Abstract
Many differences in brain activity have been reported between persons who stutter (PWS) and typically fluent controls during oral reading tasks. An earlier meta-analysis of imaging studies identified stutter-related regions, but recent studies report less agreement with those regions. A PET study on adult dextral PWS (n = 18) and matched fluent controls (CONT, n = 12) is reported that used both oral reading and monologue tasks. After correcting for speech rate differences between the groups the task-activation differences were surprisingly small. For both analyses only some regions previously considered stutter-related were more activated in the PWS group than in the CONT group, and these were also activated during eyes-closed rest (ECR). In the PWS group, stuttering frequency was correlated with cortico-striatal-thalamic circuit activity in both speaking tasks. The neuroimaging findings for the PWS group, relative to the CONT group, appear consistent with neuroanatomic abnormalities being increasingly reported among PWS.
Keywords: stuttering, oral reading, monologue, brain imaging, PET
1. Introduction
Numerous brain imaging investigations since the early 1990s have led to a general acceptance that developmental stuttering is associated with various signs of abnormal neuroanatomy and neurophysiology (see Ingham, Cykowski, Ingham, & Fox, 2008). A meta-analysis by Brown, Ingham, Ingham, Laird, and Fox (2005), for example, concluded from CBF data averaged across two fMRI and six PET studies (Braun et al., 1997; De Nil, Kroll, Kapur, & Houle, 2000; De Nil, Kroll, Lafaille, & Houle. 2003; Fox et al., 1996; Fox, Ingham, Ingham, Zamarripa, Xiong, & Lancaster, 2000; Ingham et al., 2004; Neumann et al., 2005; Preibisch et al., 2003;) that
motor areas were over-activated in stuttering, including primary motor cortex, supplementary motor area, cingulate motor area, and cerebellar vermis. Frontal operculum, rolandic operculum, and anterior insula showed anomalous right laterality in stutterers. Auditory activations, assumedly due to hearing one's own speech, were essentially undetectable in stutterers. (Brown et al., 2005, p. 105)
It is important to recognize, however, that not one of the 11 regional clusters identified by Brown et al. as differentiating persons who stutter (PWS) from controls was identified as significantly associated with stuttering in all eight of the studies they reviewed (see Brown et al., 2005, p. 111). Recent diffusion tensor imaging (DTI) studies (Chang, Synnestvedt, Ostuni, & Ludlow, 2010; Cykowski, Fox, Ingham, Ingham, & Robin, 2010) have also highlighted the variability across studies with respect to white matter abnormalities.
This dissimilarity across studies has not received the attention it deserves, particularly because these disparities persist across recent fMRI studies with adult PWS. Consider a recent report by Kell et al. (2009) with adult male PWS (n = 13, measured before and after 3 weeks of treatment), controls (n = 13), and recovered PWS (n = 13). During scanning all participants read sentences aloud or silently during 3-s intervals. Pretreatment activations for the first group were substantially different (see Kell et al.'s Table 1, p. 2753) from those reported by Brown et al. (2005); only 4/24 occurred in similar regions. Specifically, SMA was not significantly over activated in Kell et al.'s data, and right rather than left STG activity was substantially more active in PWS than controls.
TABLE 1.
Means and standard deviations (SD) for percent syllables stuttered (%SS) and syllables produced per minute (Syl/min) for persons who stutter (PWS) and normally fluent controls (CONT) during PET scanning. Each participant completed six scanning tasks: two monologues, two oral readings and two eyes-closed-rest conditions.
| Mean %SS | Mean Syl/min | |||||
|---|---|---|---|---|---|---|
| Monologue | Oral reading | Monologue | Oral reading | |||
| PWS (n = 18) (SD) | 9.75 (10.39) | 8.84 (10.05) | p = NS | 177.4 (47.6) | 175.2 (42.9) | p = NS |
| CONT (n = 12) (SD) | NA | NA | 189.4 (31.3) | 223.5 (20.8) | p = .003 | |
| p = NS | p = .0002 | |||||
Many complications exist, however, in attempting to compare findings across studies, not the least being that some studies use a mixture of right and left sided subjects (Knetch et al., 2000) and mixed genders. An fMRI study by Lu et al. (2010) that used picture naming with only dextral PWS (n = 12; 10 males) and dextral nonstuttering controls (n = 12; 7 males) reported only 4/20 activations that were greater in the PWS than in controls, for regions reported by Brown et al. (2005) as stutter-related. Marginally better agreement was reported by Chang, Kenney, Torrey, Loucks, and Ludlow (2009), who compared dextral PWS adults (n = 20; 11 females) with dextral controls (n = 20; 9 females) on listening and speech production tasks. Chang et al. found that in the overt speech production tasks only 7/24 of their PWS>control activations were in regions reported by Brown et al. However, this difference between these studies and the Brown et al. findings might be at least partly due to gender differences in neural regions that correlate with stuttering frequency (Ingham et al., 2004). Despite these differences in subject characteristics it is evident across a number of recent fMRI studies that during overt speech tasks relatively stronger activations have been reported in adult PWS in SMA, pre-SMA, posterior insula, STG (Chang et al., 2009; Lu et al., 2010) and basal ganglia (Lu et al., 2010; Giraud et al., 2008), which Giraud et al. (2008) found was strongly correlated with stuttering frequency.
It becomes increasingly obvious, in fact, that there are numerous reasons why the identification across studies of stable neural regions that are consistently associated with stuttering might be problematic. In addition to handedness (sidedness) and gender, these include differences in imaging techniques, tasks, and data analysis. Perhaps the most important might be the fact that in most fMRI studies (Chang et al., 2009; Kell et al., 2009; Lu et al., 2010) the PWS groups’ activation data are derived mostly from brief nonstuttered utterances; any stuttering events that do occur are either ignored (Lu et al., 2010) or carefully excluded from analyses (Chang et al., 2009; Salmelin, Schnitzler, Schmitz, & Freund, 2000). This means that the analyses are based on stutter-free utterances, despite ample evidence that stuttering frequency correlates with many regional activations that differ from those that occur during fluent word production (Fox et al., 2000; Ingham et al., 2000, 2004). Furthermore, stuttering-event related activations were not isolated from nonstuttering-event related activations in these recent studies. This would seem to make it difficult to claim that any differences between stutter-free activations and those produced by fluent controls are functionally related to stuttering behavior.
Another important potential source of variance across studies, which has been almost completely ignored, is the role of speech rate. One of the most well-established facts about PWS is that in adulthood their oral reading and spontaneous speech rates are generally slower than the rates of control speakers (Bloodstein & Ratner, 2008). Equally well established is that variations in the rate of syllable production will have differential effects on brain regions involved in speech production (e.g., Riecker, Kassubek, Gröschel, Grodd, & Ackermann, 2006). Kell et al. (2009), for instance, acknowledged that their PWS group spoke more slowly than their controls and recovered stutterers. Similarly, Lu et al. (2010) recognized that response latency was considerably slower for their PWS group than it was for their controls - consistent with an established feature of stuttering (Bloodstein & Ratner, 2008). Neither study, however, controlled for the potential effects of this differentiating variable. These differences appear to make it almost inevitable that neuroimaging findings will differ across these reports as well as in comparison to the results of Brown et al. (2005).
Furthermore, it is yet to be firmly established that stuttering related regions, such as those identified by Brown et al. (2005), are sufficiently robust that they will be stable across different speaking tasks, such as oral reading and spontaneous speech. The studies reviewed by Brown et al. used oral reading, with the exception of one PET study by Braun et al. (1997) that used “spontaneous narrative speech” (among different speech and language tasks). The problem is that oral reading and spontaneous speech are fundamentally different speaking tasks that require different methods of language formulation and may produce very different stuttering frequencies and severities within PWS (see Bloodstein & Ratner, 2008). In the present study, for example, even though mean stuttering frequency was similar across tasks for the PWS group, the correlation between percentage of syllables stuttered (%SS) during oral reading and monologue speech tasks was only 0.184 for the members of this group (see Results). The importance of differences across tasks is magnified in light of findings from such powerful “fluency-inducing conditions” (see Ingham, 1984) as chorus reading or rhythmic speech. These conditions can modify the ostensibly abnormal neural regions isolated during oral reading (Fox et al., 1996) or during spontaneous speech (Stager, Jeffries, & Braun, 2003). Conceivably, this occurs because these classic fluency-inducing conditions directly influence brain regions common to oral reading and spontaneous speech that functionally control stuttering. The Stager et al. (2003) PET study may be of interest in this regard because, like Braun et al., it employed a narrative task (in conjunction with rhythmic speech and singing to reduce stuttering). Unfortunately, the regional differences between the PWS and controls during this spontaneous speaking task were not reported; only the activations that were similar in both groups.
Finally, it is possible that the differences in brain activity between PWS and control groups could also be due to physiologic changes altering both task related activity and rest activity. Most prior studies have only tested for an interaction of task versus baseline and patient group. Significant alterations of brain activity at rest could impact the magnitude of this interaction and obscure the identification of task invariant effects due to stuttering. A recent report by Xuan et al. (2012) highlights this possibility. They analyzed the amplitude of low-frequency fluctuations (ALFF) in fMRI signals during an eyes-closed rest condition with 44 adult male PWS and 46 age- and gender-matched controls. They reported that the PWS group, relative to controls, showed ALFF increases “in left brain areas related to speech motor and auditory functions and bilateral prefrontal cortices related to cognitive control.” But in comparison with controls they displayed “decreased ALFF in the left posterior language reception area and bilateral non-speech motor areas” (2012, p. 2). In turn, this finding is in contrast with those obtained in earlier PET studies (Braun et al., 1997; Ingham et al., 1996) that failed to find differences between PWS and controls during rest conditions.
