Abstract
The unique effects of neighborhood-level economic deprivation on survival, recurrence, and second primary malignancy development were examined using adjusted Cox proportional hazards regression models among 1151 incident squamous cell carcinomas of the head and neck (SCCHN) patients. Cancer site was examined as a potential moderator. Main analyses yielded null results; however, interaction analyses indicated poorer overall survival [HR=1.59 (1.00-2.53)] and greater second primary malignancy development [HR=2.99 (1.46-6.11)] among oropharyngeal cancer patients from highly deprived neighborhoods relative to less deprived neighborhoods. Results suggest a dual focus on individual and neighborhood risk factors could help improve clinical outcomes among oropharyngeal cancer patients.
Keywords: neighborhood deprivation, head and neck cancer, survival, second primary malignancy, oropharyngeal cancer
Introduction
Squamous cell carcinomas of the head and neck (SCCHN) are cancers of the epithelial cells in the head and neck (excluding the eyes, ears, brain, thyroid, and esophagus), and are the main histological subtype of head and neck cancers. One of the strongest behavioral risk factors for SCCHN incidence is tobacco use, followed to a lesser extent by excessive alcohol use (Blot et al., 1988; Diaz, Holsinger, Zuniga, Roberts, & Sorensen, 2003; Lewin et al., 1998). Other factors related to SCCHN incidence include male gender, older age, African American race, and oncogenic human papillomavirus (HPV) status. Oncogenic HPV status is particularly relevant to oropharyngeal cancer (i.e., cancers that include the middle part of the throat including the soft palate, the base of tongue, the tonsils, and the back and side throat wall), with HPV-positive patients being younger, less likely to have a significant tobacco and alcohol use history, and more likely to have a better prognosis than HPV-negative patients (Chaturvedi, Engels, Anderson, & Gillison, 2008). In addition to these demographic and clinical factors, research suggests that individuals of lower socioeconomic status have higher rates of SCCHN incidence, even when controlling for tobacco and alcohol use status (S. Johnson, McDonald, & Corsten, 2008).
In total, SCCHN account for between 3% -5% of incident cancer cases in the US [cf. (NCI, 2011)], and approximately 6% of cancers worldwide (Lefebvre, 2005). Standard treatment options for SCCHN include surgery and/or radiotherapy for early stage or localized disease, with chemotherapy as an adjuvant treatment in advanced stages of disease (ACS, 2011). Despite relatively low incidence rates, treatment costs associated with SCCHN are significant, with recent estimates topping $3.1 billion dollars annually in the US alone [cf. (NCI, 2011)]. Survival rates for SCHHN vary based on disease stage at clinical presentation, and disease stage at presentation does not appear to be related to patients’ socioeconomic status (S. Johnson, Corsten, McDonald, & Chun, 2010). Stage I and II disease represent localized cancers, Stages III, IVa, and IVb represent locally advanced disease with metastasis to local lymph nodes, and Stage IVc represents distant metastasis. Five-year survival rates for patients with SCCHN are about 91% for Stage I disease, 77% for Stage II, 61% for Stage III, 32% for Stage IVa, 25% for Stage IVb, and <4% for Stage IVc. [cf.(Lefebvre, 2005)]. Although 5-year survival rates for SCCHN are relatively high, patients with SCCHN frequently suffer mortality from comorbid illnesses and relatively high rates of second primary malignancy development [4% , cf. (Lefebvre, 2005)]. In addition, functional morbidities resulting from treatment that affects speech, swallowing, and facial aesthetics may result in social and occupational impairments and a potentially lower quality of life (Bonanno, Esmaeli, Fingeret, Nelson, & Weber, 2010; Buckwalter, Karnell, Smith, Christensen, & Funk, 2007; Givens et al., 2009; Goldstein, Hynds Karnell, Christensen, & Funk, 2007; Mowry, Tang, Sadeghi, & Wang, 2010; Terrell, Fisher, & Wolf, 1998; Teymoortash, Hoch, Eivazi, & Werner, 2010; Yao et al., 2007). Thus, the fiscal, social, and clinical consequences of SCCHN are significant, warranting the need to comprehensively understand the factors that affect treatment outcome among these patients.
Thus far, studies have cited a number of sociodemographic and clinical factors that affect SCCHN clinical outcomes (i.e., prognosis and survival), including age, sex, race/ethnicity, tumor stage, tobacco use, comorbidity (Piccirillo, Tierney, Costas, Grove, & Spitznagel, 2004), HPV status, treatment modality (Chaturvedi et al., 2008; Fakhry et al., 2008; Molina et al., 2008; Pytynia et al., 2004; Schrank, Han, Weiss, & Resto, 2011), and cancer site (Chaturvedi et al., 2008; Schrank et al., 2011). A number of studies have also focused on the significant relations of socioeconomic status (SES) with SCCHN outcomes, with lower SES predicting poorer clinical outcomes [e.g., (Arbes et al., 1999; Chu et al., 2011; Molina et al., 2008)}. However, these studies have relied on neighborhood-level SES as a proxy for patient-level SES, largely because individual-level SES information is often unavailable within population-based survival databases (e.g., cancer registries). As a result, the true influence of neighborhood SES on SCCHN outcomes, over and above the influence of individual-level SES, remains unstudied. Clarifying whether neighborhood-level SES plays a unique role in SCCHN outcomes is important to improving interventions, as significant associations would suggest that a dual focus on individual and neighborhood factors would be desirable for improving outcomes (Winkleby, Cubbin, & Ahn, 2006). Also, the clinical landscape of head and neck cancers has been changing in recent years, with rising rates of oropharyngeal cancers and declining rates of non-oropharyngeal cancers (Sturgis & Ang, 2011). A better understanding of contextual influences on SCCHN by cancer site is needed, as site is associated with prognosis and survival outcomes (Chaturvedi et al., 2008; Schrank et al., 2011).
The purpose of the current study was to examine the unique effect of neighborhood-level SES on survival, recurrence, and the development of second primary malignancies among patients from Texas and Louisiana who were assessed and treated for incident SCCHN at a single, large multidisciplinary cancer center in the southwest. To our knowledge, this is the first study to examine the associations between neighborhood-level SES and SCCHN outcomes, while controlling for individual-level income as well as other sociodemographic and clinical variables. In addition to examining relations of neighborhood-level SES with clinical outcomes among all SCCHN patients, the potential for moderation by cancer site was also examined.
