Abstract
The success of cellular therapies for Parkinson’s disease (PD) will depend not only a conducive growth environment in vivo, but also on the ex vivo amplification and targeted neural differentiation of stem/progenitor cells. Here, we demonstrate the in vitro proliferative and differentiation potential of stem/progenitor cells, adult human neural progenitor cells (“AHNPs”) isolated from idiopathic PD postmortem tissue samples and, to a lesser extent, discarded deep brain stimulation electrodes. We demonstrate that these AHNPs can be isolated from numerous structures (e.g. substantia nigra, “SN”) and are able to differentiate into both glia and neurons, but only under particular growth conditions including co-culturing with embryonic stem cell-derived neural precursors; this suggests that PD multipotent neural stem/progenitor cells do reside with the SN and other areas, but by themselves appear to lack key factors required for neural differentiation. AHNPs engraft following ex vivo expansion and transplantation into the rodent brain, demonstrating their regenerative potential. Our data demonstrate the presence and capacity of endogenous stem/progenitor cells in the PD brain.
Keywords: Parkinson’s disease, neurogenesis, neural stem cell, neural progenitor cell, cell culture
Introduction
Current pharmacological and surgical treatments for PD have been successful in addressing several motor symptoms, but to date have not been shown to be capable of modifying disease, or address levodopa resistance associated symptoms. Cell transplantation therapies have also faced numerous obstacles including ethical, safety and technical issues. It has been hypothesized that novel cell-based therapies aimed at stimulation of endogenous dopamine production within the brain may provide a more physiological and more elegant way to overcome the cardinal symptoms of PD (Baquet et al., 2005; Arrias-Carrion et al., 2007; Geraerts et al., 2007; Preynat-Seauve et al., 2009).
While the existence of stem cells in the adult brain is widely accepted in the scientific community, the magnitude, pathogenetic relevance and restorative potential of neurogenesis in the diseased brain is far less understood. The number of studies on these processes in the adult human brain is limited. While several studies have suggested the presence of precursor cells within the adult rodent substantia nigra (SN) (Lie et al., 2002) in general there is a lack of evidence for adult human neural progenitor cells (AHNPs) (Walton et al., 2006) in the Parkinsonian brain, including the SN (Srivastava et al., 2008). It also remains uncertain as to whether AHNPs possess the capacity to produce dopaminergic neurons (DA neurons) within the PD brain (Geraerts et al., 2007; Storch et al., 2004). In this study, we isolated and cultured AHNPs from the SN and three other regions of postmortem PD brains. PD AHNP primary cell lines were found to differentiate into neurons and astrocytes when cultured under specific growth conditions. We conclude that adult stem/progenitor cells exist in the SN and other regions within the PD brain, and that these cells represent a potentially valuable source for bioassays aimed at both understanding disease course, and screening for potential cellular and pharmacologic therapies.
RESULTS
Isolation and culture of AHNPs from PD brain
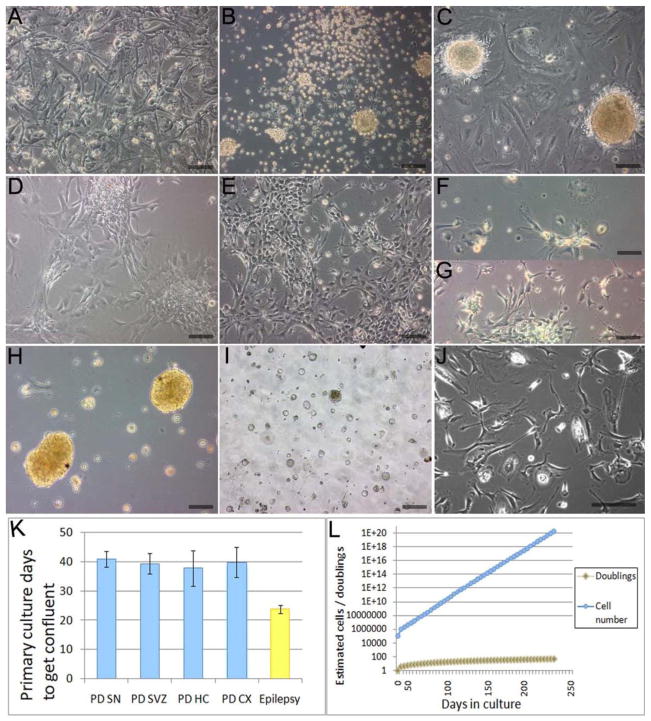
Compared to other non-PD AHNP cultures (surgical resections for temporal lobe epilepsy, n=5), PD AHNPs in primary culture exhibited a lower adherence to a polyornithine-coated substrate with more than 50% of cells detaching after a few days (data not shown). The surviving neural progenitor cells formed a confluent monolayer after 4–7 weeks (Fig 1A). AHNP cultures appeared to represent a stable expanding population in two or three passages (Figs 1B,C). Figures 1D,E document phenotypic changes that occurred within this cell population during expansion, including the appearance of many cells with neuronal morphology. Larger numbers of round and flat cells were observed in SN AHNP primary cultures (Fig 1F), while more spindle-shaped and bipolar cells were present in cultures from other brain regions (Fig 1G), but all of the cells became morphologically uniform after two passages. Compared to epilepsy cultures, PD cells in primary culture grew significantly slower (p<0.05)(Fig 1K). PD AHNPs were obtained from tissue samples derived from cortex (CX), hippocampus (HC), subventricular zone (SVZ), and SN (see Table 1), and could be expanded for more than 50 population doublings (Fig 1L). Under neurosphere (Laywell et al., 1999) conditions, cells from all regions also grew as spheres (Fig 1H). However, spheres decreased in size over 6 passages, when sphere cultures were aborted (Fig 1I).
Fig. 1. Isolation and culture of AHNPs from the PD brain.