In short, the literature suggests both similarities and differences across studies of PWS, but the reasons for those differences (which could also include different statistical significance levels or even different baseline levels of activation), and their implications for a complete understanding of stuttering, have not been explored. A within-group investigation that controls for task conditions, speech rate, and gender differences would help rectify this situation by determining whether there are neural regions that are consistently functionally associated with stuttering during different speaking tasks. In addition, a comparison of groups during eyes-closed rest conditions is now of paramount importance in order to understand the significance of recent evidence of task independent pathophysiologic stuttering substrates. The present study provided that opportunity, using a previously employed oral reading task and a monologue production task in PWS (Fox et al., 1996; 2000).
2. Method
2.1. Participants
Eighteen male PWS (age range 20-67 years; mean = 33.5 years) and 12 male controls (CONT) (age range 20-65 years; mean 34.8 years) participated in this study. All were healthy adult volunteers, including PWS who were identified from treatment waiting lists and via advertisements in San Antonio, Austin, and Houston. All PWS self-reported a stuttering problem since early childhood, self-reported that they stuttered, and displayed stuttering as confirmed by the principal investigator and a certified speech-language pathologist (SLP) using standard clinical assessments. All participants in both groups were right-handed [> +80 on the Edinburgh Handedness Inventory (Oldfield, 1971)] displayed no signs of any neurologic disorder (other than stuttering-related regional differences); reported no other current speech, language, cognitive, or behavioral disorder; and passed a hearing screening.
All PWS had experienced various therapies, but no participant reported receiving therapy for stuttering in the preceding 3 years. All produced at least three percent syllables stuttered (%SS) during each of three 3-min speaking tasks (oral reading, monologue, and telephone conversations) during within-clinic assessments (see below, Table 1). All CONT participants met the same selection criteria except that they produced 0%SS during each of the three speaking tasks and did not report the presence or a history of stuttering.
2.2. Procedures
Each participant completed a total of six scanning trials: two eyes-closed rest (ECR), two oral readings (reading aloud continuous text from Abbey, 1975) (ORA), and two monologues (continuous self-formulated speech on self-selected topics) (MON). They were presented in a counterbalanced sequence for participants in each group.
2.2.1 PET image acquisition
Cerebral blood flow (CBF) measurements using PET (GE 4096 WB scanner, with 15 slices, each 6.5 mm) were obtained while participants performed speech tasks or rested with eyes closed. CBF was measured with 15O-labeled water with a half-life of 123 s. The isotope was administered as an intravenous bolus of 8-10 ml of saline containing 60 mCi. A 90-s scan was initiated at the point in time the tracer bolus entered the brain. During ORA and MON scans participants spoke for 60 s from the onset of the 90 s and were then instructed to close their eyes. Prior to each ECR condition participants were instructed to think of a pleasant countryside scene. Speech and imaging data obtained during the first 40 s were used in the data analysis. A 10-min interscan interval, sufficient for isotope decay (five half-lives), was used.
2.2.2. MRI
A prior anatomical MRI was obtained so as to optimize spatial normalization of PET images. MRI was acquired on a 1.9-Tesla Elscint Prestige scanner using a high-resolution 3D gradient-recalled acquisition in the steady state (GRASS) sequence: repetition time = 33 ms; echo time = 12 ms; flip angle = 60°; voxel size = 1mm3; matrix size = 256 × 192 × 192; acquisition time = 15 min.
2.2.3. Image preprocessing
PET images were reconstructed into 60 slices, each 2 mm thick with an image matrix size of 60 × 128 × 128 mm, using a 5-mm Hann filter, resulting in images with a spatial resolution of approximately 7 mm at FWHM and value normalized to a whole-brain mean of 1,000. For each subject, PET images were registered to each other and coregistered to the same subject's MRI scan. These aligned data were then spatially normalized to the Montreal Neurologic Institute (MNI) atlas in SPM8 using the default method. PET images were smoothed with a Gaussian 12 mm filter. Spatially normalized MRI images were averaged together to generate a population based reference atlas. Talairach coordinates (Talairach & Tourneux, 1988) were derived from MNI coordinates by using the Talairach Client (Lancaster, Rainey et al., 1997; Lancaster, Woldorff et al., 2000). Cerebellar locations and labels were derived using the MRI atlas of human cerebellum (Schmahmann et al., 1999).
2.2.4. Image analysis
Changes of brain activity as a function of task were assessed using statistical parametric mapping, version SPM8 (from the Wellcome Functional Imaging Laboratory, London, UK). Images were proportionally rescaled to each other, calibrated to a global CBF of 50 ml/dl/min using a common volume mask. A single primary multiple regression model was used to test for task related differences in rCBF, both within and across groups. This was followed by three secondary regression models that used reduced design matrices based on one group (PWS or CONT) to estimate effects of stutter frequency and syllable rate.
Primary regression model
The design matrix included eight parameters of interest, or covariates. Model parameters 1-3 denoted scans for the three tasks (ECR, MON, ORA) in CONT participants; parameters 4-6 delineated the same three tasks in the PWS participants. Parameter 7 was a vector of the number of syllables uttered per scan for all scans, subjects, and conditions. Because there are no syllables expressed during the ECR task, a “dummy” constant variable was used for all of these scans. This was calculated from the average number of all syllables produced over all MON and ORA trials for both groups. The resulting vector was center mean normalized and accounts for variance in CBF associated with the number of syllables in the MON and ORA tasks uttered on a scan by scan basis. Parameter 8 designated whether it was the first or second repetition for each trial type. The resulting design matrix was estimated using restricted maximum likelihood in SPM8. Planned comparisons between the conditions and groups were calculated as post hoc t tests.
Inclusive main effect
Any brain area that was active during either the ORA or MON task compared to ECR, for either group, was identified and used to define an inclusive mask of speech related activity. To do this, four contrasts [each task versus baseline (ECR), for each group] were calculated. Any voxel from any of the four contrasts that was significant at a confirmatory threshold of p < 0.05 FDR corrected for multiple comparisons was then included in a pooled task related volume of speech related activity that was used as a mask in all subsequent analyses. Within this mask, all subsequent analyses were tested at a threshold of p < 0.005 uncorrected and cluster size ≥10 voxels. In addition, some task and group differences were also tested at a threshold of p < 0.05 to facilitate comparison with the meta-analysis of Brown et al. (2005) (see Results). Hemispheric cerebellar and cerebral differences in the total number of activated voxels (for each speech task versus control) and for each group at a threshold of p < 0.005 are summarized in Table 3.
TABLE 3.
Comparison between total number of voxels that were significantly more activated in the PWS than the CONT (PWS>CONT) and vice versa (CONT>PWS) across tasks in left and right hemispheres of cerebrum and cerebellum. The hemisphere location is based on local maxima location and shows the mean number of activated voxels per hemisphere (clusters ≥ 10). Using data from either contrast (PWS>CONT or CONT>PWS) the PWS shows a consistently stronger right hemisphere activation.
| Monologue | Oral Reading | |||
|---|---|---|---|---|
| PWS>CONT | ||||
| Hemisphere | Left | Right | Left | Right |
| Cerebrum | 55 | 494 | 67 | |
| Cerebellum | 0 | 53 | 0 | 39 |
| CONT>PWS | ||||
|---|---|---|---|---|
| Cerebrum | 64 | 0 | 116 | 24 |
| Cerebellum | 0 | 0 | 224 | 18 |
Task and Group Differences
Individual contrasts between the two groups (PWS>CONT; CONT>PWS) for each task and between the two speech tasks (MON>ORA; ORA>MON) for each group are included in Table 2a and 2b (reported below in 3.2.1). The data for Table 2a used an exploratory threshold of p < 0.05 uncorrected and cluster size ≥10 voxels, limited to voxels within the mask of speech related activity. As discussed below, this facilitated a comparison with previous findings (Brown et al. 2005).
Table 2a.
Result of univariate analysis showing Talairach coordinates for local maxima regions in each speaking task where voxel clusters ( ≥ 10; p < .05) were significantly more activated for PWS group than for CONT group (PWS>CONT Group) and where regions were significantly more activated in CONT group than PWS group (CONT>PWS Group).
| PWS>CONT Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Monologue vs ECR | Oral Reading vs ECR | ||||||||
| Lobe | Region | X | Y | Z | Clust | X | Y | Z | Clust |
| Frontal | |||||||||
| Left | SMA (6) | -9 | -11 | 61* | |||||
| Precentral gyrus (4) | -55 | -20 | 33* | 30 | |||||
| Right | SMA (6) | 4 | -3 | 58* | 434 | 6 | -2 | 60* | 43 |
| Precentral gyrus (4) | 5 2 | -13 | 38* | 60 | |||||
| Parietal | |||||||||
| Right | Post central gyrus (3) | 52 | -17 | 38 | 24 | ||||
| Occipital | |||||||||
| Left | Cuneus (17) | -11 | -78 | 13 | 25 | ||||
| Cuneus (18) | -7 | -75 | 26* | 24 | |||||
| Cerebellum | |||||||||
| Right | Lobule V | 5 | -64 | -36 | 52 | ||||
| Lobule VI | 36 | -60 | -19* | 39 | |||||
| CONT>PWS Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Monologue vs ECR | Oral Reading vs ECR | ||||||||
| X | Y | Z | Clust | X | Y | Z | Clust | ||
| Sub-lobar | |||||||||
| Left | Claustrum | -27 | -9 | 19 | 58 | ||||
| Insula (13) | -29 | -11 | 21 | 70 | |||||
| Insula (13) | -27 | -30 | 23 | 31 | |||||
| Caudate | -22 | -17 | 24 | ||||||
| Subthalamic nucleus | -16 | -12 | -4 | 15 | |||||
| Right | Globus pallidus | 16 | -2 | 4 | 14 | ||||
| Occipital | |||||||||
| Right | Cuneus (19) | 9 | -87 | 27 | 10 | ||||
| Cuneus (18) | 10 | -85 | 23 | ||||||
| Brainstem | |||||||||
| Left | Red nucleus | -5 | -23 | -11 | 26 | ||||
| Cerebellum | |||||||||
| Left | Lobule V | -8 | -64 | -18 | |||||
| Lobule VI | -63 | -9 | 152 | ||||||
| Lobule VI | -25 | -48 | -37 | 31 | |||||
| Lobule VI | -32 | -58 | -14 | 41 | |||||
Submaxima coordinates are italicized. Regional coordinates with correspond to those identified in Brown et al's (2005) ALE analysis as stutter-related. All activations were derived after a correction was made for the number of syllables (i.e., speaking rate) produced per scan.
