Abstract
Concussion (mild traumatic brain injury (mTBI)) is a significant pediatric public health concern. Despite increased awareness, a comprehensive understanding of the acute and chronic effects of concussion on central nervous system structure and function remains incomplete. Here we review the definition, epidemiology, and sequelae of concussion within the developing brain, during childhood and adolescence, with current data derived from studies of pathophysiology and neuroimaging. These findings may contribute to a better understanding of the neurological consequences of traumatic brain injuries, which in turn, may lead to the development of brain biomarkers to improve identification, management and prognosis of pediatric patients suffering from concussion.
Keywords: Children, TBI, concussion, adolescents, brain, cortex, cognitive, hypothalamus, autonomic, brain imaging, fMRI
1. Introduction
No single definition of mild traumatic brain injury (mTBI) contains the entire spectrum of diagnostic considerations involved in defining mTBI partly because there is no consensus of objective criteria. Here we will use the definition conceptualized in 2009 (McCrory et al., 2009): “Concussion is defined as a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces. Several common features that incorporate clinical, pathologic and biomechanical injury constructs that may be utilized in defining the nature of a concussive head injury include: (1) Concussion may be caused either by a direct blow to the head, face, neck or elsewhere on the body with an “impulsive” force transmitted to the head. (2) Concussion typically results in the rapid onset of short-lived impairment of neurologic function that resolves spontaneously. (3) Concussion may result in neuropathological changes but the acute clinical symptoms largely reflect a functional disturbance rather than a structural injury; (4) Concussion results in a graded set of clinical symptoms that may or may not involve loss of consciousness. Resolution of the clinical and cognitive symptoms typically follows a sequential course, however, it is important to note that in a small percentage of cases, post-concussion symptoms may be prolonged; (5) No abnormality on standard structural neuroimaging studies is seen in concussion”. In children, the issue includes mostly sports and recreational activity related injuries, shaken baby syndrome and motor vehicle accidents with flexion-extension injuries. As noted below (see Section 5) more recent imaging studies are changing the definition of mTBI and the constructs on which it is based, as we begin to understand that early measures of change in brain function and structure, including white matter integrity, are now available (e.g., see (Henry et al., 2011; Sharp and Ham, 2011) and below), and that resolution of clinical and subclinical neuropsychiatric impairment does not always follow a typical sequential course of recovery. Alterations may indeed be permanent (De Beaumont et al., 2009; Nicholl and LaFrance, 2009; Ponsford et al., 2011), although this risk still not well understood (McCrory, 2011).
Symptoms following a concussion are highly variable and include mainly somatic, vestibular, affective, and vegetative features (Figure 1). The symptoms may evolve over the hours to days after a concussion; therefore, the severity of concussion cannot be determined at the time of injury. Attitudes to concussion have changed dramatically in past years, and parallel with our increased awareness and understanding of the condition (Kirkwood et al., 2006). Recently the need for new research, guidelines, and treatment of this condition have reached the highest levels of media, health and government institutions (CDC, 2010; NINDS, 2012a, b; Schwarz, 2010). Current studies in concussion include the investigation of underlying neurobiological mechanisms that affect brain function and structure. Even subtle changes in neuronal function may be associated with persistent and profound clinical disability, cognitive impairment, and behavioral alterations (Kirkwood et al., 2006; Konrad et al., 2010; McAllister and Stein, 2010; Sroufe et al., 2010).
Figure 1.
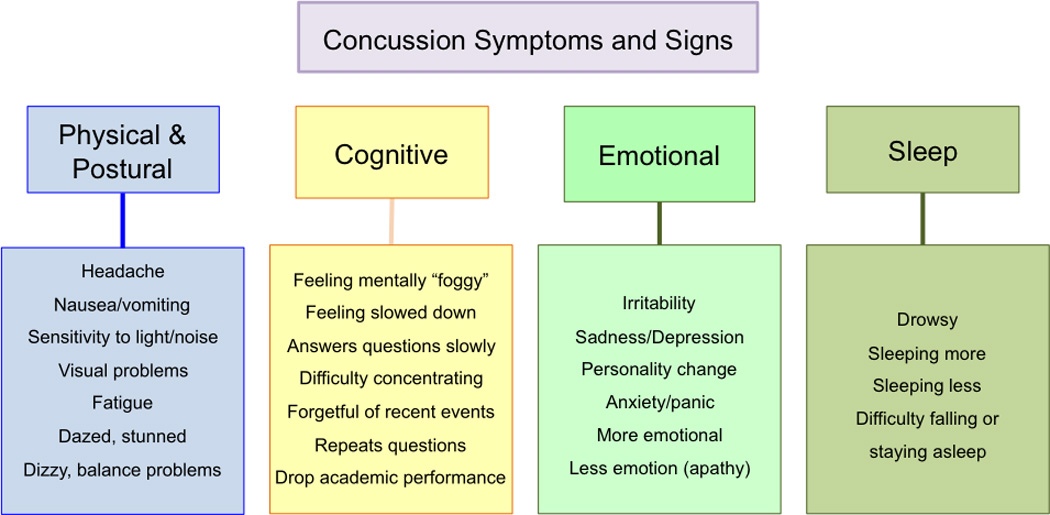
Post-concussive signs and symptoms. Physical, cognitive, emotional and sleep signs and symptoms potentially present after sustaining a concussion.
The pediatric population is of particular interest since the brain is still developing (Konrad et al., 2010), with potential for more rapid changes in reorganization following trauma than the adult brain. In addition, the consequences of mTBI may have crucial impact on lives of children as they may experience behavioral changes of miss a lot of school days or activities that may impact their normal intellectual and social development. As summarized in Figure 2, there are aspects of brain changes that may confer different levels of disease burden in individuals suffering from concussion. The diagnosis and choice of appropriate and efficacious treatment approach remains a significant challenge, there are no reliable, valid, and cost-effective objective measures to evaluate concussion severity, the potential for long-term neurological impairment, the point at which brain recovery has actually occurred, or whether and when it is actually safe for the individual to return to activity. Unfortunately, even simple and accepted recommendations such as a symptom-free waiting period are not enforced and may result in premature return-to-activity decisions, while our treatments are symptomatic and not disease modifying. Therefore long-term effects (symptomatic or non-symptomatic) that are currently not well defined may be permanent and possibly contribute to the development of neuropsychiatric disorders later in life (De Beaumont et al., 2009; Nicholl and LaFrance, 2009; Ponsford et al., 2011), although this risk still not well understood (McCrory, 2011).
Figure 2.
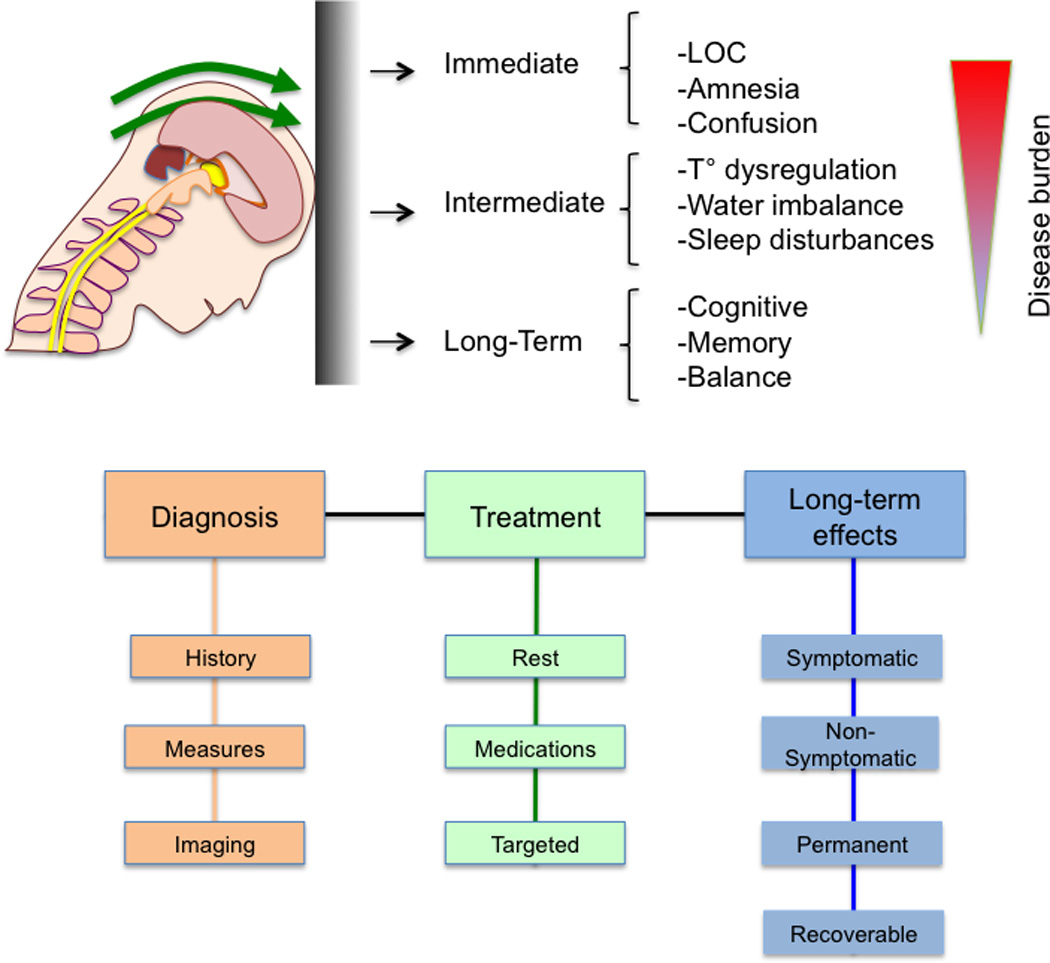
Temporal Consequences of Concussion. The figure shows the current understanding of the implications of mTBI and its chronologic burden.
1.1. The Developing Brain
The pediatric brain differs during development compared with the mature adult (reached at around 21 years) (Paus, 2010; Perrin et al., 2008; Schmithorst and Yuan, 2010; Thompson et al., 2005b). Neuronal systems develop at different rates during childhood and brain changes are dramatic with significant pruning of many connections or networks (Giedd and Rapoport, 2010). Initially those areas involved in primary senses and motor skills are developed by age 4 and areas for language develop through age 10, with further development of specific areas involved in complex thinking (e.g., parietal lobes) and fine motor skills. However areas involved in abstract processes, reasoning, judgment, and emotion, including impulsivity, which are controlled principally by frontal areas, remain less developed through the teenage years and into the early 20’s. The development of subcortical systems, such as the hypothalamus, is even less well understood. Patient age and associated brain maturation at the time of mTBI may influence prognosis. Specific neuronal systems (frontal vs. parietal) are generally developed at specific ages, may be variably adaptive or resilient post-concussion, and may be modulated by gender and pubertal changes (Lenroot and Giedd, 2010).
1.2. Epidemiology of mTBI in Childhood
In the past ten years there has been a marked increase in awareness of concussion among physicians and clinical researchers, evidenced by the increasing number of emergency department visits related to concussion and by the number of published studies now available in this field. The estimated incidence of concussions in young children is reported to be 304 cases per 100,000 child-years (Koepsell et al., 2011). In this study, incidence was highest in preschoolers and lowest in 5–9 years; most were mild in nature and boys suffered at a higher rate than girls. For ages 0–19 years, 173,285 individuals are treated annually in the US for non-fatal concussion related to sports, most mild in origin, and the numbers increase if motor vehicle accidents, prevalent in ages 15–19, are added to the statistics (CDC, 2010; Konrad et al., 2010). High school sports-related concussion involves approximately 135,901 students in a school year among all sports, most of them related to football, hockey, and soccer (Gessel et al., 2007). Rates seem to be on the increase. An estimated 502,000 emergency department visits for concussion have been reported annually in the US (Bakhos et al., 2010), an increase of 200% in ten years (1997–2007). Furthermore, recent evidence demonstrates that up to 50% of high school football players experiencing symptoms of concussion do not report them (McCrea et al., 2004). These provocative statistics have made concussion a significant and urgent public health issue. There is still a need for reliable statistics in younger than high school-age populations as well as better study, documentation, and analysis of post-concussion sequelae.
In this review of mTBI in pediatric patients, discussion will focus on: underlying pathophysiology of concussion in the section Pathophysiology mTBI that underscores changes in brain systems that may provide a better understanding for evaluating brain function; in the section on Brain Regions Affected by Concussion - Basis for Behavioral Changes we discuss specific brain regions that may be affected in concussion and observed behaviors related to these regional effects; a brief overview of Clinical Management, suggesting that the lack of objective markers of brain dysfunction may limit our ability to evaluate and treat these patients in an optimal manner; and that this may change as noted in the section on Measuring Brain Changes. We suggest that imaging measures may provide useful insights into immediate and long-term effects of concussion on the child’s brain (viz., diffuse axonal injury; persistence or improvement of functional deficits; and more importantly an assay of how patients are doing, since sub-clinical measures as detected by imaging may be significant regarding long term outcomes). While improvement is clear in most children who suffer from a concussion, the issue of how far along the line of complete improvement we really are, remains ill-defined.
2. Pathophysiology of mTBI
The pathophysiology of concussion has been reviewed in detail elsewhere (Len and Neary, 2011; McCrory et al., 2001). During the childhood and adolescent periods, a critical increase in gray and white matter occurs and, depending on activities or environment of the individual, selective elimination of this overproduction of synapses occurs (Giedd et al., 1999). Some of the skills critical to this time frame include motor dexterity, problem solving, memory, language, abstract thinking, and social and emotional skills (Dahl, 2004). An interruption during this critical period of learning could have significant consequences (Cernak et al., 2010). A number of important changes occur in TBI, including effects of the physical insult/trauma on the brain, increased propensity for decreased blood flow and ischemia, and the consequences of axonal injury and neuroinflammation. In 2001, Giza and Hovda suggested that following concussion there is a perturbation of brain physiology that include neuronal depolarization, release of excitatory neurotransmitters, altered cerebral blood flow, and altered axonal function and metabolic changes (e.g., glucose metabolism) (Giza and Hovda, 2001) (Figure 3A). Some of these are discussed in detail below. Such a notion has been further supported by more recent observation of magnetic resonance spectroscopy (MRS) measures of brain metabolic imbalance (Vagnozzi et al., 2010; Vagnozzi et al., 2008).
Figure 3.
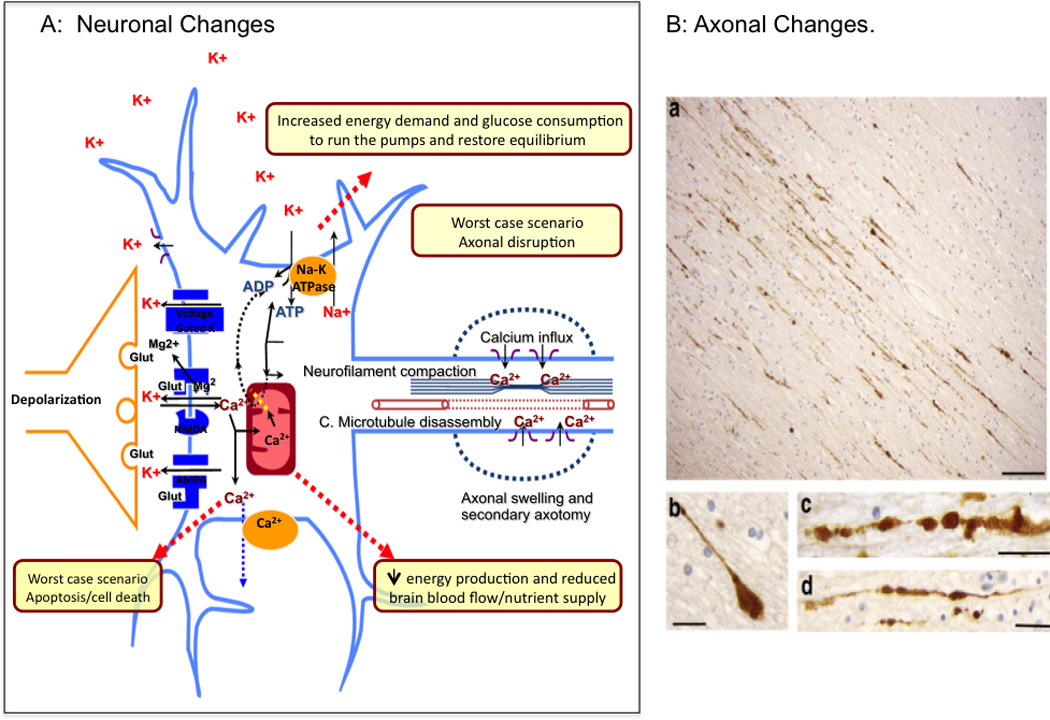
Metabolic and Anatomical Changes. A: Neuronal Changes. Schematic depiction of the process that lead to neuronal cell injury after a concussion(Adapted from (Giza and Hovda, 2001)). B: Axonal Changes. Postmortem APP immunohistochemistry of the corpus callosum after TBI showing axonal bulb formation, and points of interruption (a–d). From (Johnson et al., 2012b); permission pending.
2.1. Physics of Concussion
The biomechanics of the brain, the cerebrospinal fluid, and dural attachments to the cranial bones contribute to our understanding as to how a blow to the head may result in concussion. The density of the brain is less that of the CSF and on impact, the brain is displaced within the skull. The physics of concussion have focused on a few changes including (i) non-uniform compressive stress; (ii) brain lag and rotation (Dawson et al., 1980); (iii) coup-contre-coup impact injury; and (iv) acceleration/deceleration (Barth et al., 2001). These changes lead to complex alterations, including functional deafferentation of the cortex (Shaw, 2002) and changes in the brainstem that seems key to loss of consciousness (Browne et al., 2011). The immediate effects include neuronal swelling, sterile inflammation, axonal disruption, as well as metabolic and autonomic changes (see below). The biomechanics of adult and pediatric head injury have been modeled and scaled (Ommaya et al., 2002), but equivalent measures of TBI in adults and children are obviously difficult to determine. The biomechanics of brain injury may also be influenced by a still immature musculoskeletal system, skull geometry, suture elasticity, cerebral blood volume and level of myelination (Bauer and Fritz, 2004; Goldsmith and Plunkett, 2004; Shaw, 2002). However, the brain areas affected (see below) are directly related to the impact site and its severity.
2.2. Diffuse Axonal Injury
Diffuse axonal injury (DAI) is damage to axons as a result of mechanical loading during TBI and has been recently reviewed elsewhere (Johnson et al., 2012b; Len and Neary, 2011). DAI includes mechanical disruption of axonal cytoskeleton, altered axonal transport (Tang-Schomer et al., 2012), axonal swelling (Hemphill et al., 2011) and other changes that may include proteolysis, die-back disconnection and reorganization (Wang et al., 2011). Damage to white matter tracts seems to affect unmyelinated fibers more than myelinated fibers (Reeves et al., 2005; Staal and Vickers, 2011), and the damage in the former may be more severe in terms of effects such as disconnection/interruption. Membrane disruption can only account for a portion of injured neurons (Farkas et al., 2006; Kilinc et al., 2009), and excitotoxicity due to alterations in ion channel homeostasis likely do not account for the retraction of axons (Spaethling et al., 2008). Altered energy processing through mitochondrial dysfunction (Buki et al., 1999), altered ionic flux (Wolf et al., 2001) and demyelination (Ng et al., 1994) may contribute to DAI. Figure 3B shows axonal changes in human tissue following TBI (Johnson et al., 2012b). Differences in DAI reversal in younger vs. older brains is not well studied. However, in studies of young rats and mice, mTBI is associated with sustained cognitive changes (Creed et al., 2010; Prins et al., 2010), while clinically in more severe cases of DAI, dysautonomia and other measures are observed (Chelly et al., 2011). Thus, the behavioral consequences of mTBI relate in large part to DAI.