Materials and Methods
Study Design
The current study was a retrospective analysis of an ongoing epidemiological study of SCCHN patients at The University of Texas MD Anderson Cancer Center. All procedures were approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Participants
Participants were adults with pathologically confirmed, previously untreated, incident SCCHN, who were evaluated (and later treated) at The University of Texas MD Anderson Cancer Center. Patients with salivary gland, nasopharyngeal, or lip carcinoma, and patients with a prior malignancy were excluded from participation. Data relevant to the current study consisted of the subset of patients from the two most highly represented states (Texas and Louisiana; N=1784). These participants were enrolled between February of 1996 and November 2009.
Procedure
Patients were approached about the study upon initial clinical presentation to The University of Texas MD Anderson Cancer Center with pathologically confirmed, previously untreated, incident SCCHN. Interested persons were screened for eligibility and consented for the study at this initial clinic visit, whereupon they completed a standardized questionnaire that elicited information about sociodemographic variables and medical history. Information about diagnosis and treatment plan, as applicable, was added to the medical record by the treating clinician following the initial visit. This clinical information, as well as survival/recurrence outcomes, was updated by the treating clinician at every subsequent patient visit. Patients’ medical records were systematically reviewed for cancer site, cancer stage, cancer treatment, outcomes and dates for the present study. Additional updates were procured annually from institutional cancer registry data.
Participants’ residential addresses were gathered at the time of study enrollment and were used to ascertain information on neighborhood deprivation. First, the North American Address Locator geocoding service provided by Environmental Systems Research Institute’s ArcGIS Online web site was utilized within ArcGIS Desktop software (version 9.3.1; ESRI, Redlands, CA) to set parameters for batch geocoding of participants’ addresses. Geocoding refers to the process of associating an address with a point in geographic space. The majority of the participants’ residential addresses were successfully geocoded (n=1334) using the address locator. The remaining 450 addresses required manual geocoding, which was the process of reviewing each of the geocoding address failures and attempting to find their location through a more intensive search. Overall, 202 of these addresses could be located through manual geocoding. The remaining geocoding failures (n=249) were primarily due to missing addresses and the use of P.O. Box or route addresses. This yielded a sample of 1535 patients for whom neighborhood characteristics could be gathered by associating their locations in space with a Census tract identifier. Census tracts are administratively-created units that have been supported as suitable neighborhood proxies in previous health-related research (Jones, van Sluijs, Ness, Haynes, & Riddoch, 2010; Krieger, Chen, Waterman, Rehkopf, & Subramanian, 2003; Ross, Tremblay, & Graham, 2004; Stafford, Duke-Williams, & Shelton, 2008). The Census tract identifier was then used to link each participant to the associated area-level data from the US Census (2000).
Measures
Patient variables
Patient variables of interest included sociodemographic and clinical factors. Sociodemographic variables collected were: age, sex, race/ethnicity (Non-Hispanic White vs. Others), annual household income (<$25,000, $25,000-$74,900, ≥$75,000), and smoking status (current/former smoker vs. never smoker). Clinical factors included: cancer site (oropharyngeal versus non-oropharyngeal), cancer stage (I/II versus III/IV), and cancer treatment (single modality versus multiple modality). A total of 384 patients were dropped as a result of unknown primary cancer sites (n=28), insufficient treatment data (n = 303; e.g., no treatment, palliative care only, received treatment elsewhere), and/or because no follow-up data were available (n=291). Dropped patients differed significantly from remaining patients only on cancer sites. Specifically, fewer oropharyngeal than non-oropharyngeal cancer patients were dropped (22.4% versus 28.9%; p=0.004). The remaining patients (N=1151) were included in the analyses. Of these patients, 32 declined to provide income data and 17 had missing cancer site data.
Neighborhood SES
Five widely-used tract-level Census variables were selected to represent different aspects of patients’ neighborhood SES (e.g., education, employment status, poverty): the percentage of the total population 25 years or older with less than high school/GED degree, the percentage of residents ≥16 years old who were unemployed, the percentages of households with income below the poverty level in 1999, the percentage of single parent households, and the percentage of households with no vehicle available for use. The internal reliability of these area-level variables was good (α=.86). An index of neighborhood economic deprivation (hereafter referred to as neighborhood deprivation) was created for each patient by summing the z-scores of these area-level variables and dividing by five. Higher scores indicated greater neighborhood disadvantage (i.e., deprivation). Next, the resulting neighborhood deprivation scores were rank-ordered and quartiled. Because we were interested in the effects of the most deprived neighborhoods on survival, a bivariate predictor variable was created from these data that was comprised of the top 25% (High Neighborhood Deprivation) versus the remaining 75% (Low to Moderate Neighborhood Deprivation).
Survival/Recurrence/Second Primary Malignancy outcomes
There were four clinical outcomes of interest in the present study: overall survival, disease-specific survival, disease-free survival, and second primary malignancy development. Analyses were completed for time from first appointment, using death, recurrence, or second primary malignancy development as censoring variables. Death in overall survival analyses was defined as death from any cause. Death in disease-specific survival analyses was defined as death from incident disease only. Disease-free survival was defined as the absence of disease recurrence (relapse). Finally, second primary malignancy development was defined as the development of a second malignant cancer following the SCCHN index cancer diagnosis. Second lesions were considered second primary malignancies if they were of different histopathologic type, occurred >5 years after treatment for the index tumor, and/or were clearly separated by normal epithelium based on clinical and radiographic assessment. Pulmonary lesions were considered second primary malignancies if they had a non-squamous histology, or if they were isolated squamous lesions appearing >5 years after the initial SCCHN and designated as second primary malignancies by the thoractic oncologist or surgeon. Any discrepancies in opinions regarding the origin of the tumor as recurrence versus second primary malignancies were handled by classifying the second lesion as a local recurrence. If none of the four events of interest occurred over the period of analysis, participants were automatically censored at the end of the study.