Table 2b.
Result of univariate analysis showing bolded Talairach coordinates for local maxima regions in each speaking task where voxel clusters (≥ 10; p < .005) were significantly more activated during the monologue than the oral reading task (MONO>ORA), and the reverse (ORA>MONO) in the PWS group. Similar contrasts were obtained for the CONT group. Submaxima coordinates are italicized.
| PWS Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MONO>ORA (no ECR) | ORA>MONO (no ECR) | ||||||||
| Lobe | Region | X | Y | Z | Clust | X | Y | Z | Clust |
| Frontal | |||||||||
| Left | Pre-SMA (6) | -9 | 2 | 66 | 206 | ||||
| Right | Pre-SMA (6) | 2 | 4 | 59 | |||||
| Occipital | |||||||||
| Left | Lingual gyrus (18) | -7 | -82 | -4 | 251 | ||||
| Cuneus (17) | -7 | -83 | 5 | ||||||
| Right | Lingual gyrus (18) | 2 | -84 | 0 | |||||
| Cerebellum | |||||||||
| Left | Lobule VI | -10 | -76 | 8 | 25 | ||||
| Right | CR I | 29 | -61 | -26 | 22 | ||||
| CONT group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MONO>ORA (no ECR) | ORA>MONO (no ECR) | ||||||||
| Frontal | |||||||||
| Left | Pre-SMA (6) | -9 | 2 | 66 | 82 | ||||
| Right | Pre-SMA (6) | 4 | 3 | 68 | |||||
| Occipital | |||||||||
| Left | Lingual gyrus(18) | -7 | -82 | -4 | |||||
| Cuneus (18) | -5 | -75 | 8 | ||||||
| Cuneus (17) | -3 | -83 | 9 | ||||||
| Right | Lingual gyrus (18) | 1 | -82 | 2 | 171 | ||||
| Cerebellum | |||||||||
| Right | Lobule VI | 40 | -59 | -26 | 46 | ||||
Group differences common to both speech tasks
: The purpose of this contrast was to identify brain regions that were different between PWS and CONT groups, irrespective of what speech task was being performed. To do this, a conjunction analysis was calculated using SPM8. It was based on an inclusive conjunction of those areas where PWS>CONT in both the MON and ORA tasks. The opposite conjunction, where CONT>PWS for both tasks was also calculated. The results of this conjunction analysis (masked by the global mask of speech production) are shown in Table 4.
TABLE 4.
Result of conjoint analysis showing local maxima Talairach coordinates for voxel clusters ( ≥ 10; p < .005) that were significantly activated only during eyes-closed-rest (ECR). They are compared with voxel clusters and local maxima coordinates that remained significant after variance due to ECR has been removed [MON + ORA(no ECR)]. Submaxima coordinates within proximate voxel clusters are italicized. Table shows only local maxima coordinates for voxel clusters significantly more activated for PWS than for CONT group (PWS>CONT) and for the reverse (CONT>PWS).
| PWS > CONT GROUP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eyes closed rest (ECR) | MON + ORA (no ECR) | ||||||||
| Lobe | Region | X | Y | Z | Clust | X | Y | Z | Clust |
| Frontal | |||||||||
| Left | Pre-SMA (6) | -9 | 10 | 72 | 29 | ||||
| Inferior frontal gyrus (44) | -47 | 9 | 13 | 16 | |||||
| Parietal | |||||||||
| Right | Postcentral gyrus (43) | 53 | -7 | 15* | 29 | ||||
| Postcentral gyrus (43) | 54 | -9 | 19* | 54 | -9 | 19* | 17 | ||
| Temporal | |||||||||
| Left | Superior temporal gyrus (22) | -40 | -40 | 14* | 35 | -40 | -40 | 12* | 69 |
| Sub-lobar | |||||||||
| Left | Putamen | -29 | -12 | 13 | 25 | ||||
| Right | Globus pallidus | 19 | -7 | 2 | 14 | ||||
| Globus pallidus | 23 | -13 | 3 | ||||||
| Posterior insula (13) | 38 | -32 | 22 | 40 | |||||
| Occipital | |||||||||
| Left | Cuneus (18) | -13 | -78 | 16 | 122 | -11 | -78 | 17 | 255 |
| Right | Cuneus (18) | ||||||||
| Cerebellum | |||||||||
| Left | Vermis III | -3 | -30 | -15 | 51 | ||||
| Lobule VI | -23 | -69 | -22* | 10 | |||||
| Lobule VI | -23 | -71 | -17* | -21 | -71 | -19* | 62 | ||
| Right | Lobule VI | 36 | -69 | -20* | 48 | 36 | -69 | -20* | 98 |
| Lobule VI | 8 | -74 | -12* | 75 | 8 | -72 | -10* | 68 | |
| Vermis III | 6 | -68 | -8* | 7 | -53 | -28* | 28 | ||
| Lobule IX | 4 | -60 | -36 | ||||||
| CONT>PWS GROUP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frontal | |||||||||
| Left | SLPrM (6) | -39 | -5 | 41* | 23 | -39 | -5 | 41* | 53 |
| SLPrM (6) | -42 | -4 | 43* | ||||||
| Right | Pre-SMA (6) | 8 | 4 | 55 | 22 | ||||
| Temporal | |||||||||
| Right | Transverse temporal gyrus (41) | 54 | -22 | 12 | 10 | ||||
| Sub-lobar | |||||||||
| Left | Globus pallidus | -18 | -12 | -3 | 20 | ||||
| Posterior insula (13) | -28 | -25 | 19 | 33 | |||||
| Right | Posterior insula (13) | 26 | -30 | 18 | 13 | ||||
| Posterior insula (13) | 28 | -29 | 15 | ||||||
| Occipital | |||||||||
| Left | Lingual gyrus (18) | -5 | -82 | -4 | 16 | ||||
| Right | Cuneus (18) | 19 | -81 | 24 | 12 | ||||
| Cerebellum | |||||||||
| Left | Lobule VI | -27 | -48 | -40 | 26 | ||||
| Lobule VI | |||||||||
| Right | Lobule VI | 27 | -60 | --32* | 36 | 25 | -62 | -32* | 29 |
| Lobule VI | 25 | -57 | -33* | ||||||
All activations were derived after a correction was made for the number of syllables produced per scan. Coordinates in both the PWS and CONT groups with occur within regions identified by Brown et al. (2005) as typifying stuttered speech.
Group differences in the resting state
A separate contrast was calculated to identify areas where PWS>CONT and CONT>PWS were significantly different for the ECR task. The results of this (masked by the global mask of speech production) are shown in Table 4.
Secondary regression models
(i) Stuttering rate in PWS
To estimate brain activity associated with the rate of stuttering, the primary design matrix was reduced to include only scans from the PWS group. The design matrix included four parameters: Task (MON or ORA), Trial repetition (1 or 2), Stuttering rate per scan (see 2.2.6) and a constant. Conjunction analysis was used to identify brain areas that correlated with stuttering frequency for both tasks. Both positive and negative correlations between stuttering rate and brain activity were estimated and are summarized in Tables 5a and 5b.
TABLE 5a.
Positive correlations between stuttering frequency and brain activity in the PWS group. Talairach coordinates are provided for regions where voxel clusters ( ≥ 10; p <.005) were significantly and positively correlated with stuttering frequency following a conjoint analysis of MON+ORA for the PWS group. Local maxima coordinates shown with cluster size. Z scores showing the relative intensity of regions functionally related to stuttering frequency are shown in Figure 4.
| Z score | Region | X | Y | Z | Cluster |
|---|---|---|---|---|---|
| 5.62 | Thalamus | -23 | -26 | 1 | 35 |
| 5.40 | Precentral gyrus (4) M1 | 62 | -7 | 35 | 131 |
| 5.37 | SMA (6) | 4 | -16 | 61 | 38 |
| 5.25 | Posterior insula (13) | 34 | -35 | 16 | 183 |
| 5.08 | Caudate | -22 | -17 | 22 | 125 |
| 4.96 | Superior temporal gyrus (22) | -53 | -40 | 14 | 176 |
| 4.93 | Claustrum | -25 | -26 | 18 | 51 |
| 4.15 | Lateral globus pallidus | 21 | -11 | 2 | 40 |
| 4.09 | Lingual gyrus (18) | -5 | -84 | -6 | 14 |
| 3.96 | Medial globus pallidus | -18 | -12 | -3 | 15 |
TABLE 5b.
