Abstract
Cajal-Retzius cells are thought to play an important role for cortical development, and receive primarily spontaneous GABAergic input mediated by GABAA receptors. However, neither the effects of synaptically-released GABA on their excitability nor the cellular source(s) of spontaneous GABAergic currents have been yet determined. By directly recording electrophysiological responses from identified Cajal-Retzius cells of the CXCR4-EGFP mouse, we show that GABAergic input can trigger supra-threshold responses, and that the pharmacological activation of mGlu1α receptors with the group I agonist DHPG powerfully increases the frequency of spontaneous GABAergic currents. These effects appeared mediated by a network mechanism, because responses to DHPG were completely prevented both by surgical disconnection of layer I from lower layers and by exposure of slices to TTX.
We propose that the cellular source underlying the observed effect of DHPG are layer I-targeting Martinotti-like interneurons, which we show express functional group I mGluRs and respond to DHPG with supra-threshold depolarization already at early developmental stages.
In conclusion, our work suggests that conditions of enhanced glutamate release may be critical at early developmental stages for the recruitment of an mGlu1α-dependent micro-circuit, which then leads to the activation of Cajal-Retzius cells.
1. INTRODUCTION
The embryonic marginal zone/postnatal layer I has been suggested to play a critical role in orchestrating the development of the neocortex (Marín-Padilla, 1998). In fact, after completing their radial migration, developing pyramidal neurons first make synaptic contacts with the marginal zone/layer I (Ramón y Cajal, 1904; Marín-Padilla and Marín-Padilla, 1982), and then are displaced towards deeper layers by newly arrived migrating cells, thus generating an inside-out pattern of cortical development (Angevine and Sidman, 1961). Therefore, all pyramidal cells seem to receive progressively, as they mature, the same kind of information from layer I. However, the detailed functions performed by the micro-circuitry operating within the marginal zone/layer I are not completely understood.
The principal neuron of the marginal zone/layer I is the Cajal-Retzius cell (reviewed by Soriano and Del Río, 2005), which has been the subject of numerous studies as a cellular source of the glycoprotein reelin (Tissir and Goffinet, 2003), which is essential for several functions ranging from the correct organization of cortical layers (D’Arcangelo et al., 1995), to the maturation of dendritic arbors (via different signaling pathways, see Niu et al., 2004; 2008, and Chameau et al., 2009) and their synaptic channels (Qui and Weeber, 2007; Campo et al., 2009). Cajal-Retzius cells appear spontaneously active both at embryonic and postnatal stages. In fact, spontaneous calcium transients in Cajal-Retzius cells have been found to be synchronous in correlated networks including other Cajal-Retzius cells and/or different types of local neurons (Schwartz et al., 1998; Aguiló et al., 1999). This pattern of activity has been suggested to play computational roles, which have been postulated to be important for the development of the cortex.
Intriguingly, several studies have indicated that Cajal-Retzius cells receive predominant, if not exclusive, spontaneous excitatory synaptic input mediated by GABAA receptors (Kilb and Luhmann, 2001; Soda et al. 2003). Synchronized calcium oscillations are sensitive both to tetrodotoxin, which blocks axonal conduction (Narahashi et al., 1964), and to bicuculline, which blocks GABAA receptors (Curtis et al., 1970). Thus, GABAergic input to Cajal-Retzius cells of the marginal zone/layer I could play a critical role for the recruitment of assemblies of Cajal-Retzius cells involved in this type of spontaneous activity.
Various types of GABAergic fibers have been shown to target the developing layer I: axons of local interneurons (Hestrin and Armstrong, 1996; Zhou and Hablitz, 1996; Marín-Padilla, 2011a, 2011b; Wonzy and Williams, 2011), subplate cells (Friauf et al., 1990; Myakhar et al., 2011, Marín-Padilla, 2011a, 2011b) and thalamic zona incerta neurons (Lin et al., 1990). Paired recording between layer I interneurons and Cajal-Retzius cells have revealed a very low degree of connectivity, suggesting that GABAergic pathways originating from lower layers may provide a more significant input (Soda et al. 2003).
Here, we unravel a massive source of GABAergic input to Cajal-Retzius cells, which is powerfully activated by pharmacological agonists of group I metabotropic glutamate receptors (mGluRs) via mGlu1α. We propose that activation of GABAergic interneurons expressing mGlu1α, possibly Martinotti cells, plays an important role in generating synchronous network activity in Cajal-Retzius cells of the developing layer I, and hence, in contributing to their computational functions.
2. MATERIALS AND METHODS
2.1. Slice preparation
All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals and approved by Northwestern University Animal Care and Use Committtee. Slices were prepared from juvenile (P5–P10) CXCR4-EGPF mice (www.gensat.org) as described previously (Marchionni, 2010), or, in a few cases, from GIN mice, which identify somatostatin-expressing interneurons (strain: FVB-Tg(GadGFP)45704Swn/J, Jackson Labs, see Oliva et al., 2000). Mice were deeply anaesthetized using isoflurane, quickly decapitated and the brain dissected out in ice-cold modified ACSF (in mM: 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1 CaCl2, 2 MgCl2, 10 glucose oxygenated with 95% O2/5% CO2 at pH 7.4). Horizontal slices were cut at 250 μm on a vibratome (Leica, VT 1000 S), and then transferred to a warm (30 °C) holding chamber filled with ACSF (in mM: 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 10 glucose oxygenated with 95% O2/5% CO2 at pH 7.4) for 30 minutes, and then at room temperature until use. For slices that had layer I surgically dissected (Fig. 5), before recording, a small incision was made between layer I and layer II/III with the aid of a dissection microscope.
Figure 5.