2.3. Altered Autoregulation of Blood Flow
Immediately after mTBI cerebral blood flow (CBF) decreases and can remain so for some time in adults (Werner and Engelhard, 2007); in children and young adults, there may be a delayed decrease in CBF (Becelewski and Pierzchala, 2003), this relative hypoxia can further augment the excitotoxic damage (Choi and Rothman, 1990; DeLellis et al., 2009; Packard and Ham, 1997). Decreases in CBF have been supported in animal studies (Golding et al., 1999). The autoregulation of CBF is sensitive to trauma (Junger et al., 1997) and it may be significantly impaired but gradually resolved in days and weeks following mTBI (Rangel-Castilla et al., 2008). Altered autoregulation may be symptomatic of changes in hypothalamic function via a number of mechanisms that include metabolic control (Kalsbeek et al., 2010) and spinal projecting neurons that affect sympathetic tone (Nunn et al., 2011). An uncoupling between the autonomic nervous system and the cardiovascular system, following mTBI has been suggested (Gall et al., 2004a; Gall et al., 2004b; Goldstein et al., 1998).
2.4. Neuroinflammation, the Immune Response
Currently little or no information regarding this topic is available in pediatric or adult patients suffering from mTBI. Preclinical data indicates that inflammation may be observed including increased microglia/macrophages and reactive astrogliosis (Kelley et al., 2007; Shultz et al., 2012; Shultz et al., 2011). Evidence for a neuroinflammatory component to TBI comes from preclinical work in a model of diffuse brain injury, showing increased pro-inflammatory cytokines (production of the pro-inflammatory cytokines IL-6, IL-1beta and TNF) (Yan et al., 2011). Neurioinflmmation may play a role in persistent neurocognitive and neurobehavioral sequelae of mTBI (Whitney et al., 2009) as in some patients, even months post incidence of the injury a neuroinflammatory response may be present (Mayer et al., 2010). In addition, anti-inflammatory treatments including minocycline, (Siopi et al., 2011) lithium (Yu et al., 2011) or N-acetylcysteine (NAC) (Chen et al., 2008) have shown promising results in animal models in preventing, ameliorating and improving TBI detrimental outcomes. Some of these effects involve complex regulation related to neurotransmitter changes including altered glutamatergic tone (Dai et al., 2010). Neuroinflammatory responses also differ with age of the developing brain (Claus et al., 2010), and may become persistent as it’s described in other conditions (Banati et al., 2001).
3. Brain Regions Affected by Concussion - Basis for Behavioral Changes
Any part of the brain may be affected following concussion with some dominating the clinical picture. Multiple regions may be affected in the individual patient (Figure 4). By understanding the association of regional brain function with behavioral manifestations, the approach to defining altered brain biology in mTBI including imaging biomarkers can be further advanced. Below, we summarize many of these effects in specific brain regions.
Figure 4.
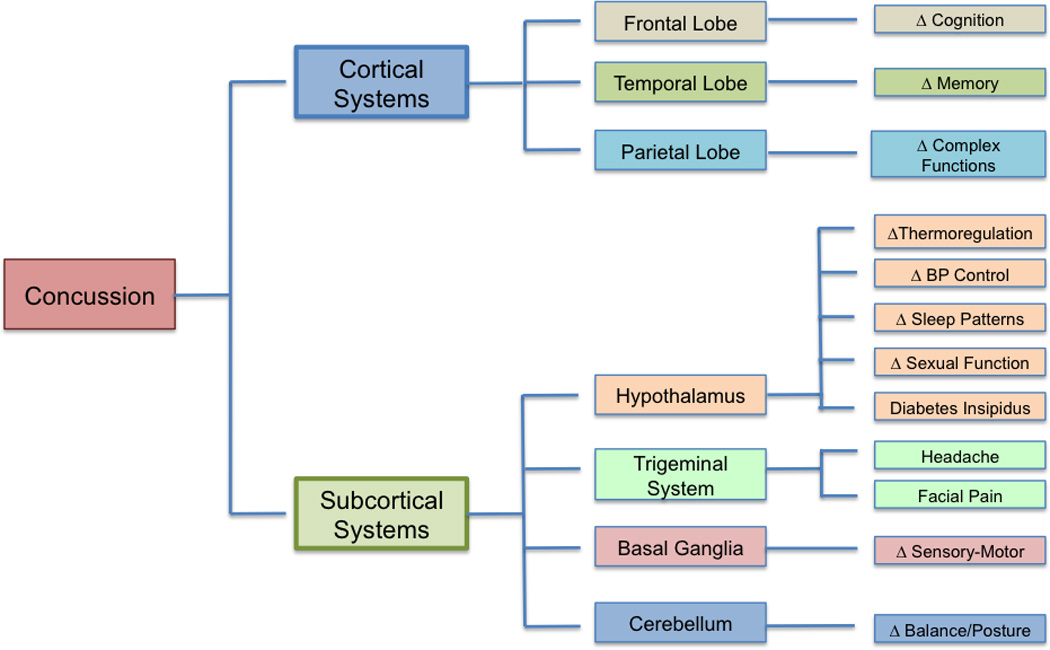
Brain-Related Behavioral Changes. Conceptualized regional brain involvement and the potential consequences of concussion.
3.1. Changes in Cortical Systems
3.1.1. Frontal Lobe in mTBI – Neurocognitive Changes
Due to the nature of many head injuries, the frontal lobe is commonly affected. Problems with neurocognitive function are the most disturbing consequences after mTBI and are almost universally present in the acute phase following the trauma. Such deficits have been identified through neurocognitive and neuropsychological testing, proving to be a useful tool in identifying changes in cerebral functions after mTBI. Neurocognitive testing in inpatient children (11–17 years) who had sustained mTBI showed deficits in executive functioning (Nance et al., 2009). Studies in older subjects (collegiate athletes) also reported similar patterns especially in cognitive processing, speed, verbal fluency, and memory. These difficulties usually persist for a limited period of time, and on average resolve by day seven (McCrea et al., 2003).
Neurocognitive testing is used to define acute cognitive dysfunction in mTBI patients (Covassin et al., 2010; McCrea et al., 2003; Nance et al., 2009) (Figure 5) but long-term effects are still under investigation. Past misconceptions about a benign course of recovery without residual deficits (Carroll et al., 2004) are now being challenged. A study from the United Kingdom reported that out of 2995 young people and adults who sustained mild TBI, 1400 subjects (47%) had disabilities after one year of the head injury (Thornhill et al., 2000). Others have reported that high school athletes may require up to 21 days to recover baseline levels of functioning, on measures such as reaction time, while other deficits such as verbal memory and motor processing speed return to normal levels by 14 days post injury (Covassin et al., 2010). Additional related changes (cognitive, emotional, somatic) have been observed among children 10 to 17 years of age, persisting up to 5 weeks after injury (Sroufe et al., 2010). Of note, subclinical changes may be present and may confer subtle changes in an individual’s interactions with society and the environment. Examples include impulsiveness, a normal behavior in teenagers owing to underdeveloped inhibitory controls of frontal regions, which may become a persistent problem beyond those years following mTBI (Boy et al., 2011). Subtle long term impairments of psychomotor speed and visual spatial skills after mTBI in childhood and adolescence have been described (Beers, 1992) persisting over 6 years after a concussion (Chuah et al., 2004; Konrad et al., 2010). These findings contribute significantly in the identification and discrimination of mTBI groups and the possible consequences of subtle cognitive impairments in daily life.
Figure 5.
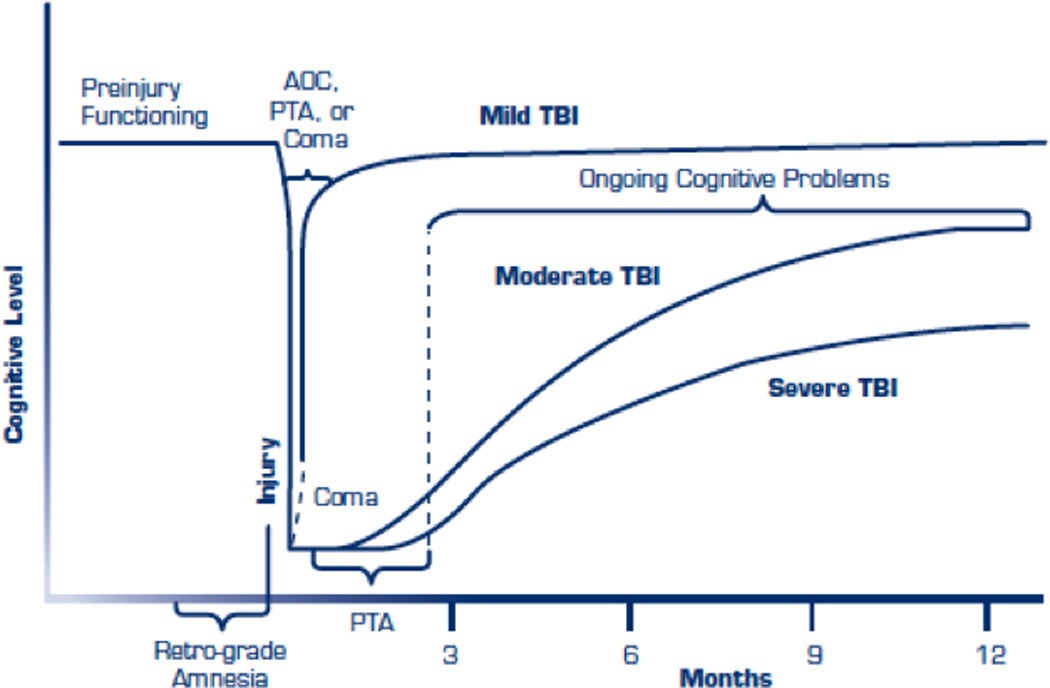
Acute and chronic consequences of different grades of severity of Concussion. Notice that even though mTBI seems to recover quickly, it continues to present cognitive problems along time (from http://www.publichealth.va.gov/docs/vhi/traumatic-brain-injury-vhi.pdf; permission pending).
A number of factors influence cognitive function including age and multiple concussions. Eleventh and 12th grade athletes perform better on information processing, motor dexterity, and attention in neurocognitive tests compared with 9th grade athletes. An assessment of baseline cognitive function in this age group which reflects a period of rapid cognitive growth, may be of use for evaluating long term deficits individually and for the population at large (Hunt and Ferrara, 2009). Multiple concussions and learning disabilities have been noted as factors that negatively affect cognitive function (Collins et al., 1999); athletes with history of 2 or more concussions and learning disabilities perform worse in neurocognitive testing than athletes with concussions without preexisting learning disabilities. It has been hypothesized that this altered recovery in patients with premorbid learning and behavioral difficulties may reflect less brain reserve capacity, as well as limit a patient’s ability to learn proper safety techniques, making it hard to assess actual concussion consequences (Collins et al., 1999). Another aspect that is related to increased neurocognitive dysfunction is the presence of post traumatic migraine, although this relationship is still not clear (Mihalik et al., 2005). Patients that self report post-concussion symptoms display more cognitive decline and longer time to recovery than asymptomatic patients (Lau et al., 2009), while the presence and duration of loss of consciousness and posttraumatic amnesia were not predictive of neurocognitive function after concussion (Collie et al., 2006; Sroufe et al., 2010).
3.1.2. Temporal Lobe - Memory Disruption
Temporal lobe dysfunction should always be considered as part of the initial assessment of the concussed patient. Temporal lobe injury may result in significant disability and possible long-term deficits in memory and language. During visual and verbal memory testing, PET and SPECT imaging have shown that up to 75% of patients after mTBI demonstrate medial temporal lobe abnormalities, the majority of these being bilateral and significantly correlated with decreased memory (Umile et al., 2002). Imaging studies following mild head injuries have shown significant hypoperfusion within the temporal lobes (Abu-Judeh et al., 2000) and reduced medial temporal functionality contributing to low memory performance after mTBI (Stulemeijer et al., 2010). Morphological changes have also shown temporal lobe involvement: TBI produces a disproportionate white matter loss and substantial hippocampal atrophy in patients with severe memory impairment (Bigler et al., 2002).
Language deficits are pathologic features of temporal lobe lesions. In adults, involvement of the left temporal lobe during concussion may produce aphasia (Junque, 1999). Data in the pediatric population is not well described. One study showed that children with a history of closed head injury 12 months previously had a significantly lower performance in speech/language battery tests than children with no injury (Jordan et al., 1988). The same group of children was reevaluated 12 months after the initial assessment, and even though the group itself showed improvement, their performance was still worse when compared with the control group (Jordan and Murdoch, 1990). Over time, language skills may normalize: language/speech in children who had sustained closed head injury 10 years prior showed no difference compared with the control group (Jordan et al., 1992).
3.1.3. Parietal Lobe – Complex Dysfunctions
Although information about specific deficits of the parietal lobe following mTBI is more limited than other regions of the brain, some of the potential sequelae following injury include anomia, apraxia, alexia, agraphia and dyscalculia, all potentially consistent with significant disability (Garcia Pena and Sanchez Cabezas, 2004). As with temporal lesions, hypoperfusion of the parietal lobes may also be present (Abu-Judeh et al., 2000). A group of 20 mTBI patients performed significantly lower than a control group in a naming object test (Kerr, 1995). Mild traumatic brain injury could also cause parietal-parasagittal traumatic intracranial hemorrhage resulting in more profound deterioration and possible coma, as compared with patients with traumatic hemorrhage elsewhere in the brain (Kinoshita et al., 2003).
3.2. Changes in Subcortical Systems
3.2.1. Hypothalamus and Autonomic Dysregulation
The hypothalamus is involved in a number of functions including autonomic (blood pressure, temperature regulation), endocrine, analgesic and circadian (Benarroch, 1993; Middleton et al., 2010; Nishino et al., 2000; Samuels, 2007). Hypothalamic pituitary dysfunction after mTBI is a well recognized complication by now (Acerini et al., 2006; Agha et al., 2004; Behan et al., 2008; Chou et al., 2009; Su et al., 2005; Tsagarakis et al., 2005; Yoshida et al., 1990), often related to damage to the hypothalamic-pituitary stalk due to shearing forces (Behan et al., 2008). While little is known about the specifics of hypothalamic-pituitary damage, a number of well-defined changes are observed that may persist for years (Kempf et al., 2010). Benarroch and colleagues described a central autonomic network, identifying the hypothalamus and its interconnections to structures such as the insula, amygdala, and medulla as important components of dysregulation of the autonomic nervous system (ANS) in various neurologic conditions including head trauma (Benarroch, 1993; Samuels, 2007). Most hypothalamic changes seem to follow temporo-parietal impact, middle fossa fractures and, of note, more frequently present in a younger population (Crompton, 1971). Delayed clinical intervention may account for the unrecognized early changes in ANS, especially with mTBI, resulting in an underreporting of autonomic dysfunction (<10% incidence of autonomic dysregulation in one comprehensive public health report on TBI) (Baguley et al., 2007). Autonomic dysregulation can occur in any form of TBI, but a correlation has been described between the severity of the insult, represented by the Glascow Coma Scale (GCS), and the degree of autonomic dysregulation. As the GCS score decreases, symptoms such as tachycardia, diaphoresis, hyperpyrexia and dystonia are more prominent (Baguley et al., 2009; Hilz et al., 2011; Thorley et al., 2001). Middleton and colleagues reported persistent neurocognitive symptoms and autonomic dysfunction described as an absence of late phase rise in blood pressure, in a patient with two previous concussions and early return to practice. Concussed athletes have reduced heart rate variability during exercise (Middleton et al., 2010). Kanjwal and colleagues studied a small group of patients who had sustained a previous TBI, and subsequently reported a number of nonspecific symptoms, such as fatigue, lightheadedness, palpitations, and presyncope. They were ultimately diagnosed to postural orthostatic tachycardia syndrome, defined as orthostatic intolerance with a heart rate increase of at least 30beats/min that occurs within the 10 minutes of head upright tilt table testing (Kanjwal et al., 2010).
Altered Sleep-Wake Cycles/Circadian Rhythm
Sleep disturbances following head trauma have by now been identified as a direct consequence of TBI in children (Milroy et al., 2008), and range from insomnia and hypersomnia to an uncategorized decrease in sleep quality. Significant numbers of patients (92.7%) perceive onset of sleep difficulties following concussion (Ouellet et al., 2004). After 1 month post concussion 55% patients recognized one or more sleep problems, and at one year 27% could still identify some difficulty with sleep (Watson et al., 2007). Objective measures of sleep, such as polysomnographic (PSG) and multiple sleep latency test (MSLT), do not correlate with the patient’s perceptions but still may be useful for diagnosis. Many studies show sleep wake cycle disturbances of up to 68% with prolonged hospital stays, including prolonged sleep initiation (Fichtenberg et al., 2002), circadian rhythm disorders with stage 2 non-rapid eye movement (NREM) sleep higher than rapid eye movement (REM) in mTBI patients (Makley et al., 2008) . Long-term sleep difficulties (28 months post injury) have also been found, patients had shorter REM onset latencies and longer sleep onset latencies than controls (Williams et al., 2008). Factors that affect sleep disturbances and its duration following concussion include: (i) the injury itself may damage sleep centers; (ii) sleep changes may increase concurrent levels of anxiety and depression (Gosselin and Tellier, 2010; Parcell et al., 2006); (iii) cognitive changes, such as inattention, that may potentiate insomnia (Bloomfield et al., 2010); (iv) symptomatic medication used to treat post-concussion symptoms (e.g. amitriptyline for headache); and (v) insomnia may be enhanced due to alterations in hypothalamic hormones including hypocretin (Baumann et al., 2005; Baumann et al., 2007) and melatonin (Shekleton et al., 2010) often released following concussion.
Altered Appetite
Appetite and food control is a multifaceted process regulated not only by hormones such as insulin, glucagon, leptin, ghrelin and somatostatin, but is also influenced by physical and psychological factors (Rowell and Faruqui, 2010). Hypothalamic obesity is a term that has been used to express several secondary causes of obesity found after trauma, in post-operative excision of hypothalamic and pituitary tumors as well as in monogenic syndromes of morbid obesity due to mutations in genes expressed in the hypothalamus (Hochberg and Hochberg, 2010). Preclinical data support lesions in the ventro-medial hypothalamus (VMH) involved in hyperphagia and obesity (Hochberg and Hochberg, 2010; King, 2006; Morrison, 1968; Nakao et al., 2007). Gastroparesis, another potential contributing factor, in patients with closed head injury has been correlated with GCS scores (Thor et al., 2003). Obesity itself may also be a side effect of medications (e.g., amitriptyline for headache) (Ness-Abramof and Apovian, 2005; Taylor, 2008).