Negative correlations between stuttering frequency and brain activity in the PWS group. Talairach coordinates are provided for regions where voxel clusters ( ≥ 10; p <.005) were significantly and negatively correlated with stuttering frequency following a conjoint analysis of MON+ORA for the PWS group. Local maxima coordinates shown with cluster size.
| Z score | Region | X | Y | Z | Cluster |
|---|---|---|---|---|---|
| 5.26 | Lobule VI | -23 | -65 | -19 | 221 |
| 4.71 | Lobule VI | 23 | -66 | -14 | 165 |
| 3.88 | Lobule VI | 38 | -58 | -15 | 132 |
| 3.86 | SMA (6) | 5 | -13 | 75 | 183 |
| 3.74 | Cuneus (17) | -14 | -79 | 11 | 38 |
| 3.70 | Transverse temporal gyrus (41) | 45 | -16 | 13 | 105 |
| 3.64 | Superior temporal gyrus (22) | -59 | -14 | 13 | 10 |
| 3.54 | Cuneus (18) | 19 | -84 | 22 | 15 |
| 3.43 | SLPrM (6) | 41 | -7 | 57 | 23 |
| 3.16 | Lobule IV | 8 | -50 | -22 | 11 |
| 2.95 | Lobule VII B | 12 | -68 | -34 | 14 |
| 2.93 | Cuneus (17) | 10 | -70 | 12 | 25 |
(ii) Syllable rate in PWS
To estimate brain activity associated with the rate of syllable production, the primary design matrix was reduced to include only scans from the PWS group. The design matrix included four parameters: Task (MON or ORA), Trial repetition (1 or 2), Syllables per minute per scan (see 2.2.6) and a constant. Conjunction analysis was used to identify brain areas that correlated with syllable rate for both tasks. Both positive and negative correlations between syllable rate and brain activity were estimated and are summarized in Tables 6a and 6b.
TABLE 6a.
Positive correlations between syllable rate and brain activity for both MON and ORA tasks in the PWS and CONT groups. Talairach coordinates are provided for regions where voxel clusters ( ≥ 10; p <.005) were significantly and positively correlated with syllable rate following a conjunction analysis combining the effects of rate for MON and ORA tasks in the PWS group. Local maxima coordinates shown with cluster size.
| PWS | |||||
|---|---|---|---|---|---|
| Z score | Region | X | Y | Z | Cluster |
| 4.98 | Lobule IV | 7 | -50 | -22 | 70 |
| 4.94 | Transverse temporal gyrus (41) | 49 | -14 | 11 | 152 |
| 4.27 | Lobule VI | -23 | -64 | -17 | 188 |
| 4.09 | Lobule VI | 23 | -64 | -14 | 128 |
| 3.92 | Lobule VI | 38 | -64 | -14 | 59 |
| 3.87 | Lobule VI | -42 | -61 | -26 | 22 |
| 3.85 | Cuneus (17) | 13 | -79 | 8 | 70 |
| 3.70 | Lobule X | -17 | -31 | -40 | 15 |
| 3.61 | Postcentral gyrus (2) | -51 | -20 | 36 | 99 |
| 3.45 | Middle frontal gyrus (6) | 34 | -10 | 45 | 127 |
| 3.38 | Precuneus (31) | 2 | -67 | 18 | 15 |
| 3.28 | Lobule VIIIB | 9 | -65 | -38 | 62 |
| 3.04 | Pre-SMA (6) | 6 | 1 | 68 | 44 |
| 2.97 | Transverse temporal gyrus (41) | -38 | -31 | 12 | 11 |
| CONT | |||||
|---|---|---|---|---|---|
| 3.88 | Cr I | 45 | -58 | -20 | 44 |
| 3.76 | Lobule IV | -7 | -49 | -15 | 22 |
| 3.73 | Pre-SMA | 8 | 1 | 51 | 116 |
| 3.66 | Superior temporal gyrus (22) | -44 | -45 | 16 | 189 |
| 3.51 | Cuneus (23) | 13 | -72 | 10 | 71 |
| 3.36 | Lobule V | -18 | -52 | -17 | 27 |
| 3.28 | Posterior insula (13) | 41 | -22 | 14 | 12 |
| 3.01 | Lobule VI | -19 | -73 | -21 | 15 |
| 3.00 | SLPrM (6) | -50 | -12 | 33 | 93 |
| 2.97 | Postcentral gyrus (3) | 50 | -17 | 40 | 20 |
| 2.73 | Postcentral gyrus (3) | -44 | -22 | 43 | 25 |
TABLE 6b.
Negative correlations between syllable rate and brain activity in the MON and ORA tasks in the PWS and CONT groups. Talairach coordinates are provided for regions where voxel clusters ( ≥ 10; p <.005) were significantly and negatively correlated with syllable rate following a conjunction analysis combining the effects of rate for MON and ORA tasks, calculated separately for the PWS and CONT groups. Local maxima coordinates shown with cluster size.
| PWS | |||||
|---|---|---|---|---|---|
| Z score | Region | X | Y | Z | Cluster |
| 5.92 | Posterior insula (13) | 34 | -31 | 16 | 296 |
| 5.78 | Precentral gyrus (6) | 58 | -2 | 20 | 143 |
| 4.75 | Postcentral gyrus | -42 | -23 | 46 | 74 |
| 4.66 | Posterior insula (13) | -25 | -29 | 19 | 51 |
| 4.55 | Thalamus | -25 | -29 | -1 | 15 |
| 4.49 | Medial globus pallidus | 14 | 0 | 5 | 18 |
| 4.42 | Posterior insula (13) | -38 | -35 | 22 | 33 |
| 4.32 | Lobule VI | -5 | -64 | -11 | 359 |
| 4.32 | Medial frontal gyrus | 6 | -13 | 59 | 39 |
| 4.17 | Caudate | -20 | -15 | 21 | 127 |
| 3.99 | Postcentral gyrus (3) | -57 | -13 | 20 | 40 |
| 3.90 | Lingual gyrus (18) | -5 | -84 | -6 | 41 |
| 3.79 | Cuneus (19) | 8 | -87 | 27 | 29 |
| 3.78 | Medial globus pallidus | -18 | -12 | -3 | 21 |
| 2.99 | Superior temporal gyrus (22) | -55 | -24 | 5 | 12 |
| CONT | |||||
|---|---|---|---|---|---|
| 4.05 | Precentral gyrus (6) | -59 | -10 | 37 | 185 |
| 4.01 | Cuneus (19) | -13 | -91 | 28 | 11 |
| 3.81 | SMA | -11 | -7 | 74 | 38 |
| 3.28 | Lobule VI | -29 | -69 | -15 | 18 |
| 3.23 | Lobule VI | -7 | 67 | -4 | 59 |
| 3.09 | SMA | 7 | -3 | 74 | 12 |
(iii) Syllable rate in CONT
To estimate brain activity associated with the rate of syllable production, the primary design matrix was reduced to include only scans from the CONT group. The design matrix included four parameters. Task (MON or ORA), Trial repetition (1 or 2), Syllables per minute per scan (see 2.2.6) and a constant. Conjunction analysis was used to identify brain areas that correlated with syllable rate for both tasks. Both positive and negative correlations between syllable rate and brain activity were estimated and are summarized in Tables 6a and 6b.
2.2.5. Speech data
Audio-visual recordings were obtained from each scanning trial and assessed for stuttering frequency and speech rate. Stuttering frequency was measured as %SS by two independent judges for each recording and according to the definitions and methods available in a standard and freely available audiovisual stuttering judgment training program (Ingham, Bakker, Ingham, Kilgo, & Moglia, 1999). Reliability (replicability) of experimental data was assured by using as the data for all analyses the mean of the two independent judges’ ratings; in addition, all recordings for which the two judges’ data differed by more than 10% were identified and re-rated before the experimental data were finalized.
Speech rate was measured for all speakers and all tasks using behavioral definitions of speech production that included all syllables, syllabic nuclei, and stutter-related extra iterations produced during the first 40 s of speech in a scan trial. This was accomplished by instructing 16 judges assigned in pairs to transcribe independently every perceived syllable or syllable-like production in each of a set of speech recordings. For five of the 60 ORA trials, one from each of five speakers, the initial ratings differed by more than 10%; these were re-rated and then resolved by one expert judge with access to the recordings and all judgments. No differences greater than 10% occurred for the 60 MON samples. This process resulted in mean differences of 2.74% and 3.40% in total syllable counts by judge pairs for the ORA samples and the MON samples, respectively.
3. Results
3.1. Behavioral performance
An important consideration in this study was to obtain imaging data under two different speaking conditions during which similar frequencies of stuttering and speaking rate could be expected. Table 1 shows the mean percent syllables stuttered (%SS) and mean syllables spoken per minute (Syl/min) scores across the two MON and the two ORA PET scans by each group. The PWS group produced a mean of 9.75%SS and 177.4 Syl/min during MON and 8.84%SS and 175.2 Syl/min during ORA. A paired t test showed the %SS and Syl/min mean scores were not significantly different. The correlation between individual participants’ %SS scores (r = .184) and Syl/min scores (r = .526) on the two tasks, however, showed there were substantial within-subject across-task differences for the PWS group. For the CONT group, the correlation between their Syl/min scores on the two tasks (r = .133) also showed within-subject differences across tasks. The CONT group's ORA rate was significantly faster than their mean Syl/min rate during MON. As Table 1 shows, both speaking tasks were produced at a faster speaking rate for the CONT when compared with the PWS group, but only during ORA was the rate difference significant. In addition, the CONT group's mean speaking rate was significantly faster during ORA (15% faster) than during their MON (223.5 vs 189.4 Syl/min). As a result of these differences, a multiple regression analysis (see Section 2.2.5) was used to derive normalized CBF data for each scan, thereby correcting for speaking rate differences.
3.2. Imaging
3.2.1. Main effect of speech
The initial contrasts (MON minus ECR and ORA minus ECR) using the .005 threshold identified essentially no differences between the groups, a result that was interpreted as potentially including Type II error because of previous reports of differences. We therefore chose to explore these data using the less conservative .05, fully recognizing the potential for Type I error but allowing comparisons with previous research, especially the findings reported by Brown et al. (2005). Those findings, incidentally, had been derived within an ALE analysis (Turkeltaub, Eden, Jones, & Zeffiro, 2002) that had employed a p < .05 threshold. This issue is revisited in the Discussion.