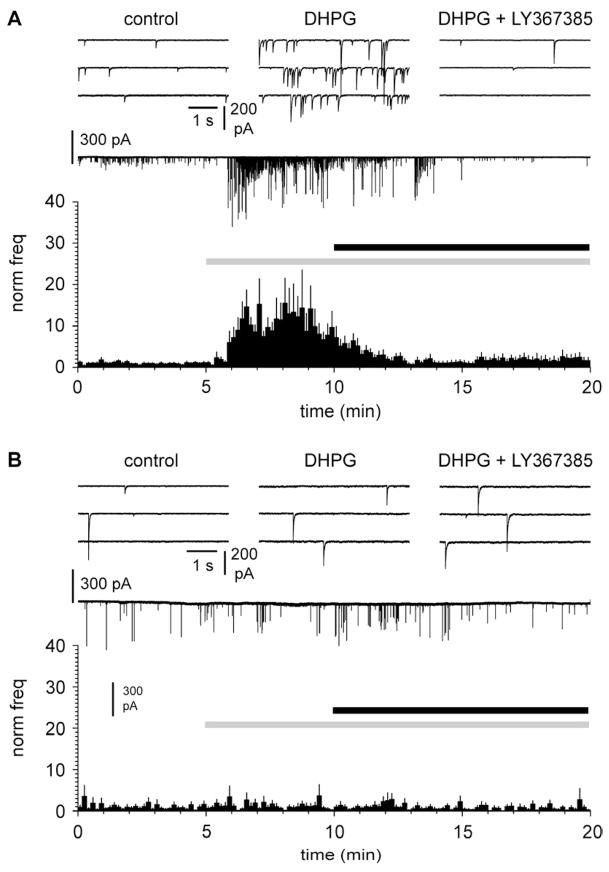
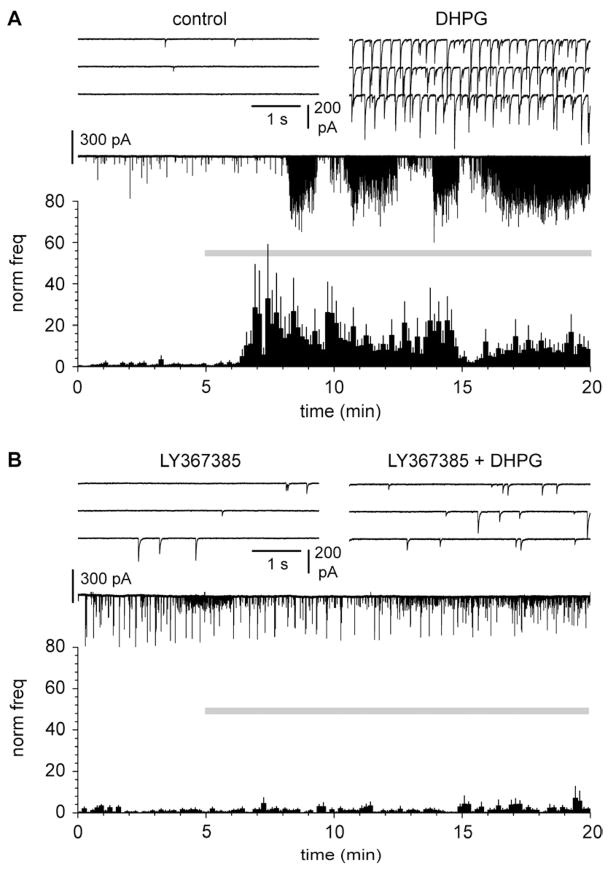
DHPG-induced increase of the frequency of spontaneous GABAergic currents is mediated by metabotropic glutamate receptors expressed by neurons located deeper than layer I. A: summary graph of the effect of DHPG (50 μM, gray bar) and subsequent application of LY367385 (100 μM) in control slices. Notice the similarity of the effects to the ones shown in Figure 3. The upper insets show traces in control (left), in the presence of DHPG (middle), and in the presence of both DHPG and LY367385 (right). Glutamatergic ionotropic synaptic transmission was blocked by NBQX (20 μM) and D-AP5 (50 μM). B: as in A, but in surgically-treated slices to cut ascending GABAergic axon targeting layer I. Notice the complete abolishment of DHPG effects.
2.2. Electrophysiological methods
During recording, slices were superfused with preheated ACSF maintained at a constant temperature of 33 ± 1°C. Cajal-Retzius cells were identified by their location in layer I, stereotypical “tadpole” shape visualized with a 60 X IR immersion DIC objective, and EGFP fluorescence excited by X-Cite Series 120 light source visualized with a VE1000 camera (DAGE-MIT, Michigan City, IN, USA). Interneurons were identified by their location in layers I and II/III, shape, firing patterns, and, when using the GIN mouse, fluorescence in layer II/III.
Conventional whole-cell and cell-attached patch-clamp recordings were performed. Patch pipettes (~3–5 MΩ resistance) were pulled from thin-walled borosilicate glass capillaries and filled with (in mM) 125 KCl, 10 NaCl, 16 KHCO3, 4 MgATP, 0.3 NaGTP, 10 QX-314 equilibrated with 95% O2/5% CO2 to a pH=7.3 for voltage clamp recordings of postsynaptic currents in Cajal-Retzius cells. Interneurons were recorded with a modified version of the above intracellular solution (KCl reduced to 20 mM, and inclusion of 115 mM KMeSO4). For cell-attached recordings, either ACSF or a modified internal solution with KCl reduced to 40 mM, and inclusion of 85 mM KMeSO4 was used.
Recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA). Holding potentials were −60 mV for experiments involving whole-cell voltage-clamp recordings of Cajal-Retzius cells. When cell-attached configuration was used, the pipette was set at 0 mV when filled with ACSF and at −45 mV when the modified internal solution was used. Data were filtered at 3 kHz, and digitized at 10–20 kHz using a Digital A/D board and pClamp 9 software suite (Molecular Devices). Series resistance was monitored throughout recordings via a −5 mV step in voltage clamp, or a −5 pA current pulse in current clamp configurations, and balanced via a bridge circuit in current-clamp mode. Drugs were applied through bath perfusion. Field stimulation was achieved through use of monopolar electrodes (FHC Inc., Bowdoin, ME, USA) connected to a constant current isolation unit (A360, World Precision Instruments, Sarasota, FL, USA) and positioned at the border of layer I/layer II/III.
2.3. Statistical methods
Data are presented as mean ± SEM, and statistical significance was determined using Student’s paired t-test, and repeated measures ANOVA with multiple comparisons post hoc Bonferroni test. Significance level was set at 0.05.
2.4. Drugs
D-AP5, NBQX, gabazine, DHPG and LY367385 were obtained from Ascent Scientific. Tetrodotoxin was obtained from Sigma Aldrich, and QX-314 was obtained from Alomone Labs.