Tissue samples of SN, SVZ, HC and CX from post-mortem PD patients were dissociated and plated into proliferative media. (A–J) Phase-contrast photomicrographs of PD AHNPs in culture. (A) Neural progenitor cells survive plating and remain attached to polyornithine coated dishes, forming a confluent monolayer after 4–7 weeks. Stably expanding populations of PD AHNPs have been established after 2–3 passages. (B) Clusters/spheres are generated during the initial stages (e.g. 3 days after subculture) of adherence to polyonithine substratata. (C) Confluent, adhesive culture with clusters of small, round cells overlying medium-sized bipolar, spindle-shaped and large cells with polygonal and multipolar morphologies. (D) and (E) Morphological changes occur within AHNP cell populations, many medium-sized or large cells evolve into neuron-like cells during expansion. (F,G) Characteristic cell morphologies in AHNP cultures. Larger numbers of round and flat cells were observed in SN AHNP primary cultures (F), while more spindle-shaped and bipolar cells are present in cultures from other brain regions (G); all of the cells become morphologically uniform after two passages. (H,I) Primary neurosphere cultures. PD AHNPs can grow as spheres under our neurosphere growth conditions (H), but they decrease in size over 6 passages (I). (J) Shows a primary PD AHNP culture generated from DBS lead electrodes removed for electrode replacement. (K) Compared to epilepsy AHNP cultures, PD cells in primary cultures grew significantly slower (p<0.02). (L) PD AHNPs do not exhibit growth rate reductions after more than 50 population doublings. All the data presented without labels in Fig. 1 are from PD SN AHNP cultures except for (G), which shows an SVZ AHNP culture. (Scale bars = 100 μm)
Table 1.
Isolation and derivation of neural progenitors
| Patient’s Age/Sex | Clinical Diagnosis | Pathological Diagnosis | Regions Sampled | Stable cell lines established | Neuron generation |
|---|---|---|---|---|---|
| 65/male | PD | Idiopathic PD | SN, SVZ, HC | SN, SVZ, | Yes |
| 84/female | PD | Idiopathic PD | SN, SVZ, HC, Primary Motor CX | SN, SVZ, CX | Yes |
| 57/female | PD & GBM | Idiopathic PD,GBM* | SN, DBS Rec SVZ, HC, Frontal CX | CX | Not determined |
| 86/male | PD | Possible PD** | SN, SVZ, HC, Primary Motor CX | SN, SVZ, HC, | Yes |
| 57/male | PD | Idiopathic PD | SN, SVZ, HC, Primary Motor CX | SN, SVZ, HC, CX | Yes |
| 75/female | PD | SN, SVZ, HC, Primary Motor CX | SN, SVZ, HC, CX | Yes | |
| 65/male | PD | SN, SVZ, HC, Primary Motor CX | SN, SVZ | Not determined |
GBM: Glioblastoma Multiforme
: A neurodegenerative disorder with possible Parkinson’s disease.
We also generated cell cultures from DBS lead electrodes removed during lead replacement procedures (n=3). Primary cells were observed growing off of the electrodes within 72 hours of culture initiation. These cells continued to grow for 3–5 weeks (Fig 1J), but could not be expanded beyond 2 passages.
Identification of AHNPs from the Parkinson’s Brain
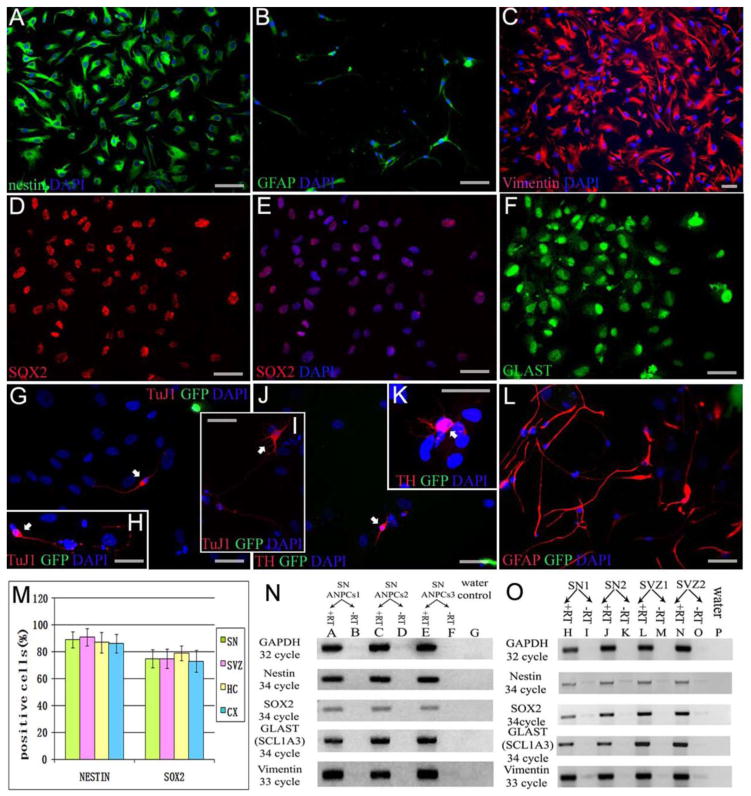
Immunocytochemistry was performed to identify the cultured PD cells as neural stem/progenitor cells. Most cells from the all brain regions sampled expressed neural stem cell markers nestin (Fig 2A) and SOX2 (Brazel et al., 2005) (Fig 2D,E), and the majority of the nestin/Sox2 positive cells were also immunoreactive for the radial glial marker vimentin (Fig 2C) (Bramanti et al., 2010), and the glutamate transporter “GLAST” (glutamate astrocyte-specific transporter, Zecevic et al., 2004, or EAAT1; Fig 2F). The GLAST labeling of all these PD cells was both nuclear and cytoplasmic, while concurrent immunolabeling studies showed that murine adult neural progenitor cells exhibited exclusive cytoplasmic localization (data not shown). Nuclear location of the glutamate astrocyte-specific transporter GLAST protein had been reported in human glioma cells (Ye et al., 1999), but their labeling pattern did not include a cytoplasmic component whereas the PD AHNP cells did exhibit light cytoplasmic in addition to strong nuclear labeling. The basis for this labeling pattern is not known, but it suggests the possibility that PD cells might have altered glutamate metabolism. We did not observe expression of CD133 (data not shown), which is thought to be predominantly expressed in S, G2 or M phase stem cells but down-regulated in slow-cycling or dormant stem cells (Sun et al., 2009). These cells were also negative for multipotent mitotic glial markers A2B5 and NG2 (data not shown). No TuJ1 positive neurons or GFAP positive astrocytes were detected in any proliferating cell cultures after subculture. Though -synuclein aggregates were observed in PD SN tissue from these patients, we did not detect -synuclein in cultured AHNPs or their progeny (data not shown). We compared the proportion of nestin and SOX2 positive cells among populations from the four brain regions studied here, but did not find a significant difference (ANOVA, p=0.47 and 0.49, respectively) (Fig 2M). We detected mRNA expression of progenitor markers in SN-derived cell cultures as well as in SVZ and SN tissue by RT-PCR. Both cells (Fig 2N) and tissue (Fig 2O) expressed nestin, SOX2, vimentin and GLAST, supportive of the existence of a progenitor cell pool in the PD SN. Interestingly, only GLAST mRNA showed a marked difference between SN and SVZ in tissue samples. Whether the weak expression of GLAST in SN is one of the pathological characteristics of PD will require further study.