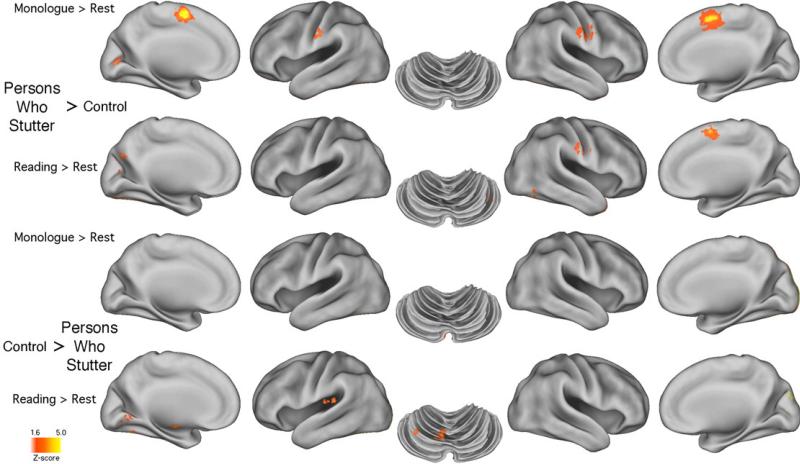
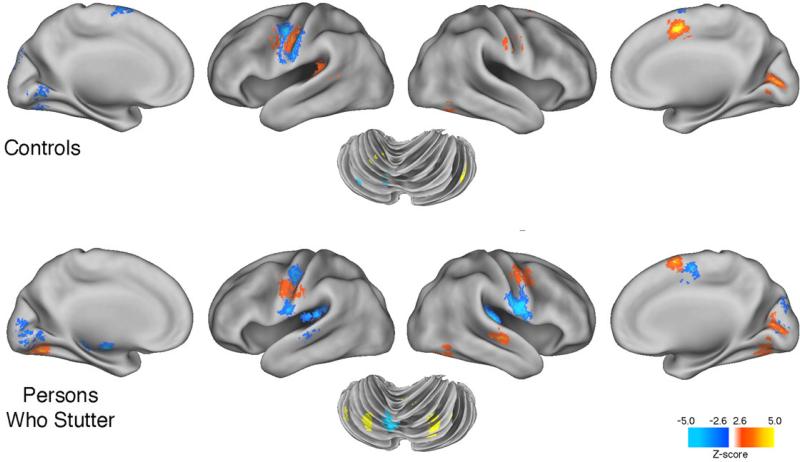
Table 2a (and Figure 1) summarizes the two contrasts for each group: MON minus ECR and ORA minus ECR for both PWS and CONT. Inspection of regional activations that were relatively larger in the PWS than in the CONT group's MON and ORA tasks revealed some important similarities and expected differences. The striking lack of similarity between the local maxima locations and the voxel cluster sizes within the PWS group during both tasks shows the differential effect of each task on the PWS. During MON the PWS group produced much stronger activations in L and R SMA and precentral gyrus than the CONT group. These regions were obviously not as overactive during oral reading. But the resemblance between the findings of activations in regions that Brown et al. (2005) identified as related to stuttering is only modest at best. Those activations are noted with asterisks within Table 2a, which shows that for the MON task 4/6 of the relatively overactivated regions were identified as stutter-related by Brown et al.'s ALE analysis, while 3/4 regions were so identified during the ORA task (1of the 9, R SMA, being common across tasks).
Figure 1.
The figure provides brain surface rendering of the data shown in Table 2a. Images show most regions where the PWS group's activated voxels were significantly greater than those produced by the CONT group, and vice versa, for the monologue and oral reading tasks. A threshold of p < 0.05 was used to as a threshold (see 2.2.4).
Table 2a (and Figure 1) also reveals a more complex picture for the CONT group. There were no similarities between the regions more activated in the CONT than the PWS group across the MON and ORA tasks. One interesting finding, however, was that the sub-lobar regions, including insula and basal ganglia, were more strongly activated in the CONT than the PWS group, but only during oral reading. This was also true for cerebellar regions, including Lobule VI.
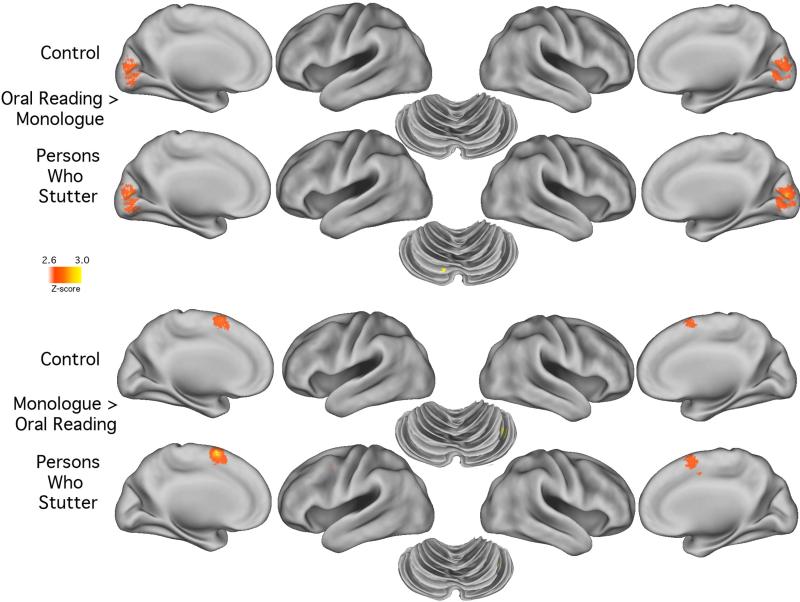
Table 2b and Figure 2 show the task differentiating effects from an alternative perspective - the within-group differences. By using a more conservative threshold (p = .005) the lack of difference between the groups becomes obvious. In the PWS and CONT group it is evident that the monologue condition again produced comparatively stronger activations in Pre-SMA than in oral reading conditions. And, as expected, the occipital lobe regions were more strongly activated during oral reading than during a monologue for both groups. But the most striking finding revealed in Table 2b is the similarity in local maxima and submaximal activations for both groups.
Figure 2.
The figure shows the brain surface rendering of the data shown in Table 2b. Images show cortical and cerebellar regions where, for each group, the activated voxels were significantly greater during the monologue task than during the oral reading task as well as vice versa. A threshold of p < 0.005 was used to enhance visualization.
3.2.2. Hemispheric asymmetries of speech related activity
Table 3 shows data derived from Table 2a in order to identify hemispheric differences in regions that were relatively more strongly activated in the PWS than the CONT group per task. The trend of differences in mean voxel activations between the L and R hemispheres within the MON and ORA tasks favored stronger R hemisphere activation in the PWS group for both tasks in both the cerebrum and cerebellum. Such overactivation is a previously reported feature of PWS (Brown et al., 2005). However, given that this finding was derived from Table 2a it cannot necessarily be regarded as powerful support for claims that a strong right hemisphere activation typifies adult PWS, because these data were derived after the significance threshold was adjusted from p < .005 to p < .05.
3.2.3. Group differences in both speech tasks and at rest
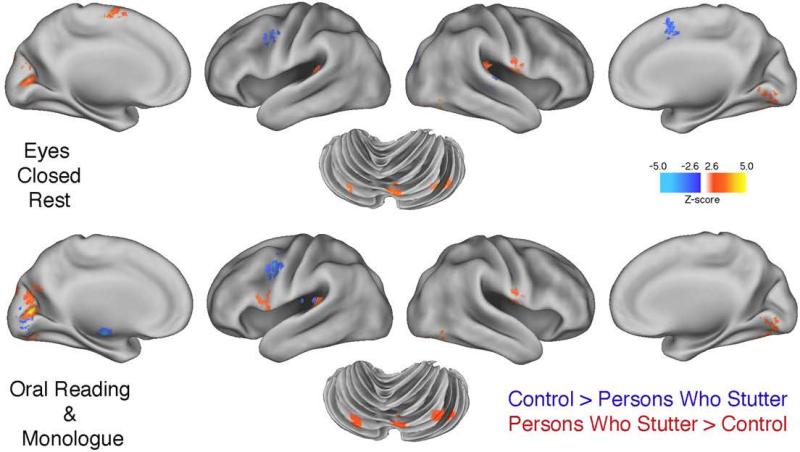
Table 4 shows the results of the conjunction analysis. This analysis identified voxels that differentiated between groups, for both the MON and ORA tasks. The identified voxels were then partitioned in two ways. First, voxels significantly activated conjointly for monologue and oral reading (MON+ORA voxels) were partitioned into those significantly more activated in the PWS than the CONT group, and vice versa. Table 4 (right column), therefore, shows the maximally and submaximally activated coordinates and associated voxel clusters that remained after variance attributable to ECR was accounted for in the regression model. Second, a direct comparison of activity between groups during the ECR condition was calculated. In this case, variance attributed to the MON and ORA tasks was accounted for within the regression model. These regional clusters are shown in Table 4 (left column).
As mentioned, Table 4 shows the local maxima coordinates for voxel clusters that were activated in both speaking tasks for a given group, but significantly more activated in PWS than CONT, as well as those more activated in the CONT than the PWS group. Eight regional voxel clusters were identified as significantly more active across the two speech tasks in the PWS group (note: Vermis III and Lobule IX clusters overlap). These include 7 activation sites in regions that were identified by Brown et al. (2005): R BA 43, L STG 22, L/R Lobule VI, R Vermis III and a second Lobule VI site. Fewer instances of stutter-related regions (Brown et al., 2005) occurred among regions more activated by the CONT group [only L SLPrM (6) and R Lobule VI].