Thermoregulation
Abnormal of temperature regulation is quite common after mTBI, including both hypothermia and hyperthermia (De Tanti et al., 2005). Animal models using a technique with fluid percussion have shown a decrease in temperature in the first 24 hours, followed by a period of hyperthermia. These findings were related pathologically with inflammation, particularly in the periventricular hypothalamus (Thompson et al., 2005a).
Diabetes Insipidus
Among the hypothalamic disorders that may develop after mTBI, diabetes insipidus (DI) is one of the most critical, as water and electrolyte imbalance could exacerbate patient morbidity post-trauma (Tsagarakis et al., 2005). In some reports, following acute trauma, up to 26% of patients have been reported to have acute DI, and approximately 7% are predicted to develop chronic DI (Agha et al., 2005; Agha et al., 2004). There is a significant correlation between post-concussion symptoms and water metabolism disorders (Bohnen et al., 1993). It is worth mentioning that based on postmortem studies of patients with severe TBI, anterior hypothalamic damage is a consistent finding (Acerini et al., 2006; Crompton, 1971). However, in mild TBI DI usually resolves quickly or is controlled with desmopressin acetate (DDAVP) treatment (Chou et al., 2009). Rarely the condition may persist for years (Hadani et al., 1985).
Sexual Dysfunction after mTBI
Sexual dysfunction after mTBI has been another well-defined but often neglected consequence of brain injury. Dysfunction includes not only specific physiologic problems but also behavioral issues, including impulsiveness and inappropriateness, global emotional sexual difficulties, changes in libido, and sexual frequency (Elliott and Biever, 1996). In adults after TBI, more than 50% of subjects report reduced sex drive, inability to satisfy their partner, along with less enjoyment and capacity to reach climax. It was also noted that the importance of sexuality in their lives was also reduced, reflected by decreased frequency of engaging in sexual activities (Ponsford, 2003). Erectile dysfunction and poor self-image have also been reported, with subjects who identify themselves as having a decline in sex appeal, less confidence, depression, and impaired communication levels with their partner (Kreutzer and Zasler, 1989; Ponsford, 2003). Information on sexual dysfunction in older children is not well documented. However, other related features has been identified in children including precocious puberty (Blendonohy and Philip, 1991). Additional studies regarding sexual dysfunction in adolescents with mTBI would be an important contribution.
3.2.2. Trigeminal System – Headache and Facial Pain Syndromes
Within the spectrum of post-concussion symptomatology, migraine, and headache in general, are prominent an often significantly contribute to patient disability. Although this symptom complex predominantly affects subcortical functioning and the trigemino-vascular system, it also is associated with neuronal changes in multiple brain regions. Descriptions of post-traumatic headache in the recent literature date from 1898 to the present (Haas and Lourie, 1988). Symptoms have been described in isolation, where trauma may be the only causative event (Solomon, 1998), or as part of the post-concussion syndrome which encompasses somatic, emotional/behavioral and cognitive symptoms (Kirkwood et al., 2006).
The prevalence of posttraumatic headache in children ranges from 2.3% to 6.8% (Barlow et al., 2010; Kirk et al., 2008), a low estimate when compared with the adult literature. In military populations, estimates reach 78%, with most headaches classified as migraine without aura and tension type headaches (Theeler and Erickson, 2009; Theeler et al., 2010). In the civilian population, the incidence and prevalence is varied, ranging from 22.5 – 71% (Bettucci et al., 1998; Hoffman et al., 2011). The features of posttraumatic headache are many and varied, and differ regarding severity, pain characteristics (tension type, occipital, migraine cluster, supraorbital), location, and occurrence in time (Gilkey et al., 1997; Seifert and Evans, 2010). With post-traumatic migraine, trauma could be the actual trigger in a susceptible person, such as an individual with a family history of migraine (Haas and Lourie, 1988) or with previous precursors of migraine, including cyclic vomiting, motion sickness, and recurrent abdominal pain. Prior history of tension type headache and female gender are associated with a higher rate of posttraumatic headache (Hoffman et al., 2011; Theeler and Erickson, 2009). One study suggests that less severe head injury is related to a higher risk of developing posttraumatic daily headache than is severe TBI, although the explanation for this relationship is unclear (Couch and Bearss, 2001). The specific pathophysiology of post-traumatic headache and its clinical implications are yet to be defined.
Figure 6 is a conceptual figure of a particular symptom in concussion using, the example of post-concussion headache. The figure attempts to make two points (i) that a symptom may spontaneously resolve or persist and (ii) that a variety of stimuli may provoke headaches following mTBI. Of these, concentration is commonly observed clinically, but its mechanism of inducing or increasing headache is unknown. One plausible explanation may be increased activation in frontal areas (seen as increased blood flow on imaging studies) that activates a system that diminishes endogenous pain modulation.
Figure 6.
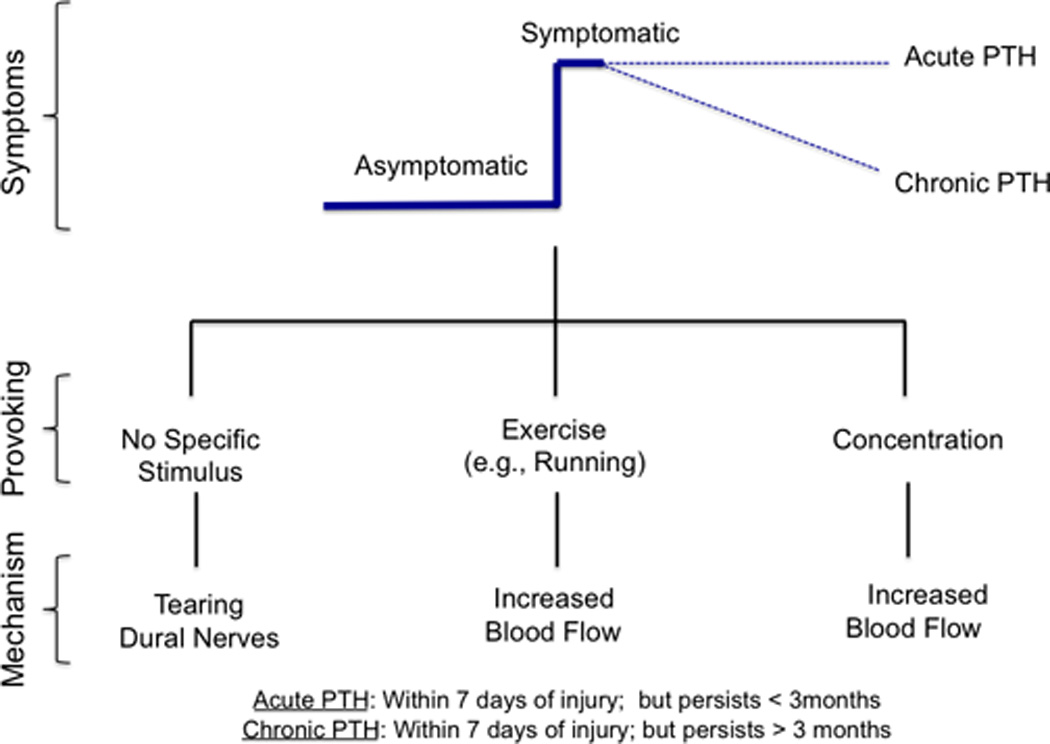
Temporal changes following Concussion. Onset and persistency of post-concussive headache due to different mechanisms involving anatomical and blood flow changes.
Although the pathogenesis of post-traumatic headache is yet to be defined, current theories include; (i) a similar process to migraine (Packard and Ham, 1997) but provoked by damage to the trigemino-vascular system (viz., structures such as the circle of Willis, extracranial cephalic blood vessels, basilar artery, vessels of the dura and pia matter that receive innervation from the trigeminal ganglion) (Sakas and Whitwell, 1997); (ii) ‘fragility’ of the cranium in children whereby the skull is more malleable and dural damage thus more likely because the dura is tightly adherent to the cranium (Sakas and Whitwell, 1997); (iii) tearing of extracranial dural afferents (Levy et al., 2009; Zhang et al., 2010b); (iv) increased propensity to cortical spreading depression (Ayata, ; Leao, 1947; Oka et al., 1977; Sakas and Whitwell, 1997); and (iv) involvement of upper cervical trauma that affects the area of spinal nerves C1–C3 (Grgic, 2007). Of note whiplash associated disorder (WAD) patients report similar symptoms to those observed in mTBI including headache, cognitive disturbances such as impaired memory, poor concentration, mental fatigue, sleep impairment, sensory sensitivity, visual disturbances and vertigo, possibly linked to perfusion abnormalities (Linnman et al., 2009; Otte et al., 1998), but see also Evans (Evans, 2010).
Even though there is a pattern in the origin of headache, there is no clear explanation for the perpetuation of headache and its chronicity. There is some evidence that cerebral blood flow alterations can be found even after 6–18 month of the original insult, with regional and hemispheric asymmetries (Gilkey et al., 1997; Seifert and Evans, 2010) and predominance of dysfunction within the frontal lobes (Lyczak and Lyczak-Rucinska, 2005). Whether this alteration in CBF plays a primary role in the pathogenesis of posttraumatic headache or reflects alteration in cortical and subcortical networks is unclear. However, long-term structural gray matter changes in structures such as the anterior cingulate cortex (ACC) and the dorsolateral prefrontal cortex (DLPFC), may later the function of descending antinociceptive networks and increase the transmission of afferent signals from trigeminovascular and dural afferents. These changes were present after 3 months in patients with persistent injury. While other morphological and chemical changes in cortical and subcortical structures are noted in migraineurs (Maleki et al., 2011; Prescot et al., 2009) such data is not currently available for headache following mTBI.
Unfortunately, there has not been a single randomized placebo-controlled trial evaluating any modality for the treatment of post-traumatic headache. Post traumatic headache treatment has been best treated with a multifactorial approach, using multiple and combined modalities, including, biofeedback, psychotherapy, and chiropractic therapy as well as NSAIDS, ergotamine, triptans, opioids, SSRIS and muscle relaxants (Meehan, 2011; Sheftell et al., 2007). Current treatment is mainly symptomatic, and more effective therapy awaits improved understanding of pathophysiologic mechanisms, and proper, rigorous controlled clinical trials.
3.2.3. Cerebellum in Concussion – Disorders of Balance
Because of its location, and especially in coup-contre coup injuries, the potential for cerebellar involvement should be considered (Potts et al., 2009). A particular vulnerability of the cerebellum during TBI has also been documented. Purkinje cells of the cerebellum appear to be selectively susceptible, as significant Purkinje neuronal loss is observed within a 24-hour period after injury. Purkinje synapse connections with parallel fibers, consisting of glutamate input, make potential exitotoxic damage likely (Park et al., 2007; Potts et al., 2009). Clinical manifestations of cerebellar concussion (Fumeya and Hideshima, 1994) following head injury include those commonly defined for non-specific cerebellar dysfunction, including a positive Rhomberg test, finger-nose intention tremor, incoordination, dysdiadochokinesia, and even stuttering (Yeoh et al., 2006). Many of these cerebellar abnormalities may be better defined through imaging. Approximately 33% of patients with TBI may experience severe ataxia, positively correlated with post-traumatic amnesia, and up to 11% these patients also have normal CT scans (Mysiw et al., 1990).
Perhaps the main issue relates to imbalance within neuronal systems, and the potential for this network’s dysfunction to contribute to further ‘accidents’ that may affect the brain. Through static and dynamic balance testing and analysis of center of pressure fluctuations, it has been explained that patients that have suffered mTBI show reduced postural control in both components (Geurts et al., 1999). In addition to these tests, Cavanaugh, et al, used approximate entropy and identified concussed athletes that, despite demonstrating normal stability, displayed subtle changes in their postural control (Cavanaugh et al., 2005), which could remain altered even after 48 to 96 hours post injury (Cavanaugh et al., 2006). Lesions of the cerebellum in children and correlations with TBI have been identified. White matter degeneration within the cerebellum and its projections to the dorsolateral prefrontal cortex, thalamus, and pons has been found in children with TBI (Spanos et al., 2007). It has also been reported that the degree of white matter deterioration in the cerebellum, posterior thalamic radiation and corticospinal tract was highly associated with balance deficits among a group of children and adolescents with TBI (Caeyenberghs et al., 2010a). The evidence suggests that postural control and stability should be tested routinely in patients after mTBI, at baseline and after the injury. In the case of young athletes, it should be done at the sidelines in the setting in which the insult occurred (Onate et al., 2007).
3.2.4. Basal ganglia Injury – Altered integration of motor-sensory-cognitive functions
Basal ganglia injury after concussion is one of the many understudied and underreported consequences, possibly due to its low and imprecise incidence, currently reported in approximately 2 to 3 % (Macpherson et al., 1986; Shaffer et al., 2003). Symptoms referable to basal ganglia dysfunction are usually reported in case studies with few patients. However, even though such symptoms are rare, they are often quite disturbing for patients and families. The concept of basal ganglia injury after traumatic events has been described in a number of studies in severe TBI where obvious dysfunction was present. In one report, 3 post-adolescent patients, after recovering of severe brain injury presented with choreoathetotic movements of the upper limbs, attributed to lesions in the basal ganglia (Drake et al., 1986). In another report of twenty one pediatric patients that sustained high velocity trauma and falls, 52% had an isolated basal ganglia lesion and an inverse correlation between the GCS and the patient’s outcome (Kurwale et al., 2010). More subtle alterations in basal ganglia function may nevertheless be present in mTBI.
Landi describes a 10-month old patient that after a minor head trauma developed a right facio-brachio-crural hemiparesis, with evidence of a left lenticular nucleus ischemic lesion after mTBI (Landi et al., 2011). Numerous other case reports show basal ganglia involvement following mTBI (Kieslich et al., 2002; Landi et al., 2011; Sobani and Ali, 2011; Yilmaz et al., 2011). The mechanism underlying damage to the basal ganglia is thought to be provoked by stretching, distortion, and even rupture of the lenticulostriate branches of the middle cerebral artery as well as a decreased cerebral blood flow (Kieslich et al., 2002; Yilmaz et al., 2011). Most reports suggest a good prognosis with no apparent permanent damage, but there are currently no studies with long-term follow-up.
4. Clinical Management
Clinical management has been reviewed by a number of authors (Grubenhoff et al., 2011; Kirkwood et al., 2008; Meehan et al., 2011; Vos et al., 2002) and is not the focus of this review. Briefly, clinical management has been empirical focused on (1) Immediate care (Kamerling et al., 2003); (2) restorative processes e.g., rest and cognitive training (Cozzarelli, 2010); motor improvement (Bland et al., 2011); (3) symptom management e.g., treating symptoms such as headache (Lucas, 2011). The most common symptoms include symptoms are headache, dizziness, decreased concentration, memory problems, irritability, fatigue, visual disturbances, sensitivity to noise, judgment problems, depression, and anxiety (Ryan and Warden, 2003). To date, there are no randomized controlled trials that have compared strategies of treatment for any symptom to placebo or against each other. Moreover, there are no well established rehabilitation techniques which have been shown to hasten or lead to a more complete recovery, from either a symptom or objective marker standpoint. At this time we do not fully understand factors (premorbid state and injury related), that place some children with mild traumatic brain injuries at risk for post-concussive symptoms that even in mTBI may last for over a year (Fay et al., 2009; Ryan and Warden, 2003) and (4) preventive measures (Franklin and Weiss, 2012).
One of the vexing issues in the clinical treatment is that of “return to play” following a concussion. Return to play or school (cognitive demands) and protocols have been suggested to implement these guidelines (Kissick and Johnston, 2005). However, evidence exists that children and adolescents take longer to recover than adults after a concussion (Guskiewicz and Valovich McLeod, 2011). Reliance on the self-report of the athlete is inadequate. Objective indices would help with this issue. Baseline and post-concussion cognitive assessments can be helpful, but their sensitivity and specificity is incomplete, and clinicians must be aware that head trauma may result in a wide array of clinical signs and symptoms, that may not be evident on these computerized cognitive assessment tests (Standaert et al., 2007). Clearly while clinical measures have their role, given the lag between the resolution of clinical symptoms and the metabolic recovery of the brain, more objective measures would help clinicians by providing a more accurate and objective assessment of when there has been metabolic and structural brain recovery, the window of vulnerability to a repeat concussion has passed, and when there is cumulative damage or impairment that should preclude the child or adolescent’s return to contact sports, for example.
The problem we face is to understand the mechanism(s) by which mTBI in children may lead to long term neurological consequences and the discovery of clinical, imaging, or other biomarkers that identify the child that is at risk (Lee, 2007). Indeed, “Intervention by a qualified rehabilitation team does not appear to impact on the long-term outcome for persons with symptoms related to mild traumatic brain injuries” (Anderson et al., 2011). Recent studies suggest that TBI may induce long-term neurodegenerative processes. Some of these may be clinical (e.g., chronic traumatic encephalopathy from repetitive concussion (Stern et al., 2011)) or subclinical involving more subtle changes resulting from progressive axonal pathology and neurodegenerative changes. Sustained perturbation of axonal function, even with recovery as shown in animal experiments (Creed et al., 2010) for up to 1-year post injury (Bramlett and Dietrich, 2002). The issue relates to how the asymptomatic status is defined. Simple disappearance of symptoms, while clearly a good outcome, does not take into account that the brain is highly susceptible to subsequent concussions, suggesting that abnormalities are not fully recovered. Such data implicate underlying anatomical and physiological alterations that can increase the brain's vulnerability to repeat injury and long-term disability (Barkhoudarian et al., 2011). Indeed, this debate of whether mTBI can cause permanent brain changes is ongoing (McHugh et al., 2006; Ruff, 2011). However, the issues is clearly complex as noted in a comparison of mild moderate and severe TBI where post concussion symptoms were reported to a greater degree in persons with mild TBI at 3 months post-injury but at 1 year after injury, no differences were found between TBI groups on the presence of post-concussion syndrome (Sigurdardottir et al., 2009). Furthermore, in adults at least, certain premorbid conditions are predictors of persistent post-concussion syndrome; e.g. anxiety in women (Dischinger et al., 2009).
Significant clinical issues do relate to prevention of potential long-term process such as chronic traumatic encephalopathy and the susceptibility of second impact syndrome. Second impact syndrome is a potentially lethal condition in which an individual who has had blunt cranio-cerebral trauma and whose symptoms have not subsided, sustains a second head injury (Cantu, 1998b; McCrory, 2001). It is believed, thanks to forensic studies, that the second blow has a synergistic effect on the initial pathology which itself may have predisposed the brain to an increased injury response, causing diffuse cerebral swelling and brainstem herniation, leading to death (Byard and Vink, 2009; McCrory, 2001; Wetjen et al., 2010). Even though it is a reality, the virtually nonexistent statistics, the yet-to-be validated inclusion and exclusion criteria, with predisposing factors being difficult, if not impossible to evaluate, make this disorder a matter of continuous debate (Byard and Vink, 2009; McCrory et al., 2012; Wetjen et al., 2010). Though new return to play guidelines have been established in order to protect the athlete (Cantu, 1998a; Kissick and Johnston, 2005), the extent of injury and the consequences of a second hit, even after months of the first impact, need to undergo further study with more objective measures that include imaging studies.