Fig. 2. Identification and In Vitro differentiation of PD AHNPs.
Immunocytochemistry was utilized to establish PD AHNPs as neural stem/progenitor cells (A–F from SN cultures). Cells in expanding cultures express neural stem cell markers nestin (A; green) and SOX2 (Brazel et al., 2005; E; red), and the majority of the nestin/SOX2 positive cells are also immunoreactive for radial glial markers vimentin (C; red) and GLAST (EAAT1) (F; green). (B) GFAP (green) expression in a subpopulation within these primary cultures. (M) There is no significant difference (p=0.47, 0.49) in the proportions of nestin and SOX2 positive cells within AHNP populations from the four brain regions studied here (SN, SVZ, HC, CX). RT-PCR was performed on SN-derived cells and SVZ and SN tissue to detect mRNA for progenitor cell markers. Both cell (N) and tissue (O) RT-PCR results show nestin, SOX2, vimentin and GLAST expression, suggesting a progenitor cell pool in the PD patients’ SN and SVZ. The expression of GLAST in SN tissue is markedly weaker than that seen in SVZ tissue. Under appropriate differentiation-inducing conditions, PD AHNPs have been shown to have the capacity to differentiate into both neurons and astrocytes, revealing their multipotent neurogenic potential (G–L images were taken from human PD SN AHNPs and mouse GFP-ESNPs co-cultures). Co-cultured with GFP expressing mouse ESNPs in DM containing SHH, FGF8b, BDNF, NT-3, PDGF, ascorbic acid and retinoid acid, PD SN AHNPs (not GFP+) gave rise to TuJ1 positive neurons (G,H,I; labeled with white arrow, red is Tuj1), TH positive DA neurons (J,K; labeled with white arrow, red is TH) and GFAP positive astrocytes (L; red is GFAP). GFP green immunofluorescence in (G–L). Nuclei are counterstained with DAPI (blue). (Scale bars = 50 μm)
Limited Potential of In Vitro-Expanded PD AHNPs
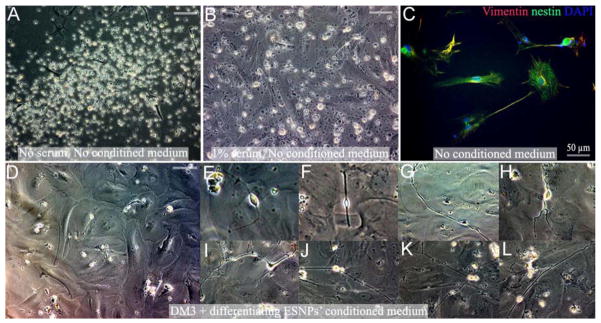
To determine the neural lineage potential of the PD stem/progenitor cells, adherent AHNPs were induced to differentiate on polyornithine coated glass coverslips with 3 types of differentiation media (DM). After 4–7 days culture in DM, in all the 3 groups of differentiation systems, approximately 50–60% of cells detached from the polyornithine coated surface (Fig 4A), and there were another 10–25% cells detached at later stages. Addition of 1% fetal bovine serum to the differentiation media only saved the later period of cell death (Fig 4B), and in both conditions, immunocytochemical analysis on the surviving cells did not reveal any TuJ1 positive neurons or GFAP positive astrocytes. We detected only weak nestin and vimentin expression (Fig 4C).
Fig. 4. In Vitro-Expanded PD AHNPs fail to develop into neurons and astrocytes in differentiation media with or without conditioned medium.
Adherent AHNPs were induced to differentiate using 3 types of DM. After 4–7 days culture in DM, in all 3 groups of differentiation systems, approximately 50–60% of cells detached from the polyornithine coated surface, and there were another 10–25% of cells that detach in later stages. (A) PD SN AHNPs in DM3 after five days. (B) Addition of 1% fetal bovine serum to DM does not prevent cells from detaching, but rescues cells from a subsequent cell death period. (C) Immunocytochemical analysis of surviving cells does not reveal the presence of TuJ1 positive neurons or GFAP positive astrocytes under both growth conditions (with or without serum). Weak nestin and vimentin expression is apparent within these cultures. PD AHNPs were cultured with conditioned medium from differentiating murine ESNPs. No obvious detachment is observed in differentiating PD AHNPs cultures exposed to conditioned medium (D), and 5–10% of these cells gradually differentiate into cells morphologically resembling neurons with extending very long processes (E–L); however, none of these cells could be labeled with our battery of neuronal or glial markers. All the data presented in Fig. S1. are from PD SN AHNP cultures. (All scale bars without labels are 100 μm)
In Vitro Evidence of Improved Neural Differentiation of PD AHNPs Following Co-culture with Rodent Embryonic and Adult Neural Precursor Cells
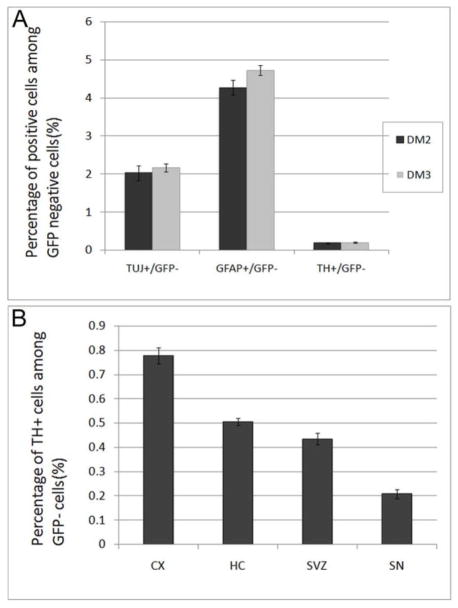
In contrast to isolated differentiation, AHNPs from PD patients’ brain gave rise to both neurons and astrocytes when co-cultured with mouse ESNP cells. We hypothesized that a more conducive environment to inducing neuronal differentiation from PD AHNPs might be generated from supporting astroglial cells (Wang et al., 1994; Song et al., 2002). Since the embryonic brain presents the most hospitable environment for neurogenesis and neuronal differentiation, including for cells of the dopaminergic lineage, we tried co-culturing PD AHNPs with cells that had the capacity to both support neuronal fate and survival as well as differentiate into TH+ neurons: ESNPs. This notion was based on, and supported by the finding that adult precursor cells showed the potential to give rise to cells of all germ layers when co-cultured with embryoid bodies (EBs) (Clarke et al., 2000), thus inductive signals for differentiation to diverse lineages should be present in EB cultures. We therefore performed co-culture studies of PD AHNPs with mouse ESNP cells to test the neurogenic potential of these PD AHNPs. Few of the co-cultured cells stayed adherent to the polyornithine coated surfaces when incubated in DM1, while most cells attached on the coverslips in DM2 or DM3, indicating that BDNF and/or NT-3 might play important roles in cell survival and differentiation. In a DM2- and DM3- incubated human SN AHNPs and mouse GFP-ESNPs co-culture system, mouse ESNPs differentiated into GFP+ neurons (identified by TuJ1 and TH) and astrocytes (GFAP) (data not shown). We observed some GFP−/TuJ1+(Fig 2G–I), GFP−/TH+(Fig 2J,K) and GFP−/GFAP+(Fig 2L) cells, which represented human PD cells. Many GFP− cells were still Nestin positive after 8 days of incubation with DM. This co-culture experiment demonstrated that there were multipotent neural progenitor cells in the SN AHNP population, which can differentiate into neurons, including DA neurons, and astrocytes in vitro when provided with an appropriate environment. Both DM2 and DM3 incubated cultures gave rise to neurons and astrocytes, suggesting that ESNPs could produce some critical differentiation and survival factors. Thus FGF8 and SHH, two factors which are thought to be essential in generating DA neurons from adult neural stem cells, are nonessential in the presence of ESNPs. The presence of FGF8 and SHH led to an immense increase in the numbers of TuJ1 positive cells and TH positive cells among a GFP positive population (data not shown), but did not distinctly enhance TuJ1 positive and TH positive cell numbers among the GFP negative PD population (p>0.05, see Fig 5A).