2.5. Visualization and reconstruction of biocytin – filled cells
Biocytin-filled cells were visualized using a slightly modified version of the protocol described by Lubke et al. (2000). Slices were fixed overnight in 4% paraformaldehyde in 0.1 M PB. Endogenous peroxidase activity was quenched using a 3% H2O2 solution for 15 minutes. Slices were incubated overnight at 4 °C in avidin-biotinylated-HRP complex (Vectastain ABC Elite kit) with 0.1% Triton X-100 in PB, followed by a peroxidase reaction with 3′3-diaminobenzidine tetrahydrochloride (DAB) as a chromogen, and intensified with 1% NiNH4SO4 and 1% CoCl2. Cells were then checked for contrast under light microscopy, and briefly postfixed with 0.1% OsO4 in PB (1–3 mins). Slices were then mounted on slides with Moviol (Hoescht AG, Frankfurt, Germany) and coverslipped. After drying, cells were reconstructed using manual neuron tracing in Neurolucida and Neuroexplorer 9 software suite.
3. RESULTS
3.1 GABAergic input to Cajal-Retzius cells
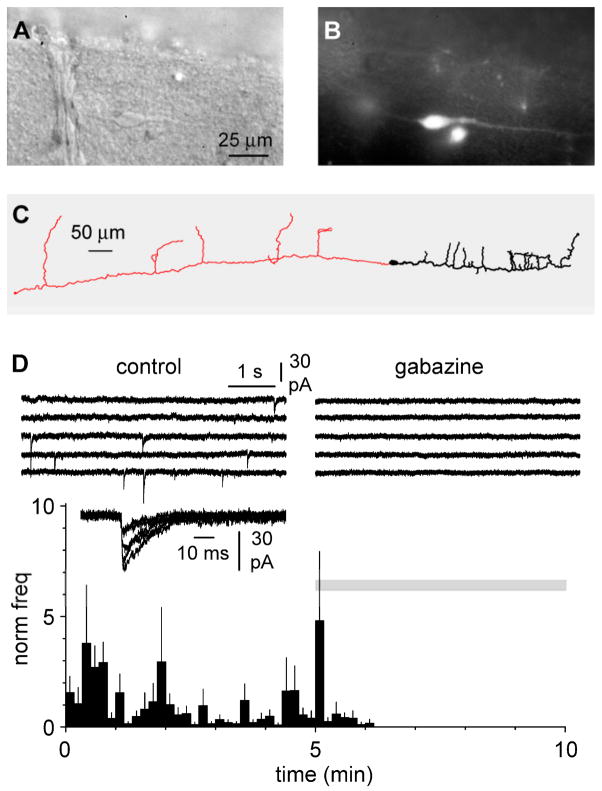
When recorded in whole-cell voltage-clamp configuration, identified neocortical Cajal-Retzius cells of the developing layer I (Figures 1A, 1B, and 1C) show rare and irregularly-occurring spontaneous synaptic events. These synaptic events were completely abolished by gabazine (12.5 μM, Figure 1D), indicating that they were mediated by GABAA receptors. Compared to a control value of 66±26 mHz, bath perfusion of gabazine decreased the spontaneous frequency to 0±0 mHz, (n=8, p<0.05, paired t-test). This indicates that, although Cajal-Retzius cells express glutamate receptors (Lu et al., 2001; Chan et al., 2003; Marchionni et al., 2010), under our experimental conditions spontaneous synaptic currents are exclusively GABAergic. This finding is in good agreement with previous work (Kilb and Luhmann, 2001; Soda et al., 2003).
Figure 1.
Anatomically identified Cajal-Retzius cells of the CXCR4-EGFP mouse receive exclusively spontaneous GABAergic currents. A: DIC image of a Cajal-Retzius cell from a slice prepared from a P6 animal. Notice the typical “tadpole” shape with a single dendrite. B: same as in A, observed under epifluorescence. C: reconstruction of a biocytin-filled Cajal-Retzius cell from a P8 CXCR4-EGFP mouse. Soma and dendrite are shown in black, the axon in red. Notice the long span of the axonal span of the overall structure. D: summary plot of the frequency of spontaneous synaptic currents recorded in control ACSF during application of the GABAA receptor antagonist gabazine (12.5 μM, grey bar). Upper insets show traces in control (left) and in the presence of gabazine (right). A few spontaneous events recorded in control conditions are superimposed and shown at a faster time scale.
The relevance of a predominant, if not exclusive, GABAergic input to Cajal-Retzius cells has been highlighted by several studies showing that neocortical and hippocampal Cajal-Retzius cells maintain a high level of intracellular chloride, which generates a depolarized EGABA (Mienville, 1998; Achilles et al., 2007; Marchionni et al., 2010). This ionic condition suggests that synaptic input mediated by GABAA receptors may generate supra-threshold responses and thus control the level of activity and synchronization of networks of Cajal-Retzius cells.
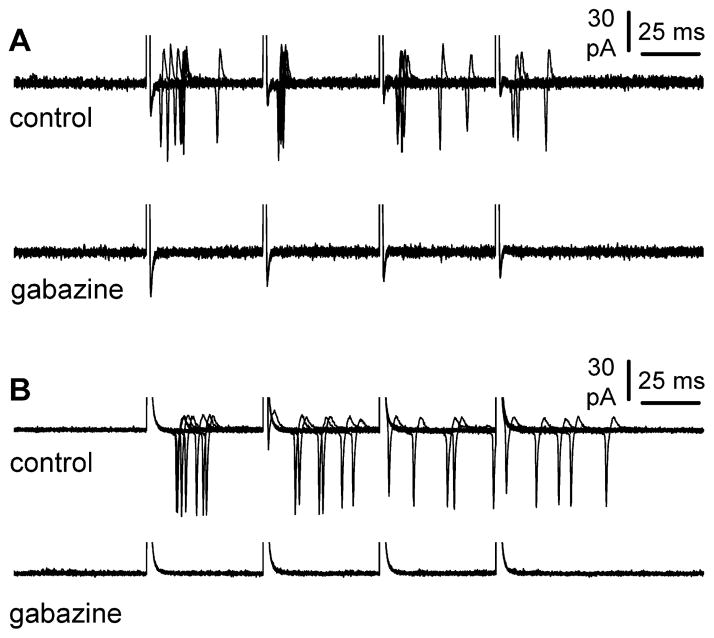
To our knowledge, despite several studies providing evidence for a depolarizing role of exogenously applied GABA, the issue of whether synaptically-evoked GABAergic input is able to trigger firing in Cajal-Retzius cells remains undetermined. We decided to take advantage of a non-invasive configuration such as cell-attached recording to observe the effect of synaptic GABAergic input on Cajal-Retzius cell excitability. Extracellular stimulation at the border of layer I/layer II/III with a four-pulse train at 20 Hz could effectively trigger action currents in Cajal-Retzius cells (Figures 2A and 2B). The average probability of firing was 0.45±0.09, 0.25±0.07, 0.25±0.08, and 0.20±0.04 for the first, second, third, and fourth stimulus, respectively (n=9). Subsequent application of gabazine (n=8) resulted in the complete suppression of the observed firing.