5. Measuring Brain Changes
Alterations in brain function underlie behavioral changes observed following concussion. Evaluations have predominantly been through clinical measures, the use of specific concussion related measures (e.g., GCS), evaluation using pre and post concussion questionnaires now more commonly available at schools (McCrea et al., 1997; Sarmiento et al., 2010; Tsushima et al., 2008), and psychological testing for components such as cognitive changes (Johnson et al., 2011b; Lau et al., 2011; Pontifex et al., 2009). Newer neuroimaging measures of alteration of brain function and structure are now being used both in the clinic (e.g., measures of white matter connections) and in research programs to define objective indices of altered brain function. It is of note that most of the imaging studies are in adolescents, presumably because imaging can be more easily obtained. The entire age range of mTBI patients awaits further scrutiny.
Non-invasive imaging is completely changing our understanding of the process, offering new insights into morphological, functional, and chemical changes in the brain. For example, MacKenzie and colleagues demonstrated whole brain atrophy is present at 11 months after injury (MacKenzie et al., 2002). As such the development of true biomarkers of the condition may be integrated with current tools used for assessment and treatments for the constellation of brain-induced behavioral and physiological changes. Clearly there are 3 diagnostic domains that imaging facilitates (i) initial assessment of the severity of the concussion; (ii) temporal window of metabolic brain vulnerability and early prognostic changes; (iii) long term evaluations. These measures are destined to change our approaches to treating children who suffer from or who have suffered from mTBI. Rehabilitation attitudes may take the form of specific drivers related to specific brain function (e.g., cognitive therapy (Limond and Leeke, 2005)), to non-invasive activation of brain systems (e.g., TMS (Demirtas-Tatlidede et al., 2011)) or processes that allow enhancing normalization of systems, including exercise (Leddy et al., 2011). New findings will, hopefully, lead to improvements in neurorehabilitation and understanding of neuroplasticity (Chen et al., 2002; Dancause and Nudo, 2011). Given that the pediatric brain is more ‘plastic’ than that in the adult, one concern to consider is whether changes are more adaptive or deleterious over time. The development of imaging biomarkers may contribute to improved understanding and thus the development of specific and effective therapies.
5.1. Current approaches
5.1.1. Computerized Tomography (CT) and standard Magnetic Resonance Imaging (MRI)
In the past decades, CT scan has become readily available in most healthcare facilities, making it the method of choice to detect acute changes in the brain, such as hemorrhage, uncommon in mTBI, as well as significant contusions and external fractures. However, CT has continually failed to show structural changes in the brain after injury (Fiser et al., 1998; Suskauer and Huisman, 2009). MRI has equal sensitivity in detecting these acute changes, and better sensitivity than CT in detecting some anomalies, such as cerebral edema, hemosiderin deposition, and minor cerebral contusion (Lee et al., 2008), and avoids the substantial radiation exposure that accompanies CT scanning – an important factor in children and adolescents.
5.2. Enhanced Neuroimaging Approaches
5.2.1. Magnetic Resonance Imaging – Functional, Morphological, and Chemical Measures of mTBI
Multimodal MRI, in which several techniques are used to determine abnormalities in function and structure, are being used concurrently to characterize brain changes. Specifically:
Functional Magnetic Resonance Imaging (fMRI): Brain changes in evoked responses in mTBI
fMRI, evaluates measures of brain function based on changes in blood flow in capillary beds in the brain (Raichle and Mintun, 2006). In particular, the Blood Oxygenation Level Dependent (BOLD) technique is highly utilized in neuroimaging. A number of studies have utilized fMRI in concussion. For example, Chen and colleagues evaluated regional brain activations associated with a working memory task from a group of concussed athletes and matched control subjects; athletes had weaker BOLD changes within the right mid-dorsolateral prefrontal cortex, a crucial area for monitoring information in working memory (Chen et al., 2004). Other fMRI BOLD studies, again in concussed athletes, showed that such individuals, while performing visual and spatial memory tasks, had a significantly higher activation at the parietal cortex, right dorsolateral prefrontal cortex, and right hippocampus, all findings that were not seen in healthy controls (Slobounov et al., 2010). Such results suggest that the concussed brain ‘compensates’ for disrupted areas by activating different ones, possibly reflecting chronic changes in brain networks. Figure 7 is an example of utilizing fMRI to evaluate changes in brain function. Such data provides insights into specific brain regions that may be functionally deregulated or abnormal and can be monitored and evaluated over time. Table 1A lists examples of other fMRI studies in concussion in the pediatric population.
Figure 7.
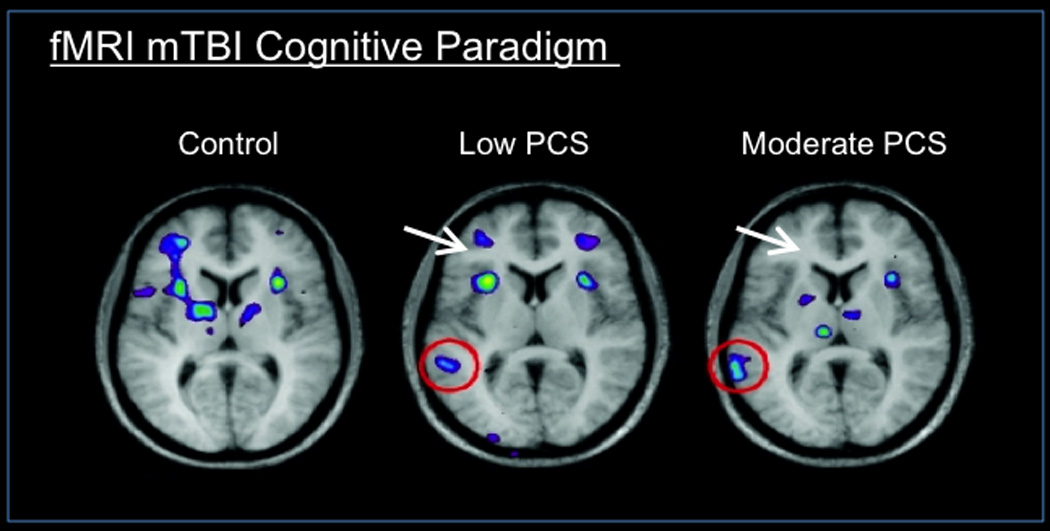
Functional Imaging - fMRI (Evoked Measures): Functional MRI image showing brain activation during a verbal working memory task in healthy controls, mTBI patients with low post concussive symptoms (PCS) and moderate PCS. The image shows additional activation in the posterior brain regions, and less activation in the frontal regions, when patients had low and moderate post concussive symptoms (PCS), compared to control subjects. These changes depict poorer brain activation in frontal areas in patients with PCS while performing neurocognitive testing. (From (Chen et al., 2007); permission pending).
Table 1.
| A: fMRI measures of concussion. | |||||
|---|---|---|---|---|---|
| Assessment | Subjects | Age (years) |
TBI severity | Results | Reference |
| Working memory Response inhibition. |
13 c;13 hc | 12 to 13 | Mild | Additional activation in the posterior cerebellum additional demand for inhibitory control. |
(Krivitzky et al., 2011) |
| Verbal working memory. |
40 c;41 hc | 7 to 17 | Mild, Moderate and Severe |
Additional activation in the LPC, inferior parietal lobule, superior parietal lobule, and precuneus. |
(Wilde et al., 2011) |
| Spatial memory. | 15 c;15 hc | mean 21 | Mild | Increased activation in the right and left DLPFC, left parietal cortex, cerebellum and right hippocampus. |
(Slobounov et al., 2010) |
| Mathematical, memory and sensorimotor processing. |
8 athletes (4 sustained mTBI during the study) |
19 to 23 | Mild | Increased activation in the supplementary motor area, bilateral premotor cortex, superior and inferior parietal regions and bilateral cerebellum. |
(Jantzen et al., 2004) |
| Working memory. | 16 c;8 hc | mean 27 | Mild | Weaker changes in MDLPFC Additional activation in temporal and parietal lobes. |
(Chen et al., 2004) |
| Declarative memory. | 43 c;20 hc | 18 to 50 | Mild | Inverse correlation between MTL activity and injury severity. MTL functionality contributes to poorer memory. |
(Stulemeijer et al., 2010) |
| Cognitive impairment withouth clinically diagnosed concussion |
24 athletes | 15 to 18 | Mild | Athletes with no clinically-observed concussion symptoms, but who demonstrated measurable neurocognitive- neurophysiologic impairments. (primarily visual working memory and altered activation in DLPFC) |
(Talavage et al., 2010) |
| Attentional dysfunction. |
16 c;16 hc | mean 27 | Mild | Hypoactivation within cerebellum and RPPC, presupplementary motor area, bilateral frontal eye fields and RVLPFC during attentional disengagement. |
(Mayer et al., 2009) |
| Balance and motor network. |
12 c; 9hc | 19 to 53 | Mild, Moderate and Severe |
Less activation of left primary motor cortex (M1), right cerebellum, and bilateral SMA with negative interaction with the left frontal cortex. |
(Kasahara et al., 2010) |
| B: RSN measures of concussion. | |||||
|---|---|---|---|---|---|
| Assessment | Subjects | Age (years) |
TBI severity | Results | Reference |
| Fluctuations of default mode network DMN) |
27 c;26 hc | mean 27 | Mild | Decreased functional connectivity within the DMN and hyper- connectivity between the DMN and lateral prefrontal cortex. |
(Mayer et al., 2011) |
| Thalamic resting state networks (RSN). |
27 c;20 hc | 22 to 62 | Mild | Disrupted 'healthy' pattern with increased thalamic networks and decreased symmetry |
(Tang et al., 2011) |
| Default mode network alterations with light physical stress. |
14c; 17 hc | mean 20 | Mild | Light exercise disrupted the DMN with reduced connectivity between the PCC, MPFC, and left and right LPC. |
(Slobounov et al., 2011) |
| Default mode network in the subacute phase. |
15 c;14 hc | mean 20 | Mild | Reduced strength and number of connections in the PCC and LPC, increased strength and number of connections in the MPFC. |
(Johnson et al., 2011a) |
| C: DTI measures of concussion. | |||||
|---|---|---|---|---|---|
| Assessment | Subjects | Age (years) |
TBI severity | Results | Reference |
| Tractography of corpus callosm. |
10 c;10 hc | 14 to 19 | Mild | Increased FA and decreased RD correlated with severity of postconcussion symptoms. |
(Wilde et al., 2008) |
| White matter changes and postconcussive symptoms. |
11 c;10 hc | 14 to 19 | Mild | Injury-affected regions showed decreased RD, and increased FA correlated with increased PCS. |
(Chu et al., 2010) |
| White matter integrity and memory functioning. |
12 c;11 hc | mean 15 | Mild | Lower ADC in the cingulate bundles and increased FA in the left cingulate bundle correlated with poorer memory. |
(Wu et al., 2010) |
| White matter integrity and congnition. |
14 c; 14 hc | 10 to 18 | Mild | Lower FA in the inferior frontal, superior frontal, and supracallosal regions. Correlated with lower processing speed, working memory and executive deficits, and greater behavioral dysregulation. |
(Wozniak et al., 2007) |
| White matter integrity and upper- limb visuomotor tracking performance. |
17 c; 14 hc | 8 to 20 | N/A | Decreased FA in corticospinal tract, posterior thalamic radiation, and optic radiation. Associated with inferior visuomotor tracking performance. |
(Caeyenberghs et al., 2010b) |
| Changes in white matter before and after concussion. |
10 c;10 controls (orthopedic injuries/healthy) |
Teenage | Mild | Significant changes in FA and MD in the right corona radiata and right inferior longitudinal fasciculus over time. |
(Bazarian et al., 2011) |
| White matter integrity and postconcussive symptoms. |
10 c;10 hc | mean 19 | Mild | Increased MD in WM fiber tracts in the inferior/superior longitudinal and frontooccipital fasciculi, the retrolenticular part of the internal capsule, posterior thalamic and acoustic radiations. Associated with mild TBI and postconcussive symptoms. |
(Cubon et al., 2011) |
| Postural control. | 12 c;14 hc | 8 to 20 | Moderate and Severe |
Reductions in FA within the cerebellum, posterior thalamic radiation, and corticospinal tract. Degree of white matter deterioration was highly correlated with balance deficits. |
(Caeyenberghs et al., 2010a) |
| White matter integrity in the semi acute stage. |
20 c;21 hc | 20 to 37 | Mild | Increased FA as a result of reduced RD in the corpus callosum and left hemisphere tracts. |
(Mayer et al., 2010) |
| White matter integrity and postconcussive symptoms. |
19 c;12 hc | 18 to 50 | Mild | Reduced FA in the uncinate fasciculus, the inferior fronto- occipital fasciculus, the internal capsule, the CC, the parietal and frontal subcortical white matter. Associated with severity of postconcussive symptoms. |
(Smits et al., 2011) |
| Prefrontal axonal injury and executive functioning. |
20 c; 20 hc | 19 to 49 | Mild | Lower DLPFC FA was significantly correlated with worse executive function performance. |
(Lipton et al., 2009) |
| Development and integrity of corpus callosum. |
41 c; 31 hc | 0 to 15 | Reduction in FA and increased RD in the genu, posterior body, isthmus and splenium with disruption in interhemispheric tracts. Arrested development of CC. Associated with poor performance in neuropsychological testing. |
(Ewing-Cobbs et al., 2008) | |
| White matter integrity and neurobehavioral outcome in the subacute stage |
23 c; 23 hc | 18 to 55 | Mild | Higher MD in the corpus callosum, right anterior thalamic radiations, superior and inferior longitudinal fasciculus and the fronto-occipital fasciculus bilaterally. Associated with poorer outcome. |
(Messe et al., 2011) |
| Traumatic axonal injury |
15 c;15 hc | mean 21 | Mild | Greater variability of FA in the genu and body of the CC.decreased diffusivity at the left and right DLPFC |
(Zhang et al., 2010a) |
| Predictive white matter measures of cognitive performance. |
17 c;29 hc | 18 to 61 | Mild | Average MD higher and average FA lower. Correlated with poorer performance in cognitive tasks. |
(Miles et al., 2008) |
| D: MRS measures of concussion. | |||||
|---|---|---|---|---|---|
| Assessment | Subjects | Age (years) |
TBI severity | Results | Reference |
| Glutamate levels as predictor of outcome. |
38 c; 10 hc | 1 to 17 | Mild, Moderate and Severe |
Glutamate/glutamine increased in all TBI patients compared to controls. No direct correlation to outcome |
(Ashwal et al., 2004) |
| Injury markers to predict neuropsychological outcome |
20 c | 1 to 18 | N/A | NAA levels and its ratios (NAA/Cre, NAA/Cho) showed strong positive correlations with intellectual and neuropsychological functioning |
(Babikian et al., 2006) |
| Injury markers to predict neuropsychological performance |
36 c; 14 hc | 6 to 18 | Mild | NAA/Cre was reduced and Cho/Cre increased in anterior and posterior parts of a selected supraventricular spectroscopic imaging slice. The ratio predicted aspects of neuropsychological performance |
(Yeo et al., 2006) |
| Injury markers and correlation with neuropsychological deficits. |
29 c; 52 hc | 18 to 42 | Mild and moderate |
Decrease of NAA and NAA/Cre, and increases of Cho and Cho/NAA, in all lobes. Correlate with measures of cognitive performance. |
(Govind et al., 2010) |
| Minimal detectable metabolite differences. |
20 c;17 hc | 19 to 59 | Mild | Subtle metabolite concentration changes are characteristic in mTBI. |
(Kirov et al., 2007) |
| E: SWI measures of concussion. | |||||
|---|---|---|---|---|---|
| Assessment of | Subjects | Age (years) |
TBI severity | Results | Reference |
| Presence of cerebral microbleeds. |
21 c;42 headache |
mean 37 | Mild | Cerebral microbleeds where found and more frequently in white matter. |
(Park et al., 2009) |
| CT vs. MRI vs. SWI | 48 c. | 5 to 14 | Mild | SWI detected additional lesions 30% of the time compared to CT and MRI. |
(Beauchamp et al., 2011) |
| F: Volumetric measures of concussion. | |||||
|---|---|---|---|---|---|
| Assessment | Subjects | Age (years) |
TBI severity | Results | Reference |
| Volume brain parenchyma |
14 c;10 hc | mean 35 | Mild and moderate |
Whole-brain atrophy occurs after mild or moderate TBI and is evident at an average of 11 months after trauma |
(MacKenzie et al., 2002) |
| Volumetric changes in grey matter concentration. |
9 c; 9 hc. | Mean 29 | Mild, moderate and severe |
Decreased GMC in frontal and temporal cortices, cingulate gyrus, subcortical grey matter, and cerebellum. Correlate with attention deficits and lower GCS. |
(Gale et al., 2005) |
| Whole Brain volume loss |
16 c;16 hc | 9 to 16 | Moderate and Severe |
Globally reduced cortical thicknes, related to reported deficits in working memory. |
(Merkley et al., 2008) |
Key: LPC, left posterior cingulate. DLPFC, dorso-lateral pre-frontal cortex. MDLPFC, mid dorso-lateral pre-frontal cortex. MTL, medial temporal lobe. RPPC, right posterior parietal cortex. RVLPFC, right ventrolateral prefrontal cortex. SMA, supplementary motor areas
Key: DMN, default mode network. PCC, posterior cingulate cortex. MPFC, medial prefrontal cortex. LPC, lateral parietal cortex.
Key: FA, fractional anisotropy. RD, radial diffusivity. PCS, postconcussive symptoms. ADC, apparent diffusion coefficient. WM, white mater. DLPFC, dorso lateral pre-frontal cortex. CC, corpus callosum. MD, mean diffusivity.
Key: NAA, N-acetyl aspartate. Cre, creatine. Cho, choline
Key: SWI, Susceptibility weighted imaging. CT, computed tomography. MRI, magnetic resonance imaging.
Key: GMC, grey matter concentration. GCS, Glasgow coma score.
Resting State Networks-Measures of mBTI alterations in brain basal state
Recently, there has been increasing interest in the concept of understanding brain networks in a default functional activity without stimulation (Raichle and Mintun, 2006; Raichle and Snyder, 2007). Resting state networks (RSNs) have been identified in healthy children and are similar to those observed in adults. A core group of networks have been identified and associated with functions such as self-monitoring, emotional processing, interoception and exteroception. Several studies have demonstrated that disease states, such as depression, and chronic pain alter several of the resting state networks (Balenzuela et al., 2010; Jin et al., 2011). Mayer et al and Tang et al have depicted abnormal resting state networks in patients with mild TBI that correlate with cognitive symptoms (Mayer et al., 2009; Tang et al., 2011). A combination of evoked cognitive tasks with resting state analysis could help identify structures associated with functional deficits. Figure 8A and 8B show an example of differentiating changes in mTBI using this approach. See Table 1B, for studies utilizing RSN’s.
Figure 8.