Fig. 5. Expression of neuronal and glial markers by GFP-negative PD SN AHNPs under differentiating conditions in DM2 and DM3, and a comparison of the yield of TH+ neurons among PD AHNPs from four brain regions.
(A) Both DM2 and DM3 incubated cultures gave rise to neurons and astrocytes. The presence of FGF8 and SHH did not distinctly enhance TuJ1-positive, GFAP-positive and TH-positive cell numbers among the GFP-negative PD population (p>0.05). (B) PD AHNPs from SN, SVZ, HC, and CX were co-cultured with mouse GFP-ESNPs in DM3 media to induce differentiation. GFP–/TuJ1+, GFP–/TH+ and GFP–/GFAP+ cells were found in PD cell co-cultures derived from all four brain regions after 8 days of induced differentiation. SN-derived PD AHNPCs generated the lowest amount of TH+ neurons, while the highest proportion of TH+ neurons was found in CX-derived PD AHNPs (CX>HC>SVZ>SN, n=5 per region, p<0.01).
To investigate whether conditioned medium from ESNPs alone could induce differentiation of PD AHNPs into neurons and glia in vitro, we cultured PD AHNPs with conditioned medium from differentiating murine ESNPs. No obvious detachment was observed in differentiating PD AHNP cultures containing conditioned medium, and approximately 5–10% of these cells gradually differentiated into cells with apparent morphological characteristics of neurons, e.g. extending very long processes (Fig 4E–L). However, none of these cells were immunopositive for neuronal or glial markers. Co-culturing with ESNPs could successfully induce the PD AHNPs to differentiate into neurons and astrocytes, but ESNP-conditioned medium could not, indicating that not only soluble factors are necessary for AHNPs differentiation but also cell-cell contact between the PD cells and supporting cells.
PD AHNPs from SVZ, HC, and CX were co-cultured with mouse GFP-ESNPs in DM3 media to induce differentiation. GFP−/TuJ1+, GFP−/TH+ and GFP−/GFAP+ cells were found in PD cell co-cultures derived from the three brain regions after 8 days of induced differentiation. This suggests that not only the progenitors from PD SN, but also progenitors from other brain regions including the SVZ, HC, and CX have the potential to develop into neurons, even DA neurons, and astrocytes in vitro when provided with an appropriate cellular/molecular environment. We quantified the numbers of GFP−/TH+ cells among four PD brain regions (n=5 per region), and found that SN-derived PD AHNPCs generated the lowest amount of TH+ neurons. The highest proportion of TH+ neurons was found in CX-derived PD AHNPs (CX>HC>SVZ>SN, p<0.01, see Fig 5B). This difference in differentiation capacity could reflect a different level of disease severity within distinct brain regions in PD brains.
Co-cultured with adult mouse SVZ stem cells, progenitors from the Parkinsonian brain can differentiate into neurons
To determine whether co-culturing with adult neural stem cells could stimulate PD AHNPs’ differentiation, we also tried to co-culture the PD AHNPs with adult mouse SVZ stem cells. Co-cultured cells were fixed for immunocytochemistry after 8 days in DM3 media. At this time point, TuJ1 positive neurons were observed, some of them being GFP negative, demonstrating that SN-derived PD AHNPs have the potential to differentiate into neurons in the presence of adult neural stem cells. The majority of the GFAP positive astrocytes in the co-culture system were GFP positive, suggesting that these cells originated from adult mouse SVZ stem cells. We noticed some differences in these PD AHNPs’ differentiation profile when compared to PD cells and ESNPs co-cultures: co-culturing PD cells with ESNPs in DM3 medium rarely resulted in detachment, but in a PD AHNP and adult SVZ stem/progenitor cell co-culture system about 30–40% cells detached during the first week in DM3 medium. Immunocytochemical analysis detected very few GFAP-positive/GFP-negative cells, and most of them appeared to be dying cells or cellular debris. These data show that progenitor cells from PD SN may be impaired in their ability to form GFAP positive astrocytes, although co-culturing with ESNPs could somewhat abrogate the defect, while the presence of adult neural stem cells could not completely help overcome this.
