23.45* | 8.83 | 15 | 28.24* | 8.29 |
| 12≤age<13 | 19 | 23.71* | 4.90 | 34 | 24.71* | 6.85 | 46 | 24.70* | 7.69 | 17 | 27.87* | 8.83 |
| 13≤age<14 | 26 | 22.3* | 6.62 | 34 | 24.28* | 8.01 | 41 | 24.06* | 8.84 | 17 | 25.13* | 6.8 |
| 14≤age<15 | 30 | 23.98* | 7.72 | 32 | 22.77* | 7.4 | 41 | 24.93* | 8.23 | 15 | 30.91* | 6.73 |
| 15≤age<16 | 22 | 27.25* | 7.49 | 42 | 23.74* | 6.3 | 28 | 26.35* | 6.89 | 17 | 30.18* | 9 |
| 16≤age<17 | 27 | 25.74* | 7.69 | 39 | 25.21* | 7.64 | 40 | 27.79* | 8.24 | 10 | 30.08* | 7.83 |
| 17≤age<18 | 30 | 25.58* | 8.15 | 36 | 25.17* | 7.07 | 46 | 30.08* | 7.79 | 10 | 31.46* | 7.78 |
Mean BMI significantly less than mean BMI for 1990–2000 (p-value < 0.05).
Figure 3.
Estimated mean Percent Body Fat (PBF) by childhood age and birth decade for boys and girls. Childhood age measured in years (8 to 18), while birth decade categorized as 1960 through 1969, 1970 through 1979, 1980 through 1989, and 1990 through 1999.
Fat-Free Mass/Height2
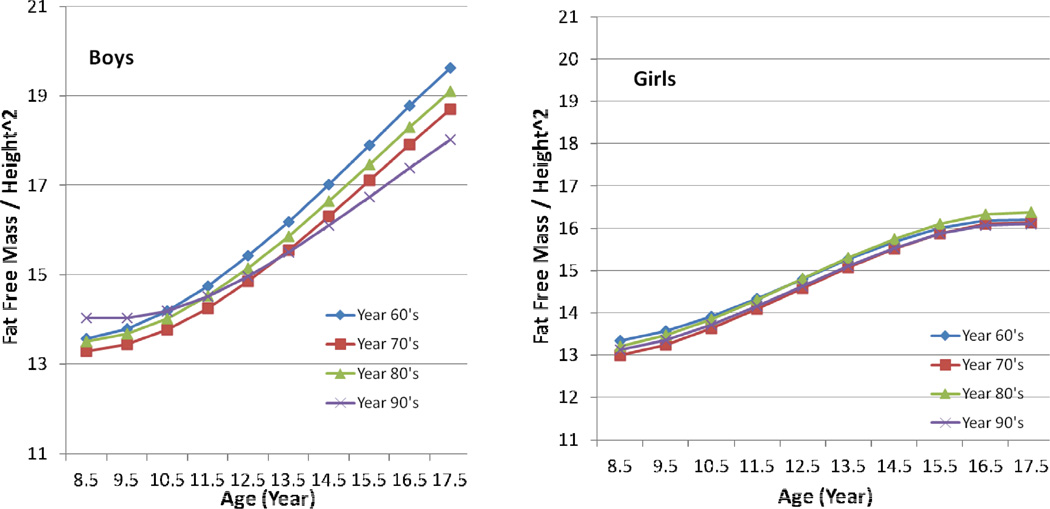
The means and standard deviations of FFM/Ht2 for ages 8 to 18 years by decade of birth are shown in Table 4 for boys and girls, and the trajectories for FFM/Ht2 are presented in Figure 4. FFM/Ht2 did not differ significantly among girls of any age in any birth decade, and their trajectories are nearly identical over a period of forty years. Boys older than 13 years who were born between 1966 and 1970 had significantly higher mean FFM/Ht2 than boys of the same ages who were born in the 1990s, and boys older than 15 years who were born in the 1980s had significantly greater mean FFM/Ht2 than did boys of the same age who were born in the 1990s.
Figure 4.