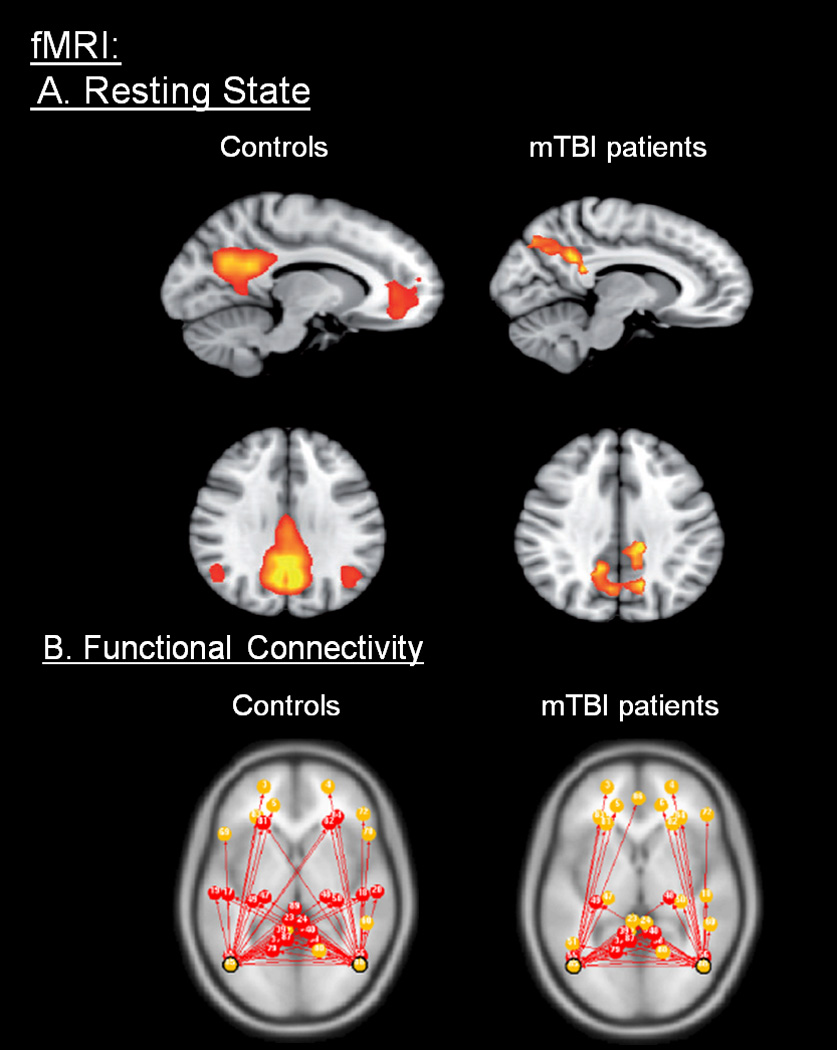
A: Functional Imaging - fMRI (Resting State). Functional MRI comparing healthy controls with patients with traumatic brain injury: Patients showed increased posterior cingulate cortex and precuneus functional connectivity activation than controls. The results hypothesize that such variations are a direct reflection of brain injury and also a representation of adaptive response to cognitive impairment. (From (Sharp et al., 2011); permission pending).
B: Functional connectivity. Functional MRI showing comparing resting state networks of normal volunteer vs. mTBI patients. The figure indicates the differences between shared (red) and non-shared (yellow) connections from left and right parietal lobes. (From (Johnson et al., 2012a); permission pending).
Perfusion Imaging- Assessing physiological brain changes
Arterial Spin Labeling (ASL) perfusion MRI is a newer technique that measures cerebral perfusion using arterial blood water as an endogenous contrast agent, discarding the need for contrast agents. Although not commonly used in children with mTBI, it has been used in adults with moderate to severe TBI showing global, regional and diffuse cerebral blood flow (CBF) reductions in the TBI subjects, demonstrating a potential use in the wide spectrum of TBI. Most notably hypoperfusion was found in the posterior cingulate cortices, the thalamus, and frontal cortex (Kim et al., 2010). Such findings may reflect and contribute to neurocognitive changes. Further studies of mild TBI studies using ASL should be encouraged.
5.2.2. Magnetic Resonance Spectroscopy (MRS)-Measuring changes at the chemical level
Proton MR spectroscopy has been used to identify concentration of neurometabolites that can be markers of brain injury, such as lactate, in order to visualize structures that have sustained damage (Suskauer and Huisman, 2009). Figure 9 shows and example of MRS in mTBI. Kirov, using this method, encountered minimum metabolite concentration changes (N-acetylaspartate (NAA), choline (Cho) and creatine (Cr)) in the thalamus between patients with mTBI and healthy controls, making this region of particular interest in the definition of mTBI (Kirov et al., 2007) (See Table 1D). It may also be potentially used to evaluate chemical changes in response to treatments. Proton magnetic resonance spectroscopy (1H-MRS) may provide an objective biomarker for complete resolution of concussion induced metabolic derangements, and may be significantly more reliable than resolution of clinical symptoms or deficits on cognitive testing. In a recent study of 13 concussed non-professional adult athletes, substantial neurochemical alterations is present in the injured brain and detectable by measuring NAA, despite the complete resolution of symptoms and normal routine brain MR imaging (Vagnozzi et al., 2008). Such an objective biomarker in the days to weeks after concussion could facilitate safe return-to-activity decisions. The extent to which this may prevent early repeat concussions and long-term sequelae is an important area for future research.
Figure 9.
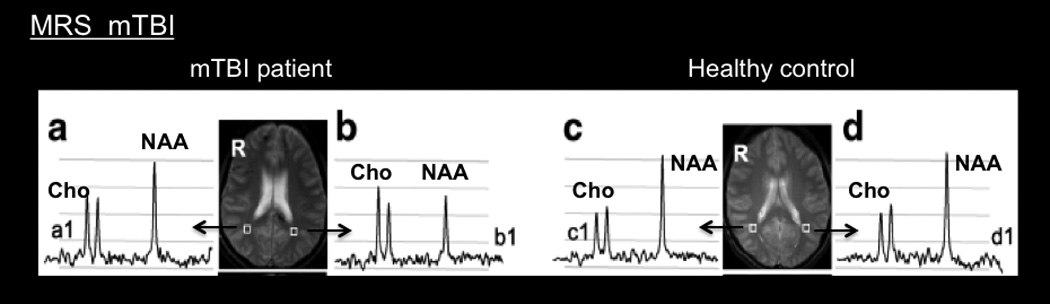
Magnetic Resonance Spectroscopy. MRS image comparing the right and left parieto occipital regions, (a and b, c and d respectively) between a mTBI patient and a healthy control: N-acetyl aspartate (NAA) and total choline (Cho) are significantly altered in b1 compared to the control subject at c1 and d1. (From (Govind et al., 2010); permission pending).
5.2.3 Magnetic Resonance Elastography (MRE)
MRE synchronizes mechanical excitations with a phase contrast imaging pulse sequence to noninvasively register shear wave propagation, from which local values of tissue viscoelastic properties can be deduced. MRE has been proposed as a potentially useful marker of TBI since concussion and other types of TBI may involve significant compression of brain tissue, brain edema, and possibly tissue out of its elastic range, the mechanical behavior of brain tissue may be altered. In animal models, MRE signal has been found to be affected by TBI, with significantly reduced shear stiffness in injured brain regions, suggesting that shear stiffness may be used as a marker of TBI and MRE may play an increasing role in the diagnosis and prognostication of TBI (Boulet et al., 2011).
5.2.4. Morphometric Imaging – Measures of Gray matter and White matter integrity
Diffusion weighted imaging (DWI)
DWI is an imaging technique that can be used to measure white matter integrity. Given the nature of concussion affects on axonal injury, the use of this technique has become more frequently used even as a standard of care. The technique shows apparent probability of the direction of movement of water molecules in the brain, and it has helped differentiate cytotoxic edema from vascular edema with the potential benefit of monitoring outcome after brain injury. Susceptibility weighted imaging (SWI), as its name implies, it uses the magnetic susceptibilities of extracellular and extravascular blood products in the brain. It detects lesions by the finding the products associated with shearing injuries such as diffuse axonal injury and can be correlated with neurocognitive outcomes of TBI (Suskauer, 2009; Suskauer and Huisman, 2009). This approach has demonstrated small alterations in white matter within the brains of patients suffering concussion (Park et al., 2009). It is assumed that such abnormalities alter effective functional communication between brain regions and diminish overall brain function in patients. Measure of how these alterations resolve over time will contribute to better understanding of treatments. For example, membrane stabilizers (including Imipramine and Amitriptyline that have a sodium channel blocking function (Gerner et al., 2003; Yang and Kuo, 2002)) may provide benefit by stabilizing activity in these damaged regions. Table 1F lists examples of studies utilizing DWI in concussion studies.
Wilde et al., discovered through diffusion tensor imaging (DTI) that despite normal computer tomographic (CT) findings, unremarkable conventional MRI findings in all but one patient, and a GCS score of 15, adolescents who sustained MTBI had increased Fractional Anisotropy (FA) and decreased diffusivity in the Corpus Callosum (CC) within 6 days post-injury. Cognitive, affective, and somatic post-concussion symptoms were all related to DTI indices of CC integrity including fractional anisotropy. DTI indices are sensitive to pathologic processes of mTBI that may correlate with the post-concussion symptom severity of patients (Wilde et al., 2008). In another study, using DTI, measures of white matter (WM) fiber tract integrity was assessed in varsity level college athletes with sports related concussion without loss of consciousness, who experienced symptoms for at least 1 month after injury. Notable abnormalities in structural integrity were present in subjects after sustaining concussion. The main structures affected where the left temporal lobe, the retrolenticular part of the internal capsule, and the posterior thalamic radiation, which contain fibers that connect the frontal and occipital lobes as well as the temporal and occipital lobes. Brain-injured subjects tended to have increased Mean Diffusivity (MD) compared to controls and a decreased FA compared to controls. The authors propose that MD may be more sensitive at detecting mild injury, whereas FA captures more severe injuries (Cubon et al., 2011). Similar results were reported in another study of concussed athletes, both acute (1–6 days post injury) and chronic (6 months) changes were measured. FA was increased in dorsal regions of both corticospinal tracts and the corpus callosum at both scanning sessions, while MD was decreased in the same regions at both time points (Henry et al., 2011). Such data support the use of DTI in detecting subtle changes in the brain. Figure 10 is an example of DTI measures in an mTBI patient.
Figure 10.
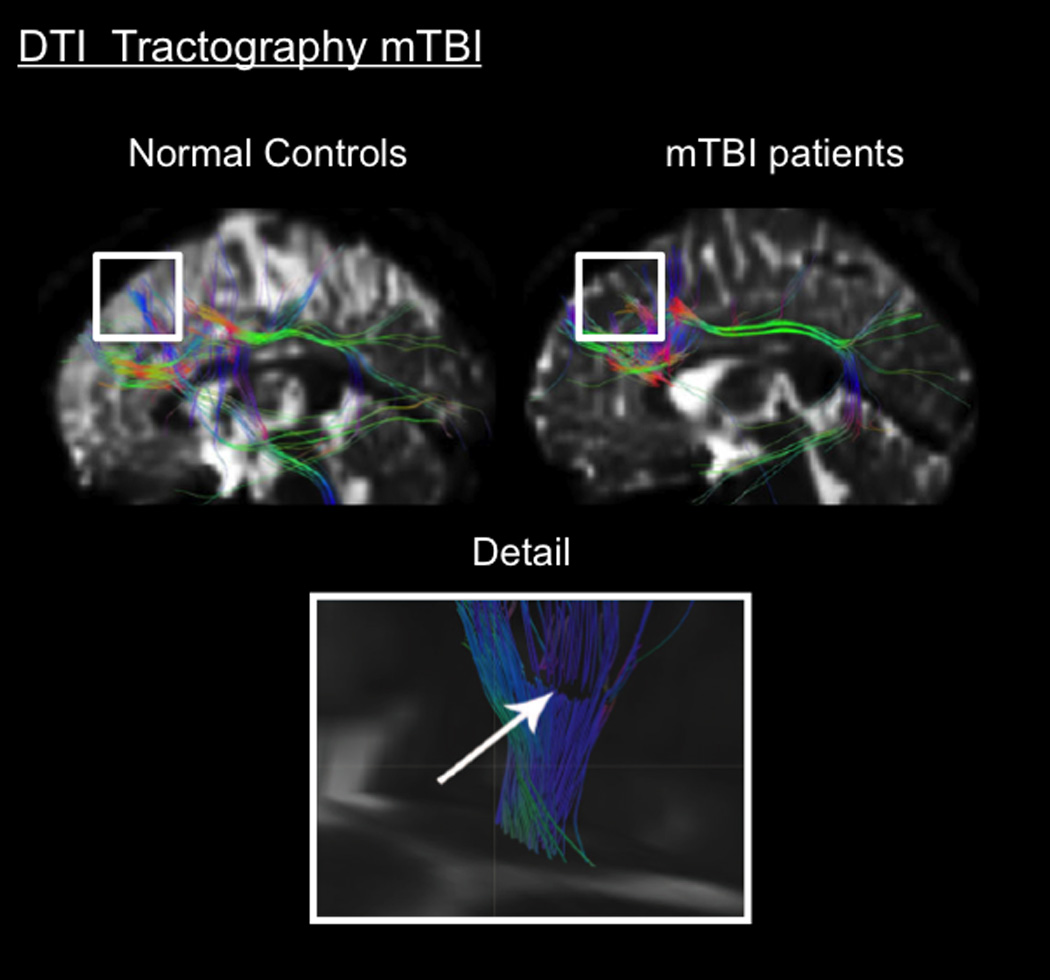
Morphometric Imaging in Concussion. Top. DTI fiber tractography showing track fiber pattern to the left DLPFC in healthy controls and mTBI patients: 15 student-athletes (mean age 20.8 ± 1.7 years) who suffered from sport-related mTBI (collegiate rugby, ice hockey and soccer players). Statistical analysis demonstrated that these different patterns involved significant variations in diffusivity between these two groups; the mTBI group had decreased diffusivity. (From (Zhang et al., 2010a); permission pending).
Bottom. DTI fiber tractography detail of a mTBI patient at the level of the right semiovale center: Mild TBI patients possessed a GCS of 13–15 after a traffic accident, blow to the head or fall. Note the discontinuous characteristics of the fibers, hypothesized to be caused by trauma. (From (Rutgers et al., 2008); permission pending).
The correlation of severity of post-concussive symptoms with changes in white matter integrity are becoming a focus in a number of DTI studies: the severity of post-concussive symptoms after minor head injury is significantly correlated with a reduction of white matter integrity, as manifested by increases in diffusivity and reduced anisotropic diffusion (Smits et al., 2011); widespread primary changes in the acute stage and secondary changes after 6 months in the white matter can be detected on DTI, even when conventional imaging appears normal and correlate significantly with the results of some of the neuropsychological tests (Kumar et al., 2009); similarly, Chu et al, also reported significant alteration in DTI metrics in a group of patients with mTBI in several brain regions, such as the corpus callosum, the thalamus, where white matter tracts were significantly affected and these changes were highly correlated with postconcussive symptom severity and emotional distress (Chu et al., 2010); and in DTI studies of mTBI in 10 adolescents (14 to 19 years of age) with mTBI 1 to 6 days post-injury with Glasgow Coma Scale score of 15 and negative CT, increased FA and decreased apparent diffusion coefficient (ADC) and radial diffusivity (RD), were correlated with intense post-concussion symptoms and emotional distress compared to the control group and also correlated with severity of post-concussion symptoms in the mTBI group (Mayer et al., 2010). Table 1C summarizes prior studies of DTI in concussed patients.
Volumetric Changes Indices of Gray Matter Alterations
High-resolution structural images couple with sophisticated analysis processes permit the determination of volumetric change in subcortical structures as well as determination of cortical thickness alterations. As previously mentioned whole brain atrophy can be evident after 11 months post trauma (MacKenzie et al., 2002), and it has been found that in the entire range of TBI (mild to severe), patients show decreased grey matter concentration in the frontal and temporal cortices, subcortical grey matter, cingulate gyrus, and the cerebellum. These findings being correlated with reduced attention and lower GCS (Gale et al., 2005). In children with TBI, it’s been reported that significant cortical thinning occurs which is also related to deficits in working memory (Merkley et al., 2008).
6. Future directions
The recognition of mTBI and its significance has evolved throughout the years, and for this reason, the means to diagnose this condition must evolve as well. In spite of the increased awareness of the problem, there is still some evidence that, clinically, mTBI is under-recognized by emergency department staff, even when patients reported findings consistent with such lesion (Powell et al., 2008). The main issues that need to be addressed are the ability to diagnose and treat the initial brain changes following concussion, to measure the effectiveness of these treatments (neurocognitive training, pharmacotherapy, TMS, etc.), and to provide a predictive and measurable outcome of the long term changes following concussion. Clearly, objective methods that can determine a unambiguous diagnosis and guidelines for recovery would be enormously useful (d'Hemecourt, 2011). In addition, we know relatively little about the neurobiology of concussion and the optimal therapeutic treatments. Current treatment recommendations include cognitive rest and pharmacological treatments for symptoms such as headache.
The use of brain imaging biomarkers for mTBI is provocative given the nature of the injury and the ability to now measure subclinical consequences of the disease (De Beaumont et al., 2011; Theriault et al., 2009). Figure 11 summarizes the use of imaging across three main domains – neural systems biology, diagnostics (biomarker) and the implications of potential biomarkers evaluate therapeutic measures. It is worrisome that abnormal changes in brain function may be identified in athletes with no previous diagnosis of concussion (Talavage et al., 2010) (see Figure 12) or in athletes with no deficits in behavioral performance (Jantzen et al., 2004). Recent studies have utilized fMRI and neurocognitive testing; both have proven to be useful in identifying brain adaptability to injury and potential recognition of long-term markers of persistent neurological sequelae (Schutze et al., 2008).
Figure 11.
Imaging uses. The figure shows the broad range of current potential uses of imaging in this field, with physiological, diagnostic and therapeutic implications.
Figure 12.
Brain alterations shown on functional imaging without behavioral changes. fMRI image of highschool football players without clinically diagnosed concussion, performing neurocognitive testing before football season and during football season: Even in the absence of concussion [in 8 out of 21 athletes], fMRI shows significantly different changes in the athletes brain, such changes are correlated with a poorer performance in neurocognitive testing. (From (Talavage et al., 2010); permission pending).
Like other CNS conditions, the emergence of imaging as a modality in concussion to identify the underlying anatomy and biology of disease and as a biomarker of recovery and long-term neurological sequelae (Ellemberg et al., 2009) (Suskauer and Huisman, 2009) holds great promise for advancing our understanding of this public health crisis and developing more informed and specific strategies for treatment and rehabilitation (McCullough and Jarvik, 2011) (Borsook et al., 2011a, b) (Figure 13).
Figure 13.