Table 4 and Figure 3 also show that the common voxel activations associated with speech often emerged from regions identical to those activated by the PWS group during rest; for instance, R BA 43, L STG 22, cuneus (BA 18), and L/R Lobule VI. Perhaps the most interesting differences in that regard occurred with respect to basal ganglia and posterior insula (BA 13). In the PWS group during rest Table 4 shows L/R basal ganglia and posterior insula were significantly more activated than in the CONT group, but these areas failed to show significant activations during speech. Even when the threshold for significance was lowered from p < .005 to p < .05 (a simple control over Type II error), neither putamen nor globus pallidus overactivity was detected during overt speech by the PWS group. By contrast, at this lower threshold level, the CONT group did show significantly greater L globus pallidus and L/R posterior insula activations than the PWS group.
Figure 3.
The figure provides brain surface rendering of group differences in the three tasks. The upper figure shows regions more active in the PWS group (and vice versa) during eyes-closed rest (ECR) scanning conditions. The bottom panel shows group differences that are common to both speech tasks (oral reading and monologue) based on a conjunction analysis. Note the similarities of group differences for both the ECR and speech tasks in the parietal and temporal lobes, cuneus of the occipital cortex. Group differences in the cerebellum are also similar for ECR and speech tasks. A threshold of p < 0.005 was used.
3.2.4. Stuttering rate and brain activity
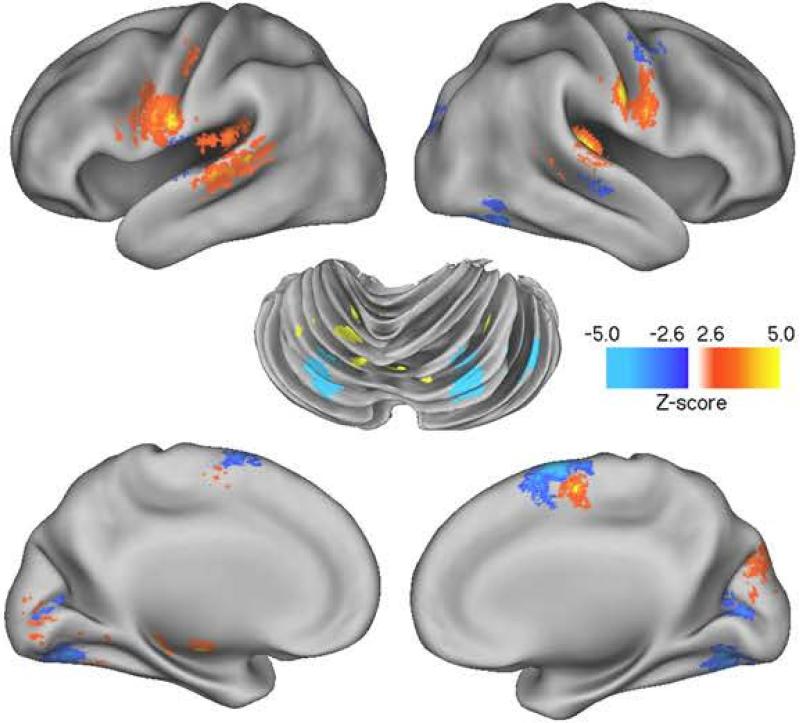
Based on a multiple regression model involving only the PWS group, the relationship between stuttering rate and brain activity was estimated after accounting for task (MON or ORA) and repetition (1 or 2). Tables 5a and 5b summarize regions that together account maximally for a positive or negative relationship between stuttering frequency and regional brain activity (these relationships are rendered within Figure 4). The positive relationship is clearly dominated by regions associated with the classic cortico-striatal-thalamic loop (thalamus, SMA, caudate, globus pallidus) (Alexander, DeLong, & Strick, 1986). Regions showing a negative relationship with stuttering frequency (Table 5b) are located in L and R cerebellum.
Figure 4.
The figure provides brain surface rendering of the correlation between brain activity and stuttering frequency in the PWS group. The images relate to Table 5a and 5b. They show regions where there is a strong positive (red/yellow) and negative (blue) correlation with the frequency of stuttering in regions for both the MON and ORA tasks. Prominent positive activations are evident in SMA, precentral gyrus, STG and basal ganglia. Negatively correlated activations are evident in pre-SMA and cerebellum. A threshold of p < 0.005 was used.
3.2.5. Syllable rate and brain activity in PWS
Based on a multiple regression model involving only the PWS group, the relationship between syllables per minute and brain activity was estimated after accounting for task (MON or ORA) and repetition (1 or 2). Interestingly, the cluster of regions that positively correlated with speech rate in the PWS group (Table 6a) did not include basal ganglia or other components of that loop. By contrast, the regions negatively correlated with speech rate (Table 6b) in the PWS group did incorporate components of the loop (these relationships are rendered within Figure 5).
Figure 5.
The figure provides brain surface rendering of the correlation between brain activity and syllable rate in the PWS and CONT groups. The images relate to Table 6a and 6b. They show regions where there is a strong positive (red/yellow) and negative (blue) correlation with syllable rate for both the MON and ORA tasks. Prominent positive activations are evident in cerebellum, frontal gyrus and pre-SMA for the PWS group, but not so prominent in similar regions for the CONT group. precentral gyrus, STG and basal ganglia. Negatively correlated activations are much more extensive in the PWS group than the CONT group, especially in posterior insula. A threshold of p < 0.005 was used.
3.2.6. Syllable rate and brain activity in CONT
In the CONT group the cluster of regions positively and negatively correlated with syllable rate (Tables 6a and 6b; also Figure 5) incorporated few regions found in the PWS group and essentially none of the cortico-striatal-thalamic loop regions. These striking differences between groups are consistent with the possibility that in the PWS group the activation source for stuttering resides within the cortico-striatal-thalamic loop.
4. Discussion
The findings of this study are of interest for a number of reasons. For the most part the findings of regional activations that were significantly greater in the PWS than in CONT group in the condition contrast data (Table 2a) were only partially consistent with those reported in an earlier ALE meta-analysis of brain imaging studies of stuttering (Brown et al., 2005) and in other studies conducted since that time. Stronger frontal lobe activation occurred in the PWS than the CONT group within SMA and BA 4 during monologue, but only R SMA was more active during oral reading. Cuneus (18), another stuttering signature region, was relatively more active in the PWS group, but there were extensive activations in cuneus that were occasionally stronger in the CONT group than in the PWS group. Also, R Lobule VI in cerebellum was overactive in oral reading, but not in monologue. In fact, the most notable feature in the present study was the minimal difference between the PWS and CONT group activation for monologue and oral reading. A comparison using a p < .005 threshold showed no difference in cerebrum or CBM activations. This is illustrated in Table 2b (figure 2) where the differences between the monologue and oral reading activations for both groups were minimal. However, at a less conservative p<.05 uncorrected, the threshold differences between the PWS and CONT groups were extensive (see Table 2a) and a majority did overlap those identified in Brown et al. as stutter-related. Previous findings from this lab (see Ingham et al., 2008) have also reported an absence of temporal lobe activity during stuttering, but if this was the case in the present study, then it was not significantly different in the PWS and CONT groups.
The across-task activations in the PWS group (see Table 4) were only slightly more consistent with Brown et al. (6/20 sites) than those that occurred within the normally fluent control group (4/20 sites). The lack of across-task consistency in the regional activations is to be expected across these different speaking tasks, but the differences in relation to the findings of other imaging studies of developmental stuttering raise a number of interesting issues. Part of the reason for those differences may reside in the lack of attention that has been given to differences that may be due to speech rate. However, the effect of using regression analysis to correct for the differences in rate, as was done in the present study, would still suggest that other factors may be contributing to the present study's findings.
The within-group consistency in regions that were significantly activated in the PWS group during ORA and MON was derived using conjunction or conjoint analysis - and served as the basis for identifying regional commonalities as well as neural regions that correlated with stuttering and speaking rate. Ostensibly these commonly activated regions are of most immediate interest because they are robust across quite different speaking tasks. Thus Table 4 shows that a set of 9 brain regions was identified as potentially stutter-related [L BA 44, R BA 43, L STG 22, L Cuneus 18, and CBM lobules L VI (2 sites), R VI, Lobule IX, and R Vermis III]. These were regions that were also significantly more activated in the PWS group than in the CONT group. The contrast, regions significantly more activated in CONT than PWS, highlights regions that are relatively less activated in the PWS group. The most obvious regions that emerged as less activated in PWS were L/R posterior insula and L globus pallidus (a finding that must now be reconciled with a reverse trend during ECR).
What is missing from the regional activations that were more prominent in PWS than in the CONT group is especially interesting. The data do not include significant activations in SMA, anterior insula, or ACC, even though all have appeared in previous imaging studies with PWS (see Brown et al., 2005). The often reported lack of activation in the temporal lobe for PWS (Braun et al., 1997; Chang et al., 2009; Fox et al., 1996; 2000; Neumann et al., 2005) was also not fully reproduced in the current study. Part of the reason for the absence of significant activations or deactivations in some of these regions might be related to the removal of variance attributable to the ECR condition.
4.1. Rest-related activation
The analysis results shown in Table 4 (and displayed in Figure 3) produced some unexpected findings concerning regions that were significantly more activated at rest (ECR) in the PWS than the CONT group, and vice versa. One of the most surprising was that regions that earlier PET studies (Braun et al., 1997; Ingham et al., 1996) found to be no different in activation between PWS and CONT groups at rest may in fact differ, even without speech. This result may be attributable to the use of newer generation and more powerful PET imaging equipment in current studies. That argument gains traction from a recent fMRI study by Xuan et al. (2012) that also detected differences between PWS and CONT groups during ECR.