Figure 2.
Synaptically released GABA can trigger action potentials in Cajal-Retzius cells. A: non-invasive cell-attached recording from a Cajal-Retzius cell during a four pulse 20 Hz stimulation. Notice the presence of action currents, which are abolished in the presence of gabazine (12.5 μM). B: similar result as in A for an experiment obtained in the presence of ionotropic glutamate receptor antagonists (NBQX: 20 μM and D-AP5: 50 μM).
3.2. Activation of group I mGluRs promotes firing in Martinotti cells of layer II/III
A potential candidate originating GABAergic input to Cajal-Retzius cells is the Martinotti cell, which heavily targets layer I (Martinotti, 1889; Ramón y Cajal, 1891; Fairen et al., 1984; Marín-Padilla, 2011a, 2011b). Martinotti cells are identified by specific molecular markers such as the peptide somatostatin (Wahle, 1993; Kawaguchi and Kubota, 1996), which is associated with high levels of expression of the group I metabotropic glutamate receptor 1α (mGlu1α, see Baude et al., 1993; Cauli et al., 2000; Stinehelfer et al., 2000; Fanselow et al., 2008). Furthermore, Martinotti cells can be found in different layers and areas of the mammalian neocortex at various developmental stages. For example, early Martinotti-like cells can be recognized as a critical component of the subplate (Friauf et al., 1990), and, at later stages, new Martinotti cells are progressively integrated in the maturing cortical plate (Marín-Padilla, 2011a, 2011b).
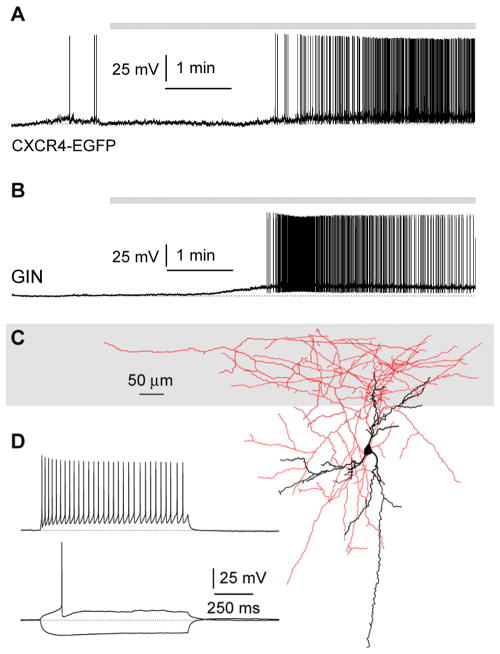
When we recorded responses to the application of the group I agonist DHPG in non-pyramidal neurons of layers II/III (of either CXCR4-EGFP or GIN mice, Figures 3A and 3B, see Methods for details), we found depolarizing responses leading to repetitive firing in ~40% of the recorded neurons (8/21 neurons, combining 3/10 recordings from CXCR4-EGFP mice and 5/11 recordings from GIN mice). When the anatomy of the responding interneurons was examined, seven of the eight responding cells had axonal projections to layer I (2/3 recordings from CXCR4-EGFP mice and 5/5 recordings from GIN mice). In contrast, when responses to DHPG were monitored from neurons with the cell body and main dendritc tree located in layer I, only sub-threshold depolarization or no effect were observed (9/9 cells, combining 8/8 from CXCR4 mice and 1\1 from a GIN mouse).
Figure 3.
Activation of group I metabotropic gluatamate receptors generate suprathreshold depolarizations in somatostatin-expressing interneurons targeting layer I. A: example of an interneuron responding with firing to DHPG (10 μM) application recorded in a slice prepared from a CXCR4-EGFP mouse. B: similar result obtained from a DHPG responding EGPF-expressing cell in a slice obtained from a P8 GIN mouse. C: anatomical properties of the cell shown in B reveal it for a young Martinotti interneuron. Notice the localization of its soma in the lower layers and the dense axonal arborization and extensive targeting of layer I (gray). D: firing pattern of the cell shown in B and C. Current steps were +100 pA (upper trace) and +40 pA and −100 pA (lower traces).
Thus, a prediction of these experiments is that if Martinotti cells provided a significant GABAergic input to Cajal-Retzius cells, then application of DHPG should cause a large increase in the frequency of the synaptic currents occurring in Cajal-Retzius cells.
3.3. DHPG increases the frequency of spontaneous postsynaptic currents in Cajal-Retzius cells via activation of mGlu1α
We directly tested this hypothesis by recording spontaneous postsynaptic currents (sPCs) from Cajal-Retzius cells before and after application of DHPG. As shown in Figure 4A, application of DHPG massively increased the frequency of sPCs from 122±33 mHz in control to 746±235 mHz in the presence of the drug (p<0.05, n=15, paired t-test). Thus, GABAergic input to Cajal-Retzius cells can be powerfully recruited by the activation of group I mGluRs. This is consistent with synaptic events being provided by Martinotti cells and with a general role of group I mGluRs in regulating GABAergic input in cortical (Chu and Hablitz, 1998) and hippocampal networks (Mannaioni et al., 2001). Because, specifically, Martinotti cells express mGlu1α, we investigated whether the strong increase of sPCs triggered by DHPG was mediated by this receptor subtype. We applied DHPG in slices continuously exposed to the mGlu1α antagonist LY367385 (Figure 4B). Under these experimental conditions, the frequency of sPCs did not change. From a value of 192±147 mHz in the presence of the antagonist, the frequency of sPSCs non-significantly decreased to 137±82 mHz after the further addition of DHPG (p>0.05, n=6 paired t-test). This result indicates that the massive increase of frequency of sPCs is mediated specifically by the activation of mGlu1α receptors.
Figure 4.
Massive increase of the frequency of spontaneous GABAergic currents caused by DHPG is mediated by mGlu1 receptors. A: summary graph of the effect of DHPG (10 μM, gray bar) application on the frequency of spontaneous synaptic events. Notice the massive increase in the presence of the drug. The upper insets show traces in control (left) and in the presence of DHPG (right). Ionotropic glutamatergic synaptic transmission was blocked throughout the experiment (NBQX: 20 μM, D-AP5: 50 μM). B: as in A, but in slices continuosly exposed to the mGlu1 antagonist LY367385 (50 μM). Notice the complete loss of DHPG effect.