PD progenitor cells can give rise to TH+ cells upon in vivo engraftment
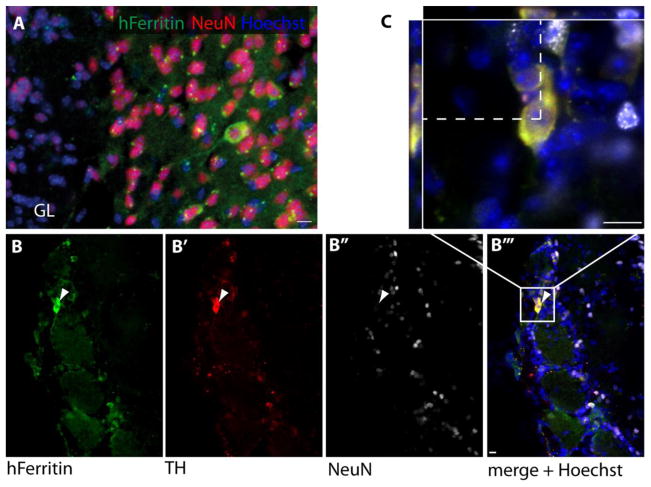
Injection of AHNPs into the ventricles of adult mice resulted in their engraftment in the SVZ and olfactory bulb. PD SVZ derived AHNPs were found in the host SVZ, and expressed GFAP. PD SVZ and SN derived AHNPs were found in the glomerular layer of the host olfactory bulb, where they expressed TH but not NeuN, in agreement with previous findings that many glomerular neurons do not express NeuN (Parrish-Aungst et al., 2007). Engrafted AHNPs were identified using human-specific CD107 and ferritin antibodies. These findings demonstrate that progenitor cells isolated from the SN or SVZ of a Parkinsonian brain can, in principle, differentiate into TH+ neuronal phenotypes. Furthermore, AHNPs from the SVZ also integrated into the neurogenic niche, while we did not observe SVZ integration from SN derived AHNPs.
Discussion
In this study, we were able to successfully isolate and propagate neural progenitor cells from the SN, SVZ, HC and CX of postmortem brain tissue of PD patients. These AHNP cells have elements in common with the floor plate radial glia-like cells that have been identified as dopaminergic neuronal progenitors in the midbrain (Bonilla et al., 2008). Neurogenic radial glia also have been reported to reside in the outer subventricular zone of the cerebral cortex (Hansen et al., 2010; Malatesta et al., 2008). PD AHNPs express nestin, SOX2, vimentin and GLAST, which are reactive astrocyte markers as well, supportive of their potential reactive astrocytic nature as well as their being part of a progenitor cell pool in the PD SN where reactive gliosis certainly is a hallmark of the disease. Unlike reactive rodent astrocytes following injuries in the adult rodent forebrain, that can be multipotent stem/progenitor cells giving rise to neurons and glia readily in culture (Malatesta et al., 2008), these human progenitor cells failed to develop into neurons and astrocytes in standard culture conditions that allow neuronal differentiation of adult human progenitor cells (Walton et al., 2006); we also performed electrophysiological studies of non-PD AHNPs in our original in vitro studies describing these cells and found them to exhibit immature biophysical properties of cultured neurons and glia, suggesting that the AHNPs from the PD brain are difficult to differentiate. When co-cultured with ESNPs, the AHNPs revealed their multipotent neurogenic potential, including the generation of TuJ1 and TH positive neurons, as well as GFAP positive astrocytes. The PD AHNPs used for co-culture experiments were passaged 7–10 times after their original isolation, so we believe the TuJ1 and TH positive neurons, as well as GFAP positive astrocytes are derived from the PD AHNP cells and not fully differentiated cells that might have been carried along during the original isolation. It is known that cell-cell fusion can occur under normal co-culture conditions and contribute to observed intrinsic plasticity of somatic cells. Even though compared with their production from embryonic stem cells [15] the yield of DA neurons from the PD AHNPs is quite low, it is still much higher than the documented extremely low cell fusion frequencies (Ying et al., 2002; Terada et al., 2002). In the studies here, however, we used GFP-labeled ESNPs/MASCs in co-culture with GFP-negative PD AHNPs, and thus all fused cells should have expressed GFP (which they did not; preliminary studies using FISH to check for mouse-human chromosome staining in the same cells also did not reveal fused cells). Interestingly, when co-cultured with adult neural stem cells, they were able to differentiate into TuJ1 positive neurons, but revealed a defect in forming GFAP positive astrocytes. This cell population could potentially contribute to a new in vitro model system for PD research, and may provide a potential target for therapeutic intervention to regenerate functional endogenous dopaminergic neurons in the human PD brain. Identifying the signals that can direct the differentiation of SN progenitor cells of PD patients toward DA neurons might be a useful approach in the future for novel therapies for PD. These progenitor cells may prove to be an attractive source for cell-based therapy for PD in that they both overcome the ethical issues inherent to the use of embryonic or fetal stem cells, and open the possibility for autologous transplantations. A previous study of the rodent SN has revealed the presence of a glial precursor cell that can give rise to neurons and glia in culture, but appear to be solely gliogenic in vivo (Lie et al., 2002). This cell appears to be different from the AHNPs described here since it expresses the NG2 proteoglycan (which AHNPs do not), and AHNPs here seem to be more in line with the AHNP cell we have described from the non-PD adult human fore- and midbrain (Walton et al., 2006). Nonetheless, the fact that there are neurogenic cells in the human SN suggests the potential to recruit this type of cell for cell-based therapy approaches in PD. Likewise, this cell could be compared with counterparts generated from normal, and PD induced pluripotent stem cells (iPSCs) and induced neurons/dopamine neurons (Takahashi et al., 2007; Yu et al., 2007; Park et al., 2008; Nguyen et al., 2011; Pfisterer et al., 2011) in future studies attempting to categorize and standardize the state of differentiation potential of reparative stem/progenitor cells engineered using such iPS cell technology.
AHNP cells can be isolated from various brain regions of PD patients, including the SN. In addition to their ability to differentiate into TH+ neurons in vitro, these cells show an impressive propensity for functional engraftment within the neurogenic SVZ-rostral migratory stream-olfactory bulb axis following in vivo transplantation. We chose to engraft in the SVZ-rostral migratory stream-olfactory bulb axis because of our previous studies (Deng et al., 2006) showing utility of this neurogenic pathway for testing the engraftment potential of heterologous populations of stem/progenitor cells. In our previous studies of AHNPs (Walton et al., 2006), we also observed functional engraftment in the cortex and hippocampus, and such xenograft studies of an AHNP-like cell, also from non-PD sources, described by the Langmoen group (Olstorn et al., 2007) also reveal impressive engraftment in rodent models. Grafted AHNPs here integrated and survived for at least 8 weeks after transplantation (data not shown). The fact that neural precursor cells can be isolated from the SN of idiopathic PD and that these cells can integrate into the host brain and differentiate into TH+ neurons could indicate a possible potential for self-regeneration inherent to the diseased SN region. Because of aberrant growth and differentiation patterns exhibited by PD AHNPs shown here in vitro, there may be a need for either ex vivo or perhaps even in situ gene therapy to enhance the reactive neurogenic prowess of these cells. Likewise, the poor growth ability of DBS electrode-derived cells also studied here suggests one of the following possibilities: 1) there are too few clonogenic progenitor cells that can be harvested from these discarded electrodes, 2) their proximity to the generating source of electrical current compromises their ability to expand in our culture system, or 3) there are some unrecognized bases for their poor ability to give rise to expandable, potentially therapeutic cells.