Estimated mean Fat Free Mass / Height2 (kg/m2) by childhood age and birth decade for boys and girls. Childhood age measured in years (8 to 18), while birth decade categorized as 1960 through 1969, 1970 through 1979, 1980 through 1989, and 1990 through 1999.
Sexual Dimorphism
We found sex-associated differences in the secular changes that we documented. The BMI and W/Ht of girls born in the decade 1990 through 1999 differed significantly from those of girls born in the decade 1980–1989, but in boys there was no difference in these parameters over the same period of time. The girls also had greater mean BMI and mean W/H than boys at the same ages. We did not find significant differences in mean FFM/Ht2 in girls over different birth decades, suggesting that the secular increase in BMI and W/H noted in girls was due to an increase in fat mass rather than to an increase in FFM. On the other hand, boys clearly showed a secular decrease in FFM/Ht2 from the birth decade 1960–1969 to the birth decade 1990–1999.
The patterns of change in PBF differed over time between boys and girls. During puberty body composition of both boys and girls changes markedly (Guo et al., 1997). The FLS data indicate that in girls PBF increases steadily from age 8, reaching 26% at age 18; whereas, in boys the PBF reaches a peak of 17% at age 12 and declines thereafter to 13% at age 18. FFM increases steadily in early puberty, rising from a mean of 25 kg at age 9 to 35 kg at age 13 in both sexes. Between ages 14–20 years, the rates of increase in FFM show pronounced sexual dimorphism. FFM climbs steadily in boys to reach a mean of 63 kg at age 18, but in girls FFM increases more gradually to reach a mean of 40 kg at age 18.
DISCUSSION
Analysis of the FLS longitudinal data demonstrates a secular increase in BMI from 1960 through 1999 in both sexes. In girls this secular increase in BMI can be ascribed solely to an increase in fat mass, since the mean fat-free mass did not increase in girls of any age from 8 to 18 over the forty-year period of this study. Our analysis discloses for the first time, positive secular trends in W/Ht and PBF in both boys and girls and a negative secular trend in FFM/Ht2 in boys over the last four decades of the 20th century.
The near identity of the girls’ four FFM/Ht2 trajectories from the 1960s to the 1990s (Figure 4) demonstrates the precision of the measurement techniques used in the FLS over a period of 40 years and lends confidence to the conclusion that the secular decrease seen in FFM/Ht2 in boys over the same four decades is a real phenomenon. The secular decrease in FFM/Ht2 in boys may reflect a secular decrease in physical activity from 1960 to 1999 owing to the increase in sedentary behavior over these four decades. There are not many available datasets that document a secular change in physical activity over these four decades. However, McDonald (2007) analyzed data from six US National Personal Transportation Surveys and found that the percentage of students who walked or biked to school declined from 40.7% in 1969 to 12.9% in 2001. The secular increase in BMI in boys from 1960 through 1999 can also be ascribed solely to a secular increase in fat mass, an increase which more than compensated for the secular decrease of FFM/Ht2 that occurred over the same four decades. The FLS data controvert the supposition of Eisenmann (2006) who noted that the secular increase in children’s BMI is due to an increase in both FFM and FM.
In addition to a secular change in physical activity, children’s and adolescents’ eating behavior has changed over the same period of time, with an increase in consumption of sugar-sweetened beverages (Brownell and Frieden, 2009), an increase in portion size, and an increase in intake of calorie-dense food of low nutritional value at convenience stores and fast food restaurants (Dietz et al., 2009).