However, Martinotti cells are not the exclusive neuronal type endowed with mGlu1α receptors. In addition to Martinotti cells, mGlu1α is strongly expressed by Cajal-Retzius cells themselves (Martínez-Galán et al., 2001; Lopez-Bandito et al., 2002). Therefore, an alternative interpretation could be that the increased frequency of spontaneous events may reflect the unmasking of “silent” GABAergic synapses, which would be triggered by a cascade of events initiated by the activation of mGlu1α receptors on the membrane of Cajal-Retzius cells.
We thought that this possibility was unlikely for two reasons. First, Cajal-Retzius cells are particularly sensitive to whole-cell recording conditions (Mienville and Pesold, 1999), which have been shown to depress responses triggered by other G-protein coupled receptors such as the chemokine metabotropic receptor CXCR4 (Marchionni et al., 2010; Marchionni et al., 2012). Second, the massive increase of spontaneous GABAergic events would indicate that most synapses are silent under baseline conditions, which seems unlikely.
3.4. mGlu1a-mediated effect is generated by interneurons located below layer I
Nevertheless, we decided to test this hypothesis by comparing the effect of DHPG-mediated activation of mGlu1α in naïve slices vs. preparations in which a surgical cut had been done at the lower border of layer I. We reasoned that this manipulation would completely sever the axonal projections made by GABAergic neurons of deeper locations, but would not affect much the axons and dendrites of Cajal-Retzius cells, which are mostly confined to layer I (Radnikow et al., 2002). Hence, if DHPG-mediated increase in synaptic events was originated by mGlu1α expressed in the somatodendritic membrane of Martinotti cells, no effect of DHPG should be observed. Alternatively, if the critical mGlu1α receptors were expressed by Cajal-Retzius cells themselves, then responses to DHPG should remained mostly unaffected.
In naïve slices (Figure 5A), as expected, application of DHPG strongly potentiated the frequency of sPCs from 334±167 mHz before the addition of the agonist to 1646±371 mHz in its presence (p<0.05, n=14, repeated measures ANOVA with Bonferroni posthoc test). Frequency was then reduced by the subsequent addition of LY367385 to 295±71 mHz (p<0.05, n=14, repeated measures ANOVA with Bonferroni posthoc test).
In contrast, when the same experimental protocol was applied to surgically treated slices (Figure 5B), no changes were measured in the frequencies of spontaneous events (77±24 mHz in control, vs. 86±36 mHz in the presence of DHPG, and 64±23 mHz in DHPG plus LY367385, n=11, p>0.05, repeated measures ANOVA with Bonferroni posthoc test). Therefore, we concluded that, under our experimental conditions, mGlu1α expressed by Cajal-Retzius cells did not play a significant role in the observed DHPG-induced modulation of spontaneous events.
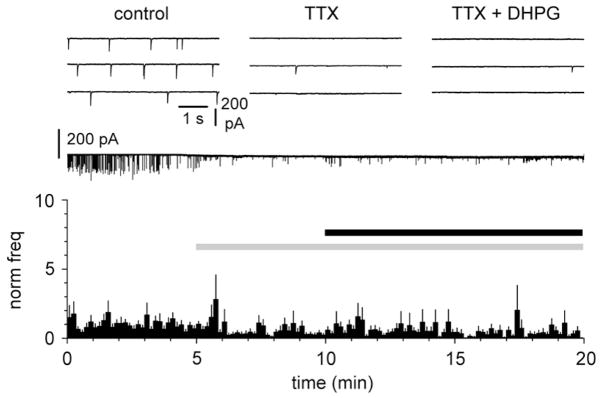
Lastly, we corroborated this result by showing that exposure to TTX, which blocks voltage-dependent sodium channels and action potentials, both reduced spontaneous synaptic activity and prevented the effect of DHPG. Application of TTX (1 μM, Figure 6) reduced the frequency of spontaneous PSCs from 155±48 mHz to 41±12 mHz (n=11, p<0.05, repeated measures ANOVA with Bonferroni posthoc test). However, when DHPG (50 μM) was added in the presence of TTX, no significant effect was observed (frequency in TTX + DHPG was 38±9 mHz, n=11, p>0.05, repeated measures ANOVA with Bonferroni posthoc test).
Figure 6.
A network mechanism underlies DHPG-induced increase of the frequency of spontaneous GABAergic currents in Cajal-Retzius cells. A: summary graph of the effect of DHPG (50 μM, black bar) following pre-exposure to TTX (1 μM, grey bar). Notice that TTX completely prevents the effect of DHPG. The upper insets show traces in control (left), in the presence of TTX (middle), and in the presence of both TTX and DHPG (right). Glutamatergic ionotropic synaptic transmission was blocked by NBQX (20 μM) and D-AP5 (50 μM).
Thus, DHPH-induced GABAergic input observed on Cajal-Retzius cells depends on firing activity of cells located below layer I, which express mGlu1α, all characteristics that fit very well with Martinotti cells.
4. DISCUSSION
To our knowledge, this work establishes for the first time two important novel results. First, we show that synaptically-released GABA is able to trigger action potentials in Cajal-Retzius cells via GABAA receptors. Second, we demonstrate that an important source of GABAergic input to Cajal-Retzius cells is massively controlled by the activation of mGlu1α receptors. Therefore, our data predict that conditions associated with sustained glutamate release, which may occur in vivo and lead to the activation of metabotropic glutamate receptors, would be especially effective in activating Cajal-Retzius cells via a disynaptic pathway.
4.1. Cajal-Retzius cells: GABAergic input and computational roles
Although Cajal-Retzius cells have been long studied as a major cellular source of the glycoprotein reelin, which is critically involved in cortical development (D’Arcangelo et al., 1995), their computational roles have received less attention and are also less understood. Yet, Cajal-Retzius cells are endowed with membrane excitability and can fire action potentials (Hestrin and Armstrong, 1996; Zhou and Hablitz, 1996; Radnikow et al., 2002), they show network- and mGluR- modulated intracellular calcium oscillations (Schwartz et al., 1998; Aguiló et al., 1999; Martine-Galan et al., 2001), and are integrated within neocortical networks (Radnikow et al., 2002; Soda et al, 2003).