Data Analyses
Because participants were clustered within Census tracts (neighborhoods), we used a robust sandwich estimate approach in all survival analyses to account for any location-based correlation in outcomes (Ying & Liu, 2006). Our main analyses examined the effect of neighborhood SES on overall survival, disease-specific survival, disease-free survival, and second primary malignancy development for the total sample of SCCHN patients. Unadjusted and adjusted analyses were performed using the Statistical Analysis Software PROC PHREG command, which is a semi-parametric procedure to fit the Cox proportional hazard regression models. Adjusted analyses included the following covariates: age, sex, race/ethnicity, income, smoking status, cancer site, cancer stage, and treatment approach. Adjusted survival curves were calculated using the corrected group prognosis method (Ghali et al., 2001). Next, cancer site (oropharyngeal versus non-oropharyngeal) was examined in each of the adjusted models as a potential moderator of the effect of neighborhood deprivation on survival. This was accomplished by including an interaction term (neighborhood deprivation x cancer site) in each adjusted model. Significant interaction results were followed up with unadjusted and adjusted survival analyses (using procedures described above) for each cancer site, respectively. All analyses were performed using Statistical Analysis Software (version 9.2; SAS Institute Inc., Cary, NC). Proportional hazard assumptions were examined for each model with no resulting violations.
Results
Patient Population
Patients (N=1151) were largely Non-Hispanic White (82%) and male (77%). Patients resided within 735 Census tracts within Texas and 82 Census tracts within Louisiana. The number of patients residing within tracts ranged from 1 to 6, with 239 tracts being home to two or more participants. Chi-square and t-tests indicated a statistically significant difference between the oropharyngeal (n=555) and non-oropharyngeal (n=579) cancer patients on several variables, including age, sex, race/ethnicity, household income, smoking status, cancer stage, cancer treatment, and neighborhood deprivation (Table 1). See Table 1 for detailed information on patient characteristics for the total sample and by cancer site.
Table 1.
Patient Characteristics for Total Sample and by Cancer Site
| Variables | Oropharyngeal (n=555) |
Non-Oropharyngeal (n=579) |
Total Sample (N=1151) |
|---|---|---|---|
|
| |||
| n (%)/M (SD) | n (%)/M (SD) | N (%)/M (SD) | |
| Neighborhood deprivation** | |||
| Low to Moderate | 451 (81.3) | 406 (70.1) | 863 (75.0%) |
| High | 104 (18.7) | 173 (29.9) | 288 (25.0%) |
| Cancer stage** | |||
| Stage I/II | 47 (8.5) | 232 (40.1) | 283 (24.6%) |
| Stage III/IV | 508 (91.5) | 347 (59.9) | 868 (75.4%) |
| Cancer Treatment** | |||
| Single Modality | 188 (33.9) | 256 (44.2) | 447 (38.8%) |
| Multiple Modalities | 367 (66.1) | 323 (55.8) | 704 (61.2%) |
| Sex** | |||
| Female | 82 (14.8) | 174 (30.1) | 260 (22.6%) |
| Male | 473 (85.2) | 405 (69.9) | 891 (77.4%) |
| Race/Ethnicity** | |||
| Other | 65 (11.7) | 141 (24.4) | 211 (18.3%) |
| Non-Hispanic White | 490 (88.3) | 438 (75.6) | 940 (81.7%) |
| Smoking status** | |||
| Current/Former | 342 (61.6) | 464 (80.1) | 821 (71.3%) |
| Never | 213 (38.4) | 115 (19.9) | 330 (28.7%) |
| Annual household income** | |||
| <$25,000 | 75 (14.0) | 185 (32.6) | 265 (23.7%) |
| $25,000-74,900 | 182 (34.0) | 250 (44.1) | 439 (39.2%) |
| ≥$75,000 | 278 (52.0) | 132 (23.3) | 415 (37.1%) |
| Age** | 55.7 (9.1) | 59.5 (12.0) | 57.7 (10.8) |
| Overall Death Event** | |||
| No | 447 (80.5) | 415 (71.7) | 870 (75.6%) |
| Yes | 108 (19.5) | 164 (28.3) | 281 (24.4%) |
| Disease-specific Death Event* | |||
| No | 489 (88.1) | 484 (83.6) | 984 (85.5%) |
| Yes | 66 (11.9) | 95 (16.4) | 167 (14.5%) |
| Cancer Recurrence Event | |||
| No | 463 (83.4) | 457 (78.9) | 933 (81.1%) |
| Yes | 92 (16.6) | 122 (21.1) | 218 (18.9%) |
| Second Primary Malignancy Event | |||
| No | 517 (93.2) | 534 (92.2) | 1054 (91.6%) |
| Yes | 38 (6.8) | 45 (7.8) | 97 (8.4%) |
Note: p< 0.05
p< 0.001: M=mean, SD=standard deviation.
Main Analyses
The median follow-up time for patients alive at last contact was 30.7 months. There were 281 incidents of death in overall survival analyses, 167 incidents of death in disease-specific analyses, 218 incidents of recurrence in disease-free survival analyses, and 97 incidents of second primary malignancy development. Unadjusted Cox proportional hazards models indicated significant differences between two neighborhood deprivation groups for each outcome examined (ps=0.001, 0.009, 0.088, and 0.002, respectively). However, this pattern of results changed in adjusted Cox proportional hazard models, which failed to reveal any significant differences by neighborhood deprivation on the outcomes of interest (ps=0.899, 0.970, 0.524, and 0.159, respectively).