These disturbing findings presage increases in the prevalence of metabolic and cardiovascular disorders known to be associated with obesity, including the components of the metabolic syndrome, type 2 diabetes, acute coronary syndrome, and congestive heart failure (Aprahamian and Sam, 2011). Franks et al., (2010) recently performed a follow-up study of American Indians whose height and weight were measured in childhood. These authors reported that the rate of death prior to age 55 years in subjects who were in the highest quartile of BMI at age 11 years was 2.3 times that of those in the lowest quartile of BMI at age 11 years, after adjusting for baseline levels of glucose, total cholesterol, and blood pressure. They found childhood BMI to be a stronger risk factor for premature death than childhood hypertension, glucose intolerance, or hypercholesterolemia. In an attempt to gain a deeper understanding of how pediatric obesity leads to inimical cardiovascular consequences, Kim et al., (2010) performed a metabolic study in 354 five-to-eight-year-old obese children and 350 non-obese children matched for age, gender and ethnicity. These authors reported finding significantly higher levels of insulin resistance, LDL-cholesterol and triglycerides in the obese children as well as significantly higher levels of cardiovascular risk factors, including high sensitivity C-reactive protein, interleukin-6, interleukin-20,myeloid related protein, P-selectin, intercellular adhesive molecule-1, retinol binding protein 4, macrophage inhibitory factor, and tumor-necrosis factor-α compared to non-obese children. These factors contribute to a systemic inflammatory state in obese children which may explain their propensity to cardiovascular consequences later in life, including premature death.
Din-Dzietham et al., (2007) analyzed NHANES data on children’s blood pressure from 1963 through 2002. They found that in 1988 the long-term downward trend in the prevalence of childhood hypertension (blood pressure > 95th percentile for age, sex and height percentile) and prehypertension (blood pressure >90th < 95th percentile for age, sex and height percentile) reached a nadir and began to rise over the subsequent decade in parallel to the increase in prevalence of childhood obesity, especially abdominal obesity. Sun et al., (2007; 2008) showed that BMI, waist circumference, and hypertension track with fidelity from childhood into adulthood and that both childhood obesity and pediatric hypertension predict the onset of the metabolic syndrome later in life. As these secondary epidemics of metabolic and cardiovascular disease ripple through the US population of young adults, the annual costs of medical care for obesity and obesity-related conditions in the US will rise well above the $148 million reported by Finkelstein et al., in 2009.
Ogden et al., (2010) documented a trend toward an increasing prevalence of obesity in childhood in data generated by successive cross-sectional population-based NHANES. These authors found positive secular trends in BMI in both boys and girls beginning in the 1970s. These findings differ from ours in that we found a positive secular trend in BMI in boys beginning in 1980 and in girls beginning in 1990. This discrepancy may be explained by the different racial and ethnic composition of the FLS and the NHANES.
The FLS is composed almost entirely of non-Hispanic whites, whereas the NHANES are population-based surveys that include blacks and Hispanic whites according to their representation in the US population. Using NHANES cross-sectional data, Din-Dzietham et al., (2007) compared the prevalence of obesity by race and ethnicity in 8–17-year-old children in 1988 to the prevalence of obesity in children of the same ages in 2002 and found striking discrepancies in the change in the prevalence of obesity: non-Hispanic white children experienced a moderate increase in prevalence, from 10% to 14%, while African American children’s prevalence increased from 14% to 22%; and Hispanic children’s prevalence increased from 16% to 24%. The greater rate of increase in the prevalence of obesity in minority children than in non-Hispanic white children may account for the earlier appearance of a secular change in prevalence of obesity in serial NHANES than in the FLS.
A recent report by Gupta et al., (2011) revealed that the phenomenon of a secular increase in BMI over a relatively short period of time is not limited to populations within the US. These authors performed two cross-sectional studies of urban Asian Indian adolescents, three years apart and found that the prevalence of obesity increased significantly in boys, from 8.9% in 2006 to 11.5% in 2009, and non-significantly in girls, from 11.4% to 12%, over the same period of time. In another Asian study, Ying-Xiu and Shu-Rong (2011) reported finding secular trends in data generated by two cross-sectional examinations performed 25 years apart in Chinese children and adolescents 7 to 18 years old. Obesity prevalence increased by two orders of magnitude, from 0.04% in 1985 to 9.3% in 2010 in boys and from 0.03% to 2.4% in girls over the same period of time.