Recent studies have shown that transplanted human neural fetal cells in PD patients are susceptible to synuclein aggregation formation over time, suggestive of an infectious potential of the PD brain milieu whereby grafted precursor cells are at risk for “contracting” the disease (Hansen et al., 2011; Kordower et al., 2008; Li et al., 2008; Olanow and Prusiner, 2009), although there is also evidence that points to a potential sparing of grafted fetal cells in the disease (Mendez et al., 2008). The potential for a synucleiopathy in AHNPs was studied here using immunolabeling of cultured cells compared to sections from the PD patient brains; no synuclein aggregates, inclusions or immunopositive Lewy neurites were observed in any of our cultures despite the presence of staining in parental tissue sections from these PD cases. AHNPs might fail to exhibit a synucleiopathy for a variety of reasons, including their state of immaturity in the studies performed here or technical difficulties with the antibodies used in our cultures, although we encountered no such problems with any of our other antibody probes. Animal models of an apparent infectious protein nature of -synuclein (mutant or wildtype) from Brundin and colleagues (Hansen et al, 2011) support previous notions of exosomal transport (Emmanouilidou et al., 2010) and transmissibility of cells in vitro and in vivo, along with transcellular uptake leading to possible toxic cellular consequences of PD-molecular species from the extracellular microenvironment that might put newly engrafted AHNPs at risk following a progressive disease course. One could certainly argue that studying the reparative or even possibly neuroprotective functions of transplanted or endogenously recruited AHNPs will lead to insights into their possible protection (using molecular therapeutics or gene therapy) and eventual positive therapeutic outcomes despite their seeming vulnerability. It is important to establish if AHNPs, despite possessing potentially genetic and molecular susceptibilities to a progressive neuropathology (in essence a “stem cell pathology” (Steindler et al., 2011), still have the potential to give rise to new neurons and glia for potential bioassays, as well as for cellular therapeutic regimens in PD. Nevertheless, the results from our experiments present valuable information that it is possible to recover progenitor cells from different regions of the diseased post-mortem brain, and this may prove to be a significant tool in the future advancement of the field. It is noteworthy that during the course of finishing this study a paper appeared (van den Berge et al., 2011) that reported the ability to isolate and expand neural stem/progenitor cells from the postmortem adult PD neurogenic subventricular zone. Even though this study did not attempt to examine clonogenic properties of cells from the SN as we have done here, and hence did not recognize an apparent disease-associated phenotype of these cells, nor did they assess the in vivo plasticity of PD stem/progenitor cells as we have done here utilizing the insinuation-friendly subventricular zone/rostral migratory stream axis, these two studies together support the notion that clonogenic cells reside in the PD brain and are worthy of further study for their potential to be mobilized for protection and repair despite disease-associated phenotypy.
In conclusion, the present study demonstrates that adult brain stem/progenitor cells exist in the PD SN and other brain regions; these could be isolated and propagated from postmortem PD brains. The PD AHNP primary cell lines failed to develop into neurons and astrocytes in standard culture conditions that allow neuronal differentiation of adult human progenitor cells, but succeeded to differentiate into both neurons (including TH+ DA neurons) and glia when co-cultured with ESNPs, while co-culture with adult neural stem cells showed defects in forming GFAP positive astrocytes. Transplantation experiments revealed that grafted PD AHNPs could integrate into the host brain and differentiate into neurons including small numbers of TH+ neurons. This finding may indicate that the neurogenic ability of PD AHNPs is possibly affected by the disease, or that there are essential factors missing in the growth conditions. PD AHNPs may thus prove to be a valuable tool for both disease modeling and cellular replacement strategies.
Experimental Procedure
Isolation and culture of AHNPs from PD brain
Donor tissue was obtained at autopsy from PD patients (n=7, see Table 1, data presented in this paper are representative and primarily from a 65-year-old male) with research consent and ethical approval following established guidelines within the University of Florida Institutional Review Board. SN, SVZ, HC, and CX (primary motor CX, n=5; frontal CX, n=1) were identified and dissected from the left hemisphere (2–12 hours post-mortem) (Laywell et al., 1999). AHNP cultures from PD brain tissue were established as previously described (Walton et al.,2006; Scheffler et al., 2005), with some modifications. In brief, tissue from different brain regions (see Table 1) was placed separately in ice-cold DMEM/F12 with antibiotics (Invitrogen), and immediately prepared for cell culture, while some tissue was cryopreserved for molecular biology or fixed with 4% paraformaldehyde and then embedded in paraffin for immunohistochemistry. For adhesive culture, tissue samples were manually dissociated into <1mm3 pieces and plated onto polyornithine (15 μg/ml, Sigma) coated plastic dishes (Goetz et al., 2006), covered with proliferative media [6] containing 10ng/mL hLIF (Chemicon) (Oshima et al., 2007), 1μg/mL laminin (Invitrogen) (Hall et al., 2008) and the media was partly changed every other day. For long-term storage, cultures were dissociated with trypsin-Versene (0.2%trypsin + 0.008% EDTA), and cryopreserved in growth medium containing 10% DMSO. For primary neurosphere cultures, we dissociated primary tissue into single cells using trypsin-versene and plated them with 2x proliferative media (without laminin)/2%methylcellulose (Sigma) (1:1) at a density of 100,000 cells/mL onto 6 well ultra low attachment dishes (Costar) (Laywell et al., 1999). Cultures were supplemented with 10ng/mL of bFGF and EGF every other day. Spheres were dissociated with trypsin-versene into single cells and replated every 4 weeks.
Cultures from DBS electrodes were obtained by collecting DBS lead electrodes (n=3) or microelectrodes (n=3) from patients undergoing elective surgery for lead replacement at the University of Florida Shands hospital (indication for replacement was lead fractures). Ethical approval and informed consent was obtained prior to surgery. The electrodes were placed on polyornithine/laminin coated cell culture plates in proliferative medium as described above. Medium was partially changed every other day.