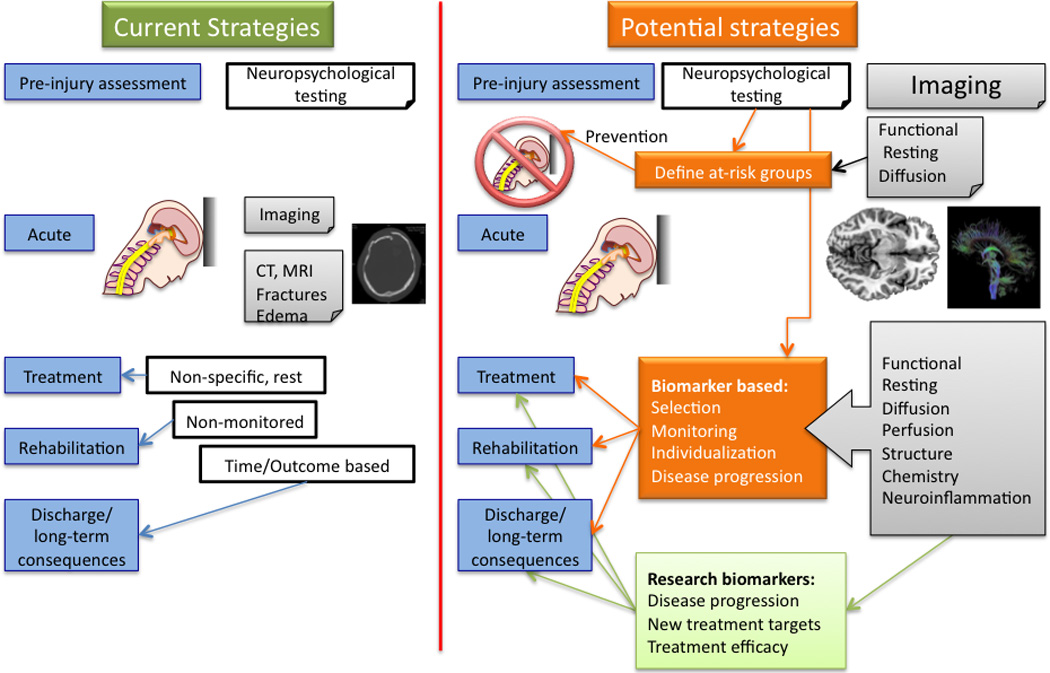
Chart depicting how a new approach for concussion can be reached with imaging and how it can change the field. Imaging encloses different and specific aspects of this pathology, which makes it a precise and sensitive tool in TBI diagnosis, pathophysiology and treatment.
The absence of any pathological findings in standard imaging methods is part of the diagnostic criteria of concussion. Novel methods of imaging could change the entire classification of mTBI with increased sensitivity to microstructural disruptions seen in Diffuse Axonal Injury (DAI). Of the emerging MR imaging techniques, Diffusion Tensor Imaging (DTI; tractography), holds promise for improving understanding of more specific brain-behavior relationships after pediatric TBI and for refining prognostic evaluation. Early evidence also suggests that these techniques may be useful for understanding mechanisms of recovery after pediatric TBI, though further longitudinal work is needed to understand the interaction between development, injury, and recovery in children with TBI (Suskauer and Huisman, 2009).
Specifically, there are 4 main areas that imaging may contribute to improving our understanding and treatment of mTBI in children:
(1) Establishing neuroimaging correlates for neurocognitive and neurobehavioral sequelae of mTBI
Estabishing such correlates will serve two purposes: First, it will provide means to understand where in the brain is responsible for a specific neurocognitive and neurobehavioral sequelae of mTBI. Second it will help to assess the success of interventions or treatment approaches and see if any improvement has been achieved. The benefit of this approach could be that the treatment responses (changes in the levels of neurotransmitters or inflammatory factors as measured by MRS for instance) could be recorded and serve as an index for recovery even before clinical symptoms are detected.
(2) mTBI and normal brain development
mTBI in children is more serious issue compared to adults, as the brain has not yet matured and it is still developing. The rate of the changes in the developing brain is not steady and it is age-dependant Therefore studying mTBI in children should be done in the context of development in order to determine how alteration in the brain as a result of mTBI could interfere with normal development or change the course or direction of it. There is a wealth of information in the literature on the application of neuroimaging techniques in studying developing brain in health and disease. Therefore the same neuroimaging approaches could be applied to image children with mTBI and compare the findings to normal children to see how the developing trajectories may vary. As such age-dependent treatment approaches may emerge which may be more efficacious.
(3) Reversibility of the damages post injury
How does the brain recover post injury? Are the damages resulting from mTBI reversible? While in mTBI there is less evidence to answer these questions, in chronic pain conditions (such as chronic migraine), neuroimaging studies have shown changes in the functional and structural plasticity as a consequence of chronic pain (Rodriguez-Raecke et al., 2009) that is reversible once the pain is adequately treated.
(4) Age-dependent individualized and optimized treatment approaches
There are inherent individual differences in the brain function and structure so as differences in brain development. Neuroimaging can provide an objective way of determining the status of the brain (e.g., by measuring myelination level using MRI techniques) and hence optimizing the intervention. The type of treatment will depend on the stage of the recovery for instance if the injury is new, the treatment should focus on neuroprotection.
7. Conclusions
Mild TBI is not a benign event and may have persistent effects on brain function and structure. This review describes the impact of mTBI and its potential effect upon brain regions and connectivity. A current paradox exists in which many studies show the extent and severity of the consequences with an increased incidence and prevalence of acute as well as chronic problems, while statistics indicate that the disease burden seems to decrease over time. The child and adolescent brain provides a challenge in this type of injury and highlights the need for specific management of the developing central nervous system. Emerging neuroimaging techniques may identify markers of severity and long-term disability and aid in delineating more precise treatments for the neurologic sequelae of mTBI.
Highlights.
Concussion is a significant pediatric public health concern.
Concussion affects several cortical and subcortical regions and systems.
The extent and chronicity of disability is yet to be elucidated in children.
Using new imaging techniques, subclinical changes are found in the concussed brain.
Neuroimaging techniques may identify markers of severity and long-term disability.
Acknowledgements
A grant from NINDS to DB [K24NS064050].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Judeh HH, Parker R, Aleksic S, Singh ML, Naddaf S, Atay S, Kumar M, Omar W, El-Zeftawy H, Luo JQ, Abdel-Dayem HM. SPECT brain perfusion findings in mild or moderate traumatic brain injury. Nucl Med Rev Cent East Eur. 2000;3:5–11. [PubMed] [Google Scholar]
- Acerini CL, Tasker RC, Bellone S, Bona G, Thompson CJ, Savage MO. Hypopituitarism in childhood and adolescence following traumatic brain injury: the case for prospective endocrine investigation. Eur J Endocrinol. 2006;155:663–669. doi: 10.1530/eje.1.02284. [DOI] [PubMed] [Google Scholar]
- Agha A, Sherlock M, Phillips J, Tormey W, Thompson CJ. The natural history of post-traumatic neurohypophysial dysfunction. Eur J Endocrinol. 2005;152:371–377. doi: 10.1530/eje.1.01861. [DOI] [PubMed] [Google Scholar]
- Agha A, Thornton E, O'Kelly P, Tormey W, Phillips J, Thompson CJ. Posterior pituitary dysfunction after traumatic brain injury. J Clin Endocrinol Metab. 2004;89:5987–5992. doi: 10.1210/jc.2004-1058. [DOI] [PubMed] [Google Scholar]
- Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. 10 years outcome from childhood traumatic brain injury. Int J Dev Neurosci. 2011 doi: 10.1016/j.ijdevneu.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M, Kido D. Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J Neurotrauma. 2004;21:1539–1552. doi: 10.1089/neu.2004.21.1539. [DOI] [PubMed] [Google Scholar]
- Ayata C. Cortical spreading depression triggers migraine attack: pro. Headache. 50:725–730. doi: 10.1111/j.1526-4610.2010.01647.x. [DOI] [PubMed] [Google Scholar]
- Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- Baguley IJ, Nott MT, Slewa-Younan S, Heriseanu RE, Perkes IE. Diagnosing dysautonomia after acute traumatic brain injury: evidence for overresponsiveness to afferent stimuli. Arch Phys Med Rehabil. 2009;90:580–586. doi: 10.1016/j.apmr.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Baguley IJ, Slewa-Younan S, Heriseanu RE, Nott MT, Mudaliar Y, Nayyar V. The incidence of dysautonomia and its relationship with autonomic arousal following traumatic brain injury. Brain Inj. 2007;21:1175–1181. doi: 10.1080/02699050701687375. [DOI] [PubMed] [Google Scholar]
- Bakhos LL, Lockhart GR, Myers R, Linakis JG. Emergency department visits for concussion in young child athletes. Pediatrics. 2010;126:e550–e556. doi: 10.1542/peds.2009-3101. [DOI] [PubMed] [Google Scholar]
- Balenzuela P, Chernomoretz A, Fraiman D, Cifre I, Sitges C, Montoya P, Chialvo DR. Modular organization of brain resting state networks in chronic back pain patients. Front Neuroinform. 2010;4:116. doi: 10.3389/fninf.2010.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Birch R, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport. 2001;12:3439–3442. doi: 10.1097/00001756-200111160-00012. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. vii-iii. [DOI] [PubMed] [Google Scholar]
- Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126:e374–e381. doi: 10.1542/peds.2009-0925. [DOI] [PubMed] [Google Scholar]
- Barth JT, Freeman JR, Broshek DK, Varney RN. Acceleration-Deceleration Sport-Related Concussion: The Gravity of It All. J Athl Train. 2001;36:253–256. [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Fritz H. Pathophysiology of traumatic injury in the developing brain: an introduction and short update. Exp Toxicol Pathol. 2004;56:65–73. doi: 10.1016/j.etp.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Stocker R, Imhof HG, Trentz O, Hersberger M, Mignot E, Bassetti CL. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging. 2011 doi: 10.1016/j.mri.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Ditchfield M, Babl FE, Kean M, Catroppa C, Yeates KO, Anderson V. Detecting traumatic brain lesions in children: CT versus MRI versus susceptibility weighted imaging (SWI) J Neurotrauma. 2011;28:915–927. doi: 10.1089/neu.2010.1712. [DOI] [PubMed] [Google Scholar]
- Becelewski J, Pierzchala K. [Cerebrovascular reactivity in patients with mild head injury] Neurol Neurochir Pol. 2003;37:339–350. [PubMed] [Google Scholar]
- Beers SR. Cognitive effects of mild head injury in children and adolescents. Neuropsychol Rev. 1992;3:281–320. doi: 10.1007/BF01108414. [DOI] [PubMed] [Google Scholar]
- Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753–759. doi: 10.1136/jnnp.2007.132837. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Bettucci D, Aguggia M, Bolamperti L, Riccio A, Mutani R. Chronic post-traumatic headache associated with minor cranial trauma: a description of cephalalgic patterns. Ital J Neurol Sci. 1998;19:20–24. doi: 10.1007/BF03028807. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 2002;23:255–266. [PMC free article] [PubMed] [Google Scholar]
- Bland DC, Zampieri C, Damiano DL. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: a systematic review. Brain Inj. 2011;25:664–679. doi: 10.3109/02699052.2011.576306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendonohy PM, Philip PA. Precocious puberty in children after traumatic brain injury. Brain Inj. 1991;5:63–68. doi: 10.3109/02699059108998513. [DOI] [PubMed] [Google Scholar]
- Bloomfield IL, Espie CA, Evans JJ. Do sleep difficulties exacerbate deficits in sustained attention following traumatic brain injury? J Int Neuropsychol Soc. 2010;16:17–25. doi: 10.1017/S1355617709990798. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Twijnstra A, Jolles J. Water metabolism and postconcussional symptoms 5 weeks after mild head injury. Eur Neurol. 1993;33:77–79. doi: 10.1159/000116907. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discov Med. 2011a;11:197–207. [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: how where, and what to look for using functional imaging. Discov Med. 2011b;11:209–219. [PubMed] [Google Scholar]
- Boulet T, Kelso ML, Othman SF. Microscopic magnetic resonance elastography of traumatic brain injury model. J Neurosci Methods. 2011;201:296–306. doi: 10.1016/j.jneumeth.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, Sumner P. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103:607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- Browne KD, Chen XH, Meaney DF, Smith DH. Mild traumatic brain injury and diffuse axonal injury in swine. J Neurotrauma. 2011;28:1747–1755. doi: 10.1089/neu.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A, Siman R, Trojanowski JQ, Povlishock JT. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J Neuropathol Exp Neurol. 1999;58:365–375. doi: 10.1097/00005072-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Byard RW, Vink R. The second impact syndrome. Forensic Sci Med Pathol. 2009;5:36–38. doi: 10.1007/s12024-008-9063-7. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Geurts M, Taymans T, Linden CV, Smits-Engelsman BC, Sunaert S, Swinnen SP. Brain-behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Hum Brain Mapp. 2010a;31:992–1002. doi: 10.1002/hbm.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Geurts M, Taymans T, Vander Linden C, Smits-Engelsman BC, Sunaert S, Swinnen SP. Brain-behavior relationships in young traumatic brain injury patients: fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia. 2010b;48:1472–1482. doi: 10.1016/j.neuropsychologia.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Cantu RC. Return to play guidelines after a head injury. Clin Sports Med. 1998a;17:45–60. doi: 10.1016/s0278-5919(05)70060-0. [DOI] [PubMed] [Google Scholar]
- Cantu RC. Second-impact syndrome. Clin Sports Med. 1998b;17:37–44. doi: 10.1016/s0278-5919(05)70059-4. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, Pepin M. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer V, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39:805–811. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41:305–313. [PMC free article] [PubMed] [Google Scholar]
- CDC. Traumatic Brain Injury in the United States. 2010 [Google Scholar]
- Cernak I, Chang T, Ahmed FA, Cruz MI, Vink R, Stoica B, Faden AI. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2010;32:442–453. doi: 10.1159/000320085. [DOI] [PubMed] [Google Scholar]
- Claus CP, Tsuru-Aoyagi K, Adwanikar H, Walker B, Manvelyan H, Whetstone W, Noble-Haeusslein LJ. Age is a determinant of leukocyte infiltration and loss of cortical volume after traumatic brain injury. Dev Neurosci. 2010;32:454–465. doi: 10.1159/000316805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collie A, Makdissi M, Maruff P, Bennell K, McCrory P. Cognition in the days following concussion: comparison of symptomatic versus asymptomatic athletes. J Neurol Neurosurg Psychiatry. 2006;77:241–245. doi: 10.1136/jnnp.2005.073155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MW, Grindel SH, Lovell MR, Dede DE, Moser DJ, Phalin BR, Nogle S, Wasik M, Cordry D, Daugherty KM, Sears SF, Nicolette G, Indelicato P, McKeag DB. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282:964–970. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]
- Couch JR, Bearss C. Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache. 2001;41:559–564. doi: 10.1046/j.1526-4610.2001.041006559.x. [DOI] [PubMed] [Google Scholar]
- Covassin T, Elbin RJ, Nakayama Y. Tracking neurocognitive performance following concussion in high school athletes. Phys Sportsmed. 2010;38:87–93. doi: 10.3810/psm.2010.12.1830. [DOI] [PubMed] [Google Scholar]
- Cozzarelli TA. Evaluation and treatment of persistent cognitive dysfunction following mild traumatic brain injury. J Spec Oper Med. 2010;10:39–42. [PubMed] [Google Scholar]
- Creed JA, DiLeonardi AM, Fox DP, Tessler AR, Raghupathi R. Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J Neurotrauma. 2010;28:547–563. doi: 10.1089/neu.2010.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton MR. Hypothalamic lesions following closed head injury. Brain. 1971;94:165–172. doi: 10.1093/brain/94.1.165. [DOI] [PubMed] [Google Scholar]
- Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28:189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly H, Chaari A, Daoud E, Dammak H, Medhioub F, Mnif J, Hamida CB, Bahloul M, Bouaziz M. Diffuse axonal injury in patients with head injuries: an epidemiologic and prognosis study of 124 cases. J Trauma. 2011;71:838–846. doi: 10.1097/TA.0b013e3182127baa. [DOI] [PubMed] [Google Scholar]
- Chen G, Shi J, Hu Z, Hang C. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Collie A, McCrory P, Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry. 2007;78:1231–1238. doi: 10.1136/jnnp.2006.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Chou YC, Wang TY, Yang PY, Meng NH, Chou LW. Permanent central diabetes insipidus after mild traumatic brain injury. Brain Inj. 2009;23:1095–1098. doi: 10.3109/02699050903379396. [DOI] [PubMed] [Google Scholar]
- Chu Z, Wilde EA, Hunter JV, McCauley SR, Bigler ED, Troyanskaya M, Yallampalli R, Chia JM, Levin HS. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol. 2010;31:340–346. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah YM, Maybery MT, Fox AM. The long-term effects of mild head injury on short-term memory for visual form, spatial location, and their conjunction in well-functioning university students. Brain Cogn. 2004;56:304–312. doi: 10.1016/j.bandc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- d'Hemecourt P. Subacute symptoms of sports-related concussion: outpatient management and return to play. Clin Sports Med. 2011;30:63–72. doi: 10.1016/j.csm.2010.08.008. Viii. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dai SS, Zhou YG, Li W, An JH, Li P, Yang N, Chen XY, Xiong RP, Liu P, Zhao Y, Shen HY, Zhu PF, Chen JF. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci. 2010;30:5802–5810. doi: 10.1523/JNEUROSCI.0268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–295. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SL, Hirsch CS, Lucas FV, Sebek BA. The contrecoup phenomenon. Reappraisal of a classic problem. Hum Pathol. 1980;11:155–166. doi: 10.1016/s0046-8177(80)80136-5. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Mongeon D, Tremblay S, Messier J, Prince F, Leclerc S, Lassonde M, Theoret H. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46:234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- De Tanti A, Gasperini G, Rossini M. Paroxysmal episodic hypothalamic instability with hypothermia after traumatic brain injury. Brain Inj. 2005;19:1277–1283. doi: 10.1080/02699050500309270. [DOI] [PubMed] [Google Scholar]
- DeLellis SM, Kane S, Katz K. The neurometabolic cascade and implications of mTBI: mitigating risk to the SOF community. J Spec Oper Med. 2009;9:36–42. doi: 10.55460/0OI6-W6Z7. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive Brain Stimulation in Traumatic Brain Injury. J Head Trauma Rehabil. 2011 doi: 10.1097/HTR.0b013e318217df55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma. 2009;66:289–296. doi: 10.1097/TA.0b013e3181961da2. discussion 296–287. [DOI] [PubMed] [Google Scholar]
- Drake ME, Jr, Jackson RD, Miller CA. Paroxysmal choreoathetosis after head injury. J Neurol Neurosurg Psychiatry. 1986;49:837–838. doi: 10.1136/jnnp.49.7.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellemberg D, Henry LC, Macciocchi SN, Guskiewicz KM, Broglio SP. Advances in sport concussion assessment: from behavioral to brain imaging measures. J Neurotrauma. 2009;26:2365–2382. doi: 10.1089/neu.2009.0906. [DOI] [PubMed] [Google Scholar]
- Elliott ML, Biever LS. Head injury and sexual dysfunction. Brain Inj. 1996;10:703–717. doi: 10.1080/026990596123972. [DOI] [PubMed] [Google Scholar]
- Evans RW. Persistent post-traumatic headache, postconcussion syndrome, and whiplash injuries: the evidence for a non-traumatic basis with an historical review. Headache. 2010;50:716–724. doi: 10.1111/j.1526-4610.2010.01645.x. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr, Fletcher JM, Barnes M, Zhang X, Hasan KM. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas O, Lifshitz J, Povlishock JT. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury? J Neurosci. 2006;26:3130–3140. doi: 10.1523/JNEUROSCI.5119-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Taylor HG, Bangert B, Dietrich A, Nuss KE, Rusin J, Wright M. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J Int Neuropsychol Soc. 2009;16:94–105. doi: 10.1017/S1355617709991007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenberg NL, Zafonte RD, Putnam S, Mann NR, Millard AE. Insomnia in a post-acute brain injury sample. Brain Inj. 2002;16:197–206. doi: 10.1080/02699050110103940. [DOI] [PubMed] [Google Scholar]