The innovative approach we took in this study includes the application of a longitudinal model to 40 years of serial data that include indirect and direct measurements of body composition. Our approach contrasts with studies which compared BMI values in successive cross-sectional analyses (Ali et al., 2000; Castilho and Lahr 2001; Danker-Hopfe and Roczen 2000; Gupta et al., 2011; Ogden et al., 2010; Ying-Xiu and Shu-Rong, 2011). These cross-sectional studies employed two analytic approaches: (1) A regression approach to estimate rates of change per birth decade of the cohorts, while ignoring the age of the cohorts; (2) A comparison of average values among subjects over several decades of birth or between two periods of time. The first approach is suitable for cross-sectional data but does not permit consideration of the patterns of change in BMI during children’s growth. Furthermore, with a regression approach, differential changes from decade to decade are not revealed. The advantage of the statistical modeling approach we used in our investigation is that longitudinal models applied to serial data can capture age-related growth trends and simultaneously evaluate secular trends over decades of birth.
Our findings in the FLS have corroborated findings from serial cross sectional studies in this country and abroad, all of which have shown a positive secular trend in children’s BMI. We have also provided evidence of concomitant positive secular trends in direct measures of body fat and a worrisome negative secular trend in fat-free mass in boys.
Limitations
Since the children in the FLS who were born in the first decade of the 21st century are not old enough to provide data from 10–18 years old, we are unable to determine if the secular trends we observed in children born from 1960 through 1999 have continued unabated or have begun to wane in children born in the past decade. Although we were able to ascertain when secular changes began in various measures of adiposity, we were not able to determine the causes of the secular changes that we detected because the FLS, does not include the variables necessary to determine such causation.
Factors such as birth weight, dietary behavior, physical activity and parental adiposity that could influence body composition are not as well documented in FLS subjects as body size and composition. Therefore, the analyses performed in this study could not be appropriately adjusted for such confounding variables. Another limitation of our study is that since the FLS decadal birth cohorts consist mainly of non-minority Caucasian subjects, our observations may not apply to other races or ethnicities.
ACKNOWLEDGEMENTS
Sources of Funding: Supported by Grants U01HL101064-02, R01HD060913-03, R01HD038356, DK 071485, HL 072838 and HD 12252 from the National Institutes of Health, Bethesda, Maryland. We thank all the data collection staff and our co-investigators at the Fels Longitudinal Study.
Footnotes
DISCLOSURE
Dr. Sun, Mrs. Deng, Mr. Carrico, Dr. Schubert, Dr. Wan, Mrs. Sabo, and Dr. Sabo declare no conflict of interest.
LITERATURE CITED
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Ali MA, Lestrel PE, Ohtsuki F. Secular trends for takeoff and maximum adolescent growth for eight decades of Japanese cohort data. Am J Hum Biol. 2000;12(5):702–712. doi: 10.1002/1520-6300(200009/10)12:5<702::AID-AJHB15>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Aprahamian TR, Sam F. Adiponectin in cardiovascular inflammation and obesity. Int J Inflam. 2011;2011 doi: 10.4061/2011/376909. 376909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock RD. Familial resemblance in patterns of growth in stature. Prog Clin Biol Res. 1978;24A:211–216. [PubMed] [Google Scholar]
- Brownell KD, Frieden TR. Ounces of prevention--the public policy case for taxes on sugared beverages. N Engl J Med. 2009;360(18):1805–1808. doi: 10.1056/NEJMp0902392. [DOI] [PubMed] [Google Scholar]
- Castilho LV, Lahr MM. Secular trends in growth among urban Brazilian children of European descent. Ann Hum Biol. 2001;28(5):564–574. doi: 10.1080/03014460110045146. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics. 1997;99(6):804–807. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- Danker-Hopfe H, Roczen K. Secular trends in height, weight and body mass index of 6-year-old children in Bremerhaven. Ann Hum Biol. 2000;27(3):263–270. doi: 10.1080/030144600282154. [DOI] [PubMed] [Google Scholar]