For immunocytochemistry, cells were plated on polyornithine coated glass coverslips and grown to confluence, fixed with 4% paraformaldehyde and processed for immunocytochemistry as previously described (Walton et al., 2006). Primary antibodies were: anti-nestin (1:200, Millipore), anti-SOX2 (10 μg/mL, R&D), anti-GFAP (1:200, Chemicon), anti-vimentin (10 μg/mL, R&D), anti-Glutamate Transporter GLAST (1:1000, Chemicon), anti CD133 (1:50, MiltenyiBiotec), anti-A2B5 (1:500, Chemicon), anti-NG2 (1:1,000, Chemicon), anti-βIII Tubulin (TuJ1, 1:2000, Promega; 1:2000, Covance), anti-Tyrosine Hydroxylase (TH, 1:250, Chemicon), anti-Green Fluorescent Protein (GFP, 1:700, Millipore; 1:700, Invitrogen), anti-α-synuclein (Syn1, 1:1000, BD Transduction; or human specific clone Syn211, 1:1000, Abcam). Nuclei were counterstained with DAPI (Sigma). Fluorescence microscopy and unbiased cell counting were performed on a Leica DMLB upright microscope (Bannockburn, IL) and images were captured with a Spot RT color CCD camera (Diagnostic Instruments). Unbiased cell counting data was generated from five independent experiments, with each trial comprising a minimum of 10 visual fields at 40x magnification. All values were expressed as mean±SEM.
Total RNA was extracted using the RNeasy Mini Kit (Qiagen) with DNase I treatment on the column. Eluted RNA was exposed to a second DNase I treatment in solution using Turbo DNA-free (Ambion). For each RT-PCR reaction, 500 ngoligodT(12-18), dNTP mix (0.5 mM each) and a corresponding amount of 1 μg of total RNA were added. Each RT reaction was run in triplicate using the SuperScript II kit (Invitrogen) according to the manufacturer’s instructions. Primer sequences (see data Table 2) and PCR conditions are available upon request.
Table 2.
Sequences for RT-PCR Primers
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| Nestin | TCCCTCCGCATCCCGTCAG | CCGTCACCTCCATTAGCCACAG |
| SOX2 | AACACCAATCCCATCCACACTC | GCTCCTACCGTACCACTAGAAC |
| GLAST(SCL1A3) | CTGCTCACAGTCACCGCTGT | AGCACGAATCTGGTGACGCG |
| Vimentin | TGCTGGAAGGCGAGGAGAG | AAGGTCATCGTGATGCTGAGAAG |
Co-culture Studies with Embryonic and Adult Neural Stem Cells
To test the neural lineage potential of the PD AHNPs, 3 types of DM were tested: (1) DM1: regular differentiation medium (Goetz et al., 2006) plus platelet-derived growth factor (PDGF, 20 ng/mL, R&D), ascorbic acid (200 μM, Sigma) and retinoic acid (100 nM, Sigma); (2) DM2: DM1 plus brain-derived neurotrophin (BDNF, 50 ng/mL, Chemicon) and neurotrophin-3 (NT-3, 50ng/mL, Chemicon); (3) DM3: DM2 plus SHH(100 ng/mL, Sigma) and FGF8b (100 ng/mL, Sigma) (Baquet et al., 2005; Caldwell et al., 2001; Espejo et al., 2000; Yan et al., 2005). The medium was changed every 4 days with fresh growth factors, and retinoic acid was added every 24 hours. To investigate whether conditioned medium from embryonic stem cell-derived neural precursors (ESNPs) can induce differentiation of these PD AHNPs into neurons and glia in vitro, we performed PD AHNPs culture with the presence of conditioned medium from differentiating mouse ESNPs. Mouse ESNPs were cultured using our controlled four-step neural induction protocol (Goetz et al., 2006), conditioned medium was collected every 3days in step IV (maturation of neural phenotypes), filtered through 0.2μm filter, and stored at −20 C until use. Conditioned medium was mixed 1:1 with DM3.
For co-culture experiments, 90% confluent PD progenitors were dissociated into single cells after 4 days of serum/mitogen withdrawal. PD AHNPs were mixed with mouse GFP ESNPs on the 2nd day of stage IV at a ratio of 1:1 by volume and plated onto polyornithine coated glass coverslips with DM1, DM2 and DM3 respectively. After 8 days in culture, they were fixed and processed for immunocytochemistry as described above.
To co-culture with the PD AHNPs, adult multipotent astrocytic stem cells (MASCs) were derived from SVZ of postnatal day 7 UBC/GFP mice, with isolation, preparation, and characterization performed as previously described (Laywell et al., 2000). The cell suspensions of adherent PD SN progenitors and GFP-adult mouse SVZ stem cells were mixed 1:1, plated onto polyornithine coated glass coverslips, grown until 90% confluent, and the serum and growth factors were removed. After 4 days, the culture was transferred to DM3 medium, and incubated for 8 days, then fixed with 4% paraformaldehyde and processed for immunocytochemistry.
Transplantation Experiments
For animal transplantation, 50,000 AHNPs/μl in N2 medium were injected into the lateral ventricles of 3 month old Fox Chase SCID mice following established Institutional Animal Care and Use guidelines and animals were cared for in accordance with these and NIH guidelines. Mice were anaesthetized by isoflurane inhalation, their skull exposed and a hole drilled at the coordinates Bregma −0.5mm anterior, −0.5 mm lateral. 1 μl of a cell suspension was injected using a Hamilton syringe at a depth of 2 mm, the syringe was left in place for 5 minutes before retraction. The incision was closed with surgical staples and the animals were allowed to recover before being returned to their cages.
4 and 8 weeks after transplantation, animals were deeply anesthetized using isoflurane and then perfused transcardially with 4% fresh paraformaldehyde in a 0.02 M phosphate buffer solution (PBS, pH 7.4). Brains were removed, post-fixed over night, cryoprotected in 30% sucrose in PBS and 30 μm cryostat sections were cut. Free-floating sections were immunostained as described (Zheng et al., 2006), mounted onto slides, coverslipped and examined on an Olympus IX81-DSU Spinning Disk confocal microscope (Olympus America, Melville, NY).
Fig. 3. In vivo engraftment of PD AHNPs.
AHNPs derived from SVZ and SN were injected into the lateral ventricles of adult Fox Chase SCID mice. The mice were sacrificed 4 weeks after transplantation, and engrafted cells in the olfactory bulb were visualized by immunohistochemistry for human specific ferritin and neuronal markers. (a) Confocal projection image of a mature human neuron in the periglomerular layer co-expressing human ferritin and NeuN (GL: glomerular layer). (b) Neurons in the glomerular layer co-expressed human ferritin (B) and TH (B′), but not NeuN (B″). (c) Confocal z-stack of boxed area in B‴. Scale bars 10 μm.
Highlights.
This study describes, for the first time, a population of neural stem/progenitor cells isolated and characterized from samples of Parkinson’s Disease brains, that can give rise to neurons and glia under particular conditions both in vitro and in vivo.