Moderator Analyses
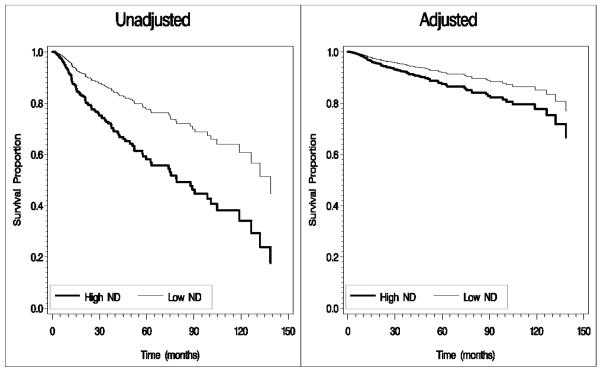
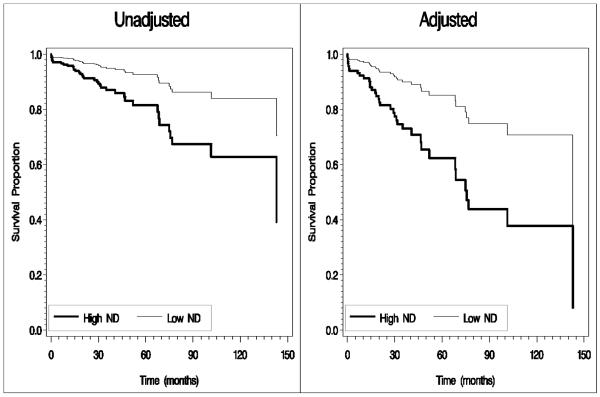
Because we were interested in the influence of cancer site on the association between neighborhood deprivation and survival/recurrence, an interaction term between neighborhood deprivation and cancer site was added to the adjusted Cox proportional hazards models. Results indicated a significant interaction term for all outcomes (ps=0.001, 0.011, 0.034, and 0.006, respectively).With the reference groups of low/moderate deprivation and oropharyngeal cancer, hazard ratios (HRs) and 95% confidence intervals for the interaction terms were as follows: overall survival = 2.91 (1.63-5.17), disease specific survival = 2.55 (1.24-5.27), disease-free survival = 1.95 (1.05-3.62), and second primary malignancy development = 3.62 (1.44-9.09). The median follow-up time for oropharyngeal patients alive at last contact was 32.8 months, and the median follow-up time for non-oropharyngeal patients alive at last contact was 27.9 months. The number of overall deaths, disease-specific deaths, recurrences, and second primary malignancy events were higher among non-oropharyngeal than oropharyngeal cancer patients, with the largest difference emerging for overall deaths (28.3% non-oropharyngeal group versus 19.5% oropharyngeal group). Therefore, follow-up analyses were conducted for each site separately. Result of unadjusted analyses indicated significant effects of neighborhood deprivation on all outcomes among oropharyngeal cancer patients (ps=0.001, 0.005, 0.040, and 0.003), but not the non-oropharyngeal group (ps=0.885, 0.706, 0.995, and 0.860). Results of adjusted analyses, however, indicated significant effects of neighborhood deprivation on overall survival and second primary malignancy among oropharyngeal cancer patients (Table 2), but not the non-oropharyngeal group (ps = 0.413 and 0.504; results not shown). Figure 1 displays the unadjusted and adjusted survival curves for overall survival, and Figure 2 displays the unadjusted and adjusted survival curves for second primary malignancy among oropharyngeal cancer patients. Results indicated that oropharyngeal cancer patients from the most deprived neighborhoods had a significantly greater risk of overall death and second primary malignancy development than oropharyngeal cancer patients from less deprived neighborhoods.
Table 2.
Effect of Neighborhood Deprivation on Survival and Second Primary Malignancy Outcomes among Oropharyngeal Cancer Patients in Adjusted Analyses
| Overall Survival |
Disease-specific Survival |
Disease-free Survival |
Second Primary Malignancy |
|
|---|---|---|---|---|
| Variables Included in Each Model | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Neighborhood Deprivation | ||||
| (Ref=Low/Moderate) | 1.59 (1.00-2.53)* | 1.38 (0.78-2.46) | 1.04 (0.63-1.74) | 2.99 (1.46-6.11)* |
| Cancer Stage | ||||
| (Ref=III/IV) | 0.55 (0.22-1.37) | 0.77 (0.22-2.74) | 1.18 (0.49-2.80) | 1.49 (0.51-4.38) |
| Cancer Treatment | ||||
| (Ref=Multiple Modalities) | 0.53 (0.34-0.85)* | 0.29 (0.14-0.62)** | 0.44 (0.26-0.77)* | 0.74 (0.36-1.50) |
| Sex | ||||
| (Ref=Male) | 0.95 (0.51-1.75) | 0.88 (0.42-1.87) | 0.94 (0.50-1.78) | 1.36 (0.52-3.56) |
| Race/Ethnicity | ||||
| (Ref=Non-Hispanic White) | 2.36 (1.47-3.79)** | 2.82 (1.62-4.90)** | 2.90 (1.73-4.87)** | 0.99 (0.33-3.01) |
| Smoking Status | ||||
| (Ref=Never Smoker) | 2.61 (1.53-4.43)** | 1.89 (1.03-3.48)* | 1.58 (0.96-2.59) | 1.78 (0.78-4.06) |
| Annual household income | ||||
| <$25,000 | 2.08 (1.19-3.64)* | 1.73 (0.85-3.53) | 1.22 (0.65-2.32) | 0.60 (0.19-1.86) |
| $25,000-74,900 | 1.06 (0.65-1.73) | 0.91 (0.50-1.67) | 0.90 (0.54-1.48) | 1.31 (0.64-2.69) |
| (Ref=≥$75,000) | ||||
| Age | 1.04 (1.02-1.06)** | 1.04 (1.01-1.07)* | 1.05 (1.02-1.08)** | 1.05 (1.01-1.08)* |
Note: “Ref” is reference group.
p< 0.05
p< 0.001: HR=hazard ratio.
Figure 1.
Survival curves for overall survival by neighborhood deprivation among oropharyngeal cancer patients (High ND = High Neighborhood Deprivation, Low ND = Low to Moderate Neighborhood Deprivation).
Note: Adjusted analyses controlled for the following: age, sex, race/ethnicity, income, smoking status, cancer site, cancer stage, and treatment approach. Age was treated as a binary variable (< 55 years and >55 years) in adjusted curve calculation due to macro specifications.
Figure 2.
Survival curves for second primary malignancy development by neighborhood deprivation among oropharyngeal cancer patients (High ND = High Neighborhood Deprivation, Low ND = Low to Moderate Neighborhood Deprivation)
Note: Adjusted analyses controlled for the following: age, sex, race/ethnicity, income, smoking status, cancer site, cancer stage, and treatment approach. Age was treated as a binary variable (< 55 years and >55 years) in adjusted curve calculation due to macro specifications.