However, both the identification of the presynaptic cells originating synaptic inputs converging onto Cajal-Retzius cells and the characterization of their postsynaptic effects on their cellular targets are still unclear, despite the importance of this type of information to understand their role(s) in the circuit. Our work, in agreement with several other studies in the neocortex (Kilb and Luhmann, 2001; Soda et al., 2003) and hippocampus (Marchionni et al., 2010), confirms that Cajal-Retzius cells receive predominantly, if not exclusively, spontaneous GABAergic input. Furthermore, using non-invasive recording techniques that maintain unperturbed chloride levels, we show that field electrical stimulation is able to trigger synaptically-evoked action potentials, which are prevented by the addition of the GABAA receptor blocker gabazine. This result fits very well with electrophysiological evidence obtained in perforated-patch (Mienville, 1998; Achilles et al., 2007) or cell-attached configuration (Marchionni et al., 2010) showing that the electrochemical gradient for GABAA receptor-induced currents is depolarized compared to resting potential, and that Cajal-Retzius cells respond with firing when exposed to exogenously applied GABA. Our finding is also consistent with the reported expression of the NKCC1 (Achilles et al., 2007), but not KCC2 transporter (Pozas et al., 2008), which would favor the maintenance of high intracellular chloride levels, as NKCC1 (Yamada et al., 2004) and KCC2 (Rivera et al., 1999) play opposite roles in regulating intracellular chloride homeostasis.
Thus, if the major synaptic input that can generate action potentials in Cajal-Retzius cells is mediated by GABAergic fibers, a critical question becomes defining the subtype of presynaptic interneurons that originate this input. Here we add an important piece of the answer to this question by showing that activation of mGlu1α massively recruits a population of interneurons that target Cajal-Retzius cells.
In the juvenile/adult mouse neocortex a strong enrichment in the expression of mGluR1α has been repeatedly demonstrated in somatostatin-expressing interneurons such as Martinotti cells. Although Martinotti cells show variable anatomical and electrophysiological properties (Whale, 1993; Kawaguchi and Kubota, 1997; Wang et al., 2004; Fanselow et al., 2008), they reliably express somatostatin and send axonal projections to layer I. In contrast, mGluR1α expression has been shown to be low in fast-spiking interneurons (Beierlein et al., 2000; Cauli et al., 2000) such as basket cells and chandelier interneurons (Kawaguchi, 1995; Kawaguchi and Kubota, 1997). Therefore, we propose that Martinotti cells are the source of the mGlu1α-dependent GABAergc input to layer I Cajal-Retzius cells observed in our experiments. Consistently, we have shown that in our slice preparation, a high proportion of neurons activated to supra-threshold levels by DHPG sent axonal projection to layer I. It is also important to note that Martinotti-like cells are among the earlier recognized local-circuit interneuron in the developing mammalian neocortex and are essential components of the subplate (Friauf et al., 1990; Marín-Padilla, 2011). Although the GABAergic input described by Myakhar et al. (2011) is likely to originate from these Martinotti-like cells, immunoreactivity for mGlu1α has been reported to be virtually absent in subplate neurons, at least at embryonic stages (Lopez-Bandito et al., 2002).
Thus, the most parsimonious explanation is that the effect we observed was either mediated by Martinotti cells of upper layers or that some subplate neurons may increase their level of expression of mGlu1α postnatally.
It needs also to be explicitly acknowledged that Martinotti cells are not the only interneuronal type expressing group I mGluRs in the neocortex. In fact, we occasionally observed depolarizing responses to DHPG in local layer I interneurons, although they never reached supra-threshold levels. Whether this is the result of our preparation, which may have disproportionally affected the axonal arborization of one class of cell vs. the other or may reflect the early developmental stage examined, remains to be determined. However, it nevertheless corroborates our suggestion that the observed increase in spontaneous synaptic events did not originate from local layer I interneurons, but from Martinotti cells of deeper layers.
Another important point that needs to be explicitly recognized is that our suggestion that the massive synaptic input unveiled by mGlu1α activation originates from Martinotti cells does not imply that, overall, Martinotti cells are the major source of GABAergic input to Cajal-Retzius cells. Similarly powerful connections may be established by other classes of GABAergic cells located in layer I (Hestrin and Armstrong, 1996; Zhou and Hablitz, 1996; Chu et al., 2003; Marín-Padilla, 2011a, 2011b; Wonzy and Williams, 2011) or in other areas (Friauf et al., 1990; Lin et al., 1990; Myakhar et al., 2011, Marín-Padilla, 2011a, 2011b). All these neurons may play an equally important role in the regulation of Cajal-Retzius cell activity. Future work based on paired recordings will hopefully provide direct information on this issue.
4.2. Functions of Martinotti→Cajal-Retzius cell connections
The axon of Martinotti cells is considered an integral part of the layer I micro-circuitry and has been shown to be present, in humans, at very early stage of development and to persist until adulthood (Marín-Padilla, 2011a, 2011b). Although recent work in adult animals has highlighted the powerful inhibitory role of Martinotti cells on the apical dendrites of pyramidal cells by modulating local calcium electrogenesis (Murayama et al., 2009), their role at early developmental stages may be different because of the immature stage of pyramidal cell dendritic excitability (Zhu, 2000).
Direct calcium imaging of layer I neurons at embryonic and early postnatal stages has revealed the presence of an active network showing correlated activity (Schwartz et al., 1998; Aguiló et al., 1999). Intriguingly, de-coupling of spontaneous calcium activity could be obtained by the pharmacological blockade of GABAA receptors and voltage dependent sodium channels. We suggest that GABA release from Martinotti cells onto postsynaptic Cajal-Retzius cells and local neurons may be critical for the selection of neuronal assemblies involved in this correlated activity. Interesting, the activation of metabotropic glutamate receptors has been shown to trigger rhythmic firing activity in a network of electrically coupled low threshold spiking neurons (Beierlein et al., 2000), which are the electrophysiological phenotype of somatostatin-expressing interneurons and of a subtype of Martinotti cells (Kawaguchi, 1995; Kawaguchi and Kubota, 1997; Goldberg et al., 2004; Wang et al., 2004).