We demonstrate the in vitro proliferative and differentiation potential of stem/progenitor cells isolated from idiopathic PD postmortem tissue samples and, to a lesser extent, discarded deep brain stimulation electrodes.
We also found that these cells engraft following ex vivo expansion and transplantation into the rodent brain, demonstrating their regenerative potential.
This study provides valuable information on the ability, albeit compromised, of PD neural stem/progenitor cells to give rise to neural cells that may be amenable to manipulation for neurotherapeutic repair and replacement strategies in the PD brain.
Acknowledgments
This work was supported by NIH/NINDS grant NS055165 and the Maren, Thompson and McKinney Regenerative Medicine Funds (DAS). M.S.O. is supported by grants from the National Parkinson Foundation, the NIH/NINDS, and the NPF Center of Excellence at the University of Florida. SNR is supported by a Citizens United for Research in Epilepsy grant. We thank Tomoko Sahara for technical assistance, and the Roper lab in the UF Department of Neurosurgery for ongoing studies of normal AHNP cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias-Carrion O, Freundlieb N, Oertel WH, Hoglinger GU. Adult neurogenesis and Parkinson’s disease. CNS Neurol Disord Drug Targets. 2007;6:326–335. doi: 10.2174/187152707783220875. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla S, Hall AC, Pinto L, Attardo A, Gotz M, Huttner WB, Arenas E. Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia. 2008;56:809–820. doi: 10.1002/glia.20654. [DOI] [PubMed] [Google Scholar]
- Bramanti V, Tomassoni D, Avitabile M, Amenta F, Avola R. Biomarkers of glial cell proliferation and differentiation in culture. Front Biosci (Schol Ed) 2010;2:558–570. doi: 10.2741/s85. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol. 2001;19:475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Deng J, Petersen B, Steindler DA, Jorgensen M, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–51. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo M, Cutillas B, Arenas TE, Ambrosio S. Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line-derived neurotrophic factor. Cell Transplant. 2000;9:45–53. doi: 10.1177/096368970000900107. [DOI] [PubMed] [Google Scholar]
- Geraerts M, Krylyshkina O, Debyser Z, Baekelandt V. Concise review: therapeutic strategies for Parkinson disease based on the modulation of adult neurogenesis. Stem Cells. 2007;25:263–270. doi: 10.1634/stemcells.2006-0364. [DOI] [PubMed] [Google Scholar]
- Goetz AK, Scheffler B, Chen HX, Wang S, Suslov O, Xiang H, Brustle O, Roper SN, Steindler DA. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:11063–11068. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PE, Lathia JD, Caldwell MA, Ffrench-Constant C. Laminin enhances the growth of human neural stem cells in defined culture media. BMC Neurosci. 2008;9:71. doi: 10.1186/1471-2202-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–25. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Kukekov VG, Steindler DA. Multipotent neurospheres can be derived from forebrain subependymal zone and spinal cord of adult mice after protracted postmortem intervals. Exp Neurol. 1999;156:430–433. doi: 10.1006/exnr.1999.7029. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Appolloni I, Calzolari F. Radial glia and neural stem cells. Cell and Tissue Research. 2008;331:165–178. doi: 10.1007/s00441-007-0481-8. [DOI] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–9. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–9. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B, Dolmetsch RE, Langston W, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Prusiner SB. Is Parkinson’s Disease a prion disorder? Proc Natl Acad Sci USA. 2009;106:12571–2. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olstorn H, Moe MC, Røste GK, Bueters T, Langmoen IA. Transplantation of stem cells from the adult human brain to the adult rat brain. Neurosurgery. 2007;60:1089–98. doi: 10.1227/01.NEU.0000255461.91892.0D. [DOI] [PubMed] [Google Scholar]
- Oshima K, Teo DT, Senn P, Starlinger V, Heller S. LIF promotes neurogenesis and maintains neural precursors in cell populations derived from spiral ganglion stem cells. BMC Dev Biol. 2007;7:112. doi: 10.1186/1471-213X-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preynat-Seauve O, Burkhard PR, Villard J, Zingg W, Ginovart N, Feki A, Dubois-Dauphin M, Hurst SA, Mauron A, Jaconi M, Krause KH. Pluripotent stem cells as new drugs? The example of Parkinson’s disease. Int J Pharm. 2009;381:113–121. doi: 10.1016/j.ijpharm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Srivastava AS, Malhotra R, Sharp J, Berggren T. Potentials of ES cell therapy in neurodegenerative diseases. Curr Pharm Des. 2008;14:3873–3879. doi: 10.2174/138161208786898617. [DOI] [PubMed] [Google Scholar]
- Steindler DA, Okun MS, Scheffler B. Stem cell pathologies and neurological disease. Modern Pathology. 2011 doi: 10.1038/modpathol.2011.165. E pub November 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch A, Sabolek M, Milosevic J, Schwarz SC, Schwarz J. Midbrain-derived neural stem cells: from basic science to therapeutic approaches. Cell Tissue Res. 2004;318:15–22. doi: 10.1007/s00441-004-0923-5. [DOI] [PubMed] [Google Scholar]
- Sun Y, Kong W, Falk A, Hu J, Zhou L, Pollard S, Smith A. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS One. 2009;4:e5498. doi: 10.1371/journal.pone.0005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- van den Berge SA, van Strien ME, Korecka JA, Dijkstraa AA, Sluijs JA, Kooijman L, Eggers R, De Fillipis L, Vescovi AL, Verhaagen J, van de Berg WD, Hol EM. The proliferative capacity of subventricular zone is maintained in the parkonsonian brain. Brain. 2011;134:3249–63. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Chen HX, Chang LJ, Roper SN, Scheffler B, Steindler DA. Derivation and large-scale expansion of multipotent astroglial neural progenitors from adult human brain. Development. 2006;133:3671–3681. doi: 10.1242/dev.02541. [DOI] [PubMed] [Google Scholar]
- Wang LC, Baird DH, Hatten ME, Mason CA. Astroglial differentiation is required for support of neurite outgrowth. J Neurosci. 1994;14:3195–3207. doi: 10.1523/JNEUROSCI.14-05-03195.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-C, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: Reduction–mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine–glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- Zheng T, Marshall GP, 2nd, Laywell ED, Steindler DA. Neurogenic astrocytes transplanted into the adult mouse lateral ventricle contribute to olfactory neurogenesis, and reveal a novel intrinsic subependymal neuron. Neuroscience. 2006;142:175–185. doi: 10.1016/j.neuroscience.2006.05.051. [DOI] [PubMed] [Google Scholar]