Discussion
To our knowledge, this was the first study to examine the unique effect of neighborhood deprivation on SCCHN recurrence and survival among patients evaluated and treated at a single, large multidisciplinary cancer center. Although results failed to support a main effect of neighborhood deprivation on SCCHN outcomes among the sample as a whole, a significant interaction effect was observed. Specifically, stratified results indicated that among oropharyngeal cancer patients, a high level of neighborhood deprivation was associated with poorer overall survival and the development of second primary malignancies, even after controlling for a number of patient-level sociodemographic and clinical variables, including individual-level annual household income. Thus, results suggest that oropharyngeal cancer patients residing in deprived neighborhoods, marked by low levels of education and income and high levels of unemployment, single-parent households, and vehicle non-availability, may be at increased risk of some negative SCCHN outcomes relative to oropharyngeal cancer patients from neighborhoods with more socioeconomic capital, even when treated at the same hospital for their incident cancer. These findings add to a growing literature supporting relations between area-level SES and survival from other cancers [e.g., colon cancer (Gorey et al., 2011), colorectal cancer (Le, Ziogas, Lipkin, & Zell, 2008)]. It also contributes to the literature linking low SES with lower survival among head and neck cancer patients in the US [e.g., (Arbes et al., 1999; Chu et al., 2011; Molina et al., 2008)]. However, the current study significantly adds to that literature by highlighting the unique role of neighborhood deprivation on clinical outcomes independent of individual-level SES. Clinicians treating oropharyngeal cancer patients may want to understand more about the places their patients live, as those living in deprived areas may be at increased risk for worse clinical outcomes regardless of personal income.
Among this sample of oropharyngeal cancer patients, who were all treated at a single cancer center using a multidisciplinary team approach, the effects of neighborhood deprivation on outcomes were limited to overall survival and second primary malignancy development. These outcomes were less related to their incident SCCHN than were disease-free or disease-specific survival outcomes. This pattern of results suggests that oropharyngeal cancer patients from economically deprived areas may be more likely to have significant comorbidities, exposures, or other risk factors that affected non-SCCHN-related overall survival or second primary malignancy development relative to oropharyngeal cancer patients from the less deprived areas. A number of previous studies have linked neighborhood deprivation to increased morbidity, mortality, and engagement in health risk behaviors among residents [e.g., (Cubbin & Winkleby, 2005; Diez-Roux et al., 1997; Diez Roux & Mair, 2010; Macintyre, Ellaway, & Cummins, 2002; Sampson, Raudenbush, & Earls, 1997)], with negative health outcomes persisting after accounting for the influence of individual-level SES (Chaix, Rosvall, & Merlo, 2007; Davidson, Bastani, Nakazono, & Carreon, 2005; Pickett & Pearl, 2001; van Lenthe et al., 2005; Wight et al., 2008).We controlled for one of these health risk behaviors in our analyses (i.e., smoking), and post-hoc analyses indicated no differences in gross alcohol use patterns (current/former weekly drinker versus never weekly drinker) among oropharyngeal cancer patients in low to moderate versus high deprivation neighborhoods in this sample. However, data on other behavioral risk factors (e.g., overweight/obesity status, dietary habits, sedentary behavior) were unknown. Recent schematic models of neighborhood effects on health and health-related behaviors highlight the importance of psychosocial and ecologic mechanisms (Diez Roux & Mair, 2010). Supporting such models are a host of theoretical and empirical studies citing associations between economically deprived neighborhoods and mechanisms that underlie health-related behaviors and health outcomes, including less social support (Black, Cook, Murry, & Cutrona, 2005; Young, Russell, & Powers, 2004), greater negative affect/depression (Cutrona et al., 2005; Cutrona, Wallace, & Wesner, 2006; Echeverria, Diez-Roux, Shea, Borrell, & Jackson, 2008; Hill & Angel, 2005; Mair et al., 2009; Wen, Hawkley, & Cacioppo, 2006), more stress (Burdette & Hill, 2008; Cutrona et al., 2006; Hill & Angel, 2005; Wen et al., 2006; Young et al., 2004), and less social capital and collective efficacy (Kawachi & Berkman, 2003; Kawachi & Berkman, 2000; Kawachi, Kennedy, & Glass, 1999). Of course, systematic differences between oropharyngeal and non-oropharyngeal cancer patients on some of these mechanisms (engagement in health risk behaviors, for example) might have contributed to null results for the non-oropharyngeal group and led to a lack of main effects for the sample as a whole. More studies are needed to better understand the mechanisms that underlie the associations between neighborhood deprivation and SCCHN outcomes among oropharyngeal cancer patients.
Previous research has suggested that the survival disparity experienced by cancer patients of lower SES is largely attributable to diagnosis at later stages of cancer and lower access to quality treatment (Byers et al., 2008; Woods, Rachet, & Coleman, 2006). However, the current study controlled for disease stage and included patients with known access to a quality treatment resource, and still found significant relations between neighborhood-level SES and clinical outcomes (albeit only among the oropharyngeal patents). Therefore, these usual suspects (i.e., later detection and lower access) may only partially explain relations between socioeconomic indicators and cancer survival outcomes for oropharyngeal SCCHN patients. In fact, at least one prior study suggested that SES was not associated with treatment stage at presentation among head and neck cancer patients (S. Johnson, Corsten, McDonald, & Chun, 2010). Therefore, it behooves clinicians and stakeholders concerned with survival disparities to better understand the role that neighborhood deprivation plays in the health status of oropharyngeal patients. However, it is important to acknowledge that the strength of relations between neighborhood deprivation and clinical outcomes may vary based on geographic area. For example, previous research has cited a Canadian advantage in cancer survival outcomes for patients from economically deprived neighborhoods relative to some US cities and states, even when controlling for cancer stage (Gorey et al., 2000a, 2000b; Gorey et al., 2011). In those cases, the geographic advantage may have to do with differences in insurance coverage, provider availability, and preventive care between the countries, but it is certainly also possible to find these variations within a single country or region of the country for reasons other than those cited. For example, distance to quality treatment resources may also affect health services utilization and cancer survival outcomes (Lamont et al., 2003). To address this possibility in our data, we conducted post-hoc analyses in which we additionally controlled for distance in miles along the road network from the patient’s residence to The University of Texas MD Anderson Cancer Center, and our pattern of results was unchanged. Nevertheless, issues such as delay in diagnosis, access to care, and geographically-varying relationships continue to be important considerations for future research linking area-level characteristics to cancer outcomes.