According to our hypothesis, Martinotti-cell driven oscillatory calcium activity could be important for several functions. First, it could regulate reelin secretion/release and hence affect a large number of pathways critical for pyramidal cell dendritic and synaptic development. Although in other neuronal types reelin secretion has been shown to follow a constitutive pathway (Lacor et al., 2000), indirect evidence for activity-dependent secretion of reelin in Cajal-Retzius cells has also been put forward. For example, Derer et al. (2001) have proposed axonal localization of reelin in specialized membrane-delimited structures they termed “axonal reelin reservoirs”. In addition, reelin levels during early development appear diminished following blockade of 5-HT3 serotoninergic excitatory input to Cajal-Retzius cells (Chameau et al., 2009), and exposure of hippocampal cultured slices to kainate (to activate glutamate receptors) can modulate reelin levels (Tinnes et al, 2011). However, these last results are complex to interpret because additional factors such as kainate-dependent depolarization block of Cajal-Retzius cells, cellular toxicity, and impact on the cleavage of the molecule need to be considered (Duveau et al., 2010; Tinnes et al., 2011).
Second, although a direct determination of the major conventional neurotransmitter released by Cajal-Retzius cells is still lacking, several studies have suggested that they are likely to be glutamatergic (Del Río et al., 1995; Radnikow et al., 2002; Hevner et al., 2003, Ina et al., 2007). Assuming that this was the case, glutamate release could play a trophic role (Balazs, 2006) during the maturation of dendrites (Lüthi et al., 2001) and synapses (Durand et al., 1996) on the apical tufts of pyramidal neurons. By directly controlling these functions, GABAergic input to Cajal-Retzius cells could be critically involved in essential developmental functions. However, the conclusive determination of the neurotransmitter released by Cajal-Retzius cells and of the receptors mediating its postsynaptic effects on target cell will be required to validate or disprove this hypothesis.
4.3. Conclusions
Our work has revealed that mGlu1α expressed by a subpopulation of neocortical interneurons, likely to be Martinotti cells, has the ability to powerfully control spontaneous GABAergic input to Cajal-Retzius cells. Although in the mature cortex Martinotti cells have been proposed to play a critical role as gatekeepers of dendritic excitability of pyramidal neurons, we propose that, at early developmental stages, they may play a different role, by synchronizing populations of Cajal-Retzius cells and potentially other layer I neurons. These functions may finally depend on reelin, glutamate or a yet unidentified output molecule as final effector(s).
HIGHLIGHTS.
Spontaneous synaptic currents in Cajal-Retzius cells (CRs) are GABAergic.
The frequency of these currents is massively increased by mGlu1α activation
This effect requires firing in cortical layers below layer I
DHPG triggers firing in layer II/III Martinotti interneurons (Ms)
Ms of layer II/III are proposed to be a critical source of synaptic input to CRs zs
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (grant number NS064135 to GM).
Footnotes
CONFLICT OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achilles K, Okabe A, Ikeda M, Shimizu-Okabe C, Yamada J, Fukuda A, Luhmann HJ, Kilb W. Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons. J Neurosci. 2007;27:8616–8627. doi: 10.1523/JNEUROSCI.5041-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiló A, Schwartz TH, Kumar VS, Peterlin ZA, Tsiola A, Soriano E, Yuste R. Involvement of cajal-retzius neurons in spontaneous correlated activity of embryonic and postnatal layer 1 from wild-type and reeler mice. J Neurosci. 1999;19:10856–10868. doi: 10.1523/JNEUROSCI.19-24-10856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Balazs R. Trophic effect of glutamate. Curr Top Med Chem. 2006;6:961–968. doi: 10.2174/156802606777323700. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One. 2009;4:e5505. doi: 10.1371/journal.pone.0005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci U S A. 2000;97:6144–6149. doi: 10.1073/pnas.97.11.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2009;106:7227–7232. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Yeh HH. Enhanced GABA(A) receptor-mediated activity following activation of NMDA receptors in Cajal-Retzius cells in the developing mouse neocortex. J Physiol. 2003;550:103–111. doi: 10.1113/jphysiol.2003.042556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Hablitz JJ. Activation of group I mGluRs increases spontaneous IPSC frequency in rat frontal cortex. J Neurophysiol. 1998;80:621–627. doi: 10.1152/jn.1998.80.2.621. [DOI] [PubMed] [Google Scholar]
- Chu Z, Galarreta M, Hestrin S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J Neurosci. 2003;23:96–102. doi: 10.1523/JNEUROSCI.23-01-00096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Duggan AW, Felix D, Johnston GA. GABA, bicuculline and central inhibition. Nature. 1970;226:1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- del Río JA, Martínez A, Fonseca M, Auladell C, Soriano E. Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb Cortex. 1995;5:13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M, Goffinet A. Axonal secretion of Reelin by Cajal-Retzius cells: evidence from comparison of normal and Reln(Orl) mutant mice. J Comp Neurol. 2001;440:136–143. doi: 10.1002/cne.1375. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Duveau V, Madhusudan A, Caleo M, Knuesel I, Fritschy JM. Impaired reelin processing and secretion by Cajal-Retzius cells contributes to granule cell dispersion in a mouse model of temporal lobe epilepsy. Hippocampus. 2011;21:935–944. doi: 10.1002/hipo.20793. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairen A, DeFelipe J, Regidor J. Nonpyramidal neurons. In: Peters A, Jones EG, editors. Cerebral Cortex: Cellular Components of the Cerebral Cortex. New York: Plenum Press; 1984. pp. 206–211. [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S, Armstrong WE. Morphology and physiology of cortical neurons in layer I. J Neurosci. 1996;16:5290–5300. doi: 10.1523/JNEUROSCI.16-17-05290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Neogi T, Englund C, Daza RA, Fink A. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- Ina A, Sugiyama M, Konno J, Yoshida S, Ohmomo H, Nogami H, Shutoh F, Hisano S. Cajal-Retzius cells and subplate neurons differentially express vesicular glutamate transporters 1 and 2 during development of mouse cortex. Eur J Neurosci. 2007;26:615–623. doi: 10.1111/j.1460-9568.2007.05703.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ. Spontaneous GABAergic postsynaptic currents in Cajal-Retzius cells in neonatal rat cerebral cortex. Eur J Neurosci. 2001;13:1387–1390. doi: 10.1046/j.0953-816x.2001.01514.x. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Grayson DR, Auta J, Sugaya I, Costa E, Guidotti A. Reelin secretion from glutamatergic neurons in culture is independent from neurotransmitter regulation. Proc Natl Acad Sci U S A. 2000;97:3556–3561. doi: 10.1073/pnas.050589597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Nicolelis MA, Schneider JS, Chapin JK. A major direct GABAergic pathway from zona incerta to neocortex. Science. 1990;248:1553–1556. doi: 10.1126/science.2360049. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Shigemoto R, Fairén A, Luján R. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex. 2002;12:625–638. doi: 10.1093/cercor/12.6.625. [DOI] [PubMed] [Google Scholar]