- Dietz WH, Benken DE, Hunter AS. Public health law and the prevention and control of obesity. Milbank Q. 2009;87(1):215–227. doi: 10.1111/j.1468-0009.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116(13):1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- Eisenmann JC. Insight into the causes of the recent secular trend in pediatric obesity: Common sense does not always prevail for complex, multi-factorial phenotypes. Prev Med. 2006;42(5):329–335. doi: 10.1016/j.ypmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garn S, Leonard W, Hawthorne V. Three limitations of body mass index. American Journal of Clinical Nutrition. 1986;44:996–997. doi: 10.1093/ajcn/44.6.996. [DOI] [PubMed] [Google Scholar]
- Guo SM, Roche AF, Houtkooper L. Fat-free mass in children and young adults predicted from bioelectric impedance and anthropometric variables. Am J Clin Nutr. 1989;50(3):435–443. doi: 10.1093/ajcn/50.3.435. [DOI] [PubMed] [Google Scholar]
- Guo SS, Chumlea WC, Roche AF, Siervogel RM. Age- and maturity-related changes in body composition during adolescence into adulthood: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 1997;21(12):1167–1175. doi: 10.1038/sj.ijo.0800531. [DOI] [PubMed] [Google Scholar]
- Guo SS, Huang C, Maynard LM, Demerath E, Towne B, Chumlea WC, Siervogel RM. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2000;24(12):1628–1635. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- Gupta DK, Shah P, Misra A, Bharadwaj S, Gulati S, Gupta N, Sharma R, Pandey RM, Goel K. Secular trends in prevalence of overweight and obesity from 2006 to 2009 in urban asian Indian adolescents aged 14–17 years. PLoS ONE. 2011;6(2):e17221. doi: 10.1371/journal.pone.0017221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Bhattacharjee R, Kheirandish-Gozal L, Khalyfa A, Sans Capdevila O, Tauman R, Gozal D. Insulin sensitivity, serum lipids, and systemic inflammatory markers in school-aged obese and nonobese children. Int J Pediatr. 2010;2010 doi: 10.1155/2010/846098. 846098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272(3):205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- Lohman GT, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- McDonald NC. Active transportation to school: trends among U.S. schoolchildren, 1969–2001. Am J Prev Med. 2007;32(6):509–516. doi: 10.1016/j.amepre.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Roche AF. Secular trends in human growth, maturation, and development. Monogr Soc Res Child Dev. 1979;44(3–4):1–120. [PubMed] [Google Scholar]
- Roche AF. Growth, Maturation, and Body Composition: The Fels Longitudinal Study 1929–1991. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- Roche AF, Sievogel RM, Chumlea WC, Webb P. Grading body fatness from limited anthropometric data. Am J Clin Nutr. 1981;34(12):2831–2838. doi: 10.1093/ajcn/34.12.2831. [DOI] [PubMed] [Google Scholar]
- Romaguera D, Angquist L, Du H, Jakobsen MU, Forouhi NG, Halkjaer J, Feskens EJ, van der AD, Masala G, Steffen A, et al. Dietary determinants of changes in waist circumference adjusted for body mass index - a proxy measure of visceral adiposity. PLoS ONE. 2010;5(7):e11588. doi: 10.1371/journal.pone.0011588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N, Georgiou C, Kafatos A. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord. 2000;24(11):1453–1458. doi: 10.1038/sj.ijo.0801401. [DOI] [PubMed] [Google Scholar]
- Statistical Analysis Software Institute Inc. Statistical Analysis Software® 9.2. Cary, NC: 2008. [Google Scholar]
- Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, Kuczmarski RJ, Flegal KM, Johnson CL, Hubbard VS. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77(2):331–340. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119(2):237–246. doi: 10.1542/peds.2006-2543. [DOI] [PubMed] [Google Scholar]
- Sun SS, Liang R, Huang TT, Daniels SR, Arslanian S, Liu K, Grave GD, Siervogel RM. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152(2):191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149(10):1085–1091. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- Ying-Xiu Z, Shu-Rong W. Secular trends in body mass index and the prevalence of overweight and obesity among children and adolescents in Shandong, China, from 1985 to 2010. J Public Health (Oxf) 2011 doi: 10.1093/pubmed/fdr053. [DOI] [PubMed] [Google Scholar]