- Fiser SM, Johnson SB, Fortune JB. Resource utilization in traumatic brain injury: the role of magnetic resonance imaging. Am Surg. 1998;64:1088–1093. [PubMed] [Google Scholar]
- Franklin CC, Weiss JM. Stopping sports injuries in kids: an overview of the last year in publications. Curr Opin Pediatr. 2012;24:64–67. doi: 10.1097/MOP.0b013e32834ec618. [DOI] [PubMed] [Google Scholar]
- Fumeya H, Hideshima H. Cerebellar concussion--three case reports. Neurol Med Chir (Tokyo) 1994;34:612–615. doi: 10.2176/nmc.34.612. [DOI] [PubMed] [Google Scholar]
- Gale SD, Baxter L, Roundy N, Johnson SC. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J Neurol Neurosurg Psychiatry. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc. 2004a;36:1269–1274. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- Gall B, Parkhouse WS, Goodman D. Exercise following a sport induced concussion. Br J Sports Med. 2004b;38:773–777. doi: 10.1136/bjsm.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Pena M, Sanchez Cabezas A. [Perceptive and praxic impairments in traumatic brain injury patients: significance in activities of daily living] Rev Neurol. 2004;38:775–784. [PubMed] [Google Scholar]
- Gerner P, Haderer AE, Mujtaba M, Sudoh Y, Narang S, Abdi S, Srinivasa V, Pertl C, Wang GK. Assessment of differential blockade by amitriptyline and its N-methyl derivative in different species by different routes. Anesthesiology. 2003;98:1484–1490. doi: 10.1097/00000542-200306000-00028. [DOI] [PubMed] [Google Scholar]
- Gessel LM, Fields SK, Collins CL, Dick RW, Comstock RD. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- Geurts AC, Knoop JA, van Limbeek J. Is postural control associated with mental functioning in the persistent postconcussion syndrome? Arch Phys Med Rehabil. 1999;80:144–149. doi: 10.1016/s0003-9993(99)90111-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey SJ, Ramadan NM, Aurora TK, Welch KM. Cerebral blood flow in chronic posttraumatic headache. Headache. 1997;37:583–587. doi: 10.1046/j.1526-4610.1997.3709583.x. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The Neurometabolic Cascade of Concussion. J Athl Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Robertson CS, Bryan RM., Jr The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin Exp Hypertens. 1999;21:299–332. doi: 10.3109/10641969909068668. [DOI] [PubMed] [Google Scholar]
- Goldsmith W, Plunkett J. A biomechanical analysis of the causes of traumatic brain injury in infants and children. Am J Forensic Med Pathol. 2004;25:89–100. doi: 10.1097/01.paf.0000127407.28071.63. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Toweill D, Lai S, Sonnenthal K, Kimberly B. Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am J Physiol. 1998;275:R1287–R1292. doi: 10.1152/ajpregu.1998.275.4.R1287. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Tellier M. Patients with traumatic brain injury are at high risk of developing chronic sleep-wake disturbances. J Neurol Neurosurg Psychiatry. 2010;81:1297. doi: 10.1136/jnnp.2010.222471. [DOI] [PubMed] [Google Scholar]
- Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart KL, Harris L, Jagid J, Maudsley AA. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic V. [Cervicogenic headache: etiopathogenesis, characteristics, diagnosis, differential diagnosis and therapy] Lijec Vjesn. 2007;129:230–236. [PubMed] [Google Scholar]
- Grubenhoff JA, Kirkwood MW, Deakyne S, Wathen J. Detailed concussion symptom analysis in a paediatric ED population. Brain Inj. 2011;25:943–949. doi: 10.3109/02699052.2011.597043. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Valovich McLeod TC. Pediatric sports-related concussion. PM R. 2011;3:353–364. doi: 10.1016/j.pmrj.2010.12.006. quiz 364. [DOI] [PubMed] [Google Scholar]
- Haas DC, Lourie H. Trauma-triggered migraine: an explanation for common neurological attacks after mild head injury. Review of the literature. J Neurosurg. 1988;68:181–188. doi: 10.3171/jns.1988.68.2.0181. [DOI] [PubMed] [Google Scholar]
- Hadani M, Findler G, Shaked I, Sahar A. Unusual delayed onset of diabetes insipidus following closed head trauma. Case report. J Neurosurg. 1985;63:456–458. doi: 10.3171/jns.1985.63.3.0456. [DOI] [PubMed] [Google Scholar]
- Hemphill MA, Dabiri BE, Gabriele S, Kerscher L, Franck C, Goss JA, Alford PW, Parker KK. A possible role for integrin signaling in diffuse axonal injury. PLoS One. 2011;6:e22899. doi: 10.1371/journal.pone.0022899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, Theoret H, Ellemberg D, Lassonde M. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Defina PA, Anders S, Koehn J, Lang CJ, Pauli E, Flanagan SR, Schwab S, Marthol H. Frequency Analysis Unveils Cardiac Autonomic Dysfunction after Mild Traumatic Brain Injury. J Neurotrauma. 2011 doi: 10.1089/neu.2010.1497. [DOI] [PubMed] [Google Scholar]
- Hochberg I, Hochberg Z. Expanding the definition of hypothalamic obesity. Obes Rev. 2010;11:709–721. doi: 10.1111/j.1467-789X.2010.00727.x. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Lucas S, Dikmen S, Braden CA, Brown AW, Brunner R, Diaz-Arrastia R, Walker WC, Watanabe TK, Bell KR. Natural History of Headache after Traumatic Brain Injury. J Neurotrauma. 2011 doi: 10.1089/neu.2011.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TN, Ferrara MS. Age-related differences in neuropsychological testing among high school athletes. J Athl Train. 2009;44:405–409. doi: 10.4085/1062-6050-44.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Anderson B, Steinberg FL, Kelso JA. A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR Am J Neuroradiol. 2004;25:738–745. [PMC free article] [PubMed] [Google Scholar]
- Jin C, Gao C, Chen C, Ma S, Netra R, Wang Y, Zhang M, Li D. A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neurosci Lett. 2011;503:105–109. doi: 10.1016/j.neulet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage. 2011a;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage. 2012a;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EW, Kegel NE, Collins MW. Neuropsychological assessment of sport-related concussion. Clin Sports Med. 2011b;30:73–88. doi: 10.1016/j.csm.2010.08.007. viii-ix. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2012b doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan FM, Cannon A, Murdoch BE. Language abilities of mildly closed head injured (CHI) children 10 years post-injury. Brain Inj. 1992;6:39–44. doi: 10.3109/02699059209008120. [DOI] [PubMed] [Google Scholar]
- Jordan FM, Murdoch BE. Linguistic status following closed head injury in children: a follow-up study. Brain Inj. 1990;4:147–154. doi: 10.3109/02699059009026159. [DOI] [PubMed] [Google Scholar]
- Jordan FM, Ozanne AE, Murdoch BE. Long-term speech and language disorders subsequent to closed head injury in children. Brain Inj. 1988;2:179–185. doi: 10.3109/02699058809150943. [DOI] [PubMed] [Google Scholar]
- Junger EC, Newell DW, Grant GA, Avellino AM, Ghatan S, Douville CM, Lam AM, Aaslid R, Winn HR. Cerebral autoregulation following minor head injury. J Neurosurg. 1997;86:425–432. doi: 10.3171/jns.1997.86.3.0425. [DOI] [PubMed] [Google Scholar]
- Junque C. [Neuropsychological sequelae of head injury] Rev Neurol. 1999;28:423–429. [PubMed] [Google Scholar]
- Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann N Y Acad Sci. 2010;1212:114–129. doi: 10.1111/j.1749-6632.2010.05800.x. [DOI] [PubMed] [Google Scholar]
- Kamerling SN, Lutz N, Posner JC, Vanore M. Mild traumatic brain injury in children: practice guidelines for emergency department and hospitalized patients. The Trauma Program, The Children's Hospital of Philadelphia, University of Pennsylvania School of Medicine. Pediatr Emerg Care. 2003;19:431–440. doi: 10.1097/01.pec.0000092590.40174.1f. [DOI] [PubMed] [Google Scholar]
- Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Autonomic dysfunction presenting as postural tachycardia syndrome following traumatic brain injury. Cardiol J. 2010;17:482–487. [PubMed] [Google Scholar]
- Kasahara M, Menon DK, Salmond CH, Outtrim JG, Taylor Tavares JV, Carpenter TA, Pickard JD, Sahakian BJ, Stamatakis EA. Altered functional connectivity in the motor network after traumatic brain injury. Neurology. 2010;75:168–176. doi: 10.1212/WNL.0b013e3181e7ca58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep-wake disturbances 3 years after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2010;81:1402–1405. doi: 10.1136/jnnp.2009.201913. [DOI] [PubMed] [Google Scholar]
- Kerr C. Dysnomia following traumatic brain injury: an information-processing approach to assessment. Brain Inj. 1995;9:777–796. doi: 10.3109/02699059509008234. [DOI] [PubMed] [Google Scholar]
- Kieslich M, Fiedler A, Heller C, Kreuz W, Jacobi G. Minor head injury as cause and co-factor in the aetiology of stroke in childhood: a report of eight cases. J Neurol Neurosurg Psychiatry. 2002;73:13–16. doi: 10.1136/jnnp.73.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp Neurol. 2009;219:553–561. doi: 10.1016/j.expneurol.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Avants B, Europa E, Wang J, Slattery J, Gee JC, Coslett HB, Detre JA. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J Neurotrauma. 2010;27:1399–1411. doi: 10.1089/neu.2009.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Kushi H, Hayashi N. Characteristics of parietal-parasagittal hemorrhage after mild or moderate traumatic brain injury. Acta Neurochir Suppl. 2003;86:343–346. doi: 10.1007/978-3-7091-0651-8_73. [DOI] [PubMed] [Google Scholar]
- Kirk C, Nagiub G, Abu-Arafeh I. Chronic post-traumatic headache after head injury in children and adolescents. Dev Med Child Neurol. 2008;50:422–425. doi: 10.1111/j.1469-8749.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood MW, Yeates KO, Taylor HG, Randolph C, McCrea M, Anderson VA. Management of pediatric mild traumatic brain injury: a neuropsychological review from injury through recovery. Clin Neuropsychol. 2008;22:769–800. doi: 10.1080/13854040701543700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117:1359–1371. doi: 10.1542/peds.2005-0994. [DOI] [PubMed] [Google Scholar]
- Kirov I, Fleysher L, Babb JS, Silver JM, Grossman RI, Gonen O. Characterizing 'mild' in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Brain Inj. 2007;21:1147–1154. doi: 10.1080/02699050701630383. [DOI] [PubMed] [Google Scholar]
- Kissick J, Johnston KM. Return to play after concussion: principles and practice. Clin J Sport Med. 2005;15:426–431. doi: 10.1097/01.jsm.0000186683.59158.8b. [DOI] [PubMed] [Google Scholar]
- Koepsell TD, Rivara FP, Vavilala MS, Wang J, Temkin N, Jaffe KM, Durbin DR. Incidence and descriptive epidemiologic features of traumatic brain injury in king county, washington. Pediatrics. 2011;128:946–954. doi: 10.1542/peds.2010-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, Geburek AJ, Rist F, Blumenroth H, Fischer B, Husstedt I, Arolt V, Schiffbauer H, Lohmann H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med. 2010:1–15. doi: 10.1017/S0033291710001728. [DOI] [PubMed] [Google Scholar]
- Kreutzer JS, Zasler ND. Psychosexual consequences of traumatic brain injury: methodology and preliminary findings. Brain Inj. 1989;3:177–186. doi: 10.3109/02699058909004550. [DOI] [PubMed] [Google Scholar]
- Krivitzky LS, Roebuck-Spencer TM, Roth RM, Blackstone K, Johnson CP, Gioia G. Functional magnetic resonance imaging of working memory and response inhibition in children with mild traumatic brain injury. J Int Neuropsychol Soc. 2011;17:1143–1152. doi: 10.1017/S1355617711001226. [DOI] [PubMed] [Google Scholar]
- Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, Pandey CM, Narayana PA. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- Kurwale NS, Gupta DK, Mahapatra AK. Outcome of pediatric patients with traumatic basal ganglia hematoma: analysis of 21 cases. Pediatr Neurosurg. 2010;46:267–271. doi: 10.1159/000321541. [DOI] [PubMed] [Google Scholar]
- Landi A, Marotta N, Mancarella C, Marruzzo D, Salvati M, Delfini R. Basal ganglia stroke due to mild head trauma in pediatric age -clinical and therapeutic management: a case report and 10 year literature review. Ital J Pediatr. 2011;37:2. doi: 10.1186/1824-7288-37-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19:216–221. doi: 10.1097/JSM.0b013e31819d6edb. [DOI] [PubMed] [Google Scholar]
- Lau BC, Collins MW, Lovell MR. Sensitivity and specificity of subacute computerized neurocognitive testing and symptom evaluation in predicting outcomes after sports-related concussion. Am J Sports Med. 2011;39:1209–1216. doi: 10.1177/0363546510392016. [DOI] [PubMed] [Google Scholar]
- Leao AA. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol. 1947;10:409–414. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Leddy JJ, Baker JG, Kozlowski K, Bisson L, Willer B. Reliability of a graded exercise test for assessing recovery from concussion. Clin J Sport Med. 2011;21:89–94. doi: 10.1097/JSM.0b013e3181fdc721. [DOI] [PubMed] [Google Scholar]
- Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma. 2008;25:1049–1056. doi: 10.1089/neu.2008.0566. [DOI] [PubMed] [Google Scholar]
- Lee LK. Controversies in the sequelae of pediatric mild traumatic brain injury. Pediatr Emerg Care. 2007;23:580–583. doi: 10.1097/PEC.0b013e31813444ea. quiz 584-586. [DOI] [PubMed] [Google Scholar]
- Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging. 2011;31:85–93. doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM, Burstein R. A critical view on the role of migraine triggers in the genesis of migraine pain. Headache. 2009;49:953–957. doi: 10.1111/j.1526-4610.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- Limond J, Leeke R. Practitioner review: cognitive rehabilitation for children with acquired brain injury. J Child Psychol Psychiatry. 2005;46:339–352. doi: 10.1111/j.1469-7610.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- Linnman C, Appel L, Soderlund A, Frans O, Engler H, Furmark T, Gordh T, Langstrom B, Fredrikson M. Chronic whiplash symptoms are related to altered regional cerebral blood flow in the resting state. Eur J Pain. 2009;13:65–70. doi: 10.1016/j.ejpain.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, Gold T, Shifteh K, Ardekani BA, Branch CA. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- Lucas S. Headache management in concussion and mild traumatic brain injury. PM R. 2011;3:S406–S412. doi: 10.1016/j.pmrj.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Lyczak P, Lyczak-Rucinska M. [Chronic post-traumatic headache and brain perfusion changes assessed using magnetic resonance imaging] Neurol Neurochir Pol. 2005;39:S49–S54. [PubMed] [Google Scholar]
- MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP, Grossman RI. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am J Neuroradiol. 2002;23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- Macpherson P, Teasdale E, Dhaker S, Allerdyce G, Galbraith S. The significance of traumatic haematoma in the region of the basal ganglia. J Neurol Neurosurg Psychiatry. 1986;49:29–34. doi: 10.1136/jnnp.49.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley MJ, English JB, Drubach DA, Kreuz AJ, Celnik PA, Tarwater PM. Prevalence of sleep disturbance in closed head injury patients in a rehabilitation unit. Neurorehabil Neural Repair. 2008;22:341–347. doi: 10.1177/1545968308315598. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, Burstein R, Borsook D. Migraine attacks the Basal Ganglia. Mol Pain. 2011;7:71. doi: 10.1186/1744-8069-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, Reichard R, Yeo RA. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Elgie R, Gasparovic C, Phillips JP, Doezema D, Yeo RA. Auditory orienting and inhibition of return in mild traumatic brain injury: a FMRI study. Hum Brain Mapp. 2009;30:4152–4166. doi: 10.1002/hbm.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Stein MB. Effects of psychological and biomechanical trauma on brain and behavior. Ann N Y Acad Sci. 2010;1208:46–57. doi: 10.1111/j.1749-6632.2010.05720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- McCrea M, Kelly JP, Kluge J, Ackley B, Randolph C. Standardized assessment of concussion in football players. Neurology. 1997;48:586–588. doi: 10.1212/wnl.48.3.586. [DOI] [PubMed] [Google Scholar]
- McCrory P. Does second impact syndrome exist? Clin J Sport Med. 2001;11:144–149. doi: 10.1097/00042752-200107000-00004. [DOI] [PubMed] [Google Scholar]
- McCrory P. Sports concussion and the risk of chronic neurological impairment. Clin J Sport Med. 2011;21:6–12. doi: 10.1097/JSM.0b013e318204db50. [DOI] [PubMed] [Google Scholar]
- McCrory P, Davis G, Makdissi M. Second impact syndrome or cerebral swelling after sporting head injury. Curr Sports Med Rep. 2012;11:21–23. doi: 10.1249/JSR.0b013e3182423bfd. [DOI] [PubMed] [Google Scholar]
- McCrory P, Johnston KM, Mohtadi NG, Meeuwisse W. Evidence-based review of sport-related concussion: basic science. Clin J Sport Med. 2001;11:160–165. doi: 10.1097/00042752-200107000-00006. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43(Suppl 1):i76–i90. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- McCullough BJ, Jarvik JG. Diagnosis of concussion: the role of imaging now and in the future. Phys Med Rehabil Clin N Am. 2011;22:635–652. doi: 10.1016/j.pmr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- McHugh T, Laforce R, Jr, Gallagher P, Quinn S, Diggle P, Buchanan L. Natural history of the long-term cognitive, affective, and physical sequelae of mild traumatic brain injury. Brain Cogn. 2006;60:209–211. [PubMed] [Google Scholar]
- Meehan WP., 3rd Medical therapies for concussion. Clin Sports Med. 2011;30:115–124. doi: 10.1016/j.csm.2010.08.003. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan WP, 3rd, d'Hemecourt P, Collins CL, Comstock RD. Assessment and management of sport-related concussions in United States high schools. Am J Sports Med. 2011;39:2304–2310. doi: 10.1177/0363546511423503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkley TL, Bigler ED, Wilde EA, McCauley SR, Hunter JV, Levin HS. Diffuse changes in cortical thickness in pediatric moderate-to-severe traumatic brain injury. J Neurotrauma. 2008;25:1343–1345. doi: 10.1089/neu.2008.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messe A, Caplain S, Paradot G, Garrigue D, Mineo JF, Soto Ares G, Ducreux D, Vignaud F, Rozec G, Desal H, Pelegrini-Issac M, Montreuil M, Benali H, Lehericy S. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp. 2011;32:999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton K, Krabak BJ, Coppel DB. The influence of pediatric autonomic dysfunction on recovery after concussion. Clin J Sport Med. 2010;20:491–492. doi: 10.1097/JSM.0b013e3181fac088. [DOI] [PubMed] [Google Scholar]
- Mihalik JP, Stump JE, Collins MW, Lovell MR, Field M, Maroon JC. Posttraumatic migraine characteristics in athletes following sports-related concussion. J Neurosurg. 2005;102:850–855. doi: 10.3171/jns.2005.102.5.0850. [DOI] [PubMed] [Google Scholar]
- Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- Milroy G, Dorris L, McMillan TM. Sleep disturbances following mild traumatic brain injury in childhood. J Pediatr Psychol. 2008;33:242–247. doi: 10.1093/jpepsy/jsm099. [DOI] [PubMed] [Google Scholar]