Our results suggest that different strategies may be necessary to improve outcomes among oropharyngeal versus non-oropharyngeal cancer patients, and that attention to interventions directed at the neighborhood-level would be most relevant among the former group. The current study is an important preliminary step to better understanding the role of neighborhood SES on SCCHN outcomes, but additional research in this area is needed to inform such interventions. However, results may have some immediate relevance for clinical practice. For example, clinicians treating oropharyngeal SCCHN patients should be aware that neighborhood deprivation has the potential to affect clinical outcomes independent of patients’ personal income levels, and patients from deprived areas may face additional challenges to their health following the SCCHN treatment protocol. Thus, clinicians might want to identify comorbidities that are likely to contribute to mortality and secondary primary malignancy development, and facilitate appropriate referrals for these conditions in conjunction with and following incident oropharyngeal SCCHN cancer treatment. Clinicians might also stress the importance of regular cancer screenings and work with institutional social workers or other personnel to identify free or low cost and accessible screening and treatment resources in the patient’s local community. Information on neighborhood deprivation can be accessed with a patient’s address via publically available national and local resources (e.g., American FactFinder), and these data might be used to identify at-risk patients.
The inability to account for the influence of oncogenic HPV status is a major limitation of this study, as HPV status was assessed in only a minority of patients in this sample. Oncogenic HPV is most strongly associated with the development and prognosis of oropharyngeal cancers, and it has been suggested that HPV-positive oropharyngeal cancer is a “clinically, biologically, and epidemiologically distinct disease” from other SCCHN types (Schrank et al., 2011; Sturgis & Ang, 2011). Specifically, patients with HPV-positive oropharyngeal cancers tend to present at a later stage of the disease, are less likely to be smokers, and have a better treatment response to and survival than HPV-negative oropharyngeal cancer patients (Schrank et al., 2011; Sturgis & Ang, 2011). Estimates suggest that approximately 50-70% of oropharyngeal cancers are HPV-positive (Sturgis & Ang, 2011). Of the oropharyngeal cancer patients in our analyses with known HPV status (n=190), 76% had HPV-positive tumors. However, there were no significant differences in the proportion of HPV-positive patients in the high versus the low to moderate neighborhood deprivation groups [x2 =.728 (1), p=.399]. Also, although we were unable to control for the HPV-status in the current study, smoking status (a covariate in our analyses) is known to be a strong proxy for HPV-related cancers.
Other limitations of the current study include the use of Census data gathered at a single point in time, despite a rolling enrollment period for patients. Future studies in this area can make use of American Community Survey data, which would allow for a better temporal matching of area-level to patient-level data. Participants in the present study were residing in Texas and Louisiana at the time they were assessed and treated at a large, multidisciplinary cancer center in the southwest. Thus, our results may not be generalized to untreated patients, patients from other areas of the country, or patients treated at other facilities. Also, selection and referral biases may exist. We estimate that approximately 90% of incident SCCHN patients at MD Anderson Cancer Center were recruited and agreed to participate in this study; unfortunately, information about those who refused to participate was not collected. Thus, systematic differences between participants and non-participants are unknown.
Future studies in this area should include more information about patients’ engagement in health risk behaviors (e.g., detailed alcohol use patterns, overweight/obesity status, dietary habits, and sedentary behavior) in order to better understand their association with neighborhood effects on clinical outcomes. Our independent variable was comprised of five Census variables that were selected based on a priori ideas about important and complementary components of neighborhood deprivation (e.g., education, employment status, poverty) rather than in response to empirical estimation [i.e., principal components analysis (Messer et al., 2006)]. In addition, our neighborhood deprivation index was based only on Census data from the tracts in which our patients resided. A comparison of these tracts to all tracts in Texas and Louisiana suggests that, on average, our patients resided in areas with a larger population size and less deprivation across all five Census variables included in the index. Thus, our methodology for developing the neighborhood deprivation index has some limitations that might affect the generalizability of results. Relatedly, the extent to which other area-level variables, such as racial/ethnic segregation, might contribute to our results was not assessed. Finally, although our analyses had adjusted for several potential confounders, the presence of unknown and unmeasured confounders might have influenced the results.
In conclusion, this study was the first to link neighborhood SES to overall survival and second primary malignancy development among oropharyngeal cancer patients treated at a multidisciplinary cancer center, independent of individual-level SES and other sociodemographic and clinical variables. Results are in need of replication, but the unique influence of the neighborhood context in survival outcomes among oropharyngeal cancer patients suggests directions for future research, which can better inform clinical practice and intervention development.
Highlights.
 Neighborhood economic deprivation may have a unique effect on cancer outcomes.
Neighborhood economic deprivation may have a unique effect on cancer outcomes. We examine this among head and neck cancer patients from a large treatment center.
We examine this among head and neck cancer patients from a large treatment center. Neighborhood deprivation predicted poorer survival among oropharyngeal patients.
Neighborhood deprivation predicted poorer survival among oropharyngeal patients. It also predicted more secondary malignancies among oropharyngeal patients.
It also predicted more secondary malignancies among oropharyngeal patients. Oropharynx cancer patients from deprived areas may need additional interventions.
Oropharynx cancer patients from deprived areas may need additional interventions.
Acknowledgements
We are grateful to the contributions of (omitted for blind review), who performed the geocoding and procured the US Census data for this project. We also greatly appreciate the guidance of (name omitted for blind review) and the assistance of (name omitted for blind review) in addressing concerns raised by the anonymous reviewers of this manuscript.
Grant Support This manuscript was supported by the National Institutes of Health through The University of Texas MD Anderson’s Cancer Center Support Grant (CA016672), the National Cancer Institute (R03CA128110 to xxx, K07CA133099 and R03CA135679 to xxx, and R01CA131274 to xxx), the National Institute of Environmental Health Sciences (R01ES011740 to xxx), and The University of Texas MD Anderson Cancer Center start-up funds (xxx). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or The University of Texas MD Anderson Cancer Center.