The implications of the surprising rest-state findings are difficult to gauge without further research, but an obviously interesting finding was the differential effect of the speaking task on basal ganglia activations in the PWS and CONT groups. There have been strong suggestions that basal ganglia abnormalities are intimately involved in stuttering (e.g., Alm, 2004; Giraud et al., 2008), suggestions that are relevant to the findings of the present study in an interesting way. This is evident by taking account of the ECR data shown in Table 4 (and Figure 1). This table shows that basal ganglia regions (putamen and globus pallidus) and R insula in the PWS were actually significantly more activated during ECR than they were in the CONT group. This may help to explain why basal ganglia and R insula could still be abnormally activated during speech in some studies (e.g., Fox et al., 1996), or, in the case of R insula, even when PWS simply imagine stuttering (Ingham, Fox, Ingham, & Zamarripa, 2000). Furthermore, as shown below, despite the relative inactivity of basal ganglia during speech by PWS it still appears to be functionally activated by changes in stuttering frequency. Conceivably, it is this aberrant activity that is diminished during fluency-inducing conditions.
Another interesting ECR finding concerned SMA, especially L Pre-SMA. Strong SMA activity has been regularly associated with stuttering (see Brown et al., 2005; Ingham et al., 2008), but it could be that it is active before speech occurs (during ECR) and is then conflated with speech production. That may also be true for the CONT group who showed significant R pre-SMA activity. This activity during conditions preceding oral reading and monologues would not be surprising and is in keeping with conclusions by Nachev, Kennard, and Hussain (2008) on the status of pre-SMA. They argue that pre-SMA “is more likely to be active in more-complex or more-‘cognitive’ situations than the more caudal end (the SMA), which seems to be more tightly related to actions” (2008, p. 866). That would also be consistent with basal ganglia activity during rest by the PWS group because of the established connections between pre-SMA and basal ganglia (see Nachev et al., 2008).
The link between ECR activations and those during speech is a previously undocumented finding in imaging research on stuttering. The conjoint analysis result shown in Table 4 reveals that the 7/9 regions that were more significantly activated in PWS than CONT during speech were also activated during ECR and at almost identical local maxima sites. This was only true for 3/9 regions where the CONT group showed significantly more activation than the PWS group. In other words, the activated voxel clusters associated with speech - especially by PWS - appear mainly to have emerged from a select set of neural regions significantly activated when no speech is present. Of course, in the context of this experiment, this unusual level of ECR activation might have occurred more in the PWS group because speech was anticipated (see below). Unfortunately, the limitations of PET in regard to temporal factors make it possible only to speculate about this interaction.
It is of interest that Xuan et al. (2012), who used low frequency fMRI signals during ECR with large PWS (n = 46) and CONT (n = 44) groups, also found relatively greater activation in the PWS group in, for example, L STG and L IFG, but relatively less activation in R SMA. Those findings are partially consistent with the present study's findings. Unfortunately the Xuan et al. study did not include the imaging of cerebellum which would have made possible a more complete comparison between the studies.
4.2. Current findings in relation to previous research
Multiple fMRI studies have reported speech production findings that differ from those obtained in the present study (see Brown et al., 2005). These differences may now deserve far more attention than they have received. They can be dismissed as unimportant by invoking the “methodological differences among studies” caveat, but they may be also a predictable result of neuroanatomic differences that have been found in recent diffusion tensor imaging studies (Beal, Gracco, Lafaille, & De Nil, 2007; Sommer, Koch, Paulus, Weiller, & Büchel,, 2002; Watkins, Smith, Davis, & Howell, 2008). Several studies have now suggested that white matter (WM) abnormality is a principal causal factor for developmental stuttering. The site of this abnormality seems to vary somewhat among studies, but there is growing agreement that it is present in adults and also children who stutter (Chang, Erickson, Ambrose, Hasegawa-Johnson, & Ludlow, 2008). A study by Cykowski et al. (2010) found indications of reduced FA values in remarkably similar sites across their adult PWS subjects (n = 13). Their data show “that robust FA reductions in (PWS) were largely focal, left hemispheric, and within late-myelinating associative and commissural fibers (division III of the left superior longitudinal fasciculus, callosal body, forceps minor of the corpus callosum)” (Cykowski et al., 2010, p. 1495). Complementary findings were recently reported by Chang, Horwitz, Ostuni, Reynolds, and Ludlow (2011). Using probabilistic tractography (Behrens et al., 2003) with dextral adult PWS (n = 15; 6 females) and dextral controls (n = 14; 7 females), they found significantly decreased tract density in left superior longitudinal fasciculus, which led them to conclude that there was likely “a deficient left inferior frontal to premotor connection in stuttering” (Chang et al., 2011, p. 9). In addition, recent findings by Choo et al. (2011) using voxel-based morphometry with adult male dextral PWS (n = 11) and controls (n = 12) showed that the absolute area of the corpus callosum was significantly larger in the PWS cohort, which may relate as well to the Cykowski et al. (2010) findings. Against this background of neuroanatomic abnormality among adult PWS, it seems plausible that various compensatory strategies or forms of plasticity (see Grafman, 2000) could emerge in PWS - a factor that might also result in marked differences in CBF measures during speech production by PWS.1
One other difference between the current and previous research findings concerned the regional correlations with stuttering frequency (see Tables 5a and 5b). Previous studies by two of the current authors (RJI and JCI) and colleagues (Fox et al., 2000; Ingham et al., 2004) have reported strong positive correlations between stuttering frequency and cerebellar activations. But, as Tables 5a and 5b show, a multiple regression analysis of the performance of the PWS group derived from the conjoint analysis showed cerebellum was associated with significant negative correlations in lobules IV, VI and VIIB. It is not absolutely clear why this difference occurred, but one reasonable hypothesis is because of the way the correlations were derived in the Fox et al. and Ingham et al. studies. In both studies the correlations were calculated by including resting state activations when there was no speech and obviously zero stuttering. Arguably, this may have distorted the calculations because a correlation between neural activity and a target speech behavior that involves no opportunity for the occurrence of the target behavior might confound the meaning of the resulting correlation. For that reason the present study did not include resting state data within the multiple regression analysis. However, the inclusion of resting state activations in a reanalysis of the results of the present study did not alter the general finding reported in Tables 5a and 5b.
As mentioned in the Introduction, comparisons between the speech performance of PWS and normally fluent speakers have consistently shown that PWS will have a slower rate of speech than normally fluent speakers (see Bloodstein & Ratner, 2008). That difference occurs even, as was shown in the present study, when the movements related to stuttering are taken into account. Conceivably, the significant difference in speaking rate between the two cohorts might be a source of differences between regions activated in PWS and CONT groups. A series of studies by Riecker, Ackermann, and colleagues (Riecker et al., 2006; Riecker, Wildgruber, Dogil, Grodd, & Ackermann, 2002; Wildgruber, Ackermann, & Grodd, 2001) appears to have provided relevant findings. Riecker et al. (2006), for instance, have shown that variations in the rate of repeating a syllable during fMRI produced a linear increase in the BOLD response of normal speakers in some areas and a concomitant BOLD response decline in others. Thus Riecker et al.'s findings may implicate speaking rate as a source of the differences between the CONT groups’ activated regions during oral reading and monologue (see Table 1). Given that normally fluent controls will always be expected to speak on average more rapidly than PWS, this is an issue that group comparison studies, such as the current study, may always have to resolve. This is especially true when they involve direct comparisons between PWS and CONT groups. In this case it may help to explain why significant neural activations in regions that often are reported as stutter-related (e.g., R anterior insula) might be masked by activations produced by much faster speaking controls.
The role of the cortico-striatal-thalamic loop in stuttering has been raised by some of the findings discussed above - notably in regard to basal ganglia regions that correlated with stuttering frequency (see Table 5a). Alm (2004, 2007) has been most recently associated with theorizing about the critical role of basal ganglia in stuttering. He has postulated that the source of the disorder resides in a failure of the integrity of a dual premotor system (Goldberg, 1985; Goldberg & Bloom, 1990). The system involves a medial and lateral premotor system - the former constituting the cortico-striatal-thalamic loop and the latter involving a premotor-cerebellum loop. Both systems presumably work in harmony to produce the fine-grained timing necessary for initiating speech movements. Recent investigations of tasks requiring ongoing abnormal movement correction, as may be the case with the speech of PWS (Max, Guenther, Gracco, Ghash, & Wallace, 2004), show that excessive globus pallidus and subthalamic nuclei activation occur in conjunction with these submovements (Grafton & Tunik, 2011; Tunik, Houk, & Grafton, 2009). Excessive basal ganglia activation, therefore, may reflect disrupted or impaired fine-grained speech movement by PWS - even during rest conditions prior to speech (see Table 4).
The present findings do suggest abnormal activity in the medial and lateral premotor systems in PWS, but they do not identify the source of the breakdown in the integrity of either loop. Alm (2004, 2007) appears to be agnostic as to the source of the system integrity breakdown. However, given the increasing evidence of white-matter abnormality in the PWS population it seems likely that the integrity of the loop is potentially corrupted by consequences of this abnormality. Furthermore, continuing research has suggested that the output from cerebellum and basal ganglia not only interacts with primary motor cortex, but also with the prefrontal, temporal, parietal, and oculomotor regions (see Bostan & Strick, 2010) and so the points of disruption seem to be almost infinite.
Because of the importance of the resting-state findings in the present study it is important to recognize some of the challenges that imaging investigations face in interpreting resting state data. Despite being instructed to simply rest and think of a pleasant scene as was the case in the present study, there is no obvious way to verify that these instructions were followed. In the present study, for instance, participants occasionally indicated that because of the perceived importance of the different tasks to the findings, some indicated that they had used that time to think of what they would say during the MON scan condition. Only by manipulating ECR instructions in some future studies will the effects be able to be judged.