- Lu SM, Zecevic N, Yeh HH. Distinct NMDA and AMPA receptor-mediated responses in mouse and human Cajal-Retzius cells. J Neurophysiol. 2001;86:2642–2646. doi: 10.1152/jn.2001.86.5.2642. [DOI] [PubMed] [Google Scholar]
- Lübke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendrites and axons of single and synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J Neurosci. 2000;20:5300–5311. doi: 10.1523/JNEUROSCI.20-14-05300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, Schwyzer L, Mateos JM, Gähwiler BH, McKinney RA. NMDA receptor activation limits the number of synaptic connections during hippocampal development. Nat Neurosci. 2001;11:1102–1027. doi: 10.1038/nn744. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni I, Takács VT, Nunzi MG, Mugnaini E, Miller RJ, Maccaferri G. Distinctive properties of CXC chemokine receptor 4-expressing Cajal-Retzius cells versus GABAergic interneurons of the postnatal hippocampus. J Physiol. 2010;588:2859–2878. doi: 10.1113/jphysiol.2010.190868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni I, Beaumont M, Maccaferri G. The chemokine CXCL12 and the HIV-1 envelope protein gp120 regulate spontaneous activity of Cajal-Retzius cells in opposite directions. J Physiol. 2012 doi: 10.1113/jphysiol.2011.224873. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Padilla M, Marín-Padilla TM. Origin, prenatal development and structural organization of layer I of the human cerebral (motor) cortex. A Golgi study. Anat Embryol. 1982;164:161–206. doi: 10.1007/BF00318504. [DOI] [PubMed] [Google Scholar]
- Marín-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Marín-Padilla M. Human motor cortex first lamina: development and cytoarchitecture. In: Marín-Padilla M, editor. The Human Brain: Prenatal Development and Structure. New York: Springer; 2011a. pp. 49–64. [Google Scholar]
- Marín-Padilla M. Human motor cortex excitatory-inhibitory neuronal systems: development and cytoarchitecture. In: Marín-Padilla M, editor. The Human Brain: Prenatal Development and Structure. New York: Springer; 2011b. pp. 65–83. [Google Scholar]
- Martínez-Galán JR, López-Bendito G, Luján R, Shigemoto R, Fairén A, Valdeolmillos M. Cajal-Retzius cells in early postnatal mouse cortex selectively express functional metabotropic glutamate receptors. Eur J Neurosci. 2001;13:1147–1154. doi: 10.1046/j.0953-816x.2001.01494.x. [DOI] [PubMed] [Google Scholar]
- Martinotti C. Contributo allo studio della corteccia cerebrale, ed all’origine central dei nervi. Ann Freniatr Sci Affini. 1889;1:14–348. [Google Scholar]
- Mienville JM. Persistent depolarizing action of GABA in rat Cajal-Retzius cells. J Physiol. 1998;512:809–817. doi: 10.1111/j.1469-7793.1998.809bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienville JM, Pesold C. Low resting potential and postnatal upregulation of NMDA receptors may cause Cajal-Retzius cell death. J Neurosci. 1999;19:1636–1646. doi: 10.1523/JNEUROSCI.19-05-01636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myakhar O, Unichenko P, Kirischuk S. GABAergic projections from the subplate to Cajal-Retzius cells in the neocortex. Neuroreport. 2011;22:525–529. doi: 10.1097/WNR.0b013e32834888a4. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Moore JW, Scott WR. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J Gen Physiol. 1964;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D’Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci. 2008;28:10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Sur la structure de l’ecorce cerebrale de quelques mammiferes. Cellule. 1891;7:3–54. [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozas E, Paco S, Soriano E, Aguado F. Cajal-Retzius cells fail to trigger the developmental expression of the Cl− extruding co-transporter KCC2. Brain Res. 2008;1239:85–91. doi: 10.1016/j.brainres.2008.08.058. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D, Lübke J. Axonal projection, input and output synapses, and synaptic physiology of Cajal-Retzius cells in the developing rat neocortex. J Neurosci. 2002;22:6908–6919. doi: 10.1523/JNEUROSCI.22-16-06908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. La Textura del Sistema Nerviosa del Hombre y los Vertebrados. Madrid (Spain) Moya 1904 [Google Scholar]
- Soriano E, Del Río JA. The cells of cajal-retzius: still a mystery one century after. Neuron. 2005;46:389–394. doi: 10.1016/j.neuron.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Qiu S, Weeber EJ. Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J Neurophysiol. 2007;97:2312–2321. doi: 10.1152/jn.00869.2006. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Rabinowitz D, Unni V, Kumar VS, Smetters DK, Tsiola A, Yuste R. Networks of coactive neurons in developing layer 1. Neuron. 1998;20:541–552. doi: 10.1016/s0896-6273(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Soda T, Nakashima R, Watanabe D, Nakajima K, Pastan I, Nakanishi S. Segregation and coactivation of developing neocortical layer 1 neurons. J Neurosci. 2003;23:6272–6279. doi: 10.1523/JNEUROSCI.23-15-06272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinehelfer S, Vruwink M, Burette A. Immunolocalization of mGluR1alpha in specific populations of local circuit neurons in the cerebral cortex. Brain Res. 2000;861:37–44. doi: 10.1016/s0006-8993(00)01952-1. [DOI] [PubMed] [Google Scholar]
- Tinnes S, Schäfer MK, Flubacher A, Münzner G, Frotscher M, Haas CA. Epileptiform activity interferes with proteolytic processing of Reelin required for dentate granule cell positioning. FASEB J. 2011;25:1002–1013. doi: 10.1096/fj.10-168294. [DOI] [PubMed] [Google Scholar]
- Wahle P. Differential regulation of substance P and somatostatin in Martinotti cells of the developing cat visual cortex. J Comp Neurol. 1993;329:519–538. doi: 10.1002/cne.903290408. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C, Williams SR. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb Cortex. 2011;21:1818–1826. doi: 10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol. 2004;557:829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Morphological properties of intracellularly labeled layer I neurons in rat neocortex. J Comp Neurol. 1996;376:198–213. doi: 10.1002/(SICI)1096-9861(19961209)376:2<198::AID-CNE3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Zhu JJ. Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol. 2000;526:571–587. doi: 10.1111/j.1469-7793.2000.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]