Financial Support: This manuscript was supported by the National Institutes of Health through The University of Texas MD Anderson’s Cancer Center Support Grant (CA016672), the National Cancer Institute (R03CA128110 to EM Sturgis, K07CA133099 and R03CA135679 to G Li, and R01CA131274 to Q Wei), the National Institute of Environmental Health Sciences (R01ES011740 to Q Wei), and The University of Texas MD Anderson Cancer Center start-up funds (LR Reitzel). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or The University of Texas MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors do not have any competing interests directly pertaining to this work.
References
- American Cancer Society (ACS) Cancer Facts & Figures. American Cancer Society; Atlanta: 2011. [Google Scholar]
- Arbes SJ, Jr., Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States) Cancer Causes Control. 1999;10(6):513–523. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- Black AR, Cook JL, Murry VM, Cutrona CE. Ties that bind: implications of social support for rural, partnered African American women’s health functioning. Womens Health Issues. 2005;15(5):216–223. doi: 10.1016/j.whi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–3287. [PubMed] [Google Scholar]
- Bonanno A, Esmaeli B, Fingeret MC, Nelson DV, Weber RS. Social challenges of cancer patients with orbitofacial disfigurement. Ophthal Plast Reconstr Surg. 2010;26(1):18–22. doi: 10.1097/IOP.0b013e3181b8e646. [DOI] [PubMed] [Google Scholar]
- Buckwalter AE, Karnell LH, Smith RB, Christensen AJ, Funk GF. Patient-reported factors associated with discontinuing employment following head and neck cancer treatment. Arch Otolaryngol Head Neck Surg. 2007;133(5):464–470. doi: 10.1001/archotol.133.5.464. [DOI] [PubMed] [Google Scholar]
- Burdette AM, Hill TD. An examination of processes linking perceived neighborhood disorder and obesity. Soc Sci Med. 2008;67(1):38–46. doi: 10.1016/j.socscimed.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113(3):582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- Chaix B, Rosvall M, Merlo J. Assessment of the magnitude of geographical variations and socioeconomic contextual effects on ischaemic heart disease mortality: a multilevel survival analysis of a large Swedish cohort. J Epidemiol Community Health. 2007;61(4):349–355. doi: 10.1136/jech.2006.047597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- Chu KP, Shema S, Wu S, Gomez SL, Chang ET, Le QT. Head and neck cancer-specific survival based on socioeconomic status in Asians and Pacific Islanders. Cancer. 2011;117(9):1935–1945. doi: 10.1002/cncr.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubbin C, Winkleby MA. Protective and harmful effects of neighborhood-level deprivation on individual-level health knowledge, behavior changes, and risk of coronary heart disease. Am J Epidemiol. 2005;162(6):559–568. doi: 10.1093/aje/kwi250. [DOI] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW, Brown PA, Clark LA, Hessling RM, Gardner KA. Neighborhood context, personality, and stressful life events as predictors of depression among African American women. J Abnorm Psychol. 2005;114(1):3–15. doi: 10.1037/0021-843X.114.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE, Wallace G, Wesner KA. Neighborhood Characteristics and Depression: An Examination of Stress Processes. Curr Dir Psychol Sci. 2006;15(4):188–192. doi: 10.1111/j.1467-8721.2006.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PL, Bastani R, Nakazono TT, Carreon DC. Role of community risk factors and resources on breast carcinoma stage at diagnosis. Cancer. 2005;103(5):922–930. doi: 10.1002/cncr.20852. [DOI] [PubMed] [Google Scholar]
- Diaz EM, Holsinger FC, Zuniga ER, Roberts DB, Sorensen DM. Squamous cell carcinoma of the buccal mucosa: One institution’s experience with 119 previously untreated patients. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck. 2003;25(4):267–273. doi: 10.1002/hed.10221. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146(1):48–63. doi: 10.1093/oxfordjournals.aje.a009191. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Echeverria S, Diez-Roux AV, Shea S, Borrell LN, Jackson S. Associations of neighborhood problems and neighborhood social cohesion with mental health and health behaviors: The Multi-ethnic Study of Atheroschlerosis. Health & Place. 2008;14:853–865. doi: 10.1016/j.healthplace.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286(12):1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- Givens DJ, Karnell LH, Gupta AK, Clamon GH, Pagedar NA, Chang KE, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(12):1209–1217. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- Goldstein DP, Karnell L. Hynds, Christensen AJ, Funk GF. Health-related quality of life profiles based on survivorship status for head and neck cancer patients. Head Neck. 2007;29(3):221–229. doi: 10.1002/hed.20507. [DOI] [PubMed] [Google Scholar]
- Gorey KM, Holowaty EJ, Fehringer G, Laukkanen E, Richter NL, Meyer CM. An international comparison of cancer survival: metropolitan Toronto, Ontario, and Honolulu, Hawaii. Am J Public Health. 2000a;90(12):1866–1872. doi: 10.2105/ajph.90.12.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorey KM, Holowaty EJ, Fehringer G, Laukkanen E, Richter NL, Meyer CM. An international comparison of cancer survival: relatively poor areas of Toronto, Ontario and three US metropolitan areas. J Public Health Med. 2000b;22(3):343–348. doi: 10.1093/pubmed/22.3.343. [DOI] [PubMed] [Google Scholar]
- Gorey KM, Luginaah IN, Bartfay E, Fung KY, Holowaty EJ, Wright FC, et al. Effects of socioeconomic status on colon cancer treatment accessibility and survival in Toronto, Ontario, and San Francisco, California, 1996-2006. Am J Public Health. 2011;101(1):112–119. doi: 10.2105/AJPH.2009.173112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TD, Angel RJ. Neighborhood disorder, psychological distress, and heavy drinking. Soc Sci Med. 2005;61(5):965–975. doi: 10.1016/j.socscimed.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Johnson S, Corsten MJ, McDonald JT, Chun J. Socio-economic factors and stage at presentation of head and neck cancer patients in Ottawa, Canada: A logictic regression analysis. Oral Oncology. 2010;46:366–368. doi: 10.1016/j.oraloncology.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Johnson S, McDonald JT, Corsten MJ. Socioeconomic Factors in Head and Neck Cancer. Journal of Otolaryngology-Head & Neck Surgery. 2008;37(4):597–601. [PubMed] [Google Scholar]