- Morrison SD. The relationship of energy expenditure and spontaneous activity to the aphagia of rats with lesions in the lateral hypothalamus. J Physiol. 1968;197:325–343. doi: 10.1113/jphysiol.1968.sp008562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysiw WJ, Corrigan JD, Gribble MW. The ataxic subgroup: a discrete outcome after traumatic brain injury. Brain Inj. 1990;4:247–255. doi: 10.3109/02699059009026174. [DOI] [PubMed] [Google Scholar]
- Nakao R, Ozaki E, Hasegawa M, Kondo A, Uezu K, Hirasaka K, Nikawa T, Kishi K. Distinct effects of anterior pyriform cortex and the lateral hypothalamus lesions on protein intake in rats. J Med Invest. 2007;54:255–260. doi: 10.2152/jmi.54.255. [DOI] [PubMed] [Google Scholar]
- Nance ML, Polk-Williams A, Collins MW, Wiebe DJ. Neurocognitive evaluation of mild traumatic brain injury in the hospitalized pediatric population. Ann Surg. 2009;249:859–863. doi: 10.1097/SLA.0b013e3181a41ae5. [DOI] [PubMed] [Google Scholar]
- Ness-Abramof R, Apovian CM. Drug-induced weight gain. Drugs Today (Barc) 2005;41:547–555. doi: 10.1358/dot.2005.41.8.893630. [DOI] [PubMed] [Google Scholar]
- Ng HK, Mahaliyana RD, Poon WS. The pathological spectrum of diffuse axonal injury in blunt head trauma: assessment with axon and myelin strains. Clin Neurol Neurosurg. 1994;96:24–31. doi: 10.1016/0303-8467(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Nicholl J, LaFrance WC., Jr Neuropsychiatric sequelae of traumatic brain injury. Semin Neurol. 2009;29:247–255. doi: 10.1055/s-0029-1223878. [DOI] [PubMed] [Google Scholar]
- Health NIo., editor. NINDS. Traumatic Brain Injury. Traumatic Brain Injuty; 2012a. [Google Scholar]
- Health NIo., editor. NINDS. Traumatic Brain Injury Reseach. 2012b. [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Nunn N, Womack M, Dart C, Barrett-Jolley R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr Neuropharmacol. 2011;9:262–277. doi: 10.2174/157015911795596531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka H, Kako M, Matsushima M, Ando K. Traumatic spreading depression syndrome. Review of a particular type of head injury in 37 patients. Brain. 1977;100:287–298. doi: 10.1093/brain/100.2.287. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br J Neurosurg. 2002;16:220–242. doi: 10.1080/02688690220148824. [DOI] [PubMed] [Google Scholar]
- Onate JA, Beck BC, Van Lunen BL. On-field testing environment and balance error scoring system performance during preseason screening of healthy collegiate baseball players. J Athl Train. 2007;42:446–451. [PMC free article] [PubMed] [Google Scholar]
- Otte A, Goetze M, Mueller-Brand J. Statistical parametric mapping in whiplash brain: is it only a contusion mechanism? Eur J Nucl Med. 1998;25:306–307. [PubMed] [Google Scholar]
- Ouellet MC, Savard J, Morin CM. Insomnia following traumatic brain injury: a review. Neurorehabil Neural Repair. 2004;18:187–198. doi: 10.1177/1545968304271405. [DOI] [PubMed] [Google Scholar]
- Packard RC, Ham LP. Pathogenesis of posttraumatic headache and migraine: a common headache pathway? Headache. 1997;37:142–152. doi: 10.1046/j.1526-4610.1997.3703142.x. [DOI] [PubMed] [Google Scholar]
- Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after traumatic brain injury. Arch Phys Med Rehabil. 2006;87:278–285. doi: 10.1016/j.apmr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Park E, Ai J, Baker AJ. Cerebellar injury: clinical relevance and potential in traumatic brain injury research. Prog Brain Res. 2007;161:327–338. doi: 10.1016/S0079-6123(06)61023-6. [DOI] [PubMed] [Google Scholar]
- Park JH, Park SW, Kang SH, Nam TK, Min BK, Hwang SN. Detection of traumatic cerebral microbleeds by susceptibility-weighted image of MRI. J Korean Neurosurg Soc. 2009;46:365–369. doi: 10.3340/jkns.2009.46.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsford J. Sexual changes associated with traumatic brain injury. Neuropsychol Rehabil. 2003;13:275–289. doi: 10.1080/09602010244000363. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A. Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J Neurotrauma. 2011;28:937–946. doi: 10.1089/neu.2010.1516. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, O'Connor PM, Broglio SP, Hillman CH. The association between mild traumatic brain injury history and cognitive control. Neuropsychologia. 2009;47:3210–3216. doi: 10.1016/j.neuropsychologia.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Potts MB, Adwanikar H, Noble-Haeusslein LJ. Models of traumatic cerebellar injury. Cerebellum. 2009;8:211–221. doi: 10.1007/s12311-009-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JM, Ferraro JV, Dikmen SS, Temkin NR, Bell KR. Accuracy of mild traumatic brain injury diagnosis. Arch Phys Med Rehabil. 2008;89:1550–1555. doi: 10.1016/j.apmr.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, Renshaw P, Burstein R, Borsook D. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain. 2009;5:34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hales A, Reger M, Giza CC, Hovda DA. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci. 2010;32:510–518. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097-1089. [DOI] [PubMed] [Google Scholar]
- Rangel-Castilla L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26:521–541. doi: 10.1016/j.ncl.2008.02.003. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell AM, Faruqui RA. Persistent hyperphagia in acquired brain injury; an observational case study of patients receiving inpatient rehabilitation. Brain Inj. 2010;24:1044–1049. doi: 10.3109/02699052.2010.489795. [DOI] [PubMed] [Google Scholar]
- Ruff RM. Mild traumatic brain injury and neural recovery: rethinking the debate. NeuroRehabilitation. 2011;28:167–180. doi: 10.3233/NRE-2011-0646. [DOI] [PubMed] [Google Scholar]
- Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LM, Warden DL. Post concussion syndrome. Int Rev Psychiatry. 2003;15:310–316. doi: 10.1080/09540260310001606692. [DOI] [PubMed] [Google Scholar]
- Sakas DE, Whitwell HL. Neurological episodes after minor head injury and trigeminovascular activation. Med Hypotheses. 1997;48:431–435. doi: 10.1016/s0306-9877(97)90042-6. [DOI] [PubMed] [Google Scholar]
- Samuels MA. The brain-heart connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- Sarmiento K, Mitchko J, Klein C, Wong S. Evaluation of the Centers for Disease Control and Prevention's concussion initiative for high school coaches: "Heads Up: Concussion in High School Sports". J Sch Health. 2010;80:112–118. doi: 10.1111/j.1746-1561.2010.00491.x. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schutze M, Kundt G, Buchholz K, Piek J. [Which factors are predictive for long-term complaints after mild traumatic brain injuries?] Versicherungsmedizin. 2008;60:78–83. [PubMed] [Google Scholar]
- Schwarz A. Congress Considers Concussion Protections. New York Times. 2010 [Google Scholar]
- Seifert TD, Evans RW. Posttraumatic headache: a review. Curr Pain Headache Rep. 2010;14:292–298. doi: 10.1007/s11916-010-0117-7. [DOI] [PubMed] [Google Scholar]
- Shaffer L, Rich PM, Pohl KR, Ganesan V. Can mild head injury cause ischaemic stroke? Arch Dis Child. 2003;88:267–269. doi: 10.1136/adc.88.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, Powell JH, Counsell SJ, Patel MC, Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Ham TE. Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol. 2011;24:558–563. doi: 10.1097/WCO.0b013e32834cd523. [DOI] [PubMed] [Google Scholar]
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol. 2002;67:281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Sheftell FD, Tepper SJ, Lay CL, Bigal ME. Post-traumatic headache: emphasis on chronic types following mild closed head injury. Neurol Sci. 2007;28(Suppl 2):S203–S207. doi: 10.1007/s10072-007-0777-1. [DOI] [PubMed] [Google Scholar]
- Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SM. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010;74:1732–1738. doi: 10.1212/WNL.0b013e3181e0438b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma. 2012;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Macfabe DF, Foley KA, Taylor R, Cain DP. Sub-concussive brain injury in the Long-Evans rat induces acute neuroinflammation in the absence of behavioral impairments. Behav Brain Res. 2011;229:145–152. doi: 10.1016/j.bbr.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir S, Andelic N, Roe C, Schanke AK. Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J Int Neuropsychol Soc. 2009;15:740–750. doi: 10.1017/S1355617709990452. [DOI] [PubMed] [Google Scholar]
- Siopi E, Cho AH, Homsi S, Croci N, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline Restores sAPPalpha Levels and Reduces the Late Histopathological Consequences of Traumatic Brain Injury in Mice. J Neurotrauma. 2011;28:2135–2143. doi: 10.1089/neu.2010.1738. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Zhang K, Johnson BD, Gay M, Sebastianelli W, Hallett M, Horovitz S. Default Mode Network in Concussed Individuals in Response to YMCA Physical Stress Test. J Neurotrauma. 2011 doi: 10.1089/neu.2011.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov SM, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp Brain Res. 2010;202:341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M, Houston GC, Dippel DW, Wielopolski PA, Vernooij MW, Koudstaal PJ, Hunink MG, van der Lugt A. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2011;53:553–563. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobani ZA, Ali A. Pediatric traumatic putamenal strokes: Mechanisms and prognosis. Surg Neurol Int. 2011;2:51. doi: 10.4103/2152-7806.80116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S. John Graham Senior Clinicians Award Lecture. Posttraumatic migraine. Headache. 1998;38:772–778. doi: 10.1046/j.1526-4610.1998.3810772.x. [DOI] [PubMed] [Google Scholar]
- Spaethling JM, Klein DM, Singh P, Meaney DF. Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J Neurotrauma. 2008;25:1207–1216. doi: 10.1089/neu.2008.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos GK, Wilde EA, Bigler ED, Cleavinger HB, Fearing MA, Levin HS, Li X, Hunter JV. cerebellar atrophy after moderate-to-severe pediatric traumatic brain injury. AJNR Am J Neuroradiol. 2007;28:537–542. [PMC free article] [PubMed] [Google Scholar]
- Sroufe NS, Fuller DS, West BT, Singal BM, Warschausky SA, Maio RF. Postconcussive symptoms and neurocognitive function after mild traumatic brain injury in children. Pediatrics. 2010;125:e1331–e1339. doi: 10.1542/peds.2008-2364. [DOI] [PubMed] [Google Scholar]
- Staal JA, Vickers JC. Selective vulnerability of non-myelinated axons to stretch injury in an in vitro co-culture system. J Neurotrauma. 2011;28:841–847. doi: 10.1089/neu.2010.1658. [DOI] [PubMed] [Google Scholar]
- Standaert CJ, Herring SA, Cantu RC. Expert opinion and controversies in sports and musculoskeletal medicine: concussion in the young athlete. Arch Phys Med Rehabil. 2007;88:1077–1079. doi: 10.1016/j.apmr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R. 2011;3:S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Stulemeijer M, Vos PE, van der Werf S, van Dijk G, Rijpkema M, Fernandez G. How mild traumatic brain injury may affect declarative memory performance in the post-acute stage. J Neurotrauma. 2010;27:1585–1595. doi: 10.1089/neu.2010.1298. [DOI] [PubMed] [Google Scholar]
- Su DH, Chang YC, Chang CC. Post-traumatic anterior and posterior pituitary dysfunction. J Formos Med Assoc. 2005;104:463–467. [PubMed] [Google Scholar]
- Suskauer SJ. The promise of evolving neuroimaging techniques for pediatric Traumatic Brain Injury. J Pediatr Rehabil Med. 2009;2:249–253. doi: 10.3233/PRM-2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer SJ, Huisman TA. Neuroimaging in pediatric traumatic brain injury: current and future predictors of functional outcome. Dev Disabil Res Rev. 2009;15:117–123. doi: 10.1002/ddrr.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage TM, Nauman E, Breedlove EL, Yoruk U, Dye AE, Morigaki K, Feuer H, Leverenz LJ. Functionally-Detected Cognitive Impairment in High School Football Players Without Clinically-Diagnosed Concussion. J Neurotrauma. 2010 doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol. 2012;233:364–372. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Ge Y, Sodickson DK, Miles L, Zhou Y, Reaume J, Grossman RI. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260:831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FR. Weight change associated with the use of migraine-preventive medications. Clin Ther. 2008;30:1069–1080. doi: 10.1016/j.clinthera.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Theeler BJ, Erickson JC. Mild head trauma and chronic headaches in returning US soldiers. Headache. 2009;49:529–534. doi: 10.1111/j.1526-4610.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Theeler BJ, Flynn FG, Erickson JC. Headaches after concussion in US soldiers returning from Iraq or Afghanistan. Headache. 2010;50:1262–1272. doi: 10.1111/j.1526-4610.2010.01700.x. [DOI] [PubMed] [Google Scholar]
- Theriault M, De Beaumont L, Gosselin N, Filipinni M, Lassonde M. Electrophysiological abnormalities in well functioning multiple concussed athletes. Brain Inj. 2009;23:899–906. doi: 10.1080/02699050903283189. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Hoover RC, Tkacs NC, Saatman KE, McIntosh TK. Development of posttraumatic hyperthermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J Cereb Blood Flow Metab. 2005a;25:163–176. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Sowell ER, Gogtay N, Giedd JN, Vidal CN, Hayashi KM, Leow A, Nicolson R, Rapoport JL, Toga AW. Structural MRI and brain development. Int Rev Neurobiol. 2005b;67:285–323. doi: 10.1016/S0074-7742(05)67009-2. [DOI] [PubMed] [Google Scholar]
- Thor PJ, Goscinski I, Kolasinska-Kloch W, Madroszkiewicz D, Madroszkiewicz E, Furgala A. Gastric myoelectric activity in patients with closed head brain injury. Med Sci Monit. 2003;9:CR392–CR395. [PubMed] [Google Scholar]
- Thorley RR, Wertsch JJ, Klingbeil GE. Acute hypothalamic instability in traumatic brain injury: a case report. Arch Phys Med Rehabil. 2001;82:246–249. doi: 10.1053/apmr.2001.18698. [DOI] [PubMed] [Google Scholar]
- Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ. 2000;320:1631–1635. doi: 10.1136/bmj.320.7250.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagarakis S, Tzanela M, Dimopoulou I. Diabetes insipidus, secondary hypoadrenalism and hypothyroidism after traumatic brain injury: clinical implications. Pituitary. 2005;8:251–254. doi: 10.1007/s11102-006-6049-x. [DOI] [PubMed] [Google Scholar]
- Tsushima WT, Oshiro R, Zimbra D. Neuropsychological test performance of Hawai'i high school athletes: Hawai'i ImPACT normative data. Hawaii Med J. 2008;67:93–95. [PubMed] [Google Scholar]
- Umile EM, Sandel ME, Alavi A, Terry CM, Plotkin RC. Dynamic imaging in mild traumatic brain injury: support for the theory of medial temporal vulnerability. Arch Phys Med Rehabil. 2002;83:1506–1513. doi: 10.1053/apmr.2002.35092. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, Ria A, Marziale S, Zoccatelli G, Tavazzi B, Del Bolgia F, Sorge R, Broglio SP, McIntosh TK, Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, Tarascio G, Amorini AM, Di Pietro V, Delfini R, Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes--part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. discussion 1295-1286. [DOI] [PubMed] [Google Scholar]
- Vos PE, Battistin L, Birbamer G, Gerstenbrand F, Potapov A, Prevec T, Stepan Ch A, Traubner P, Twijnstra A, Vecsei L, von Wild K. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207–219. doi: 10.1046/j.1468-1331.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamm RJ, Povlishock JT. Traumatic axonal injury in the optic nerve: evidence for axonal swelling, disconnection, dieback, and reorganization. J Neurotrauma. 2011;28:1185–1198. doi: 10.1089/neu.2011.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NF, Dikmen S, Machamer J, Doherty M, Temkin N. Hypersomnia following traumatic brain injury. J Clin Sleep Med. 2007;3:363–368. [PMC free article] [PubMed] [Google Scholar]
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Wetjen NM, Pichelmann MA, Atkinson JL. Second impact syndrome: concussion and second injury brain complications. J Am Coll Surg. 2010;211:553–557. doi: 10.1016/j.jamcollsurg.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, Chia J, Levin HS. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Newsome MR, Bigler ED, Pertab J, Merkley TL, Hanten G, Scheibel RS, Li X, Chu Z, Yallampalli R, Hunter JV, Levin HS. Brain imaging correlates of verbal working memory in children following traumatic brain injury. Int J Psychophysiol. 2011;82:86–96. doi: 10.1016/j.ijpsycho.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Lazic SE, Ogilvie RD. Polysomnographic and quantitative EEG analysis of subjects with long-term insomnia complaints associated with mild traumatic brain injury. Clin Neurophysiol. 2008;119:429–438. doi: 10.1016/j.clinph.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, Kiragu A, Lim KO. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, Chu Z, Li X, Hanten G, Hunter JV, Levin HS. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan EB, Hellewell SC, Bellander BM, Agyapomaa DA, Morganti-Kossmann MC. Post-traumatic hypoxia exacerbates neurological deficit, neuroinflammation and cerebral metabolism in rats with diffuse traumatic brain injury. J Neuroinflammation. 2011;8:147. doi: 10.1186/1742-2094-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Kuo CC. Inhibition of Na(+) current by imipramine and related compounds: different binding kinetics as an inactivation stabilizer and as an open channel blocker. Mol Pharmacol. 2002;62:1228–1237. doi: 10.1124/mol.62.5.1228. [DOI] [PubMed] [Google Scholar]
- Yeo RA, Phillips JP, Jung RE, Brown AJ, Campbell RC, Brooks WM. Magnetic resonance spectroscopy detects brain injury and predicts cognitive functioning in children with brain injuries. J Neurotrauma. 2006;23:1427–1435. doi: 10.1089/neu.2006.23.1427. [DOI] [PubMed] [Google Scholar]
- Yeoh HK, Lind CR, Law AJ. Acute transient cerebellar dysfunction and stuttering following mild closed head injury. Childs Nerv Syst. 2006;22:310–313. doi: 10.1007/s00381-005-1154-0. [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Pekdemir M, Sarisoy HT, Yaka E. Post-traumatic cerebral infarction: a rare complication in a pediatric patient after mild head injury. Ulus Travma Acil Cerrahi Derg. 2011;17:186–188. [PubMed] [Google Scholar]
- Yoshida J, Shiroozu A, Zaitsu A, Imazono Y, Kohrogi T, Yokohata K, Kishikawa H. Diabetes insipidus after traumata of two extremes in severity. Yonsei Med J. 1990;31:71–73. doi: 10.3349/ymj.1990.31.1.71. [DOI] [PubMed] [Google Scholar]
- Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM. Lithium Ameliorates Neurodegeneration, Suppresses Neuroinflammation, and Improves Behavioral Performance in a Mouse Model of Traumatic Brain Injury. J Neurotrauma. 2011 doi: 10.1089/neu.2011.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res. 2010a;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010b;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