4.3. Conclusion
The current findings appear to be consistent with the emerging variable results reported in other imaging studies on the neurophysiology of adult and late adolescent PWS. The findings in this paper show, at the very least, how difficult it is to argue that there exists a consistent set of neural regions that are signatures of stuttering (Brown et al., 2005). Perhaps, therefore, it is time to alter the direction of the search for a common neural system. One way in which that might occur is through more fine grained imaging investigations of individual PWS. Because the opportunities for this change in direction are growing with the rapid developments in imaging technologies, it is almost platitudinous to make this observation. However, there is now every reason to believe that careful investigations of the link between neuroanatomic abnormality and aberrant neurophysiology in PWS of all ages are needed to locate the neural processes that result in the production of stuttered speech.
HIGHLIGHTS.
PET study on stuttering across speaking tasks.
Major differences in regions activated in stutterers and controls across tasks.
Use of speech rate correction to offset rate differences between subject groups.
Activations at rest in persons who stutter overlap those activated during speech.
A cortico-striatal-thalamic loop was correlated with stuttering frequency.
Acknowledgments
This study was completed with the support of RO1 Grant DC007893 from the National Institute on Deafness and Other Communication Disorders awarded to the first author. Special thanks are offered to Peter Fox for providing the facilities for this study and for his continuing support for imaging research on developmental stuttering. Thanks are also due to Robin Selman, Krystal Purkheiser, Annie New, Kate Paolini, Gina Pecile, Amanda Petros, and Dana Bryant for their assistance in gathering data and background material.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
More than a decade ago Ludlow (2000) made some prescient observations about early indications of differences among brain imaging studies on stuttering. She drew attention to the substantive differences between findings reported by Fox et al. (1996) and Braun et al. (1997) from PET studies on stuttering and went on to suggest the following: “Brain activation patterns observed in stuttering adults are perhaps the result of individually adapted systems that evolved during childhood and early adolescence in an effort to produce fluent speech. Individually adapted systems, when combined for correlation analyses, may then lead to varying results across studies using different tasks and measures” (Ludlow, 2000, p 183).
References
- Abbey E. The monkey wrench gang. Avon Books; New York: 1975. [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking the basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders. 2004;37:325–369. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Alm PA. The Dual Premotor Model of cluttering and stuttering: A neurological framework.. Proceeding of the 1st World Conference on Cluttering; Katarino, Bulgaria. May 2007.2007. [Google Scholar]
- Beal DS, Gracco VL, Lafaille SJ, De Nil LF. Voxel-based morphometry of auditory and speech-related cortex in stutterers. Neuroreport. 2007;18:1257–1260. doi: 10.1097/WNR.0b013e3282202c4d. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bloodstein O, Ratner NB. A handbook on stuttering. 6th ed. Singular; San Diego: 2008. [Google Scholar]
- Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychological Review. 2010;20:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H215O positron emission tomography study. Brain. 1997;120:761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Human Brain Mapping. 2005;25:105–117. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39:1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Horwitz B, Ostuni J, Reynolds R, Ludlow CL. Evidence of left inferior frontal--premotor structural and functional connectivity deficits in adults who stutter. Cerebral Cortex. 2011;21:2507–2518. doi: 10.1093/cercor/bhr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Kenney MK, Torrey MJ, Loucks TMJ, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage. 2009;46:2010–212. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Synnestvedt A, Ostuni J, Ludlow C. Similarities in speech and white matter characteristics in idiopathic developmental stuttering and adult-onset stuttering. Journal of Neurolinguistics. 2010;23:455–469. doi: 10.1016/j.jneuroling.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo AL, Kraft SJ, Olivero W, Ambrose NG, Sharma H, Chang S-E, Loucks TM. Corpus callosum differences associated with persistent stuttering in adults. Journal of Communication Disorders. 2011;44:470–477. doi: 10.1016/j.jcomdis.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski M, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: A potential role for impaired myelination. Neuroimage. 2010;52:1495–1504. doi: 10.1016/j.neuroimage.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral word reading in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research. 2000;43:1038–1053. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Lafaille SJ, Houle S. A positron emission tomography study of short- and long-term treatment effects on functional brain activation in adults who stutter. Journal of Fluency Disorders. 2003;28:357–380. doi: 10.1016/j.jfludis.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch T, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–162. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL. Brain correlates of stuttering and syllable production: a PET performance-correlation analysis. Brain. 2000;123:1985–2004. doi: 10.1093/brain/123.10.1985. [DOI] [PubMed] [Google Scholar]
- Giraud A-L, Neumann K, Bachoud-Levi A-C, von Gudenberg AW, Euler HA, Lanfermann H, Preibisch C. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain and Language. 2008;104:190–199. doi: 10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function. The Behavioral and Brain Sciences. 1985;8:567–616. [Google Scholar]
- Goldberg G, Bloom KK. The alien hand sign. Localization, lateralization and recovery. American Journal of Physical Medicine & Rehabilitation. 1990;69:228–238. doi: 10.1097/00002060-199010000-00002. [DOI] [PubMed] [Google Scholar]
- Grafman J. Conceptualizing functional neuroplasticity. Journal of Communication Disorders. 2000;33:345–356. doi: 10.1016/s0021-9924(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Tunik E. Human basal ganglia and the dynamic control of force during on-line corrections. The Journal of Neuroscience. 2011;31:1600–1605. doi: 10.1523/JNEUROSCI.3301-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. The functional neuroanatomy of language. Physics of Life Reviews. 2009;6:121–143. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham RJ. Stuttering and behavior therapy. Current status and experimental foundations. College-Hill Press; San Diego CA: 1984. [Google Scholar]
- Ingham RJ, Bakker K, Ingham JC, Kilgo M, Moglia R. Stuttering Measurement System (SMS software). [April 7, 2007];1999 from http://www.speech.ucsb.edu/
- Ingham RJ, Cykowski M, Ingham JC, Fox PT. Neuroimaging contributions to developmental stuttering theory and treatment. In: Ingham RJ, editor. Neuroimaging in communication sciences and disorders. Plural Publishing; San Diego: 2008. pp. 53–85. [Google Scholar]
- Ingham RJ, Fox PT, Ingham JC, Xiong J, Zamarripa F, Hardies LJ, Lancaster JL. Brain correlates of stuttering and syllable production: gender comparison and replication. Journal of Speech, Language, and Hearing Research. 2004;4:321–341. doi: 10.1044/1092-4388(2004/026). [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Fox PT, Ingham JC, Zamarripa F. Is overt speech a prerequisite for the neural activations associated with chronic developmental stuttering? Brain and Language. 2000;75:163–194. doi: 10.1006/brln.2000.2351. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Fox PT, Ingham JC, Zamarripa F, Jerabek P, Cotton J. A functional lesion investigation of developmental stuttering using positron emission tomography. Journal of Speech and Hearing Research. 1996;39:1208–1227. doi: 10.1044/jshr.3906.1208. [DOI] [PubMed] [Google Scholar]
- Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, Giraud A-L. How the brain repairs stuttering. Brain. 2009;132:2747–2760. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Knetch S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Peng D, Chen C, Ning N, Ding G, Li K, et al. Altered effective connectivity and anomalous anatomy in the basal ganglia-thalamocortical circuit of stuttering speakers. Cortex. 2010;46:49–67. doi: 10.1016/j.cortex.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Ludlow CL. Stuttering: dysfunction in a complex and dynamic system. Brain. 2000;123:1983–1984. doi: 10.1093/brain/123.10.1983. [DOI] [PubMed] [Google Scholar]
- Max L, Guenther FH, Gracco VL, Ghash SS, Wallace ME. Unstable or insufficiently activated internal models and feedback biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary Issues in Communication Science and Disorders. 2004;31:105–122. [Google Scholar]
- Nachev P, Kennard C, Hussain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Neumann K, Preibisch C, Euler HA, von Gudenberg AW, Lanfermann H, Gall V, Giraud AL. Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorders. 2005;30:23–39. doi: 10.1016/j.jfludis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;25:965–969. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Neumann K, Raab P, Euler HA, von Gudenberg AW, Lanfermann H, Giraud AL. Evidence for compensation for stuttering by the right frontal operculum. Neuroimage. 2003;20:1356–1364. doi: 10.1016/S1053-8119(03)00376-8. [DOI] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Groschel K, Grodd W, Ackermann H. The cerebral control of speech tempo: Opposite relationship between speaking rate and BOLD signals changes at striatal and cerebellar structures. Neuroimage. 2006;29:46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Dogil G, Grodd W, Ackermann H. Hemispheric lateralization effects of rhythm implementation during syllable repetitions: An fMRI study. Neuroimage. 2002;16:169–176. doi: 10.1006/nimg.2002.1068. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund HJ. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123:1184–1202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360:380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Stager SV, Jeffries KJ, Braun AR. Common features of fluency-evoking conditions studied in stuttering subjects and controls: an H215O PET study. Journal of Fluency Disorders. 2003;28:319–335. doi: 10.1016/j.jfludis.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Tunik E, Houk JC, Grafton ST. Basal ganglia contribution to the initiation of corrective submovements. NeuroImage. 2009;47:1757–1766. doi: 10.1016/j.neuroimage.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkelbaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: Effects of syllable repetition rate evaluated by fMRI. Neuroimage. 2001;13:101–109. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
- Xuan Y, Meng C, Yang Y, Zhu C, Wang L, Yans Q, et al. Resting-state brain activity differs in adult males who stutter. PLoS One. 2012;7(1):e30570. doi: 10.1371/journal.pone.0030570. Epub 2012 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]