- Jones AP, van Sluijs EM, Ness AR, Haynes R, Riddoch CJ. Physical activity in children: Does how we define neighborhood matter? Health & Place. 2010;16:236–241. doi: 10.1016/j.healthplace.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Berkman L. Neighborhoods and health. Oxford University Press; New York: 2003. [Google Scholar]
- Kawachi I, Berkman LF. Social cohesion, social capital, and health. In: Berkman LF, Kawachi I, editors. Social Epidemiology. Oxford University Press; New York: 2000. pp. 174–190. [Google Scholar]
- Kawachi I, Kennedy BP, Glass R. Social capital and self-rated health: a contextual analysis. Am J Public Health. 1999;89(8):1187–1193. doi: 10.2105/ajph.89.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont EB, Hayreh D, Pickett KE, Dignam JJ, List MA, Stenson KM, et al. Is patient travel distance associated with survival on phase II clinical trials in oncology? J Natl Cancer Inst. 2003;95(18):1370–1375. doi: 10.1093/jnci/djg035. [DOI] [PubMed] [Google Scholar]
- Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL. Current clinical outcomes demand new treatment options for SCCHN. Annals of Oncology. 2005;16(Suppl 6):vi7–vi12. doi: 10.1093/annonc/mdi452. [DOI] [PubMed] [Google Scholar]
- Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, Biorklund A, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck - A population-based case-referent study in Sweden. Cancer. 1998;82(7):1367–1375. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55(1):125–139. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Mair C, Diez Roux A, Shen M, Shea S, Seeman T, Echeverria S, et al. Cross-sectional and longitudinal associations of neighborhood cohesion and stressors with depressive symptoms in the Multiethnic Study of Atherosclerosis. Ann Epidemiol. 2009;19:49–57. doi: 10.1016/j.annepidem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina MA, Cheung MC, Perez EA, Byrne MM, Franceschi D, Moffat FL, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113(10):2797–2806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- Mowry SE, Tang C, Sadeghi A, Wang MB. Standard chemoradiation versus intensity-modulated chemoradiation: a quality of life assessment in oropharyngeal cancer patients. Eur Arch Otorhinolaryngol. 2010;267(7):1111–1116. doi: 10.1007/s00405-009-1183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) A Snapshot of Head and Neck and Thyroid Cancers. 2011 http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/snapshots.
- Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytynia KB, Grant JR, Etzel CJ, Roberts DB, Wei Q, Sturgis EM. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22(19):3981–3988. doi: 10.1200/JCO.2004.02.133. [DOI] [PubMed] [Google Scholar]
- Ross NA, Tremblay SS, Graham K. Neighbourhood influences on health in Montreal, Canada. Soc Sci Med. 2004;59(7):1485–1494. doi: 10.1016/j.socscimed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Schrank TP, Han Y, Weiss H, Resto VA. Case-matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States--possible role for human papillomavirus in survival disparities. Head Neck. 2011;33(1):45–53. doi: 10.1002/hed.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford M, Duke-Williams O, Shelton N. Small area inequalities in health: are we underestimating them? Soc Sci Med. 2008;67(6):891–899. doi: 10.1016/j.socscimed.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Sturgis E, Ang K. The Epidemic of HPV-Associated Oropharyngeal Cancer is Here-Is It Time to Change Our Treatment Paradigms? Journal of the National Comprehensive Cancer Network. 2011;9:665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- Terrell JE, Fisher SG, Wolf GT. Long-term quality of life after treatment of laryngeal cancer. The Veterans Affairs Laryngeal Cancer Study Group. Arch Otolaryngol Head Neck Surg. 1998;124(9):964–971. doi: 10.1001/archotol.124.9.964. [DOI] [PubMed] [Google Scholar]
- Teymoortash A, Hoch S, Eivazi B, Werner JA. Postoperative morbidity after different types of selective neck dissection. Laryngoscope. 2010;120(5):924–929. doi: 10.1002/lary.20894. [DOI] [PubMed] [Google Scholar]
- van Lenthe FJ, Borrell LN, Costa G, Diez Roux AV, Kauppinen TM, Marinacci C, et al. Neighbourhood unemployment and all cause mortality: a comparison of six countries. J Epidemiol Community Health. 2005;59(3):231–237. doi: 10.1136/jech.2004.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Hawkley LC, Cacioppo JT. Objective and perceived neighborhood environment, individual SES and psychosocial factors, and self-rated health: an analysis of older adults in Cook County, Illinois. Soc Sci Med. 2006;63(10):2575–2590. doi: 10.1016/j.socscimed.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Wight RG, Cummings JR, Miller-Martinez D, Karlamangla AS, Seeman TE, Aneshensel CS. A multilevel analysis of urban neighborhood socioeconomic disadvantage and health in late life. Soc Sci Med. 2008;66(4):862–872. doi: 10.1016/j.socscimed.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby M, Cubbin C, Ahn D. Effect of cross-level interaction between individual and neighborhood socioeconomic status on adult mortality rates. Am J Public Health. 2006;96(12):2145–2153. doi: 10.2105/AJPH.2004.060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Annals of Oncology. 2006;17(1):5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- Yao M, Karnell LH, Funk GF, Lu H, Dornfeld K, Buatti JM. Health-related quality-of-life outcomes following IMRT versus conventional radiotherapy for oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2007;69(5):1354–1360. doi: 10.1016/j.ijrobp.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ying G-S, Liu C. Statistical analysis of clustered data using SAS system. 19th Annual Northeast SAS User Group Conference; Philadelphia, PA. 2006. [Google Scholar]
- Young AF, Russell A, Powers JR. The sense of belonging to a neighborhood: Can it be measured and is it related to health and well-being in older women? Social Science & Medicine. 2004;59:2627–2637. doi: 10.1016/j.socscimed.2004.05.001. [DOI] [PubMed] [Google Scholar]